Follicular Fluid Amino Acid Alterations in Endometriosis: Evidence for Oxidative Stress and Metabolic Dysregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Collection of the Follicular Fluid Samples
2.3. Amino Acid Analysis
Sample Processing and UHPLC Analysis
2.4. Data Analysis
3. Results
3.1. Patients’ Demographics and Clinical Characteristics
3.2. Amino Acid Analysis of the FF Samples
3.3. Comparison of Amino Acid Profiles Between EM and Control Groups
3.4. Comparison of Amino Acid Concentration Based on Body Mass Index (BMI)
3.5. Comparison of Amino Acid Concentration Based on the Age of the Patients
3.6. Heatmap and PLS-DA Analysis
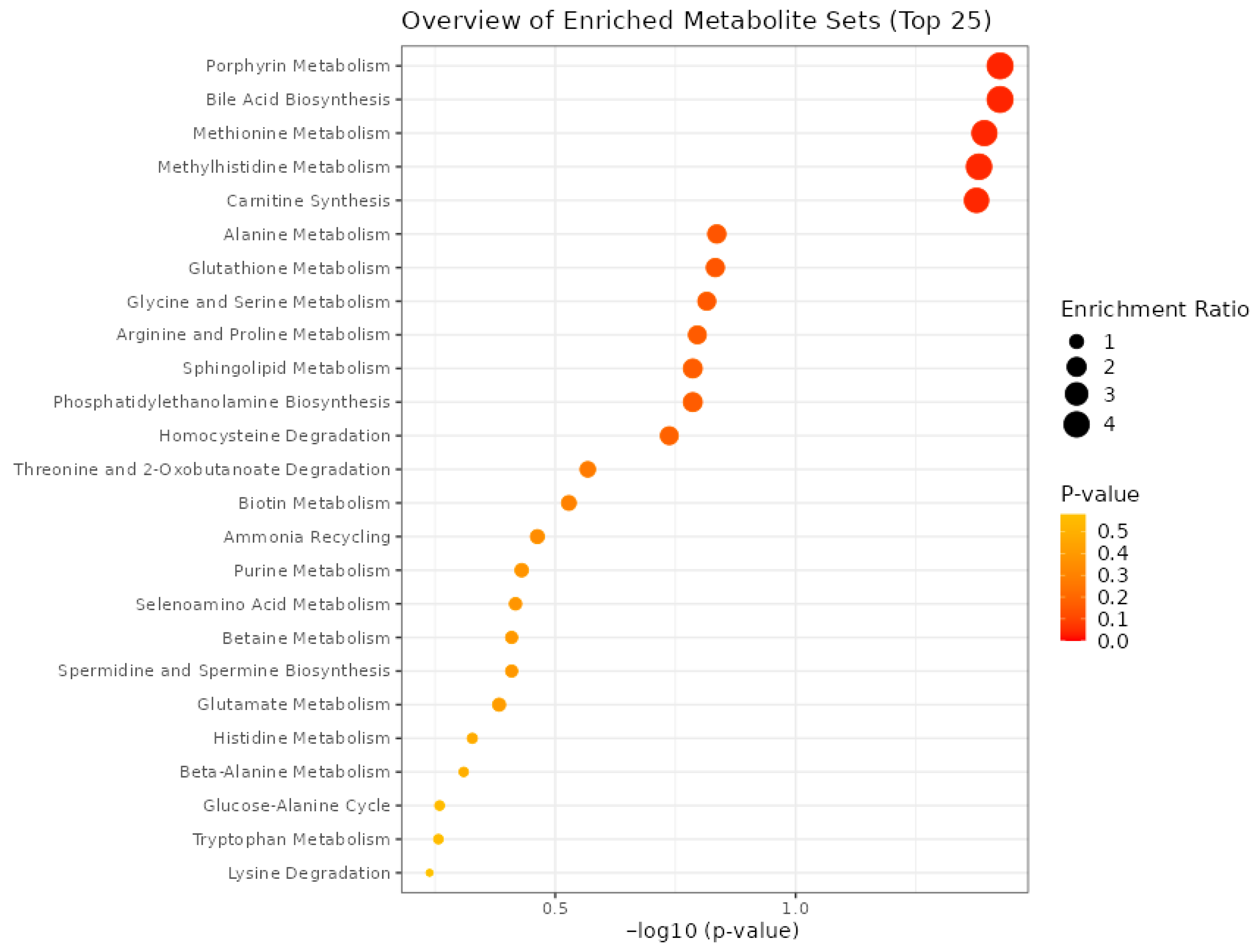
3.7. Metabolite Set Enrichment Analysis (MSEA)
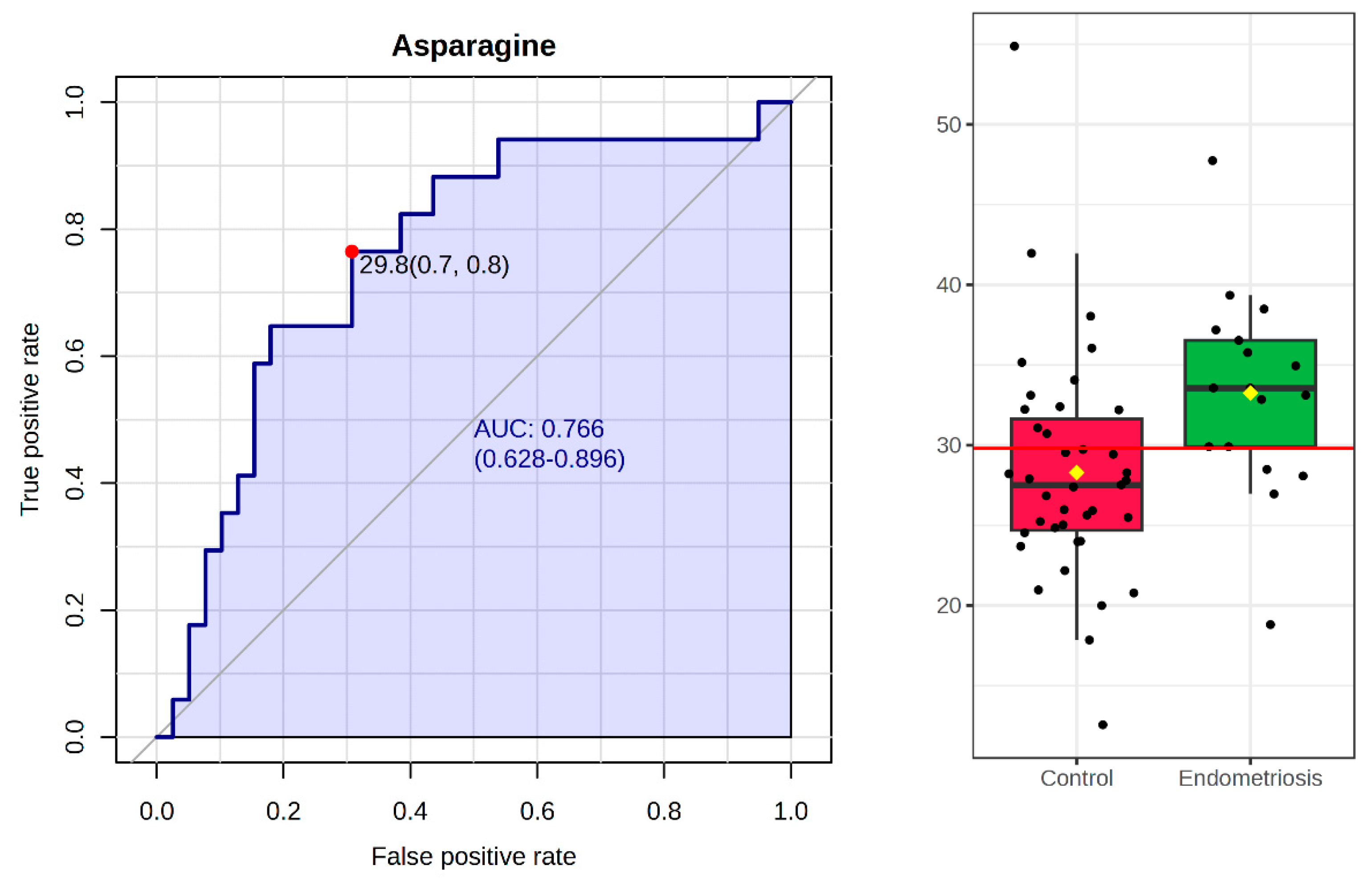
3.8. Biomarker Analysis
3.9. Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EM | Endometriosis |
| FF | Follicular fluid |
| ART | Artificial reproductive treatment |
| rFSH | Recombinant follicle-stimulating hormone |
| rLH | Recombinant luteinizing hormone |
| hMG | Human menopausal gonadotropin |
| hCG | Human chorionic gonadotropin |
| MPA | 3-mercaptopropionic acid |
| FMOC | 9-fluorenylmethyloxycarbonyl chloride |
| OPA | Ortho-phthalaldehyde |
| RT | Retention time |
| AUC | Area under the curve |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| ROC | Receiver Operating Characteristic |
| MSEA | Metabolite Set Enrichment Analysis |
| PCOS | Polycystic ovary syndrome |
| BCAAs | Branched-chain amino acids |
References
