Association Between Renal Cell Cancer and Chronic Kidney Disease: An Update on a Never-Healing Wound
Abstract
1. Introduction
2. Literature Search
3. RCC in Patients with Renal Disease
3.1. Epidemiology of RCC in Patients with Renal Disease
3.2. Risk Factors for the Development of RCC in Patients with Renal Disease
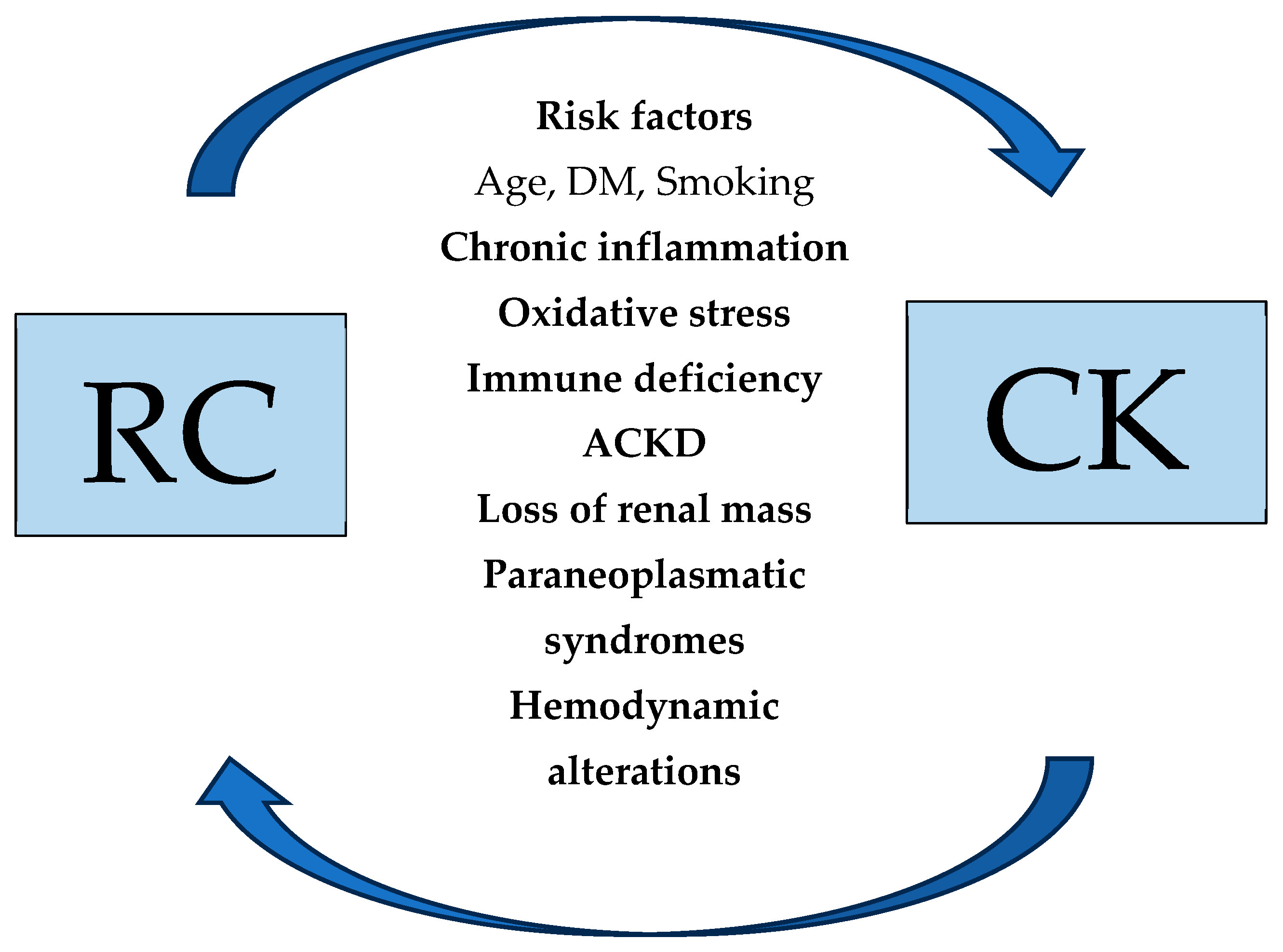
3.3. Pathophysiologic Mechanisms of RCC Development in Patients with Renal Disease
3.3.1. The Role of Chronic Inflammation, Oxidative Stress, and Immune Deficiency
3.3.2. ACKD as a Precursor to Malignancy
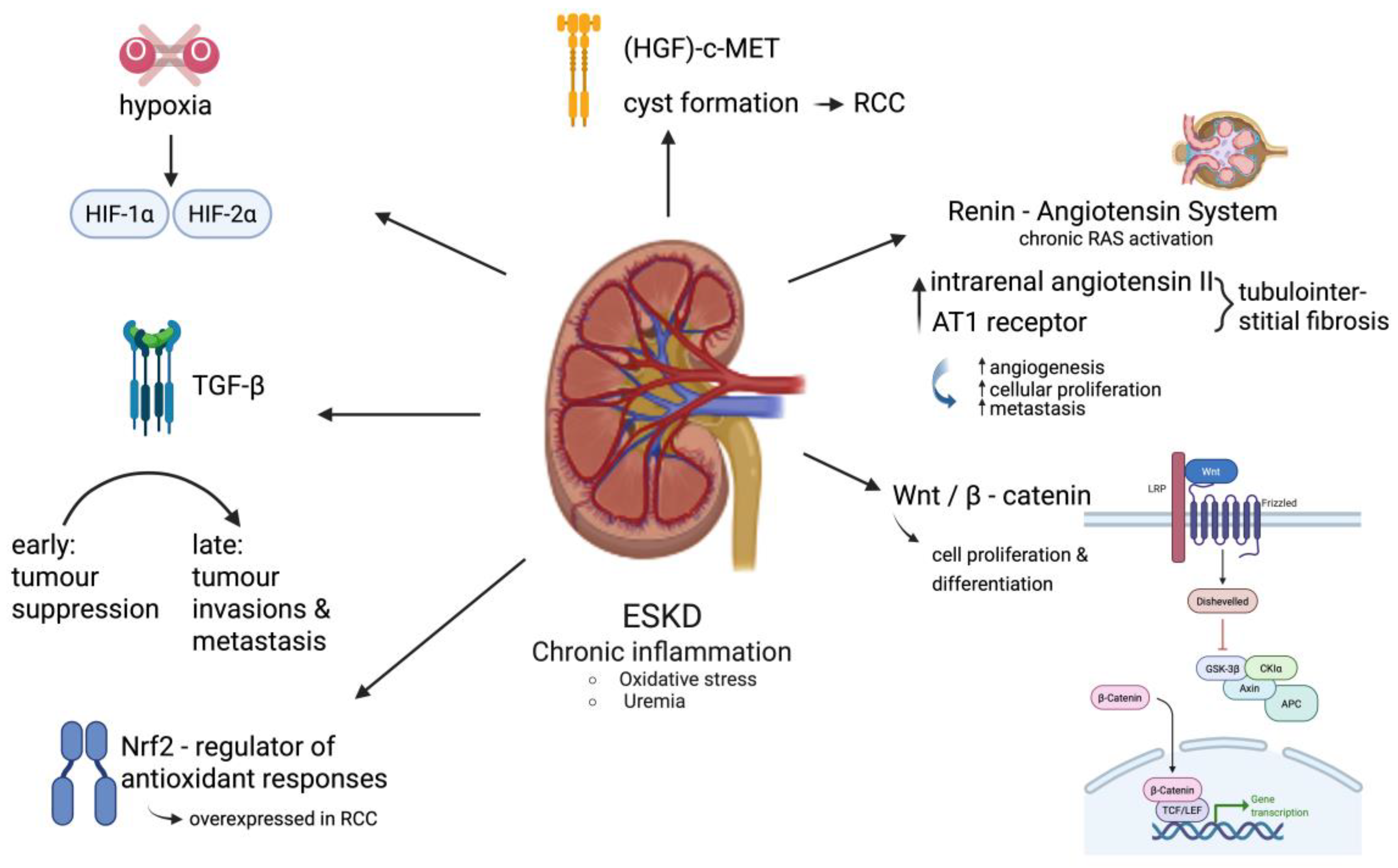
3.3.3. Epigenetic Alterations and Molecular Pathways in ESKD-Associated RCC
4. Renal Disease in Patients with RCC
4.1. Epidemiology of Renal Disease in Patients with RCC
4.2. Risk Factors for the Development of Renal Disease in RCC Patients
4.3. Pathophysiologic Mechanisms of Renal Disease Development in RCC Patients
4.3.1. Direct Tumor Effects on Kidney Dysfunction
4.3.2. Systemic Tumor Effects on Kidney Dysfunction
4.3.3. Paraneoplastic Syndromes
4.3.4. Systemic Inflammation and Oxidative Stress
4.3.5. Hemodynamic Alterations and Electrolyte Imbalances
5. Management of RCC in ESKD Patients
6. Future Directions
7. Limitations
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rose, T.L.; Kim, W.Y. Renal Cell Carcinoma: A Review. JAMA 2024, 332, 1001–1010. [Google Scholar] [CrossRef]
- Cairns, P. Renal cell carcinoma. Cancer Biomark. 2010, 9, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Saly, D.L.; Eswarappa, M.S.; Street, S.E.; Deshpande, P. Renal Cell Cancer and Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2021, 28, 460–468.e461. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A.; Dreicer, R.; Rosner, M.H. Renal cell carcinoma for the nephrologist. Kidney Int. 2018, 94, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, S.A.; Lokkegaard, H.; Storm, H.H. Cancer risk in patients on dialysis and after renal transplantation. Lancet 2000, 355, 1886–1887. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Agodoa, L.; Gellert, R.; Stewart, J.H.; Buccianti, G.; Lowenfels, A.B.; Wolfe, R.A.; Jones, E.; Disney, A.P.; Briggs, D.; et al. Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet 1999, 354, 93–99. [Google Scholar] [CrossRef]
- Park, J.; Shin, D.W.; Han, K.; Kim, D.; Chun, S.; Jang, H.R. Associations Between Kidney Function, Proteinuria, and the Risk of Kidney Cancer: A Nationwide Cohort Study Involving 10 Million Participants. Am. J. Epidemiol. 2021, 190, 2042–2052. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Corley, D.A.; Zhao, W.K.; Colt, J.S.; Shuch, B.; Chow, W.H.; Purdue, M.P. Chronic kidney disease and risk of renal cell carcinoma: Differences by race. Epidemiology 2015, 26, 59–67. [Google Scholar] [CrossRef]
