Abstract
This review synthesizes current knowledge on the roles of X-box binding protein 1 (XBP1) in development and regenerative medicine. XBP1 is defined as a key transcription factor that regulates biological processes from embryogenesis to adult tissue homeostasis via both endoplasmic reticulum(ER) stress-dependent and independent mechanisms. Evidence for its regulatory role in cell fate determination and tissue maintenance across multiple systems is presented. The therapeutic potential of targeting XBP1 is explored, particularly for the regeneration of skeletal muscle, skin, and bone. Critical future research priorities are outlined, such as deciphering the precise functions of the Inositol requiring enzyme 1 (IRE1α)/XBP1 signaling axis and evaluating the long-term safety of its modulation. XBP1 is thus confirmed as a prime target for advancing developmental biology and pioneering new regenerative therapies.
1. Introduction
The precise regulation of developmental processes and the extraordinary capacity for tissue regeneration represent fundamental biological phenomena with far-reaching implications for both basic science and clinical medicine [1,2]. These processes are governed by intricate molecular networks and regulatory mechanisms that coordinate cell fate determination, tissue homeostasis, and regenerative potential [3,4]. At the core of these networks, individual transcription factors exhibit context-dependent regulatory versatility—they can bind to multiple genomic loci, while the cis-regulatory function of enhancers requires combinatorial binding of diverse transcription factors [5]. This cooperative binding paradigm enables genes to receive sophisticated spatiotemporal-specific regulation. Among the key transcription factors, X-box binding protein 1 (XBP1) has emerged as a critical focus of recent research due to its multifaceted roles in development and regeneration [6,7].
XBP1 is a highly conserved mammalian transcription factor belonging to the basic leucine zipper (bZIP) family. As a nodal regulator, XBP1 operates at multiple hierarchical levels—from orchestrating early lineage commitment through direct transcriptional control [6], to maintaining proteostasis via its canonical unfolded protein response (UPR) function during rapid tissue expansion [8,9]. The Xbp1 gene undergoes unique post-transcriptional splicing that generates two functionally distinct isoforms (Figure 1), illustrating how alternative splicing converts the latent XBP1U isoform into the potent transcriptional activator XBP1S [10].
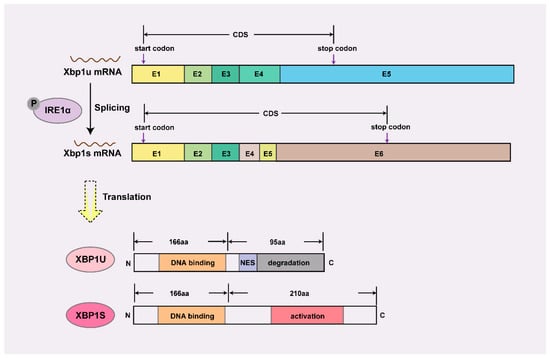
Figure 1.
Schematic diagram of mRNA and protein structure of Xbp1. XBP1U retains a DNA-binding domain but is sequestered in the cytoplasm by its C-terminal nuclear export signal (NES) and targeted for proteasomal degradation via a degradation domain, rendering it transcriptionally inactive. In contrast, XBP1S acquires a C-terminal transcriptional activation domain through alternative splicing, enabling nuclear localization and target gene regulation, thus serving as the dominant functional isoform. E, exon; CDS, coding sequence; P, phosphorylation; N, amino terminus; C, carboxy terminus.
This review synthesizes current knowledge on XBP1’s pleiotropic functions in developmental biology, with particular emphasis on its molecular mechanisms of action; tissue-specific regulatory networks; and translational potential in regenerative medicine. Through critical analysis of existing literature, we aim to provide a conceptual framework for understanding XBP1’s integrative roles in development and its emerging therapeutic applications.
2. The Regulatory Role of XBP1 in Organogenesis and Tissue Homeostasis
While early investigations primarily focused on XBP1’s canonical role in UPR-mediated stress responses, emerging evidence suggests more diverse functions in development and tissue maintenance that extend beyond its classical ER stress-related activities (Figure 2A) [8,11,12]. As illustrated in Figure 2B, XBP1 orchestrates key developmental processes including neural differentiation, heart ventricle growth, immune cell development, chondrocyte differentiation, mammary gland function, and liver metabolism. Mechanistically, XBP1 operates through two parallel pathways: during organogenesis, it predominantly utilizes ER stress-independent mechanisms to direct lineage specification (e.g., neuronal commitment, osteoblast maturation), except in secretory tissues where ER biogenesis is obligatory (Table 1). Conversely, tissue homeostasis relies principally on its ER stress-dependent activity, particularly in combating proteotoxicity in neurodegeneration and maintaining metabolic balance (Table 2).
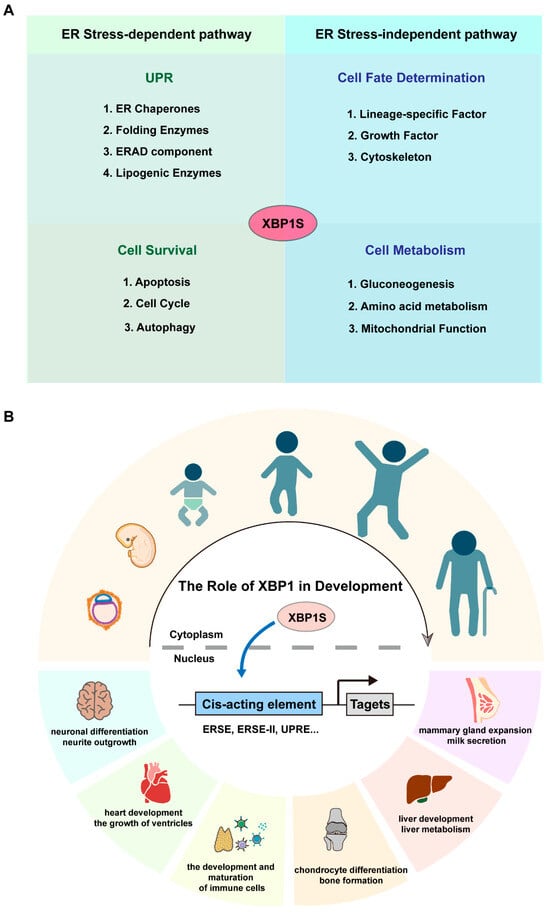
Figure 2.
The role of XBP1 in the development. (A) Schematic representation of XBP1’s regulatory mechanisms. ER, endoplasmic reticulum; ERAD, ER-associated degradation. (B) Schematic representation of XBP1’s developmental roles, showcasing its impact on processes such as neural differentiation, heart ventricle growth, immune cell development, chondrocyte differentiation, mammary gland function, and liver metabolism. ERSE, Endoplasmic Reticulum Stress Response Element; ERSE-II, Endoplasmic Reticulum Stress Response Element-II; UPRE, Unfolded Protein Response Element.