- Fan, Y.; Yang, Q.; Lin, Y.; Fu, X.; Shu, J. The effect of endometriosis on oocyte quality: Mechanisms, diagnosis and treatment. Arch. Gynecol. Obstet 2025, 311, 841–850. [Google Scholar] [CrossRef]
- Saunders, P.T.K.; Whitaker, L.H.R.; Horne, A.W. Endometriosis: Improvements and challenges in diagnosis and symptom management. Cell Rep. Med. 2024, 5, 101596. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.; Murk, W.; Arici, A. Endometriosis and infertility: Epidemiology and evidence-based treatments. In Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Malden, MA, USA, 2008; pp. 92–100. [Google Scholar] [CrossRef]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Qi, Q.; Li, Y.; Chen, Z.; Luo, Z.; Zhou, T.; Zhou, J.; Zhang, Y.; Chen, S.; Wang, L. Update on the pathogenesis of endometriosis-related infertility based on contemporary evidence. Front. Endocrinol. 2025, 16, 1558271. [Google Scholar] [CrossRef]
- La Marca, A.; Semprini, M.; Mastellari, E.; Donno, V.; Capuzzo, M.; Alboni, C.; Giulini, S. Fertility preservation in women with endometriosis. Hum. Reprod. Open 2025, 2025, hoaf012. [Google Scholar] [CrossRef]
- Bonavina, G.; Taylor, H.S. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front. Endocrinol. 2022, 13, 1020827. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Y.; Chen, G. Causal Relationship Between Endometriosis, Female Infertility, and Primary Ovarian Failure Through Bidirectional Mendelian Randomization. Int. J. Women’s Health 2024, 16, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Ran, H.; Chen, Y.; Ma, L. Lipidomics analysis of human follicular fluid form normal-weight patients with polycystic ovary syndrome: A pilot study. J. Ovarian Res. 2021, 14, 135. [Google Scholar] [CrossRef]
