Percutaneous Temporary Mechanical Circulatory Support as a Bridge to Heart Transplantation in the Current UNOS Allocation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Assessment
2.2. Institutional Standard of Care
2.3. Patient Selection
2.4. Multi-Disciplinary Impella Management
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTT | bridge to transplant |
| DBD | donation after brain death |
| DCD | donation after circulatory death |
| HFCS | heart failure cardiogenic shock |
| LOS | length of stay |
| LVAD | left ventricular assist device |
| MCS | mechanical circulatory support |
| tMCS | temporary mechanical circulatory support |
| UNOS | united network for organ sharing |
References
- Osman, M.; Syed, M.; Patibandla, S.; Sulaiman, S.; Kheiri, B.; Shah, M.K.; Bianco, C.; Balla, S.; Patel, B. Fifteen-Year Trends in Incidence of Cardiogenic Shock Hospitalization and In-Hospital Mortality in the United States. J. Am. Heart Assoc. 2021, 10, e021061. [Google Scholar] [CrossRef] [PubMed]
- Maitra, N.S.; Dugger, S.J.; Balachandran, I.C.; Civitello, A.B.; Khazanie, P.; Rogers, J.G. Impact of the 2018 UNOS Heart Transplant Policy Changes on Patient Outcomes. JACC Heart Fail. 2023, 11, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Uriel, N.; Jorde, U.P.; Woo Pak, S.; Jiang, J.; Clerkin, K.; Takayama, H.; Naka, Y.; Schulze, P.C.; Mancini, D.M. Impact of long term left ventricular assist device therapy on donor allocation in cardiac transplantation. J. Heart Lung Transplant. 2013, 32, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.R.; Kazam, J.; Edwards, P.; Maybaum, S.; Bello, R.A.; D’Alessandro, D.A.; Goldstein, D.J. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J. Card. Surg. 2010, 25, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Onwuzurike, J.; Kingsford, P.; Li, J.P.; Bradley, C.P.; Vaidya, A.S.; Wolfson, A.M.; DePasquale, E.C. Effects of the UNOS Allocation Policy Change on Impella Use as Bridge in Heart Transplant. J. Heart Lung Transplant. 2021, 40 (Suppl. S4), S277. [Google Scholar] [CrossRef]
