Employing an Artificial Intelligence Platform to Enhance Treatment Responses to GLP-1 Agonists by Utilizing Metabolic Variability Signatures Based on the Constrained Disorder Principle
Abstract
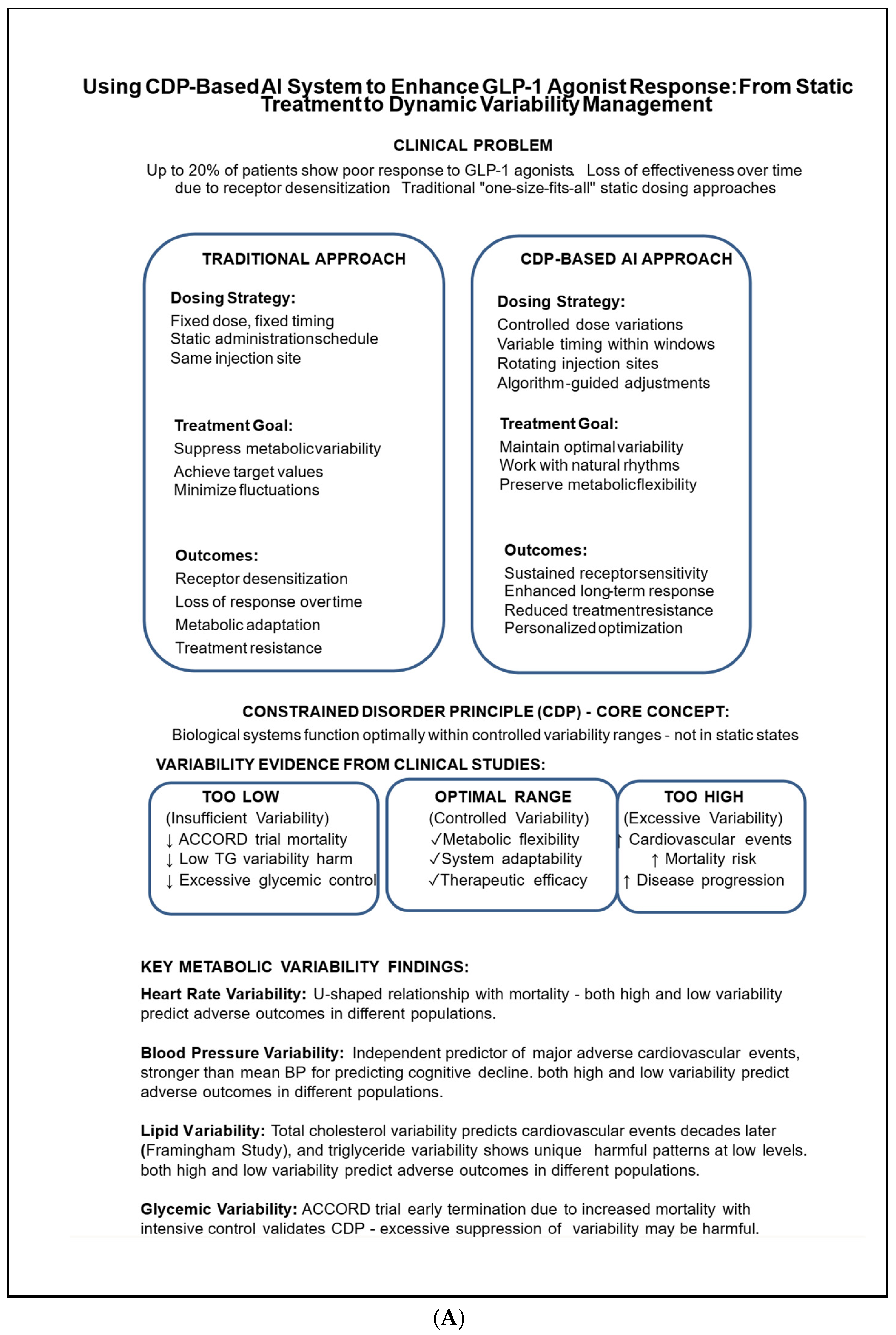
1. Introduction
2. The Constrained Disorder Principle (CDP) Offers a Framework for Leveraging Biological Variability to Enhance the Effectiveness of Chronic Therapies
3. Increased Metabolic Variability Is Linked to a Higher Incidence of Health Issues
4. Heart Rate Variability: A Subtle Equilibrium in Autonomic Regulation
5. Blood Pressure Variability and Its Effect on Prognosis
6. Blood Lipid Variability: A Multifaceted Indicator of Cardiovascular and Metabolic Risk
7. Glycemic Variability Plays a Crucial Role in Diabetes Management and Has Significant Clinical Implications, Particularly Concerning Cardiovascular Risk and the Limitations of Maintaining Tight Glucose Control
8. Weight Fluctuations and Health Risks: Reevaluating BMI as a Dynamic Metric
9. Prognostic Significance of Variability in Metabolic Rate and the Dynamics of Mitochondria
10. GLP-1 Receptor Agonists: Connecting Therapeutic Response to Metabolic Variability Profiles
11. Analysis of Metabolic Networks Based on Constraints and Integration of the Metabolome
12. Utilizing Frameworks Based on the Clinical Development Plan (CDP) to Enhance the Effectiveness of GLP-1 Agonists
13. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDP | constrained disorder principle |
| AI | artificial intelligence |
| METv | variability in metabolic parameters |
References
- McEntire, K.; Gage, M.; Gawne, R.; Hadfield, M.; Hulshof, C.; Johnson, M.; Levesque, D.; Segura, J.; Pinter-Wollman, N. Understanding Drivers of Variation and Predicting Variability Across Levels of Biological Organization. Integr. Comp. Biol. 2021, 61, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Almuwaqqat, Z.; Hui, Q.; Liu, C.; Zhou, J.J.; Voight, B.F.; Ho, Y.-L.; Posner, D.C.; Vassy, J.L.; Gaziano, J.M.; Cho, K.; et al. Long-Term Body Mass Index Variability and Adverse Cardiovascular Outcomes. JAMA Netw. Open 2024, 7, e243062. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.; Keller, F.; Siwy, J.; Latosinska, A.; Mischak, H.; Mayor, J.; Clausen, J.; Wilhelmi, M.; Brauckmann, V.; Sehmisch, S. Application of urinary peptide-biomarkers in trauma patients as a predictive tool for prognostic assessment, treatment and intervention timing. Sci. Rep. 2025, 15, 898. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Kym, D.; Cho, Y.S.; Hur, J.; Yoon, D. Longitudinal analysis of ARDS variability and biomarker predictive power in burn patients. Sci. Rep. 2024, 14, 26376. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.-L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Ilan, Y. Overcoming randomness does not rule out the importance of inherent randomness for functionality. J. Biosci. 2019, 44, 132. [Google Scholar] [CrossRef]
- Ilan, Y. Generating randomness: Making the most out of disordering a false order into a real one. J. Transl. Med. 2019, 17, 49. [Google Scholar] [CrossRef]
- Ilan, Y. Advanced Tailored Randomness: A Novel Approach for Improving the Efficacy of Biological Systems. J. Comput. Biol. 2020, 27, 20–29. [Google Scholar] [CrossRef]
- Ilan, Y. Order Through Disorder: The Characteristic Variability of Systems. Front. Cell Dev. Biol. 2020, 8, 186. [Google Scholar] [CrossRef]
- El-Haj, M.; Kanovitch, D.; Ilan, Y. Personalized inherent randomness of the immune system is manifested by an individualized response to immune triggers and immunomodulatory therapies: A novel platform for designing personalized immunotherapies. Immunol. Res. 2019, 67, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Randomness in microtubule dynamics: An error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol. Int. 2019, 43, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Microtubules: From understanding their dynamics to using them as potential therapeutic targets. J. Cell Physiol. 2019, 234, 7923–7937. [Google Scholar] [CrossRef] [PubMed]
- Ilan-Ber, T.; Ilan, Y. The role of microtubules in the immune system and as potential targets for gut-based immunotherapy. Mol. Immunol. 2019, 111, 73–82. [Google Scholar] [CrossRef]
- Forkosh, E.; Kenig, A.; Ilan, Y. Introducing variability in targeting the microtubules: Review of current mechanisms and future directions in colchicine therapy. Pharmacol. Res. Perspect. 2020, 8, e00616. [Google Scholar] [CrossRef]
- Ilan, Y. Beta-Glycosphingolipids as Mediators of Both Inflammation and Immune Tolerance: A Manifestation of Randomness in Biological Systems. Front. Immunol. 2019, 10, 1143. [Google Scholar] [CrossRef]
- Ilan, Y. Microtubules as a potential platform for energy transfer in biological systems: A target for implementing individualized, dynamic variability patterns to improve organ function. Mol. Cell. Biochem. 2022, 478, 375–392. [Google Scholar] [CrossRef]
- Ilan, Y. Enhancing the plasticity, proper function and efficient use of energy of the Sun, genes and microtubules using variability. Clin. Transl. Discov. 2022, 2, e103. [Google Scholar] [CrossRef]