Table 1.
The role of Xbp1 in the development of different systems.

Table 2.
The role of Xbp1 in tissue homeostasis.
The selection between ER stress-dependent and independent activation of XBP1 is governed by a spectrum of contextual cellular cues. Key among these is the nature and intensity of extrinsic stimuli—for instance, varying degrees of viral infection or metabolic stress can preferentially engage one pathway over the other. Furthermore, cell-type-specific signaling environments significantly influence this choice; immune cells may utilize different regulatory networks compared to neurons, even under similar stimuli. Another crucial layer of regulation arises from crosstalk with other major signaling pathways, such as NF-κB, HIF-1α, and autophagy, which can directly or indirectly modulate XBP1 activity. Notably, certain plasma membrane receptor signaling pathways can activate UPR sensors, including IRE1α, independently of classical ER stress, thereby engaging XBP1 in a non-canonical manner [36]. Additionally, post-translational modifications of XBP1 and its interactors (e.g., phosphorylation, ubiquitination) and epigenetic landscapes that determine target gene accessibility provide further fine-tuning, collectively ensuring a precise and context-appropriate cellular response.
2.1. Nervous System
XBP1 plays multifaceted roles in the nervous system, regulating neuronal differentiation, neurite outgrowth, and synapse formation during development (Figure 3). During this period, Xbp1s mRNA is highly expressed, and XBP1S facilitates neurotrophic signaling—particularly of BDNF—between neurites and the nucleus to promote neurite extension [37]. In Xbp1-knockout neurons, the upregulation of GABAergic markers such as somatostatin (Sst), neuropeptide Y (Npy), and calbindin (Calb1) is suppressed, impairing BDNF-induced neurite outgrowth [13]. Consistent with a developmental role, nervous system-specific Xbp1 knockout in mice leads to deficits in hippocampal contextual memory and long-term potentiation, with accompanying cognitive decline [14]. Conversely, constitutive neuronal expression of XBP1S induces recurrent spontaneous seizures and premature lethality [38], indicating that precise regulation of XBP1 activity is critical for normal neural circuit maturation. These findings collectively demonstrate that XBP1’s functions in neural development are largely independent of endoplasmic reticulum (ER) stress. Despite the absence of documented human neurodevelopmental disorders linked to XBP1 mutations, evidence from expression analyses and conditional knockout models underscores its importance in neural development.
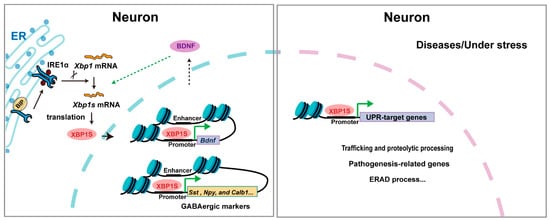
Figure 3.
The mechanism of XBP1 in neurons. Triangular arrows: positive regulation or promoting effects.
Transitioning from development to adult homeostasis, XBP1 continues to exert important functions and is implicated in the pathogenesis of diverse psychiatric and neurodegenerative disorders [39]. Recent research indicates that early senescent neurons confer neuroprotection against age-related decline by transferring heat shock proteins via extracellular vesicles to activate the IRE1α-XBP1 pathway and upregulate chondroitin synthase in glial cells [40]. In neurodegenerative diseases—such as Alzheimer’s (AD), Parkinson’s (PD), Huntington’s (HD), and amyotrophic lateral sclerosis (ALS)—synaptic dysfunction and neuronal death are linked to proteostasis disruption. Here, XBP1 exhibits context-dependent roles: in a C. elegans AD model, UPR activation aggravates Aβ toxicity, whereas XBP1 overexpression in murine AD models reduces aberrant protein aggregation and attenuates disease progression [25,41]. In PD, XBP1 supports dopaminergic neuron survival under both physiological and pathological conditions [26]. Paradoxically, Xbp1-deficient mice show slowed disease progression in models of ALS (Sod1 mutation) and HD [12,27], suggesting a complex, disease-specific involvement. Beyond neurodegeneration, XBP1 influences central metabolic regulation; constitutive expression of XBP1S in POMC neurons—key regulators of energy balance—restores body weight homeostasis in obese mice [29]. While direct evidence from human mutations linking XBP1 to common neurodegenerative diseases is still emerging, its central role in proteostatic and metabolic maintenance highlights its potential therapeutic relevance.
2.2. Cardiovascular System
XBP1 serves as a master regulator orchestrating cardiac development and maturation (Figure 4). During cardiac morphogenesis, XBP1 governs critical developmental events—its ablation in mice leads to profound structural defects including ventricular wall hypoplasia, impaired trabeculation, and disrupted septal formation, ultimately resulting in embryonic lethality [15]. Intriguingly, the postnatal heart exhibits chamber-specific XBP1 activation patterns, with predominant left ventricular expression driving its functional maturation and hemodynamic dominance [42]. While cardiomyocyte-specific Xbp1 knockout mice are viable at birth, they develop progressive contractile dysfunction and premature mortality, revealing XBP1’s essential role in maintaining cardiac homeostasis beyond development [30]. Although direct evidence of XBP1 mutations causing human congenital heart defects remains limited, its conserved expression and functional necessity across species highlight its potential contribution to cardiovascular developmental pathways.
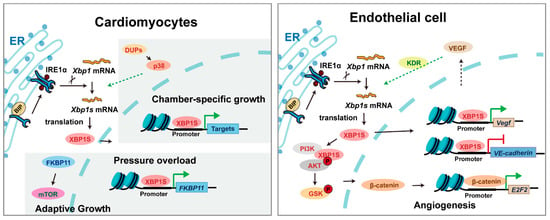
Figure 4.
The mechanism of XBP1 in cardiomyocytes and vascular endothelial cells. Triangular arrows: positive regulation or promoting effects; flat-headed arrows: negative regulation or inhibitory effects.
Beyond development, XBP1 is a critical mediator of cardiovascular adaptation under pathological stress. In conditions such as ischemia, pressure overload, or heart failure, XBP1 is robustly activated and mediates adaptive remodeling through coordinated regulation of hypertrophy, metabolism, and survival pathways. Genetic studies demonstrate its protective role, as cardiac-specific Xbp1 deficiency exacerbates dysfunction while its activation preserves contractility [30,43]. In the vasculature, XBP1 exhibits context-dependent regulation of angiogenesis—it is indispensable for VEGF-mediated neovascularization during development and ischemic repair [16,44], yet chronic activation triggers excessive autophagy and endothelial apoptosis, paradoxically promoting atherosclerotic progression [31]. These dual roles underscore XBP1’s complex integration of developmental programs and stress responses in cardiovascular biology. Emerging clinical associations suggest that specific XBP1 polymorphisms may influence susceptibility to hypertension and coronary artery disease in humans, further supporting its translational relevance in cardiovascular homeostasis and pathology.