- Mullan, C.W.; Sen, S.; Ahmad, T. Left Ventricular Assist Devices Versus Heart Transplantation for End Stage Heart Failure is a Misleading Equivalency. JACC Heart Fail. 2021, 9, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Kearns, M.J.; Chou, L.; Aguillon, M.; Passano, E.; Miller, S.; Czer, L.; Megna, D.; Emerson, D.; Esmailian, F.; Trento, A.; et al. Impella 5.0 as a Bridge to Cardiac Transplantation before and after Reclassification of the United Network for Organ Sharing Heart Allocation Criteria. J. Heart Lung Transplant. 2020, 39 (Suppl. S4), S335. [Google Scholar] [CrossRef]
- Xia, Y.; Kim, J.S.; Eng, I.K.; Nsair, A.; Ardehali, A.; Shemin, R.J.; Kwon, M.H. Outcomes of heart transplant recipients bridged with percutaneous versus durable left ventricular assist devices. Clin. Transplant. 2023, 37, e14904. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Vela, M.; Panoulas, V.; García-Saez, D.; de Robertis, F.; Stock, U.; Simon, A.R. Outcomes of heart transplantation in patients bridged with Impella 5.0: Comparison with native chest transplanted patients without preoperative mechanical circulatory support. Artif. Organs 2021, 45, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Reichenspurner, H. Impella 5.5 as an Ideal Bridge to Left Ventricular Assist Device. Interv. Cardiol. Rev. 2021, 16 (Suppl. S2), 10. [Google Scholar] [CrossRef]
- Paghdar, S.; Desai, S.; Jang, J.M.; Ruiz, J.; Malkani, S.; Patel, P.; Yip, D.S.; Leoni, J.C.; Nativi, J.; Sareyyupoglu, B.; et al. One-year survival in recipients older than 50 bridged to heart transplant with Impella 5.5 via axillary approach. J. Geriatr. Cardiol. 2023, 20, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.B.; Fritz, A.V.; Kiley, S.; Sharma, S.; Patel, P.; Heckman, A.; Martin, A.K.; Goswami, R. Comparison of Intraoperative Blood Product Use During Heart Transplantation in Patients Bridged with Impella 5.5 versus Durable Left Ventricular Assist Devices. J. Cardiothorac. Vasc. Anesth. 2024, 38, 2567–2575. [Google Scholar] [CrossRef] [PubMed]

| Impella 5.5 (n = 18) | LVAD (n = 27) | p-Value | |
|---|---|---|---|
| Age in years (Median, IQR) | 61 (54–66) | 52 (44–62) | 0.023 |
| Gender | |||
| • Male (n, %) | 16 (89%) | 19 (70%) | |
| Blood Group | |||
| • A | 4 | 10 | |
| • B | 1 | 2 | |
| • AB | 1 | 0 | |
| • O | 12 | 15 | |
| Baseline Labs and comorbidities | |||
| • Hematocrit (g/dL, Median, IQR) | 29 (26–32) | 34 (31–36) | 0.002 |