- Akerlund, J.; Holmberg, E.; Lindblad, P.; Stendahl, M.; Ljungberg, B.; Thorstenson, A.; Lundstam, S. Increased risk for renal cell carcinoma in end stage renal disease—A population-based case-control study. Scand. J. Urol. 2021, 55, 209–214. [Google Scholar] [CrossRef]
- El-Zaatari, Z.M.; Truong, L.D. Renal Cell Carcinoma in End-Stage Renal Disease: A Review and Update. Biomedicines 2022, 10, 657. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, J.; Peng, Y.; Zhou, J. Renal cell carcinoma of the native kidney in renal transplant recipients: Case report and literature review. Front. Oncol. 2025, 15, 1536411. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Ragheb, N.E.; Schwartz, A.G.; Hawthorne, V.M. Neoplasms in dialysis patients: A population-based study. Am. J. Kidney Dis. 1989, 14, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Chandhoke, P.S.; Torrence, R.J.; Clayman, R.V.; Rothstein, M. Acquired cystic disease of the kidney: A management dilemma. J. Urol. 1992, 147, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Gulanikar, A.C.; Daily, P.P.; Kilambi, N.K.; Hamrick-Turner, J.E.; Butkus, D.E. Prospective pretransplant ultrasound screening in 206 patients for acquired renal cysts and renal cell carcinoma. Transplantation 1998, 66, 1669–1672. [Google Scholar] [CrossRef]
- Gresele, P.; Harrison, P.; Bury, L.; Falcinelli, E.; Gachet, C.; Hayward, C.P.; Kenny, D.; Mezzano, D.; Mumford, A.D.; Nugent, D.; et al. Diagnosis of suspected inherited platelet function disorders: Results of a worldwide survey. J. Thromb. Haemost. 2014, 12, 1562–1569. [Google Scholar] [CrossRef]
- Weng, P.H.; Hung, K.Y.; Huang, H.L.; Chen, J.H.; Sung, P.K.; Huang, K.C. Cancer-specific mortality in chronic kidney disease: Longitudinal follow-up of a large cohort. Clin. J. Am. Soc. Nephrol. 2011, 6, 1121–1128. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Ordonez, J.; Udaltsova, N.; Russo, P.; Go, A.S. CKD and the risk of incident cancer. J. Am. Soc. Nephrol. 2014, 25, 2327–2334. [Google Scholar] [CrossRef]
- Peired, A.J.; Lazzeri, E.; Guzzi, F.; Anders, H.J.; Romagnani, P. From kidney injury to kidney cancer. Kidney Int. 2021, 100, 55–66. [Google Scholar] [CrossRef]
- Polascik, T.J.; Bostwick, D.G.; Cairns, P. Molecular genetics and histopathologic features of adult distal nephron tumors. Urology 2002, 60, 941–946. [Google Scholar] [CrossRef]
- Peired, A.J.; Antonelli, G.; Angelotti, M.L.; Allinovi, M.; Guzzi, F.; Sisti, A.; Semeraro, R.; Conte, C.; Mazzinghi, B.; Nardi, S.; et al. Acute kidney injury promotes development of papillary renal cell adenoma and carcinoma from renal progenitor cells. Sci. Transl. Med. 2020, 12, eaaw6003. [Google Scholar] [CrossRef]