2.3. Immune System
XBP1 plays a stage-specific role in adaptive immunity, with its functions becoming particularly critical under conditions of cellular stress (Figure 5). While T cell development proceeds normally in Xbp1 knockout mice, thymic defects are markedly exacerbated when combined with SEL1L deficiency, revealing XBP1’s compensatory role in ERAD-impaired conditions [32,45]. In mature T cells, XBP1 regulates subset differentiation through distinct mechanisms: (1) XBP1S drives Th2 responses via enhanced proliferation and cytokine production [33,46], (2) stress-induced XBP1 promotes Th17 differentiation in autoimmunity [47], and (3) XBP1S enhances CD8+ effector function through upregulation of killer cell lectin-like receptor G1 [48]. Although direct evidence of XBP1 mutations causing human T-cell immunodeficiencies is currently lacking, its conserved role in T-cell stress adaptation suggests potential relevance to immune dysregulation syndromes.
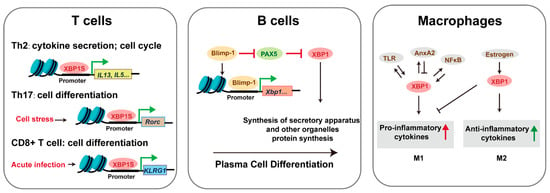
Figure 5.
The mechanism of XBP1 in immune cells. Triangular arrows: positive regulation or promoting effects; flat-headed arrows: negative regulation or inhibitory effects.
In the humoral arm of immunity, XBP1 is indispensable for terminal B-cell differentiation. It mediates organelle biogenesis required for plasma cell function and antibody secretion [49]. While Xbp1 deficiency permits initial plasmablast formation, it severely impairs bone marrow homing and sustained antibody production [17], whereas forced XBP1 expression significantly boosts immunoglobulin output [50]. NK cells similarly require XBP1 for effective anti-tumor and anti-infection responses. Among innate immune cells, TLR-activated XBP1 splicing serves as a key modulator of inflammatory responses [51]. Macrophages exhibit context-dependent responses to XBP1 activation: transient XBP1S induces autophagy and proliferation, while chronic expression triggers apoptosis [52]. The P300/XBP1S/Herpud1 axis has been identified as a driver of M2 polarization in pathological contexts such as macular degeneration [53]. Additionally, XBP1 deficiency reduces dendritic cell survival—a defect reversible by progenitor cell overexpression [18]—and selectively impairs eosinophil differentiation through disruption of secretory granule formation, without affecting neutrophil or basophil development [19]. These findings across lymphoid and myeloid lineages highlight XBP1 as a central regulator of immune cell development, function, and stress adaptation, with emerging implications for understanding human immune pathologies.
2.4. Skeletal System
XBP1 plays context-dependent roles in skeletal development, with distinct mechanisms governing chondrogenesis and osteogenesis (Figure 6). During BMP2-induced chondrocyte differentiation, IRE1α activation promotes Xbp1 mRNA splicing, upregulating granulin precursor protein to facilitate endochondral ossification [54,55]. Chondrocyte-specific Xbp1 knockout mice exhibit transient dwarfism and chondrodysplasia during development, which normalizes in adulthood despite IRE1α overactivation without triggering decay pathways [21]. Paradoxically, excessive Xbp1 expression also impairs cartilage development by inducing ER stress-mediated suppression of growth plate chondrocyte proliferation [56]. A regulatory network involving LncRNA/circRNA-miRNA interactions in Xbp1-deficient chondrocytes offers potential therapeutic targets for cartilage disorders [57]. While direct evidence of XBP1 mutations causing human skeletal dysplasia remains limited, its conserved role in cartilage development suggests potential relevance to chondrodysplasia phenotypes.
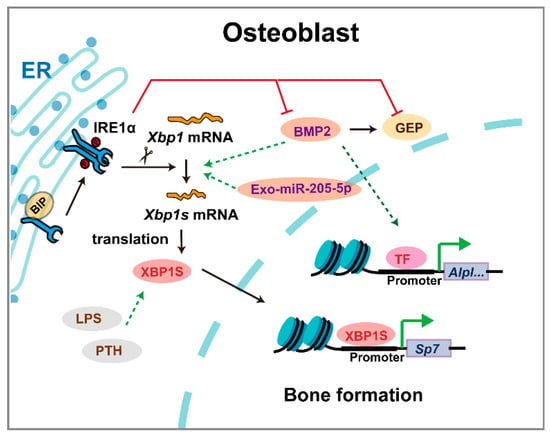
Figure 6.
The mechanism of XBP1 in osteoblasts. Triangular arrows: positive regulation or promoting effects; flat-headed arrows: negative regulation or inhibitory effects.
Beyond developmental patterning, XBP1 continues to regulate skeletal homeostasis through integrated stress response pathways. In osteoblast differentiation, Xbp1 deficiency specifically impairs late-stage maturation by suppressing Osx transcription, without affecting early differentiation [20]. IRE1α, XBP1, and BMP2 form a feedback loop fine-tuning osteogenesis [58]. Notably, chaperone protein BIP upregulation ameliorates bone loss in osteoporosis models, underscoring XBP1’s role in maintaining osteoblast viability under stress [59]. Although Xbp1−/−; LivXBP1 mice complete ossification, mineralization remains deficient [8]. XBP1 also regulates craniofacial homeostasis, with genome-wide analyses identifying it as critical for temporomandibular joint (TMJ) function and regeneration [60]. Furthermore, XBP1 emerges as a shared regulator in fibrocartilage formation and heterotopic ossification, suggesting therapeutic potential for tendon-bone interface healing [61]. These findings establish XBP1 as a key modulator of both skeletal development and maintenance, with implications for understanding and treating various bone and cartilage disorders.
2.5. Exocrine Glands
XBP1 is a master regulator of ER biogenesis and chaperone expression, essential for meeting the high protein-folding demands of exocrine glands [62,63]. Xbp1−/−; LivXBP1 mice exhibit severe postnatal growth retardation and early lethality due to pancreatic hypoplasia and deficient digestive enzyme production [8]. This phenotype is linked to acinar cell apoptosis at E18.5, though XBP1 is dispensable for endocrine pancreas development and function [8]. In salivary glands, Xbp1 expression increases during acinar cell maturation, and its loss reduces ER volume and disrupts lobular architecture [8,55]. Single-cell RNA-seq confirms XBP1 as a top transcriptional correlate of acinar development [55].
Similarly, the mammary gland—a specialized exocrine organ—relies on XBP1 for structural and functional adaptation during lactation. Epithelial-specific knockout models (hGFAP-Cre or BLG-Cre; Xbp1fl/fl) reveal that Xbp1 loss impairs ductal branching and bud formation in virgin mice, induces stromal fibrosis, and causes chronic ER stress during lactation, suppressing proliferation and differentiation without apoptosis [9,24]. Consequently, Xbp1-deficient mice show reduced milk synthesis, impaired pup growth, and increased mortality [9]. Notably, Xbp1 deletion reduces ER content by pregnancy day 18 and blocks alveolar expansion, leaving persistent adipocytes [24]. These results affirm XBP1 as a central coordinator of mammary epithelial integrity, ER homeostasis, and secretory capacity throughout reproductive stages.