| • Body Mass Index (BMI) (Median, IQR) | 28 (26–30) | 30 (25–35) | 0.169 |
| • Creatinine (mg/dL, Median, IQR) | 1.4 (1.3–1.8) | 1.4 (1.1–1.6) | 0.04 |
| • Diabetic status (n, %) | 7 (39%) | 8 (30%) | 0.268 |
| Etiology | |||
| • Ischemic | 6 (33%) | 11 (41%) | |
| • Non-ischemic | 11 (61%) | 14 (52%) | |
| • Congenital | 1 (5%) | 1 (3%) | |
| • LVAD pump exchange | 0 | 1 (3%) | |
| Average (Min–max) HLA Class 1 PRA % | 3 (0–21) | 15 (0–100) | 0.014 |
| Average (Min–max) HLA Class 2 PRA % | 17 (0–100) | 7 (0–47) | 0.119 |
| Mechanical support device (%) | |||
| • HeartWare HVAD | 0 | 13 (48%) | |
| • HeartMate 3 | 0 | 10 (37%) | |
| • HeartMate 2 | 0 | 4 (15%) | |
| • Impella 5.5 | 18 (100%) | 0 | |
| Outcome (%) | |||
| • Transplanted | 18 (100%) | 19 (70%) | 0.001 |
| INTERMACS Score at Implant | |||
| • 1 | - | 8 | 0.027 |
| • 2 | 18 | 9 | |
| • 3 | - | 2 | |
| UNOS Status at Transplant | |||
| • 1 | 2 | 1 | <0.001 |
| • 2 | 13 | 3 | |
| • 2e | 3 | 9 | |
| • 3 | 0 | 3 | |
| • 4 | 0 | 3 | |
| Complication resulting in status upgrade | 1 (5%) | 12 (63%) | |
| • RV Failure | 1 (100%) | 3 (25%) | <0.001 |
| • Stroke | 0 | 1 (8%) | |
| • Driveline infection | 0 | 6 (50%) | |
| • Pump thrombosis | 0 | 2 (16%) | |
| • Remain on waitlist | 0 | 3 (11%) | |
| • Died on waitlist | 0 | 2 (7%) |
| Impella 5.5 (n = 18) | LVAD (n = 19) | p Value | |
|---|---|---|---|
| Donor age (Median, IQR) | 32 (28–36) | 29 (22–32) | 0.078 |
| Donor Gender | |||
| • Male | 17 (94%) | 16 (84%) | |
| • Gender Mismatch | 3 | 2 | |
| Donor Distance (Median, IQR) | 336 (200–586) | 273 (113–499) | 0.087 |
| Offer Sequence (Median, IQR) | 4 (1–6) | 5 (1–32) | 0.039 |
| Downtime in minutes (Median, IQR) | 0 (0–46) | 0 (0–51) | 0.355 |
| Initially reported LVEF % (Median, IQR) | 59 (55–65) | 60 (56–65) | 0.341 |
| PHS increased risk | 3 | 5 | |
| Hepatitis C-positive donor | 0 | 2 | |
| DBD Donor | 17 | 19 | |
| DCD Donor | 1 | 0 |
| Parameter (Median, IQR) | Impella 5.5 (n = 18) | LVAD (n = 19) | p Value |
|---|---|---|---|
| Listing to Transplant (days) | 35 (15–75.25) | 696 (298–750) | <0.001 |
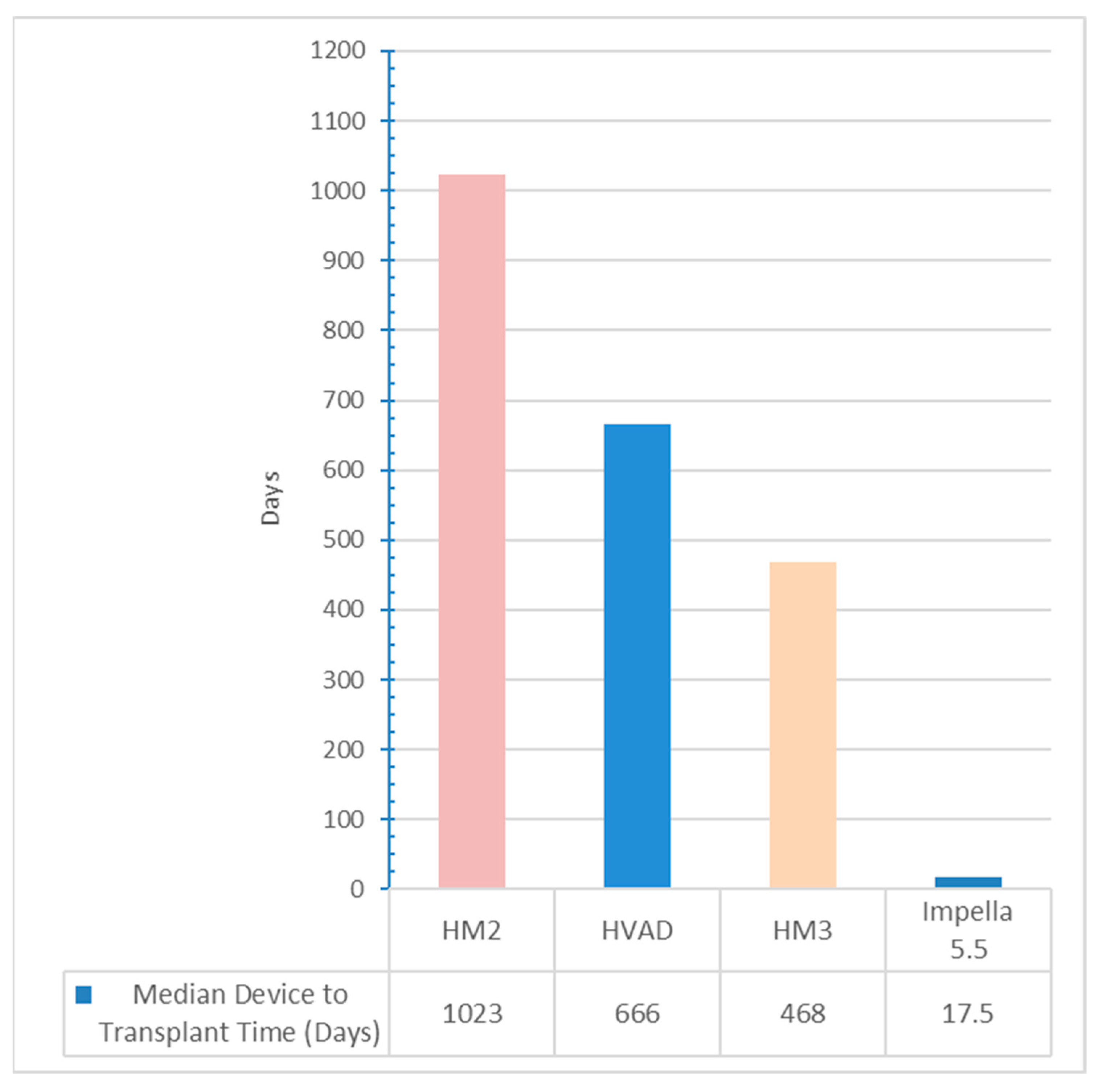
| Device to Transplant (days) | 18 (11–27) | 666 (544–914) | <0.001 |
| Cardiopulmonary bypass time (minutes) | 181 (156–197) | 219 (191–244) | 0.002 |