- Wagatsuma, K.; Satoh, K. Estimation Using an Enhancement Factor on Non Local Thermodynamic Equilibrium Behavior of High-lying Energy Levels of Neutral Atom in Argon Radio-Frequency Inductively-Coupled Plasma. Anal. Sci. 2016, 32, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Denton, M.D.; Magee, C.C.; Ovuworie, C.; Mauiyyedi, S.; Pascual, M.; Colvin, R.B.; Cosimi, A.B.; Tolkoff-Rubin, N. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: A pathologic analysis. Kidney Int. 2002, 61, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Marple, J.T.; MacDougall, M.; Chonko, A.M. Renal cancer complicating acquired cystic kidney disease. J. Am. Soc. Nephrol. 1994, 4, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Hurst, F.P.; Jindal, R.M.; Fletcher, J.J.; Dharnidharka, V.; Gorman, G.; Lechner, B.; Nee, R.; Agodoa, L.Y.; Abbott, K.C. Incidence, predictors and associated outcomes of renal cell carcinoma in long-term dialysis patients. Urology 2011, 77, 1271–1276. [Google Scholar] [CrossRef]
- Glassock, R.J. Is the presence of microalbuminuria a relevant marker of kidney disease? Curr. Hypertens. Rep. 2010, 12, 364–368. [Google Scholar] [CrossRef]
- Luo, L.; Kieneker, L.M.; van der Vegt, B.; Bakker, S.J.L.; Gruppen, E.G.; Casteleijn, N.F.; de Boer, R.A.; Suthahar, N.; de Bock, G.H.; Aboumsallem, J.P.; et al. Urinary albumin excretion and cancer risk: The PREVEND cohort study. Nephrol. Dial. Transplant. 2023, 38, 2723–2732. [Google Scholar] [CrossRef]
- Karami, S.; Yanik, E.L.; Moore, L.E.; Pfeiffer, R.M.; Copeland, G.; Gonsalves, L.; Hernandez, B.Y.; Lynch, C.F.; Pawlish, K.; Engels, E.A. Risk of Renal Cell Carcinoma Among Kidney Transplant Recipients in the United States. Am. J. Transplant. 2016, 16, 3479–3489. [Google Scholar] [CrossRef]
- Klatte, T.; Marberger, M. Renal cell carcinoma of native kidneys in renal transplant patients. Curr. Opin. Urol. 2011, 21, 376–379. [Google Scholar] [CrossRef]
- Gusev, E.; Solomatina, L.; Zhuravleva, Y.; Sarapultsev, A. The Pathogenesis of End-Stage Renal Disease from the Standpoint of the Theory of General Pathological Processes of Inflammation. Int. J. Mol. Sci. 2021, 22, 11453. [Google Scholar] [CrossRef]
- Volovat, S.R.; Volovat, C.; Miron, I.; Kanbay, M.; Goldsmith, D.; Lungulescu, C.; Badarau, S.C.; Covic, A. Oncogenic mechanisms in renal insufficiency. Clin. Kidney J. 2021, 14, 507–515. [Google Scholar] [CrossRef]
- Hu, S.L.; Chang, A.; Perazella, M.A.; Okusa, M.D.; Jaimes, E.A.; Weiss, R.H.; American Society of Nephrology Onco-Nephrology Forum. The Nephrologist’s Tumor: Basic Biology and Management of Renal Cell Carcinoma. J. Am. Soc. Nephrol. 2016, 27, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Russo, P. End stage and chronic kidney disease: Associations with renal cancer. Front. Oncol. 2012, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Semjen, D.; Denes, B.; Somoracz, A.; Fintha, A.; Forika, G.; Jenei, A.; Dobi, D.; Micsik, T.; Eizler, K.V.; Giba, N.; et al. Renal Cell Carcinoma in End-Stage Renal Disease: A Retrospective Study in Patients from Hungary. Pathobiology 2023, 90, 322–332. [Google Scholar] [CrossRef]