3. The Promises and Challenges of XBP1 in Regeneration
XBP1 serves as a master coordinator of regenerative processes across multiple organ systems through distinct yet interconnected mechanisms [7,64]. In skeletal muscle, the IRE1α-XBP1 axis promotes myoblast fusion via Mymk activation and satellite cell proliferation, with both genetic and therapeutic studies demonstrating its essential role in muscle repair [7,64]. These findings position XBP1 modulation as a promising therapeutic strategy for treating muscle degenerative diseases such as Duchenne muscular dystrophy and age-related sarcopenia. For cutaneous wound healing, XBP1S enhances tissue regeneration by upregulating critical growth factors (PDGF-BB, TGF-β3) [65], stimulating collagen synthesis through β-catenin signaling [66], and promoting angiogenesis [16,44,67,68]. The demonstrated efficacy of topical XBP1 activators in preclinical models of diabetic ulcers suggests significant translational potential for chronic wound management. The protein similarly drives bone regeneration by enhancing the osteogenic potential of periodontal ligament cells [69] and facilitating chondrogenic differentiation of mesenchymal stem cells [70,71]. This mechanistic understanding provides a foundation for developing XBP1-targeted therapies to accelerate fracture healing and treat osteoporosis. Beyond these established roles, emerging evidence reveals XBP1’s involvement in liver regeneration post-hepatectomy [72], neuroprotection through serotonin pathway modulation [73], and metabolic regulation via obesity reversal and insulin sensitivity restoration [74]. The breadth of these regenerative functions highlights XBP1’s potential as a multi-organ therapeutic target, though tissue-specific delivery systems will be crucial for clinical application.
As illustrated in Figure 7, XBP1 orchestrates multiple regenerative processes through three key mechanisms: enhancing cellular adaptive survival pathways, directing progenitor cell fate decisions, and remodeling the tissue microenvironment to facilitate repair. From a therapeutic perspective, these mechanisms offer multiple intervention points—from small molecule IRE1α/XBP1 pathway modulators to gene therapy approaches—each with distinct clinical implications. While preliminary findings highlight XBP1’s regenerative potential, critical knowledge gaps remain that require systematic investigation. First, tissue-specific mechanistic studies employing inducible genetic ablation and pharmacological modulation are needed to delineate the precise role of the IRE1α/XBP1 pathway across different regenerative contexts. These studies should specifically address optimal therapeutic windows and dosage parameters for potential clinical translation. Second, comprehensive safety assessments must address potential risks associated with sustained XBP1 activation, particularly concerning fibrotic complications and oncogenic transformation [75]. The oncogenic potential of sustained XBP1 activation is a significant concern. For instance, XBP1 has been demonstrated to drive tumorigenesis and progression in multiple myeloma by promoting the survival of malignant plasma cells within the hypoxic bone marrow niche [76]. Similarly, its overexpression is linked to poor prognosis in breast cancer, where it facilitates tumor cell proliferation and chemoresistance [77]. Prolonged XBP1 signaling may also contribute to fibrotic pathologies. Evidence from models of liver fibrosis indicates that the IRE1α-XBP1 pathway is persistently activated in fibroblasts, promoting their activation and excessive extracellular matrix deposition [78,79]. This suggests that uncontrolled XBP1 activation could similarly impede regenerative processes by favoring scar tissue formation over functional tissue restoration. Overcoming these challenges through targeted delivery systems and temporal control of XBP1 activity will be essential for realizing its full therapeutic potential.
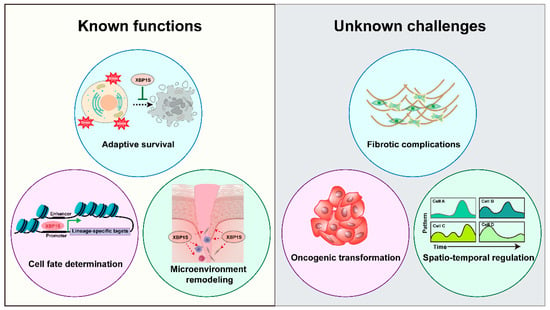
Figure 7.
XBP1 in Regeneration: Mechanisms and Challenges. Schematic representation of XBP1’s dual roles in tissue regeneration, illustrating its established functions in adaptive survival, cell fate determination, and microenvironment remodeling, alongside key challenges including fibrotic complications, oncogenic risks, and spatiotemporal regulation requirements.
Notwithstanding these challenges, XBP1 emerges as a highly promising therapeutic target for regenerative medicine. Future research directions should focus on elucidating spatiotemporal regulation of XBP1 activity during regeneration, developing tissue-selective delivery systems and optimizing activation protocols to maximize regenerative outcomes while minimizing off-target effects. These advances will facilitate translation of XBP1-based therapies into clinical applications for diverse regenerative indications.
4. Conclusions
XBP1 has emerged as a master transcriptional regulator with profound implications for both developmental biology and regenerative medicine. This review systematically synthesizes current understanding of XBP1’s multifaceted roles, elucidating its molecular mechanisms across diverse physiological contexts. Our analysis reveals XBP1’s dual functionality as a crucial developmental modulator governing tissue morphogenesis, and a potent mediator of regenerative processes through stress adaptation and cellular reprogramming. The translational potential of XBP1 is particularly noteworthy, with emerging evidence supporting its therapeutic targeting for enhanced tissue repair. Future investigations should focus on three key areas: first, delineating tissue-specific regulatory networks controlled by XBP1; second, developing precision modulation strategies to harness its regenerative capacity; and third, addressing safety considerations for clinical translation. These efforts promise to advance both fundamental understanding of developmental processes and innovative approaches for treating degenerative diseases and traumatic injuries. Synergistic application of single-cell multi-omics platforms and next-generation organoids promises to unveil the precise spatiotemporal control mechanisms of XBP1 in human tissue regeneration.
Author Contributions
D.H. and F.G.: writing the manuscript. J.M. and Z.C.: review and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Key Program No. 82230029) to Zhi Chen, National Natural Science Foundation of China (No. 62171193) to Jingzhi Ma, Key Research and Development Program of Hubei Province (No. 2022BCA033) to Jingzhi Ma, and China Postdoctoral Science Foundation (Grant Number 2024M761059) to Delan Huang.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article as it is a comprehensive review of previously published literature.