- Shabat, Y.; Lichtenstein, Y.; Ilan, Y. Short-Term Cohousing of Sick with Healthy or Treated Mice Alleviates the Inflammatory Response and Liver Damage. Inflammation 2021, 44, 518–525. [Google Scholar] [CrossRef]
- Ilan, Y. Making use of noise in biological systems. Prog. Biophys. Mol. Biol. 2023, 178, 83–90. [Google Scholar] [CrossRef]
- Rotnemer-Golinkin, D.; Ilan, Y. Personalized-Inherent Variability in a Time-Dependent Immune Response: A Look into the Fifth Dimension in Biology. Pharmacology 2022, 107, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Constrained disorder principle-based variability is fundamental for biological processes: Beyond biological relativity and physiological regulatory networks. Prog. Biophys. Mol. Biol. 2023, 180, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. The constrained-disorder principle defines the functions of systems in nature. Front. Netw. Physiol. 2024, 4, 1361915. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. Second-Generation Digital Health Platforms: Placing the Patient at the Center and Focusing on Clinical Outcomes. Front. Digit. Health 2020, 2, 569178. [Google Scholar] [CrossRef]
- Ilan, Y. Improving Global Healthcare and Reducing Costs Using Second-Generation Artificial Intelligence-Based Digital Pills: A Market Disruptor. Int. J. Environ. Res. Public Health 2021, 18, 811. [Google Scholar] [CrossRef]
- Ilan, Y. The constrained disorder principle defines living organisms and provides a method for correcting disturbed biological systems. Comput. Struct. Biotechnol. J. 2022, 20, 6087–6096. [Google Scholar] [CrossRef]
- Ilan, Y. Next-Generation Personalized Medicine: Implementation of Variability Patterns for Overcoming Drug Resistance in Chronic Diseases. J. Pers. Med. 2022, 12, 1303. [Google Scholar] [CrossRef]
- Hurvitz, N.; Ilan, Y. The Constrained-Disorder Principle Assists in Overcoming Significant Challenges in Digital Health: Moving from “Nice to Have” to Mandatory Systems. Clin. Pract. 2023, 13, 994–1014. [Google Scholar] [CrossRef]
- Sigawi, T.; Ilan, Y. Using Constrained-Disorder Principle-Based Systems to Improve the Performance of Digital Twins in Biological Systems. Biomimetics 2023, 8, 359. [Google Scholar] [CrossRef]
- Sigawi, T.; Lehmann, H.; Hurvitz, N.; Ilan, Y. Constrained Disorder Principle-Based Second-Generation Algorithms Implement Quantified Variability Signatures to Improve the Function of Complex Systems. J. Bioinform. Syst. Biol. 2023, 6, 82–89. [Google Scholar] [CrossRef]
- Kreger, B.E.; Odell, P.M.; D’Agostino, R.B.; Wilson, P.F. Long-term intraindividual cholesterol variability: Natural course and adverse impact on morbidity and mortality—The Framingham Study. Am. Heart J. 1994, 127, 1607–1614. [Google Scholar] [CrossRef]
- Wang, M.C.; Li, C.I.; Liu, C.S.; Lin, C.H.; Yang, S.Y.; Li, T.C.; Lin, C.C. Effect of blood lipid variability on mortality in patients with type 2 diabetes: A large single-center cohort study. Cardiovasc. Diabetol. 2021, 20, 228. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, K.; Park, Y.M.; Kwon, H.S.; Kang, G.; Yoon, K.H.; Lee, S.H. Associations of Variability in Blood Pressure, Glucose and Cholesterol Concentrations, and Body Mass Index With Mortality and Cardiovascular Outcomes in the General Population. Circulation 2018, 138, 2627–2637. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Han, K.; Yoon, K.H.; Kim, M.K.; Lee, S.H. Impact of Mean and Variability of High-Density Lipoprotein-Cholesterol on the Risk of Myocardial Infarction, Stroke, and Mortality in the General Population. J. Am. Heart Assoc. 2020, 9, e015493. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Choi, K.M. Association between Variability of Metabolic Risk Factors and Cardiometabolic Outcomes. Diabetes Metab. J. 2022, 46, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Nong, F.; Zhu, W.; Jiang, Y. Extreme dipping blood pressure pattern is associated with increased mortality in hemorrhagic stroke patients: A retrospective cohort study. BMC Neurol. 2025, 25, 318. [Google Scholar] [CrossRef]
- Lim, S.; Chung, S.H.; Kim, J.H.; Kim, Y.H.; Kim, E.J.; Joo, H.J. Effects of metabolic parameters’ variability on cardiovascular outcomes in diabetic patients. Cardiovasc. Diabetol. 2023, 22, 114. [Google Scholar] [CrossRef]
- Clark, D., 3rd; Nicholls, S.J.; St John, J.; Elshazly, M.B.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E.; Puri, R. Visit-to-visit cholesterol variability correlates with coronary atheroma progression and clinical outcomes. Eur. Heart J. 2018, 39, 2551–2558. [Google Scholar] [CrossRef]
- Ceriello, A.; De Cosmo, S.; Rossi, M.C.; Lucisano, G.; Genovese, S.; Pontremoli, R.; Fioretto, P.; Giorda, C.; Pacilli, A.; Viazzi, F.; et al. Variability in HbA1c, blood pressure, lipid parameters and serum uric acid, and risk of development of chronic kidney disease in type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 1570–1578. [Google Scholar] [CrossRef]