- Johnson, T.A.; Maekawa, S.; Fujita, M.; An, J.; Ju, Y.S.; Maejima, K.; Kanazashi, Y.; Jikuya, R.; Okawa, Y.; Sasagawa, S.; et al. Genomic features of renal cell carcinoma developed during end-stage renal disease and dialysis. Hum. Mol. Genet. 2023, 32, 290–303. [Google Scholar] [CrossRef]
- Ishihara, H.; Yamashita, S.; Liu, Y.Y.; Hattori, N.; El-Omar, O.; Ikeda, T.; Fukuda, H.; Yoshida, K.; Takagi, T.; Taneda, S.; et al. Genetic and epigenetic profiling indicates the proximal tubule origin of renal cancers in end-stage renal disease. Cancer Sci. 2020, 111, 4276–4287. [Google Scholar] [CrossRef]
- Banumathy, G.; Cairns, P. Signaling pathways in renal cell carcinoma. Cancer Biol. Ther. 2010, 10, 658–664. [Google Scholar] [CrossRef]
- Su, D.; Singer, E.A.; Srinivasan, R. Molecular pathways in renal cell carcinoma: Recent advances in genetics and molecular biology. Curr. Opin. Oncol. 2015, 27, 217–223. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Milanese, G.; Galosi, A.B.; Pompei, V.; Salvolini, E.; Campagna, R. Nrf2 Signaling in Renal Cell Carcinoma: A Potential Candidate for the Development of Novel Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 13239. [Google Scholar] [CrossRef]
- Miller, D.C.; Schonlau, M.; Litwin, M.S.; Lai, J.; Saigal, C.S.; Urologic Diseases in America Project. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer 2008, 112, 511–520. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Ghanem, Y.A.; Albiges, L.; Bonn, S.; Campi, R.; Capitanio, U.; Dabestani, S.; Hora, M.; Klatte, T.; Kuusk, T.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2025 Update. Eur. Urol. 2025, 87, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Akerlund, J.; Ljungberg, B.; Lundstam, S.; Peeker, R.; Holmberg, E.; Mansson, M.; Bergdahl, A.G. End-stage renal disease after renal cancer surgery: Risk factors and overall survival. Scand. J. Urol. 2024, 59, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Khurana, A.; Hirani, S.; Kidd, J.; Paul, A. Paraneoplastic Glomerulonephropathy Associated With Renal Cell Carcinoma: A Descriptive Analysis of Published Reports. Cureus 2023, 15, e36928. [Google Scholar] [CrossRef]
- Li, L.; Lau, W.L.; Rhee, C.M.; Harley, K.; Kovesdy, C.P.; Sim, J.J.; Jacobsen, S.; Chang, A.; Landman, J.; Kalantar-Zadeh, K. Risk of chronic kidney disease after cancer nephrectomy. Nat. Rev. Nephrol. 2014, 10, 135–145. [Google Scholar] [CrossRef]
- Huang, W.C.; Levey, A.S.; Serio, A.M.; Snyder, M.; Vickers, A.J.; Raj, G.V.; Scardino, P.T.; Russo, P. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. 2006, 7, 735–740. [Google Scholar] [CrossRef]
- O’Donnell, K.; Tourojman, M.; Tobert, C.M.; Kirmiz, S.W.; Riedinger, C.B.; Demirjian, S.; Lane, B.R. Proteinuria is a Predictor of Renal Functional Decline in Patients with Kidney Cancer. J. Urol. 2016, 196, 658–663. [Google Scholar] [CrossRef]