Acknowledgments
No individuals beyond the listed authors require acknowledgment in this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| XBP1 | X-box binding protein 1 |
| IRE1α | Inositol requiring enzyme 1 |
| ER | endoplasmic reticulum |
| bZIP | basic leucine zipper |
| UPR | unfolded protein response |
| NES | nuclear export signal |
| ERAD | ER-associated degradation |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| ALS | amyotrophic lateral sclerosis |
| TMJ | temporomandibular joint |
References
- Sui, B.; Zheng, C.; Zhao, W.; Xuan, K.; Li, B.; Jin, Y. Mesenchymal Condensation in Tooth Development and Regeneration: A Focus on Translational Aspects of Organogenesis. Physiol. Rev. 2023, 103, 1899–1964. [Google Scholar] [CrossRef]
- Moiseeva, A.; Nikolenko, V.; Oganesyan, M.; Nikitina, A.; Rizaeva, N.; Zharikova, T.; Pontes-Silva, A.; Zharikov, Y. Neural Crest Cells: Bridging Embryology and Regenerative Medicine. Neuroscience 2025, 579, 259–266. [Google Scholar] [CrossRef]
- Hénon, P. Key Success Factors for Regenerative Medicine in Acquired Heart Diseases. Stem Cell Rev. Rep. 2020, 16, 441–458. [Google Scholar] [CrossRef]
- Miron, R.; Shirakata, Y.; Ahmad, P.; Romandini, M.; Estrin, N.; Farshidfar, N.; Bosshardt, D.; Sculean, A. 30 Years of Enamel Matrix Derivative: Mimicking Tooth Development as a Clinical Concept. Periodontol. 2000, 2025; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Spitz, F.; Furlong, E. Transcription Factors: From Enhancer Binding to Developmental Control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Chen, H.; Ma, L.; E, W.; Wang, R.; Fang, X.; Zhou, Z.; Sun, H.; Wang, J.; Jiang, M.; et al. Systematic Identification of Cell-Fate Regulatory Programs Using a Single-Cell Atlas of Mouse Development. Nat. Genet. 2022, 54, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.S.; Tomaz da Silva, M.; Roy, A.; Koike, T.E.; Wu, M.; Castillo, M.B.; Gunaratne, P.H.; Liu, Y.; Iwawaki, T.; Kumar, A. The IRE1α/XBP1 Signaling Axis Drives Myoblast Fusion in Adult Skeletal Muscle. EMBO Rep. 2024, 25, 3627–3650. [Google Scholar] [CrossRef]
- Lee, A.; Chu, G.; Iwakoshi, N.; Glimcher, L. XBP-1 Is Required for Biogenesis of Cellular Secretory Machinery of Exocrine Glands. EMBO J. 2005, 24, 4368–4380. [Google Scholar] [CrossRef]
- Hasegawa, D.; Calvo, V.; Avivar-Valderas, A.; Lade, A.; Chou, H.; Lee, Y.; Farias, F.; Aguirre-Ghiso, J.; Friedman, S. Epithelial Xbp1 Is Required for Cellular Proliferation and Differentiation during Mammary Gland Development. Mol. Cell Biol. 2015, 35, 1543–1556. [Google Scholar] [CrossRef]
- Cox, J.; Walter, P. A Novel Mechanism for Regulating Activity of a Transcription Factor That Controls the Unfolded Protein Response. Cell 1996, 87, 391–404. [Google Scholar] [CrossRef]
- Luo, X.; Alfason, L.; Wei, M.; Wu, S.; Kasim, V. Spliced or Unspliced, That Is the Question: The Biological Roles of XBP1 Isoforms in Pathophysiology. Int. J. Mol. Sci. 2022, 23, 2746. [Google Scholar] [CrossRef] [PubMed]
- Matus, S.; Nassif, M.; Glimcher, L.; Hetz, C. XBP-1 Deficiency in the Nervous System Reveals a Homeostatic Switch to Activate Autophagy. Autophagy 2009, 5, 1226–1228. [Google Scholar] [CrossRef]
- Hayashi, A.; Kasahara, T.; Kametani, M.; Kato, T. Attenuated BDNF-Induced Upregulation of GABAergic Markers in Neurons Lacking Xbp1. Biochem. Biophys. Res. Commun. 2008, 376, 758–763. [Google Scholar] [CrossRef]
- Martínez, G.; Vidal, R.; Mardones, P.; Serrano, F.; Ardiles, A.O.; Wirth, C.; Valdés, P.; Thielen, P.; Schneider, B.L.; Kerr, B.; et al. Regulation of Memory Formation by the Transcription Factor XBP1. Cell Rep. 2016, 14, 1382–1394. [Google Scholar] [CrossRef]
- Masaki, T.; Yoshida, M.; Noguchi, S. Targeted Disruption of CRE-Binding Factor TREB5 Gene Leads to Cellular Necrosis in Cardiac Myocytes at the Embryonic Stage. Biochem. Biophys. Res. Commun. 1999, 261, 350–356. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, Q.; Chen, M.; Margariti, A.; Martin, D.; Ivetic, A.; Xu, H.; Mason, J.; Wang, W.; Cockerill, G.; et al. Vascular Endothelial Cell Growth–Activated XBP1 Splicing in Endothelial Cells Is Crucial for Angiogenesis. Circulation 2013, 127, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dougan, S.; McGehee, A.; Love, J.; Ploegh, H. XBP-1 Regulates Signal Transduction, Transcription Factors and Bone Marrow Colonization in B Cells. EMBO J. 2009, 28, 1624–1636. [Google Scholar] [CrossRef]
- Iwakoshi, N.; Pypaert, M.; Glimcher, L. The Transcription Factor XBP-1 Is Essential for the Development and Survival of Dendritic Cells. J. Exp. Med. 2007, 204, 2267–2275. [Google Scholar] [CrossRef]
- Bettigole, S.; Lis, R.; Adoro, S.; Lee, A.; Spencer, L.; Weller, P.F.; Glimcher, L.H. The Transcription Factor XBP1 Is Selectively Required for Eosinophil Differentiation. Nat. Immunol. 2015, 16, 829–837. [Google Scholar] [CrossRef]
- Tohmonda, T.; Miyauchi, Y.; Ghosh, R.; Yoda, M.; Uchikawa, S.; Takito, J.; Morioka, H.; Nakamura, M.; Iwawaki, T.; Chiba, K.; et al. The IRE1α–XBP1 Pathway Is Essential for Osteoblast Differentiation through Promoting Transcription of Osterix. EMBO Rep. 2011, 12, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Cameron, T.; Gresshoff, I.; Bell, K.; Piróg, K.; Sampurno, L.; Hartley, C.; Sanford, E.; Wilson, R.; Ermann, J.; Boot-Handford, R.; et al. Cartilage-Specific Ablation of XBP1 Signaling in Mouse Results in a Chondrodysplasia Characterized by Reduced Chondrocyte Proliferation and Delayed Cartilage Maturation and Mineralization. Osteoarthr. Cartil. 2015, 23, 661–670. [Google Scholar] [CrossRef]