- de Bruyne, M.C.; Kors, J.A.; Hoes, A.W.; Klootwijk, P.; Dekker, J.M.; Hofman, A.; van Bemmel, J.H.; Grobbee, D.E. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: The Rotterdam Study. Am. J. Epidemiol. 1999, 150, 1282–1288. [Google Scholar] [CrossRef]
- Kikuya, M.; Ohkubo, T.; Metoki, H.; Asayama, K.; Hara, A.; Obara, T.; Inoue, R.; Hoshi, H.; Hashimoto, J.; Totsune, K.; et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: The Ohasama study. Hypertension 2008, 52, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Jarczok, M.N.; Weimer, K.; Braun, C.; Williams, D.P.; Thayer, J.F.; Gündel, H.O.; Balint, E.M. Heart rate variability in the prediction of mortality: A systematic review and meta-analysis of healthy and patient populations. Neurosci. Biobehav. Rev. 2022, 143, 104907. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T., Jr.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Aftyka, J.; Staszewski, J.; Dębiec, A.; Pogoda-Wesołowska, A.; Żebrowski, J. Heart rate variability as a predictor of stroke course, functional outcome, and medical complications: A systematic review. Front. Physiol. 2023, 14, 1115164. [Google Scholar] [CrossRef]
- Magdás, A.; Szilágyi, L.; Incze, A. Can Ambulatory Blood Pressure Variability Contribute to Individual Cardiovascular Risk Stratification? Comput. Math. Methods Med. 2016, 2016, 7816830. [Google Scholar] [CrossRef]
- Rosei, E.A.; Chiarini, G.; Rizzoni, D. How important is blood pressure variability? Eur. Heart J. Suppl. 2020, 22, E1–E6. [Google Scholar] [CrossRef]
- Mancia, G.; Ferrari, A.; Gregorini, L.; Parati, G.; Pomidossi, G.; Bertinieri, G.; Grassi, G.; di Rienzo, M.; Pedotti, A.; Zanchetti, A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ. Res. 1983, 53, 96–104. [Google Scholar] [CrossRef]
- Harefa; Wijaya, I.P.; Muhadi; Rumende, C.M.; Nasution, S.A.; Koesnoe, S.; Marbun, M.B.; Shatri, H. The association between 24-h blood pressure variability and major adverse cardiac events (MACE) in hospitalized patients with acute myocardial infarction: A retrospective cohort study. Egypt. Heart J. 2021, 73, 88. [Google Scholar] [CrossRef]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef]
- McAlister, F.A.; Lethebe, B.C.; Leung, A.A.; Padwal, R.S.; Williamson, T. Visit-to-visit blood pressure variability is common in primary care patients: Retrospective cohort study of 221,803 adults. PLoS ONE 2021, 16, e0248362. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Qin, J.; Wei, X.; Yang, Y.; Yuan, Y.; Yan, F.; Huo, X.; Han, L. Blood pressure variability predicts poor outcomes in acute stroke patients without thrombolysis: A systematic review and meta-analysis. J. Neurol. 2024, 271, 1160–1169. [Google Scholar] [CrossRef]
- Wei, F.F.; Zhou, Y.; Thijs, L.; Xue, R.; Dong, B.; He, X.; Liang, W.; Wu, Y.; Jiang, J.; Tan, W.; et al. Visit-to-Visit Blood Pressure Variability and Clinical Outcomes in Patients With Heart Failure With Preserved Ejection Fraction. Hypertension 2021, 77, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Wei, W.; Pu, Y.; Zhang, L.; Cui, T.; Ma, L.; Wang, B.; Zhao, Y.; Fu, P. Blood Pressure Variability and the Progression of Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2023, 38, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- de Heus, R.A.A.; Tzourio, C.; Lee, E.J.L.; Opozda, M.; Vincent, A.D.; Anstey, K.J.; Hofman, A.; Kario, K.; Lattanzi, S.; Launer, L.J.; et al. Association Between Blood Pressure Variability With Dementia and Cognitive Impairment: A Systematic Review and Meta-Analysis. Hypertension 2021, 78, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Bartolák-Suki, E.; Suki, B. Tuning mitochondrial structure and function to criticality by fluctuation-driven mechanotransduction. Sci. Rep. 2020, 10, 407. [Google Scholar] [CrossRef]
- Manemann, S.M.; Bielinski, S.J.; Moser, E.D.; St Sauver, J.L.; Takahashi, P.Y.; Roger, V.L.; Olson, J.E.; Chamberlain, A.M.; Remaley, A.T.; Decker, P.A.; et al. Variability in Lipid Levels and Risk for Cardiovascular Disease: An Electronic Health Record-Based Population Cohort Study. J. Am. Heart Assoc. 2023, 12, e027639. [Google Scholar] [CrossRef]
- Sigawi, T.; Israeli, A.; Ilan, Y. Harnessing Variability Signatures and Biological Noise May Enhance Immunotherapies’ Efficacy and Act as Novel Biomarkers for Diagnosing and Monitoring Immune-Associated Disorders. Immunotargets Ther. 2024, 13, 525–539. [Google Scholar] [CrossRef]
- Porat, O.; Kaplan, M.; Atlibenkin, S.; Hasson-Gilad, D.; Karban, A.; Zalts, R. Differences between repeated lipid profile measurements in a tertiary hospital over a short time period. Lipids Health Dis. 2024, 23, 30. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Yu, E.; Chin, W.; Barrett, J.; Mok, A.H.Y.; Lau, C.S.T.; Wang, Y.; Wong, I.; Chan, E.W.Y.; Lam, C. Greater variability in lipid measurements associated with cardiovascular disease and mortality: 10-year diabetes cohort study. Diabetes Obes. Metab. 2020, 22, 1777–1788. [Google Scholar] [CrossRef]