- Dey, S.; Hamilton, Z.; Noyes, S.L.; Tobert, C.M.; Keeley, J.; Derweesh, I.H.; Lane, B.R. Chronic Kidney Disease Is More Common in Locally Advanced Renal Cell Carcinoma. Urology 2017, 105, 101–107. [Google Scholar] [CrossRef]
- Woldu, S.L.; Weinberg, A.C.; RoyChoudhury, A.; Chase, H.; Kalloo, S.D.; McKiernan, J.M.; DeCastro, G.J. Renal insufficiency is associated with an increased risk of papillary renal cell carcinoma histology. Int. Urol. Nephrol. 2014, 46, 2127–2132. [Google Scholar] [CrossRef]
- Lane, B.R.; Babineau, D.C.; Poggio, E.D.; Weight, C.J.; Larson, B.T.; Gill, I.S.; Novick, A.C. Factors predicting renal functional outcome after partial nephrectomy. J. Urol. 2008, 180, 2363–2368, discussion 2368–2369. [Google Scholar] [CrossRef]
- Ellis, R.J.; White, V.M.; Bolton, D.M.; Coory, M.D.; Davis, I.D.; Francis, R.S.; Giles, G.G.; Gobe, G.C.; Neale, R.E.; Wood, S.T.; et al. Tumor size and postoperative kidney function following radical nephrectomy. Clin. Epidemiol. 2019, 11, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Otaibi, M.A.; Tanguay, S. Locally advanced renal cell carcinoma. Can. Urol. Assoc. J. 2007, 1, S55–S61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazhar, H.R.; Aeddula, N.R. Renal Vein Thrombosis; StatPearls, Treasure Island (FL) Ineligible Companies: Treasure Island, FL, USA, 2025. [Google Scholar][Green Version]
- Arpita, S.; Manoj, J.; Kaushik, S.; Anish, S. Metastasis of renal cell carcinoma to urinary bladder: A rare case report with review of literature. J. Lab. Physicians 2017, 9, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Nabi, S.; Kessler, E.R.; Bernard, B.; Flaig, T.W.; Lam, E.T. Renal cell carcinoma: A review of biology and pathophysiology. F1000Res 2018, 7, 307. [Google Scholar] [CrossRef]
- Chakraborty, S.; Balan, M.; Sabarwal, A.; Choueiri, T.K.; Pal, S. Metabolic reprogramming in renal cancer: Events of a metabolic disease. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188559. [Google Scholar] [CrossRef]
- Palapattu, G.S.; Kristo, B.; Rajfer, J. Paraneoplastic syndromes in urologic malignancy: The many faces of renal cell carcinoma. Rev. Urol. 2002, 4, 163–170. [Google Scholar]
- Pepper, K.; Jaowattana, U.; Starsiak, M.D.; Halkar, R.; Hornaman, K.; Wang, W.; Dayamani, P.; Tangpricha, V. Renal cell carcinoma presenting with paraneoplastic hypercalcemic coma: A case report and review of the literature. J. Gen. Intern. Med. 2007, 22, 1042–1046. [Google Scholar] [CrossRef]
- Moyses-Neto, M.; Guimaraes, F.M.; Ayoub, F.H.; Vieira-Neto, O.M.; Costa, J.A.; Dantas, M. Acute renal failure and hypercalcemia. Ren. Fail. 2006, 28, 153–159. [Google Scholar] [CrossRef]
- Habas, E., Sr.; Eledrisi, M.; Khan, F.; Elzouki, A.Y. Secondary Hyperparathyroidism in Chronic Kidney Disease: Pathophysiology and Management. Cureus 2021, 13, e16388. [Google Scholar] [CrossRef]