- Kaser, A.; Lee, A.; Franke, A.; Glickman, J.; Zeissig, S.; Tilg, H.; Nieuwenhuis, E.; Higgins, D.; Schreiber, S.; Glimcher, L.; et al. XBP1 Links ER Stress to Intestinal Inflammation and Confers Genetic Risk for Human Inflammatory Bowel Disease. Cell 2008, 134, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Reimold, A.; Etkin, A.; Clauss, I.; Perkins, A.; Friend, D.; Zhang, J.; Horton, H.; Scott, A.; Orkin, S.; Byrne, M.; et al. An Essential Role in Liver Development for Transcription Factor XBP-1. Genes Dev. 2000, 14, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.; Giesy, S.; Long, Q.; Krumm, C.; Harvatine, K.; Boisclair, Y. XBP1 Regulates the Biosynthetic Capacity of the Mammary Gland During Lactation by Controlling Epithelial Expansion and Endoplasmic Reticulum Formation. Endocrinology 2016, 157, 417–428. [Google Scholar] [CrossRef]
- Duran-Aniotz, C.; Poblete, N.; Rivera-Krstulovic, C.; Ardiles, Á.O.; Díaz-Hung, M.L.; Tamburini, G.; Sabusap, C.M.P.; Gerakis, Y.; Cabral-Miranda, F.; Diaz, J.; et al. The Unfolded Protein Response Transcription Factor XBP1s Ameliorates Alzheimer’s Disease by Improving Synaptic Function and Proteostasis. Mol. Ther. 2023, 31, 2240–2256. [Google Scholar] [CrossRef]
- Valdés, P.; Mercado, G.; Vidal, R.; Molina, C.; Parsons, G.; Court, F.A.; Martinez, A.; Galleguillos, D.; Armentano, D.; Schneider, B.L.; et al. Control of Dopaminergic Neuron Survival by the Unfolded Protein Response Transcription Factor XBP1. Proc. Natl. Acad. Sci. USA 2014, 111, 6804–6809. [Google Scholar] [CrossRef]
- García-Huerta, P.; Troncoso-Escudero, P.; Wu, D.; Thiruvalluvan, A.; Cisternas-Olmedo, M.; Henríquez, D.R.; Plate, L.; Chana-Cuevas, P.; Saquel, C.; Thielen, P.; et al. Insulin-like Growth Factor 2 (IGF2) Protects against Huntington’s Disease through the Extracellular Disposal of Protein Aggregates. Acta Neuropathol. 2020, 140, 737–764. [Google Scholar] [CrossRef]
- Ozcan, L.; Ergin, A.; Lu, A.; Chung, J.; Sarkar, S.; Nie, D.; Myers, M.; Ozcan, U. Endoplasmic Reticulum Stress Plays a Central Role in Development of Leptin Resistance. Cell Metab. 2009, 9, 35–51. [Google Scholar] [CrossRef]
- Williams, K.; Liu, T.; Kong, X.; Fukuda, M.; Deng, Y.; Berglund, E.; Deng, Z.; Gao, Y.; Liu, T.; Sohn, J.; et al. Xbp1s in Pomc Neurons Connects ER Stress with Energy Balance and Glucose Homeostasis. Cell Metab. 2014, 20, 471–482. [Google Scholar] [CrossRef]
- Wang, X.; Deng, Y.; Zhang, G.; Li, C.; Ding, G.; May, H.I.; Tran, D.H.; Luo, X.; Jiang, D.-S.; Li, D.L.; et al. Spliced X-Box Binding Protein 1 Stimulates Adaptive Growth Through Activation of mTOR. Circulation 2019, 140, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Margariti, A.; Li, H.; Chen, T.; Martin, D.; Vizcay-Barrena, G.; Alam, S.; Karamariti, E.; Xiao, Q.; Zampetaki, A.; Zhang, Z.; et al. XBP1 mRNA Splicing Triggers an Autophagic Response in Endothelial Cells through BECLIN-1 Transcriptional Activation. J. Biol. Chem. 2013, 288, 859–872. [Google Scholar] [CrossRef]
- Liu, X.; Yu, J.; Xu, L.; Umphred-Wilson, K.; Peng, F.; Ding, Y.; Barton, B.; Lv, X.; Zhao, M.; Sun, S.; et al. Notch-Induced Endoplasmic Reticulum-Associated Degradation Governs Mouse Thymocyte Β−selection. eLife 2021, 10, e69975. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xiao, X.; Hu, S.; He, W.; Wu, G.; Geng, X.; Fan, J.; Ma, L.; Liu, J.; Liu, Z.; et al. XBP1 Is Required in Th2 Polarization Induction in Airway Allergy. Theranostics 2022, 12, 5337–5349. [Google Scholar] [CrossRef] [PubMed]
- Duwaerts, C.; Siao, K.; Soon, R.; Her, C.; Iwawaki, T.; Kohno, K.; Mattis, A.; Maher, J. Hepatocyte-Specific Deletion of XBP1 Sensitizes Mice to Liver Injury through Hyperactivation of IRE1α. Cell Death Differ. 2021, 28, 1455–1465. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, H.; Bu, Q.; Wei, S.; Li, L.; Zhou, J.; Zhou, S.; Su, W.; Liu, M.; Liu, Z.; et al. Role of XBP1 in Regulating the Progression of Non-Alcoholic Steatohepatitis. J. Hepatol. 2022, 77, 312–325. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R. Mechanisms, Regulation and Functions of the Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Hayashi, A.; Kasahara, T.; Iwamoto, K.; Ishiwata, M.; Kametani, M.; Kakiuchi, C.; Furuichi, T.; Kato, T. The Role of Brain-Derived Neurotrophic Factor (BDNF)-Induced XBP1 Splicing during Brain Development. J. Biol. Chem. 2007, 282, 34525–34534. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Kolls, B.J.; Mace, B.; Yu, S.; Li, X.; Liu, W.; Chaparro, E.; Shen, Y.; Dang, L.; et al. Sustained Overexpression of Spliced X-Box-Binding Protein-1 in Neurons Leads to Spontaneous Seizures and Sudden Death in Mice. Commun. Biol. 2023, 6, 252. [Google Scholar] [CrossRef]
- Wang, C.; Chang, Y.; Zhu, J.; Ma, R.; Li, G. Dual Role of Inositol-Requiring Enzyme 1α–X-Box Binding Protein 1 Signaling in Neurodegenerative Diseases. Neuroscience 2022, 505, 157–170. [Google Scholar] [CrossRef]