- Karimi, M.A.; Vaezi, A.; Ansari, A.; Archin, I.; Dadgar, K.; Rasouli, A.; Ghannadikhosh, P.; Alishiri, G.; Tizro, N.; Gharei, F.; et al. Lipid variability and risk of microvascular complications in patients with diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2024, 24, 4. [Google Scholar] [CrossRef]
- Teekaput, C.; Thiankhaw, K.; Wongcharoen, W.; Prasertwitayakij, N.; Gunaparn, S.; Phrommintikul, A. Visit-to-visit lipid variability on long-term major adverse cardiovascular events: A prospective multicentre cohort from the CORE-Thailand registry. Sci. Rep. 2025, 15, 1953. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Mota, S.; Zhang, B. Circadian Clock Regulation on Lipid Metabolism and Metabolic Diseases. Adv. Exp. Med. Biol. 2020, 1276, 53–66. [Google Scholar] [PubMed]
- Ferrell, J.M.; Chiang, J.Y.L. Circadian rhythms in liver metabolism and disease. Acta Pharm. Sin. B 2015, 5, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yan, H.; Zhang, H.; Wang, M.; Zhang, Q.; Zhou, X. Progress in the seasonal variations of blood lipids: A mini-review. Lipids Health Dis. 2020, 19, 108. [Google Scholar] [CrossRef]
- Shin, Y.; Park, S.; Choue, R. Comparison of time course changes in blood glucose, insulin and lipids between high carbohydrate and high fat meals in healthy young women. Nutr. Res. Pract. 2009, 3, 128–133. [Google Scholar] [CrossRef]
- Thompson, P.D.; Crouse, S.F.; Goodpaster, B.; Kelley, D.; Moyna, N.; Pescatello, L. The acute versus the chronic response to exercise. Med. Sci. Sports Exerc. 2001, 33, S438–S445; discussion S452–S453. [Google Scholar] [CrossRef]
- Ramalingam, A.; Santhanathas, T.; Shaukat Ali, S.; Zainalabidin, S. Resveratrol Supplementation Protects Against Nicotine-Induced Kidney Injury. Int. J. Environ. Res. Public Health 2019, 16, 4445. [Google Scholar] [CrossRef]
- Ordovas, J.M. Genetic influences on blood lipids and cardiovascular disease risk: Tools for primary prevention. Am. J. Clin. Nutr. 2009, 89, 1509S–1517S. [Google Scholar] [CrossRef]
- Park, J.B.; Shin, E.; Lee, J.E.; Lee, S.J.; Lee, H.; Choi, S.Y.; Choe, E.K.; Choi, S.H.; Park, H.E. Genetic Determinants of Visit-to-Visit Lipid Variability: Genome-Wide Association Study in Statin-Naïve Korean Population. Front. Cardiovasc. Med. 2022, 9, 811657. [Google Scholar]
- Simundic, A.M.; Cornes, M.; Grankvist, K.; Lippi, G.; Nybo, M. Standardization of collection requirements for fasting samples: For the Working Group on Preanalytical Phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin. Chim. Acta 2014, 432, 33–37. [Google Scholar] [CrossRef]
- Wolska, A.; Remaley, A.T. Measuring LDL-cholesterol: What is the best way to do it? Curr. Opin. Cardiol. 2020, 35, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Roeback, J.R.; Cook, J.R.; Guess, H.A.; Heyse, J.F. Time-dependent variability in repeated measurements of cholesterol levels: Clinical implications for risk misclassification and intervention monitoring. J. Clin. Epidemiol. 1993, 46, 1159–1171. [Google Scholar] [CrossRef]
- Kannel, W.B.; Castelli, W.P.; Gordon, T.; McNamara, P.M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann. Intern. Med. 1971, 74, 1–12. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Zhou, Z.; Moran, C.; Murray, A.M.; Zoungas, S.; Magnussen, C.; Chong, T.T.-J.; Shah, R.C.; Sheets, K.M.; Nelson, M.; Zhu, C.; et al. Association of Year-to-Year Lipid Variability With Risk of Cognitive Decline and Dementia in Community-Dwelling Older Adults. Neurology 2025, 104, e210247. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.Y.F.; Yu, E.Y.T.; Chin, W.Y.; Lau, C.S.T.; Mok, A.H.Y.; Wang, Y.; Wong, I.C.K.; Chan, E.W.Y.; Lam, C.L.K. Greater variability in lipid measurements associated with kidney diseases in patients with type 2 diabetes mellitus in a 10-year diabetes cohort study. Sci. Rep. 2021, 11, 8047. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, C.; An, D.W.; Zhou, Y.; Yu, Z.; Wu, Y.; Wu, D.; He, X.; He, J.; Dong, Y.; et al. Cardiovascular Outcomes and Variability in Plasma Lipid Levels Across Body Mass Index Categories: The ARIC Study. J. Nutr. Metab. 2025, 2025, 8858333. [Google Scholar] [CrossRef]
- Chen, N.; Liu, Y.H.; Hu, L.K.; Ma, L.L.; Zhang, Y.; Chu, X.; Dong, J.; Yan, Y.X. Association of variability in metabolic parameters with the incidence of type 2 diabetes: Evidence from a functional community cohort. Cardiovasc. Diabetol. 2023, 22, 183. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, S.; Shao, J.; Liu, Y.; Lu, Y.; Wu, H.; Tian, Y.; Ma, Y.; Gao, J. Metabolic syndrome parameters’ variability and stroke incidence in hypertensive patients: Evidence from a functional community cohort. Cardiovasc. Diabetol. 2024, 23, 203. [Google Scholar] [CrossRef]
- Chung, H.S.; Lee, J.S.; Kim, J.A.; Roh, E.; Lee, Y.B.; Hong, S.H.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; et al. Variability in Total Cholesterol Concentration Is Associated With the Risk of Dementia: A Nationwide Population-Based Cohort Study. Front. Neurol. 2019, 10, 441. [Google Scholar] [CrossRef]