- Singh Singh, A.; Naranjo, J.; Alonso, M.; Villanego, F.; Amaro, J.M.; Carrasco, D.; Salvatierra, C.; Mazuecos, A. Successful treatment of renal cell carcinoma with paraneoplastic nephrotic syndrome: A case report. Front. Med. 2025, 12, 1506592. [Google Scholar] [CrossRef]
- Fontes-Sousa, M.; Magalhaes, H.; da Silva, F.C.; Mauricio, M.J. Stauffer’s syndrome: A comprehensive review and proposed updated diagnostic criteria. Urol. Oncol. 2018, 36, 321–326. [Google Scholar] [CrossRef]
- Sharma, N.; Darr, U.; Darr, A.; Sood, G. Stauffer Syndrome: A Comprehensive Review of the Icteric Variant of the Syndrome. Cureus 2019, 11, e6032. [Google Scholar] [CrossRef] [PubMed]
- Algarra, M.A.; Fita, M.J.J.; Sandiego, S.; Aguilar, H.A.; Alvarez, P.; Quispe, M.; Salvador, A.; Egido, A.; Lavernia, J.; Machado, I.; et al. Advanced systemic amyloidosis secondary to metastatic renal cell carcinoma. Ecancermedicalscience 2020, 14, 1156. [Google Scholar] [CrossRef] [PubMed]
- Kruk, L.; Mamtimin, M.; Braun, A.; Anders, H.J.; Andrassy, J.; Gudermann, T.; Mammadova-Bach, E. Inflammatory Networks in Renal Cell Carcinoma. Cancers 2023, 15, 2212. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Zhang, T.; Wu, T.; Brugarolas, J. Facts and Hopes for Immunotherapy in Renal Cell Carcinoma. Clin. Cancer Res. 2022, 28, 5013–5020. [Google Scholar] [CrossRef]
- Imig, J.D.; Ryan, M.J. Immune and inflammatory role in renal disease. Compr. Physiol. 2013, 3, 957–976. [Google Scholar] [CrossRef]
- Rashid, H.; Jali, A.; Akhter, M.S.; Abdi, S.A.H. Molecular Mechanisms of Oxidative Stress in Acute Kidney Injury: Targeting the Loci by Resveratrol. Int. J. Mol. Sci. 2023, 25, 3. [Google Scholar] [CrossRef]
- Brown, R.B.; Mielke, J.G. Carcinogenesis Associated with Toxin Nephropathy: Proposed Mediation by Phosphate Toxicity. Cells 2025, 14, 952. [Google Scholar] [CrossRef]
- Hammouri, D.; Orwick, A.; Doll, M.A.; Sanchez Vega, D.; Shah, P.P.; Clarke, C.J.; Clem, B.; Beverly, L.J.; Siskind, L.J. Remote organ cancer induces kidney injury, inflammation, and fibrosis and adversely alters renal function. Am. J. Physiol. Renal Physiol. 2025, 328, F272–F288. [Google Scholar] [CrossRef]
- Steffens, J.; Bock, R.; Braedel, H.U.; Isenberg, E.; Buhrle, C.P.; Ziegler, M. Renin-producing renal cell carcinoma. Eur. Urol. 1990, 18, 56–60. [Google Scholar] [CrossRef]
- Handler, J. Renal cell carcinoma and hypertension. J. Clin. Hypertens. 2005, 7, 249–251. [Google Scholar] [CrossRef]
- Puckett, L. Renal and electrolyte complications in eating disorders: A comprehensive review. J. Eat. Disord. 2023, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Trump, D.L.; Johnson, C.S.; Feldman, D. The role of vitamin D in cancer prevention and treatment. Endocrinol. Metab. Clin. N. Am. 2010, 39, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Reungjui, S.; Roncal, C.A.; Sato, W.; Glushakova, O.Y.; Croker, B.P.; Suga, S.; Ouyang, X.; Tungsanga, K.; Nakagawa, T.; Johnson, R.J.; et al. Hypokalemic nephropathy is associated with impaired angiogenesis. J. Am. Soc. Nephrol. 2008, 19, 125–134. [Google Scholar] [CrossRef]