- Wu, J.; Yarmey, V.R.; Yang, O.J.; Soderblom, E.J.; San-Miguel, A.; Yan, D. Heat Shock Proteins Function as Signaling Molecules to Mediate Neuron-Glia Communication in C. Elegans during Aging. Nat. Neurosci. 2025, 28, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Safra, M.; Ben-Hamo, S.; Kenyon, C.; Henis-Korenblit, S. The Ire-1 ER Stress-Response Pathway Is Required for Normal Secretory-Protein Metabolism in C. elegans. J. Cell Sci. 2013, 126, 4136–4146. [Google Scholar] [CrossRef]
- Yokota, T.; Li, J.; Huang, J.; Xiong, Z.; Zhang, Q.; Chan, T.; Ding, Y.; Rau, C.; Sung, K.; Ren, S.; et al. P38 Mitogen-Activated Protein Kinase Regulates Chamber-Specific Perinatal Growth in Heart. J. Clin. Investig. 2020, 130, 5287–5301. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, Y.; Gao, N.; Pedrozo, Z.; Li, D.L.; Morales, C.; Criollo, A.; Luo, X.; Tan, W.; Jiang, N.; et al. Spliced X-Box Binding Protein 1 Couples the Unfolded Protein Response to Hexosamine Biosynthetic Pathway. Cell 2014, 156, 1179–1192. [Google Scholar] [CrossRef]
- Ghosh, R.; Lipson, K.L.; Sargent, K.E.; Mercurio, A.M.; Hunt, J.S.; Ron, D.; Urano, F. Transcriptional Regulation of VEGF-A by the Unfolded Protein Response Pathway. PLoS ONE 2010, 5, e9575. [Google Scholar] [CrossRef] [PubMed]
- von Freeden-Jeffry, U.; Solvason, N.; Howard, M.; Murray, R. The Earliest T Lineage-Committed Cells Depend on IL-7 for Bcl-2 Expression and Normal Cell Cycle Progression. Immunity 1997, 7, 147–154. [Google Scholar] [CrossRef]
- Pramanik, J.; Chen, X.; Kar, G.; Henriksson, J.; Gomes, T.; Park, J.-E.; Natarajan, K.; Meyer, K.B.; Miao, Z.; McKenzie, A.N.J.; et al. Genome-Wide Analyses Reveal the IRE1a-XBP1 Pathway Promotes T Helper Cell Differentiation by Resolving Secretory Stress and Accelerating Proliferation. Genome Med. 2018, 10, 76. [Google Scholar] [CrossRef]
- Brucklacher-Waldert, V.; Ferreira, C.; Stebegg, M.; Fesneau, O.; Innocentin, S.; Marie, J.C.; Veldhoen, M. Cellular Stress in the Context of an Inflammatory Environment Supports TGF-β-Independent T Helper-17 Differentiation. Cell Rep. 2017, 19, 2357–2370. [Google Scholar] [CrossRef]
- Kamimura, D.; Bevan, M. Endoplasmic Reticulum Stress Regulator XBP-1 Contributes to Effector CD8+ T Cell Differentiation during Acute Infection. J. Immunol. 2008, 181, 5433–5441. [Google Scholar] [CrossRef]
- Shaffer, A.; Shapiro-Shelef, M.; Iwakoshi, N.; Lee, A.; Qian, S.; Zhao, H.; Yu, X.; Yang, L.; Tan, B.K.; Rosenwald, A.; et al. XBP1, Downstream of Blimp-1, Expands the Secretory Apparatus and Other Organelles, and Increases Protein Synthesis in Plasma Cell Differentiation. Immunity 2004, 21, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Adams, N.; Xu, Y.; Cao, J.; Allan, D.; Carlyle, J.; Chen, X.; Sun, J.; Glimcher, L. The IRE1 Endoplasmic Reticulum Stress Sensor Activates Natural Killer Cell Immunity in Part by Regulating C-Myc. Nat. Immunol. 2019, 20, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Chen, X.; Lee, A.; Glimcher, L. TLR Activation of the Transcription Factor XBP1 Regulates Innate Immune Responses in Macrophages. Nat. Immunol. 2010, 11, 411–418. [Google Scholar] [CrossRef]
- Tian, P.; Jiang, Z.; Li, J.; Zhou, Z.; Zhang, Q.-H. Spliced XBP1 Promotes Macrophage Survival and Autophagy by Interacting with Beclin-1. Biochem. Biophys. Res. Commun. 2015, 463, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Zhu, L.; Du, S.; Mao, J.; Wang, Y.; Wang, S.; Bo, Q.; Tu, Y.; Yi, Q. The P300/XBP1s/Herpud1 Axis Promotes Macrophage M2 Polarization and the Development of Choroidal Neovascularization. J. Cell. Mol. Med. 2021, 25, 6709–6720. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, J.; Zhang, P.; Song, F.; Jiang, R.; Li, M.; Xia, F.; Guo, F.-J. IRE1α Dissociates with BiP and Inhibits ER Stress-Mediated Apoptosis in Cartilage Development. Cell Signal 2013, 25, 2136–2146. [Google Scholar] [CrossRef]
- Hauser, B.; Aure, M.; Kelly, M.; Hoffman, M.P.; Chibly, A.M. Generation of a Single-Cell RNAseq Atlas of Murine Salivary Gland Development. iScience 2020, 23, 101838. [Google Scholar] [CrossRef]
- Kung, L.; Rajpar, M.; Preziosi, R.; Briggs, M.; Boot-Handford, R. Increased Classical Endoplasmic Reticulum Stress Is Sufficient to Reduce Chondrocyte Proliferation Rate in the Growth Plate and Decrease Bone Growth. PLoS ONE 2015, 10, e0117016. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Liang, L.; Fan, M.; Li, X.; Feng, N.; Pan, Y.; Tan, Q.; Xu, Q.; Xie, Y.; et al. Effect Of XBP1 Deficiency In Cartilage On The Regulatory Network Of LncRNA/circRNA-miRNA-mRNA. Int. J. Biol. Sci. 2022, 18, 315–330. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, R.; Xiong, Z.; Xia, F.; Li, M.; Chen, L.; Liu, C. IRE1a Constitutes a Negative Feedback Loop with BMP2 and Acts as a Novel Mediator in Modulating Osteogenic Differentiation. Cell Death Dis. 2014, 5, e1239. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Kondo, S.; Yoshinaga, K.; Saito, A.; Murakami, T.; Kanemoto, S.; Sekiya, H.; Chihara, K.; Aikawa, Y.; Hara, H.; et al. Regulation of ER Molecular Chaperone Prevents Bone Loss in a Murine Model for Osteoporosis. J. Bone Miner. Metab. 2010, 28, 131–138. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, M.; Ricupero, C.; He, L.; Wu, J.; Chen, K.; Friedman, R.; Guarnieri, P.; Wang, Z.; Zhou, X.; et al. Profiling of Stem/Progenitor Cell Regulatory Genes of the Synovial Joint by Genome-Wide RNA-Seq Analysis. BioMed Res. Int. 2018, 2018, 9327487. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Xu, Y.; Lin, W.; Luo, Z.; Han, Z.; Liu, S.; Qi, B.; Sun, C.; Go, K.; et al. Single-Cell Integration Analysis of Heterotopic Ossification and Fibrocartilage Developmental Lineage: Endoplasmic Reticulum Stress Effector Xbp1 Transcriptionally Regulates the Notch Signaling Pathway to Mediate Fibrocartilage Differentiation. Oxid. Med. Cell Longev. 2021, 2021, 7663366. [Google Scholar] [CrossRef]