- Koh, S.M.; Chung, S.H.; Yum, Y.J.; Park, S.J.; Joo, H.J.; Kim, Y.H.; Kim, E.J. Comparison of the effects of triglyceride variability and exposure estimate on clinical prognosis in diabetic patients. Cardiovasc. Diabetol. 2022, 21, 245. [Google Scholar] [CrossRef]
- Rodbard, D. Glucose Variability: A Review of Clinical Applications and Research Developments. Diabetes Technol. Ther. 2018, 20, S25–S215. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Dovč, K. Glycemic Variability: The Danger of a Physiologically Stable Metric. J. Clin. Endocrinol. Metab. 2020, 105, e3815–e3817. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, L.E.; Alvarez, M.; Dilla, T.; Gil-Guillén, V.; Orozco-Beltrán, D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013, 4, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Sherr, J.L.; Heinemann, L.; Fleming, G.A.; Bergenstal, R.M.; Bruttomesso, D.; Hanaire, H.; Holl, R.W.; Petrie, J.R.; Peters, A.L.; Evans, M. Automated insulin delivery: Benefits, challenges, and recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association. Diabetologia 2023, 66, 3–22. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Owens, D.R. The application of simple metrics in the assessment of glycaemic variability. Diabetes Metab. 2018, 44, 313–319. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.; Deng, H.; Xu, W.; Lv, J.; Zhou, Y.; Luo, S.; Zheng, X.; Liang, H.; Yao, B.; et al. Impacts of glycemic variability on the relationship between glucose management indicator from iPro(™)2 and laboratory hemoglobin A1c in adult patients with type 1 diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820931664. [Google Scholar] [CrossRef]
- Zhou, J.J.; Schwenke, D.C.; Bahn, G.; Reaven, P. Glycemic Variation and Cardiovascular Risk in the Veterans Affairs Diabetes Trial. Diabetes Care 2018, 41, 2187–2194. [Google Scholar] [CrossRef]
- Critchley, J.A.; Carey, I.M.; Harris, T.; DeWilde, S.; Cook, D.G. Variability in Glycated Hemoglobin and Risk of Poor Outcomes Among People With Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care 2019, 42, 2237–2246. [Google Scholar] [CrossRef]
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3047. [Google Scholar] [CrossRef]
- Chen, J.; Yin, D.; Dou, K. Intensified glycemic control by HbA1c for patients with coronary heart disease and Type 2 diabetes: A review of findings and conclusions. Cardiovasc. Diabetol. 2023, 22, 146. [Google Scholar] [CrossRef]
- Buse, J.B.; Bigger, J.T.; Byington, R.P.; Cooper, L.S.; Cushman, W.C.; Friedewald, W.T.; Genuth, S.; Gerstein, H.C.; Ginsberg, H.N.; Goff, D.C., Jr.; et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: Design and methods. Am. J. Cardiol. 2007, 99, S21–S33. [Google Scholar] [CrossRef]
- Lachin, J.M. Point: Intensive glycemic control and mortality in ACCORD—A chance finding? Diabetes Care 2010, 33, 2719–2721. [Google Scholar] [CrossRef][Green Version]
- Riddle, M.C.; Ambrosius, W.T.; Brillon, D.J.; Buse, J.B.; Byington, R.P.; Cohen, R.M.; Goff, D.C., Jr.; Malozowski, S.; Margolis, K.L.; Probstfield, J.L.; et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010, 33, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Agmon, O.; Sigawi, T.; Ilan, Y. The constrained disorder principle: A novel framework for understanding glycemic variability and optimizing diabetes management. Endocr. Metab. Sci. 2025, 19, 100262. [Google Scholar] [CrossRef]
- Vrana-Diaz, C.J.; Balasubramanian, P.; Kayadjanian, N.; Bohonowych, J.; Strong, T.V. Variability and change over time of weight and BMI among adolescents and adults with Prader-Willi syndrome: A 6-month text-based observational study. Orphanet J. Rare Dis. 2020, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Massey, R.J.; Siddiqui, M.K.; Pearson, E.R.; Dawed, A.Y. Weight variability and cardiovascular outcomes: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2023, 22, 5. [Google Scholar] [CrossRef]
- Mehran, L.; Mousapour, P.; Khalili, D.; Cheraghi, L.; Honarvar, M.; Amouzegar, A.; Azizi, F. BMI variability and incident diabetes mellitus, Tehran Lipid and Glucose Study (TLGS). Sci. Rep. 2022, 12, 18370. [Google Scholar] [CrossRef]
- Shin, Y.L.; Yoo, H.; Hong, J.Y.; Kim, J.; Han, K.D.; Lee, K.N.; Kim, Y.H. Glucose Control in Korean Patients with Type 2 Diabetes Mellitus according to Body Mass Index. J. Obes. Metab. Syndr. 2023, 32, 55–63. [Google Scholar] [CrossRef]
- Ilan, Y. Using the Constrained Disorder Principle to Navigate Uncertainties in Biology and Medicine: Refining Fuzzy Algorithms. Biology 2024, 13, 830. [Google Scholar] [CrossRef]
- Ilan, Y. The Constrained Disorder Principle Overcomes the Challenges of Methods for Assessing Uncertainty in Biological Systems. J. Pers. Med. 2025, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. The constrained disorder principle and the law of increasing functional information: The elephant versus the Moeritherium. Comput. Struct. Biotechnol. Rep. 2025, 2, 100040. [Google Scholar] [CrossRef]