- Alrabiah, N.A.; Simintiras, C.A.; Evans, A.C.O.; Lonergan, P.; Fair, T. Biochemical alterations in the follicular fluid of bovine peri-ovulatory follicles and their association with final oocyte maturation. Reprod. Fertil. 2023, 4, e220090. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, H.; Lian, F.; Zhang, X.; Pang, C.; Guo, Y.; Song, J.; Wang, A.; Shi, L.; Han, L. Human Follicular Fluid Metabolomics Study of Follicular Development and Oocyte Quality. Chromatographia 2017, 80, 901–909. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, Q.; Gao, H.; Lyu, Q.; Chai, W.; Wu, L.; Li, B. Metabolomics analysis of follicular fluid in ovarian endometriosis women receiving progestin-primed ovary stimulation protocol for in vitro fertilization. Sci. Rep. 2023, 13, 5747. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef]
- Brinca, A.T.; Peiró, A.M.; Evangelio, P.M.; Eleno, I.; Oliani, A.H.; Silva, V.; Vicente, L.F.; Ramalhinho, A.C.; Gallardo, E. Follicular Fluid and Blood Monitorization of Infertility Biomarkers in Women with Endometriosis. Int. J. Mol. Sci. 2024, 25, 7177. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. Understanding the Metabolic Responses of Living Systems to Pathophysiological Stimuli via Multivariate Statistical Analysis of Biological NMR Spectroscopic Data. Available online: www.taylorandfrancis.com (accessed on 6 June 2025).