- Lee, A.; Iwakoshi, N.; Glimcher, L. XBP-1 Regulates a Subset of Endoplasmic Reticulum Resident Chaperone Genes in the Unfolded Protein Response. Mol. Cell Biol. 2003, 23, 7448–7459. [Google Scholar] [CrossRef]
- Sriburi, R.; Jackowski, S.; Mori, K.; Brewer, J.W. XBP1: A Link between the Unfolded Protein Response, Lipid Biosynthesis, and Biogenesis of the Endoplasmic Reticulum. J. Cell Biol. 2004, 167, 35–41. [Google Scholar] [CrossRef]
- Joshi, A.; Castillo, M.; Tomaz da Silva, M.; Vuong, A.T.; Gunaratne, P.; Darabi, R.; Liu, Y.; Kumar, A. Single-Nucleus Transcriptomic Analysis Reveals the Regulatory Circuitry of Myofiber XBP1 during Regenerative Myogenesis. iScience 2024, 27, 111372. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Xu, L.; Kim, H.; Qiu, Y.; Zhang, K. Boosting UPR Transcriptional Activator XBP1 Accelerates Acute Wound Healing. PNAS Nexus 2023, 2, pgad050. [Google Scholar] [CrossRef]
- Ham, S.; Pyo, M.; Kang, M.; Kim, Y.; Lee, D.; Chung, J.; Lee, S. HSP47 Increases the Expression of Type I Collagen in Fibroblasts through IRE1α Activation, XBP1 Splicing, and Nuclear Translocation of β-Catenin. Cells 2024, 13, 527. [Google Scholar] [CrossRef] [PubMed]
- Angbohang, A.; Huang, L.; Li, Y.; Zhao, Y.; Gong, Y.; Fu, Y.; Mao, C.; Morales, J.; Luo, P.; Ehteramyan, M.; et al. X-Box Binding Protein 1–Mediated COL4A1s Secretion Regulates Communication between Vascular Smooth Muscle and Stem/Progenitor Cells. J. Biol. Chem. 2021, 296, 100541. [Google Scholar] [CrossRef]
- Mallick, R.; Montaser, A.; Komi, H.; Juusola, G.; Tirronen, A.; Gurzeler, E.; Barbiera, M.; Korpisalo, P.; Terasaki, T.; Nieminen, T.; et al. VEGF-B Is a Novel Mediator of ER Stress Which Induces Cardiac Angiogenesis via RGD-Binding Integrins Independent of VEGFR1/NRP Activities. Mol. Ther. 2025, 33, 3242–3256. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Qin, R.; Feng, J.; Liu, Y.; Zhou, X.; Qin, X.; Li, Y.; Zhang, Z.; He, X. XBP1s Gene of Endoplasmic Reticulum Stress Enhances Proliferation and Osteogenesis of Human Periodontal Ligament Cells. Tissue Cell 2023, 83, 102139. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhang, F.; Feng, N.; Kuang, B.; Fan, M.; Chen, C.; Pan, Y.; Zhou, P.; Geng, N.; Li, X.; et al. IRE1α Protects against Osteoarthritis by Regulating Progranulin-Dependent XBP1 Splicing and Collagen Homeostasis. Exp. Mol. Med. 2023, 55, 2376–2389. [Google Scholar] [CrossRef]
- Shen, C.; Jiang, T.; Zhu, B.; Le, Y.; Liu, J.; Qin, Z.; Chen, H.; Zhong, G.; Zheng, L.; Zhao, J.; et al. In Vitro Culture Expansion Impairs Chondrogenic Differentiation and the Therapeutic Effect of Mesenchymal Stem Cells by Regulating the Unfolded Protein Response. J. Biol. Eng. 2018, 12, 26. [Google Scholar] [CrossRef]
- Miyazaki, K.; Saito, Y.; Ichimura-Shimizu, M.; Imura, S.; Ikemoto, T.; Yamada, S.; Tokuda, K.; Morine, Y.; Tsuneyama, K.; Shimada, M. Defective Endoplasmic Reticulum Stress Response via X Box-Binding Protein 1 Is a Major Cause of Poor Liver Regeneration after Partial Hepatectomy in Mice with Non-Alcoholic Steatohepatitis. J. Hepatobiliary Pancreat. Sci. 2022, 29, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Tsybko, A.; Eremin, D.; Ilchibaeva, T.; Khotskin, N.; Naumenko, V. CDNF Exerts Anxiolytic, Antidepressant-like, and Procognitive Effects and Modulates Serotonin Turnover and Neuroplasticity-Related Genes. Int. J. Mol. Sci. 2024, 25, 10343. [Google Scholar] [CrossRef]
- Ajwani, J.; Hwang, E.; Portillo, B.; Lieu, L.; Wallace, B.; Kabahizi, A.; He, Z.; Dong, Y.; Grose, K.; Williams, K.W. Upregulation of Xbp1 in NPY/AgRP Neurons Reverses Diet-Induced Obesity and Ameliorates Leptin and Insulin Resistance. Neuropeptides 2024, 108, 102461. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, L.; Bi, F.; Zhou, Z. ALKBH5 Inhibits YTHDF2-m6A-Mediated Degradation of RCN1 mRNA to Promote Keloid Formation by Activating IRE1α-XBP1-Mediated ER Stress. J. Cosmet. Dermatol. 2025, 24, e70177. [Google Scholar] [CrossRef]
- Tang, T.; Chan, Y.; Cheong, H.; Cheok, Y.; Anuar, N.; Looi, C.; Gan, G.; Wong, W. Regulatory Network of BLIMP1, IRF4, and XBP1 Triad in Plasmacytic Differentiation and Multiple Myeloma Pathogenesis. Cell. Immunol. 2022, 380, 104594. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, S.; Fu, Y.; Sun, J. XBP1: A Key Regulator in Breast Cancer Development and Treatment. Pathol. Res. Pract. 2025, 269, 155900. [Google Scholar] [CrossRef]
- Maiers, J.; Kostallari, E.; Mushref, M.; deAssuncao, T.; Li, H.; Jalan-Sakrikar, N.; Huebert, R.; Cao, S.; Malhi, H.; Shah, V. The Unfolded Protein Response Mediates Fibrogenesis and Collagen I Secretion through Regulating TANGO1 in Mice. Hepatology 2017, 65, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.; Kim, Y.; Kim, S. Roles of X-Box Binding Protein 1 in Liver Pathogenesis. Clin. Mol. Hepatol. 2025, 31, 1–31. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).