- Ilan, Y. The Constrained Disorder Principle Defines Mitochondrial Variability and Provides A Platform for A Novel Mechanism for Improved Functionality of Complex Systems. Fortune J. Health Sci. 2024, 7, 338–347. [Google Scholar] [CrossRef]
- Theodorakis, N.; Nikolaou, M. The Human Energy Balance: Uncovering the Hidden Variables of Obesity. Diseases 2025, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.G.; Soares, J.; Caspersen, C.J.; McCurdy, T. Examining variations of resting metabolic rate of adults: A public health perspective. Med. Sci. Sports Exerc. 2014, 46, 1352–1358. [Google Scholar] [CrossRef]
- Ndahimana, D.; Kim, E.K. Measurement Methods for Physical Activity and Energy Expenditure: A Review. Clin. Nutr. Res. 2017, 6, 68–80. [Google Scholar] [CrossRef]
- Gitsi, E.; Kokkinos, A.; Konstantinidou, S.K.; Livadas, S.; Argyrakopoulou, G. The Relationship between Resting Metabolic Rate and Body Composition in People Living with Overweight and Obesity. J. Clin. Med. 2024, 13, 5862. [Google Scholar] [CrossRef]
- Aliprandi, G.; Bissolotti, L.; Turla, D.; Vallet, M.; Scarazzato, M.; Fredi, M. The use of REE determination in a clinical setting applied to respiratory disease. Acta Diabetol. 2001, 38, 27–30. [Google Scholar] [CrossRef]
- Goedecke, J.H.; St Clair Gibson, A.; Grobler, L.; Collins, M.; Noakes, T.D.; Lambert, E.V. Determinants of the variability in respiratory exchange ratio at rest and during exercise in trained athletes. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1325–E1334. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Murison, S.D.; Duncan, J.S.; Rance, K.A.; Speakman, J.R. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am. J. Clin. Nutr. 2005, 82, 941–948. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Norton, L.E. Metabolic adaptation to weight loss: Implications for the athlete. J. Int. Soc. Sports Nutr. 2014, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Salgado Nunez Del Prado, S.; Celi, F.S. Thyroid Hormone Action and Energy Expenditure. J. Endocr. Soc. 2019, 3, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Bacha, F.; Bartz, S.K.; Puyau, M.; Adolph, A.; Sharma, S. Metabolic flexibility across the spectrum of glycemic regulation in youth. JCI Insight 2021, 6, e14600. [Google Scholar]
- Rothschild, J.A.; Kilding, A.E.; Stewart, T.; Plews, D.J. Factors Influencing Substrate Oxidation During Submaximal Cycling: A Modelling Analysis. Sports Med. 2022, 52, 2775–2795. [Google Scholar] [CrossRef]
- Brun, J.F.; Myzia, J.; Varlet-Marie, E.; Raynaud de Mauverger, E.; Mercier, J. Beyond the Calorie Paradigm: Taking into Account in Practice the Balance of Fat and Carbohydrate Oxidation during Exercise? Nutrients 2022, 14, 1605. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Badve, S.V.; Bilal, A.; Lee, M.M.Y.; Sattar, N.; Gerstein, H.C.; Ruff, C.T.; McMurray, J.J.V.; Rossing, P.; Bakris, G.; Mahaffey, K.W.; et al. Effects of GLP-1 receptor agonists on kidney and cardiovascular disease outcomes: A meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2025, 13, 15–28. [Google Scholar] [CrossRef]
- Nevola, R.; Epifani, R.; Imbriani, S.; Tortorella, G.; Aprea, C.; Galiero, R.; Rinaldi, L.; Marfella, R.; Sasso, F.C. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1703. [Google Scholar] [CrossRef]
- Arredouani, A. GLP-1 receptor agonists, are we witnessing the emergence of a paradigm shift for neuro-cardio-metabolic disorders? Pharmacol. Ther. 2025, 269, 108824. [Google Scholar] [CrossRef]
- Griffioen, K.J.; Wan, R.; Okun, E.; Wang, X.; Lovett-Barr, M.R.; Li, Y.; Mughal, M.R.; Mendelowitz, D.; Mattson, M.P. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc. Res. 2010, 89, 72–78. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, C.H.; Huang, Y.Y.; Chen, H.Y.; Tai, A.S.; Fu, S.C.; Hsieh, S.H.; Sun, J.H.; Chen, S.T.; Lin, S.H. Regimen comprising GLP-1 receptor agonist and basal insulin can decrease the effect of food on glycemic variability compared to a pre-mixed insulin regimen. Eur. J. Med. Res. 2022, 27, 273. [Google Scholar] [CrossRef]
- Moll, H.; Frey, E.; Gerber, P.; Geidl, B.; Kaufmann, M.; Braun, J.; Beuschlein, F.; Puhan, M.A.; Yebyo, H.G. GLP-1 receptor agonists for weight reduction in people living with obesity but without diabetes: A living benefit-harm modelling study. eClinicalMedicine 2024, 73, 102661. [Google Scholar] [CrossRef]
- Squire, P.; Naude, J.; Zentner, A.; Bittman, J.; Khan, N. Factors associated with weight loss response to GLP-1 analogues for obesity treatment: A retrospective cohort analysis. BMJ Open 2025, 15, e089477. [Google Scholar] [CrossRef]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.Y.; Peters, T.M.; Eisenberg, M.J. The expanding role of GLP-1 receptor agonists: A narrative review of current evidence and future directions. eClinicalMedicine 2025, 86, 103363. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Siriyotha, S.; Anothaisintawee, T.; Looareesuwan, P.; Nimitphong, H.; McKay, G.J.; Attia, J.; Thakkinstian, A. Effectiveness of glucagon-like peptide-1 receptor agonists for reduction of body mass index and blood glucose control in patients with type 2 diabetes mellitus and obesity: A retrospective cohort study and difference-in-difference analysis. BMJ Open 2024, 14, e086424. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.F.; Pusapati, S.; Anwar, M.S.; Lohana, D.; Kumar, P.; Nandula, S.A.; Nawaz, F.K.; Tracey, K.; Yang, H.; LeRoith, D.; et al. Glucagon-like peptide-1: A multi-faceted anti-inflammatory agent. Front. Immunol. 2023, 14, 1148209. [Google Scholar] [CrossRef]