- Robinson, S.; Nag, A.; Peticca, B.; Prudencio, T.; Di Carlo, A.; Karhadkar, S. Renal Cell Carcinoma in End-Stage Kidney Disease and the Role of Transplantation. Cancers 2023, 16, 3. [Google Scholar] [CrossRef]
- Fergany, A.F.; Saad, I.R.; Woo, L.; Novick, A.C. Open partial nephrectomy for tumor in a solitary kidney: Experience with 400 cases. J. Urol. 2006, 175, 1630–1633, discussion 1633. [Google Scholar] [CrossRef]
- Henriksen, K.J.; Meehan, S.M.; Chang, A. Non-neoplastic renal diseases are often unrecognized in adult tumor nephrectomy specimens: A review of 246 cases. Am. J. Surg. Pathol. 2007, 31, 1703–1708. [Google Scholar] [CrossRef]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Renal function after partial nephrectomy: Effect of warm ischemia relative to quantity and quality of preserved kidney. Urology 2012, 79, 356–360. [Google Scholar] [CrossRef]
- Gan, Z.S.; Besharatian, B.D.; Naji, A.; Pierorazio, P.M. The Case for Active Surveillance of Small Renal Masses in Renal Transplant Recipient Candidates. J. Urol. 2023, 209, 3–5. [Google Scholar] [CrossRef]
- Schaarschmidt, B.M.; Zensen, S.; Kesch, C.; Dertnig, T.; Opitz, M.; Drews, M.; Nadjiri, J.; Forsting, M.; Hadaschik, B.A.; Haubold, J. Current use of percutaneous ablation in renal tumors: An analysis of the registry of the German Society for Interventional Radiology and Minimally Invasive Therapy. Eur. Radiol. 2025, 35, 1723–1731. [Google Scholar] [CrossRef]
- Iguchi, T.; Matsui, Y.; Tomita, K.; Uka, M.; Umakoshi, N.; Kawabata, T.; Gobara, H.; Araki, M.; Hiraki, T. Ablation of Kidney Tumors in Patients with Substantial Kidney Impairment: Current Status. Curr. Oncol. Rep. 2024, 26, 573–582. [Google Scholar] [CrossRef]
- Maringhini, S.; Zoccali, C. Chronic Kidney Disease Progression-A Challenge. Biomedicines 2024, 12, 2203. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, J.; Huang, W.; Wu, Z.; Gong, J.; Wang, Q.; Liu, Q.; Wang, C.; Zhu, Y.; Ding, X.; et al. A retrospective study of CT-guided percutaneous irreversible electroporation (IRE) ablation: Clinical efficacy and safety. BMC Cancer 2021, 21, 124. [Google Scholar] [CrossRef]
- Giannini, L.; Torrisi, M.; Deantoni, C.L.; Tummineri, R.; Fodor, A. Stereotactic Body Radiotherapy for Multiple Renal Cell Carcinoma Lesions in a Patient With Polycystic Kidney Disease After Partial Nephrectomy. Cureus 2025, 17, e83080. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Sorbo, D.; Ronco, F.; Ronco, C. Key Considerations regarding the Renal Risks of Iodinated Contrast Media: The Nephrologist’s Role. Cardiorenal Med. 2023, 13, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Bander, N.H.; Nanus, D.M. Renal-cell carcinoma. N. Engl. J. Med. 1996, 335, 865–875. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Staehler, M.; Negrier, S.; Chevreau, C.; Desai, A.A.; Rolland, F.; et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J. Clin. Oncol. 2009, 27, 3312–3318. [Google Scholar] [CrossRef]