| Cold ischemic time (minutes) | 222 (201–239) | 230 (198–251) | 0.30 |
| Packed red blood cell (of units) | 4 (3–5) | 4 (3.5–6.5) | 0.204 |
| Fresh frozen plasma (mL) | 625 (500–1000) | 800 (500–1125) | 0.086 |
| Cryoprecipitate (mL) | 110 (0–200) | 200 (200–300) | 0.011 |
| Autologous transfusion (mL) | 675 (450–900) | 1125 (1013–1050) | 0.001 |
| Platelets (mL) | 350 (250–500) | 675 (425–1000) | 0.005 |
| Immediate post-operative vasoactive inotrope score | 7.9 (5–11.9) | 13 (9–16.8) | 0.003 |
| ICU length of stay (days) | 4 (3.25–6.75) | 6 (4.5–8.5) | 0.495 |
| Post-Transplant-to-discharge duration (days) | 12.5 (11.3–14.8) | 14 (12–21) | 0.498 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goswami, R.; Ruiz, J.; Desai, A.; Wlodkowski, P.; Sareyyupoglu, B.; Kiley, S.; Bhattacharyya, A.; Yip, D.; Lyle, M.; Nativi-Nicolau, J.; et al. Percutaneous Temporary Mechanical Circulatory Support as a Bridge to Heart Transplantation in the Current UNOS Allocation System. Biomedicines 2025, 13, 2637. https://doi.org/10.3390/biomedicines13112637
Goswami R, Ruiz J, Desai A, Wlodkowski P, Sareyyupoglu B, Kiley S, Bhattacharyya A, Yip D, Lyle M, Nativi-Nicolau J, et al. Percutaneous Temporary Mechanical Circulatory Support as a Bridge to Heart Transplantation in the Current UNOS Allocation System. Biomedicines. 2025; 13(11):2637. https://doi.org/10.3390/biomedicines13112637
Chicago/Turabian StyleGoswami, Rohan, Jose Ruiz, Aarti Desai, Peter Wlodkowski, Basar Sareyyupoglu, Sean Kiley, Anirban Bhattacharyya, Daniel Yip, Melissa Lyle, Jose Nativi-Nicolau, and et al. 2025. "Percutaneous Temporary Mechanical Circulatory Support as a Bridge to Heart Transplantation in the Current UNOS Allocation System" Biomedicines 13, no. 11: 2637. https://doi.org/10.3390/biomedicines13112637
APA StyleGoswami, R., Ruiz, J., Desai, A., Wlodkowski, P., Sareyyupoglu, B., Kiley, S., Bhattacharyya, A., Yip, D., Lyle, M., Nativi-Nicolau, J., Leoni, J., Sanghavi, D., Quiñones-Hinojosa, A., Chaudhary, S., Landolfo, K., Pham, S., & Patel, P. (2025). Percutaneous Temporary Mechanical Circulatory Support as a Bridge to Heart Transplantation in the Current UNOS Allocation System. Biomedicines, 13(11), 2637. https://doi.org/10.3390/biomedicines13112637