- Eghbali, M.; Alaei-Shahmiri, F.; Hashemi-Madani, N.; Emami, Z.; Mostafavi, L.; Malek, M.; Khamseh, M.E. Glucagon-Like Peptide 1 (GLP-1) Receptor Variants and Glycemic Response to Liraglutide: A Pharmacogenetics Study in Iranian People with Type 2 Diabetes Mellitus. Adv. Ther. 2024, 41, 826–836. [Google Scholar] [CrossRef]
- Saini, V.M.; Liu, K.R.; Surve, A.S.; Gupta, S.; Gupta, A. MicroRNAs as biomarkers for monitoring cardiovascular changes in Type II Diabetes Mellitus (T2DM) and exercise. J. Diabetes Metab. Disord. 2022, 21, 1819–1832. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Konishi, K.; Tsunoda, T.; Koyama, H. Significance of Glycemic Variability in Diabetes Mellitus. Intern. Med. 2022, 61, 281–290. [Google Scholar] [CrossRef]
- Heni, M.; Frühwald, L.; Karges, W.; Naudorf, M.; Niemöller, K.; Pagnia, F.; Reindel, J.; Seufert, J.; Ufer, G.; Wagner, C.; et al. Heterogeneity in response to GLP-1 receptor agonists in type 2 diabetes in real-world clinical practice: Insights from the DPV register—An IMI-SOPHIA study. Diabetologia 2025, 68, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Gulkarov, S.; Lau, R.; Klek, S.P.; Srivastava, A.; Renna, H.A.; De Leon, J. Weight Reduction with GLP-1 Agonists and Paths for Discontinuation While Maintaining Weight Loss. Biomolecules 2025, 15, 408. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.G.; da Silva, V.L.J.; de Azevedo Marques Lopes, F.; Bouskela, E.; Coelho de Souza, M.D.G.; Kraemer-Aguiar, L.G. Ghrelin and glucagon-like peptide-1 according to body adiposity and glucose homeostasis. Arch. Endocrinol. Metab. 2023, 67, e000611. [Google Scholar] [CrossRef] [PubMed]
- Nathan, B.M.; Rudser, K.D.; Abuzzahab, M.J.; Fox, C.K.; Coombes, B.J.; Bomberg, E.M.; Kelly, A.S. Predictors of weight-loss response with glucagon-like peptide-1 receptor agonist treatment among adolescents with severe obesity. Clin. Obes. 2016, 6, 73–78. [Google Scholar] [CrossRef]
- Zhu, X.; Fowler, M.J.; Wells, Q.S.; Stafford, J.M.; Gannon, M. Predicting responsiveness to GLP-1 pathway drugs using real-world data. BMC Endocr. Disord. 2024, 24, 269. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Palecek, E.J.; Kimzey, M.M.; Zhang, J.; Marsden, J.; Bays, C.; Moran, W.P.; Mauldin, P.D.; Schreiner, A.D. Glucagon-like peptide-1 receptor agonist therapy effects on glycemic control and weight in a primary care clinic population. J. Investig. Med. 2024, 72, 911–919. [Google Scholar] [CrossRef]
- Adar, O.; Shakargy, J.D.; Ilan, Y. The Constrained Disorder Principle: Beyond Biological Allostasis. Biology 2025, 14, 339. [Google Scholar] [CrossRef]
- Ilan, Y. The Relationship Between Biological Noise and Its Application: Understanding System Failures and Suggesting a Method to Enhance Functionality Based on the Constrained Disorder Principle. Biology 2025, 14, 349. [Google Scholar] [CrossRef]
- Gelman, R.; Hurvitz, N.; Nesserat, R.; Kolben, Y.; Nachman, D.; Jamil, K.; Agus, S.; Asleh, R.; Amir, O.; Berg, M.; et al. A second-generation artificial intelligence-based therapeutic regimen improves diuretic resistance in heart failure: Results of a feasibility open-labeled clinical trial. Biomed. Pharmacother. 2023, 161, 114334. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, N.; Dinur, T.; Revel-Vilk, S.; Agus, S.; Berg, M.; Zimran, A.; Ilan, Y. A Feasibility Open-Labeled Clinical Trial Using a Second-Generation Artificial-Intelligence-Based Therapeutic Regimen in Patients with Gaucher Disease Treated with Enzyme Replacement Therapy. J. Clin. Med. 2024, 13, 3325. [Google Scholar] [CrossRef] [PubMed]
- Sigawi, T.; Gelman, R.; Maimon, O.; Yossef, A.; Hemed, N.; Agus, S.; Berg, M.; Ilan, Y.; Popovtzer, A. Improving the response to lenvatinib in partial responders using a Constrained-Disorder-Principle-based second-generation artificial intelligence-therapeutic regimen: A proof-of-concept open-labeled clinical trial. Front. Oncol. 2024, 14, 1426426. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, N.; Lehman, H.; Hershkovitz, Y.; Kolben, Y.; Jamil, K.; Agus, S.; Berg, M.; Aamar, S.; Ilan, Y. A constrained disorder principle-based second-generation artificial intelligence digital medical cannabis system: A real-world data analysis. J. Public Health Res. 2025, 14, 22799036251337640. [Google Scholar] [CrossRef]
- Ilan, Y. Overcoming Compensatory Mechanisms toward Chronic Drug Administration to Ensure Long-Term, Sustainable Beneficial Effects. Mol. Ther. Methods Clin. Dev. 2020, 18, 335–344. [Google Scholar] [CrossRef]