- Hayman, S.R.; Leung, N.; Grande, J.P.; Garovic, V.D. VEGF inhibition, hypertension, and renal toxicity. Curr. Oncol. Rep. 2012, 14, 285–294. [Google Scholar] [CrossRef]
- Kao, C.C.; Liu, J.S.; Chang, Y.K.; Lin, M.H.; Lin, Y.C.; Chen, H.H.; Chang, W.C.; Hsu, C.C.; Wu, M.S. Cancer and mTOR inhibitors in kidney transplantation recipients. PeerJ 2018, 6, e5864. [Google Scholar] [CrossRef]
- Amiri, F.S. Serum tumor markers in chronic kidney disease: As clinical tool in diagnosis, treatment and prognosis of cancers. Ren. Fail. 2016, 38, 530–544. [Google Scholar] [CrossRef]
| Risk Factors for the Development | |
|---|---|
| RCC in CKD patients | CKD in RCC patients |
| Acquired cystic disease in ESKD patients | Patient characteristics |
| Renal injury | Age, Sex, Ethnicity |
| Vascular injury | Lifestyle Smoking |
| Other Comorbidities | |
| DM, Hypertension, Genetic factors | |
| Pre-surgical renal factors | |
| Proteinuria, Renal Disease, Interstitial fibrosis, Tubular Atrophy, Arteriolar Sclerosis | |
| Surgical factors | |
| Surgical Technique, Tumor Volume | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakodimos, I.; Kaltsas, A.; Koumenis, A.; Mitakidi, E.; Adamos, K.; Deligiannis, D.; Stavropoulos, M.; Kratiras, Z.; Chrisofos, M. Association Between Renal Cell Cancer and Chronic Kidney Disease: An Update on a Never-Healing Wound. Biomedicines 2025, 13, 2638. https://doi.org/10.3390/biomedicines13112638
Giannakodimos I, Kaltsas A, Koumenis A, Mitakidi E, Adamos K, Deligiannis D, Stavropoulos M, Kratiras Z, Chrisofos M. Association Between Renal Cell Cancer and Chronic Kidney Disease: An Update on a Never-Healing Wound. Biomedicines. 2025; 13(11):2638. https://doi.org/10.3390/biomedicines13112638
Chicago/Turabian StyleGiannakodimos, Ilias, Aris Kaltsas, Andreas Koumenis, Evangelia Mitakidi, Konstantinos Adamos, Dimitrios Deligiannis, Marios Stavropoulos, Zisis Kratiras, and Michael Chrisofos. 2025. "Association Between Renal Cell Cancer and Chronic Kidney Disease: An Update on a Never-Healing Wound" Biomedicines 13, no. 11: 2638. https://doi.org/10.3390/biomedicines13112638
APA StyleGiannakodimos, I., Kaltsas, A., Koumenis, A., Mitakidi, E., Adamos, K., Deligiannis, D., Stavropoulos, M., Kratiras, Z., & Chrisofos, M. (2025). Association Between Renal Cell Cancer and Chronic Kidney Disease: An Update on a Never-Healing Wound. Biomedicines, 13(11), 2638. https://doi.org/10.3390/biomedicines13112638