- Bayatra, A.; Nasserat, R.; Ilan, Y. Overcoming Low Adherence to Chronic Medications by Improving their Effectiveness Using a Personalized Second-generation Digital System. Curr. Pharm. Biotechnol. 2024, 25, 2078–2088. [Google Scholar] [CrossRef]
- Ilan, Y. Why targeting the microbiome is not so successful: Can randomness overcome the adaptation that occurs following gut manipulation? Clin. Exp. Gastroenterol. 2019, 12, 209–217. [Google Scholar] [CrossRef]
- Ilan, Y. The Co-Piloting Model for Using Artificial Intelligence Systems in Medicine: Implementing the Constrained-Disorder-Principle-Based Second-Generation System. Bioengineering 2024, 11, 1111. [Google Scholar] [CrossRef]
- Volkova, S.; Matos, M.R.A.; Mattanovich, M.; Marín de Mas, I. Metabolic Modelling as a Framework for Metabolomics Data Integration and Analysis. Metabolites 2020, 10, 303. [Google Scholar] [CrossRef]
- Akbari, A.; Yurkovich, J.T.; Zielinski, D.C.; Palsson, B.O. The quantitative metabolome is shaped by abiotic constraints. Nat. Commun. 2021, 12, 3178. [Google Scholar] [CrossRef]
- Barreda, L.; Boutet, S.; De Vos, D.; Boulard, C.; Grain, D.; Lepiniec, L.; Corso, M. Specialized metabolome and transcriptome atlas of developing Arabidopsis thaliana seed under warm temperatures. Sci. Data 2025, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, N.; Scossa, F.; Fernie, A.; Nikoloski, Z. Variability of metabolite levels is linked to differential metabolic pathways in Arabidopsis’s responses to abiotic stresses. PLoS Comput. Biol. 2014, 10, e1003656. [Google Scholar] [CrossRef] [PubMed]
- Moulin, C.; Tournier, L.; Peres, S. Combining Kinetic and Constraint-Based Modelling to Better Understand Metabolism Dynamics. Processes 2021, 9, 1701. [Google Scholar] [CrossRef]
- Nieva, A.S.; Romero, F.M.; Erban, A.; Carrasco, P.; Ruiz, O.A.; Kopka, J. Metabolic Profiling and Metabolite Correlation Network Analysis Reveal That Fusarium solani Induces Differential Metabolic Responses in Lotus japonicus and Lotus tenuis against Severe Phosphate Starvation. J. Fungi 2021, 7, 765. [Google Scholar] [CrossRef]
- Oh, S.J.; Joung, J.G.; Chang, J.H.; Zhang, B.T. Construction of phylogenetic trees by kernel-based comparative analysis of metabolic networks. BMC Bioinform. 2006, 7, 284. [Google Scholar] [CrossRef]
- Ivanov, P.C. The New Field of Network Physiology: Building the Human Physiolome. Front. Netw. Physiol. 2021, 1, 711778. [Google Scholar] [CrossRef]
- Ramon, C.; Stelling, J. Functional comparison of metabolic networks across species. Nat. Commun. 2023, 14, 1699. [Google Scholar] [CrossRef]
- Tun, K.; Dhar, P.K.; Palumbo, M.C.; Giuliani, A. Metabolic pathways variability and sequence/networks comparisons. BMC Bioinform. 2006, 7, 24. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Xin, Y.; Wang, Y. Circadian secretion rhythm of GLP-1 and its influencing factors. Front. Endocrinol. 2022, 13, 991397. [Google Scholar] [CrossRef]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Almabruk, B.A.; Alharbi, S.H.; Alsaqer, F.S.; Al Essa, A.; Eid, H.; Alqahtani, O.; Badawood, M.A.; Alzahrani, E.M.; Alzahrani, E.M.; Alshaikh, F.K.; et al. The Role of Intermittent Fasting on Metabolic Syndrome: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e71623. [Google Scholar] [CrossRef]
- Lazar, S.; Reurean-Pintilei, D.V.; Ionita, I.; Avram, V.F.; Herascu, A.; Timar, B. Glycemic Variability and Its Association with Traditional Glycemic Control Biomarkers in Patients with Type 1 Diabetes: A Cross-Sectional, Multicenter Study. J. Clin. Med. 2025, 14, 2434. [Google Scholar] [CrossRef]
- Rinott, E.; Sigawi, T.; Hurvitz, N.; Elkhateeb, N.; Rinsky-Halivni, L.; Ilan, Y. Variability in Exercise is Linked to Improved Age-related Dysfunctions, Suggesting a Potential Role for the Constrained-Disorder Principle-based Second-Generation Artificial Intelligence System. Curr. Aging Sci. 2025. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landau, J.; Tiram, Y.; Ilan, Y. Employing an Artificial Intelligence Platform to Enhance Treatment Responses to GLP-1 Agonists by Utilizing Metabolic Variability Signatures Based on the Constrained Disorder Principle. Biomedicines 2025, 13, 2645. https://doi.org/10.3390/biomedicines13112645
Landau J, Tiram Y, Ilan Y. Employing an Artificial Intelligence Platform to Enhance Treatment Responses to GLP-1 Agonists by Utilizing Metabolic Variability Signatures Based on the Constrained Disorder Principle. Biomedicines. 2025; 13(11):2645. https://doi.org/10.3390/biomedicines13112645
Chicago/Turabian StyleLandau, Jakob, Yariv Tiram, and Yaron Ilan. 2025. "Employing an Artificial Intelligence Platform to Enhance Treatment Responses to GLP-1 Agonists by Utilizing Metabolic Variability Signatures Based on the Constrained Disorder Principle" Biomedicines 13, no. 11: 2645. https://doi.org/10.3390/biomedicines13112645
APA StyleLandau, J., Tiram, Y., & Ilan, Y. (2025). Employing an Artificial Intelligence Platform to Enhance Treatment Responses to GLP-1 Agonists by Utilizing Metabolic Variability Signatures Based on the Constrained Disorder Principle. Biomedicines, 13(11), 2645. https://doi.org/10.3390/biomedicines13112645