- Li, J.; Zhang, Z.; Wei, Y.; Zhu, P.; Yin, T.; Wan, Q. Metabonomic analysis of follicular fluid in patients with diminished ovarian reserve. Front. Endocrinol. 2023, 14, 1132621. [Google Scholar] [CrossRef]
- Hood, R.B.; Liang, D.; Tan, Y.; Ford, J.; Souter, I.; Jones, D.P.; Hauser, R.; Gaskins, A.J. Characterizing the follicular fluid metabolome: Quantifying the correlation across follicles and differences with the serum metabolome. Fertil. Steril. 2022, 118, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Józwik, M.; Józwik, M.; Teng, C.; Battaglia, F.C. Amino acid, ammonia and urea concentrations in human pre-ovulatory ovarian follicular fluid. Hum. Reprod. 2006, 21, 2776–2782. [Google Scholar] [CrossRef] [PubMed]
- Kirsipuu, T.; Laks, K.; Velthut-Meikas, A.; Levkov, L.; Salumets, A.; Palumaa, P. Comprehensive elucidation of amino acid profile in human follicular fluid and plasma of in vitro fertilization patients. Gynecol. Endocrinol. 2015, 31, 9–17. [Google Scholar] [CrossRef]
- Kurdi, C.; Lelovics, V.; Hesszenberger, D.; Lajtai, A.; Lakatos, Á.; Herczeg, R.; Gödöny, K.; Mauchart, P.; Várnagy, Á.; Kovács, G.L.; et al. Amino Acid Profiling of Follicular Fluid in Assisted Reproduction Reveals Important Roles of Several Amino Acids in Patients with Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 12458. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Yi, K.W. What is the link between endometriosis and adiposity? Obstet. Gynecol. Sci. 2022, 65, 227–233. [Google Scholar] [CrossRef]
- Li, J.; Guan, L.; Zhang, H.; Gao, Y.; Sun, J.; Gong, X.; Li, D.; Chen, P.; Liang, X.; Huang, M.; et al. Endometrium metabolomic profiling reveals potential biomarkers for diagnosis of endometriosis at minimal-mild stages. Reprod. Biol. Endocrinol. 2018, 16, 42, Correction in Reprod. Biol. Endocrinol. 2019, 17, 89. https://doi.org/10.1186/s12958-019-0532-5. [Google Scholar] [CrossRef]
- Ruebel, M.L.; Piccolo, B.D.; Mercer, K.E.; Pack, L.; Moutos, D.; Shankar, K.; Andres, A. Obesity leads to distinct metabolomic signatures in follicular fluid of women undergoing in vitro fertilization. J. Physiol. Endocrinol. Metab. 2019, 316, 383–396. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Z.; Zhang, Y.; Li, J.; Ruan, X.; Wan, Q.; Yin, T.; Zou, Y.; Chen, S.; Zhang, Y. Nontargeted metabolomics analysis of follicular fluid in patients with endometriosis provides a new direction for the study of oocyte quality. MedComm 2023, 4, e302. [Google Scholar] [CrossRef]
- Adamyan, L.; Pivazyan, L.; Zarova, E.; Avetisyan, J.; Laevskaya, A.; Sarkisova, A.; Stepanian, A. Metabolomic biomarkers of endometriosis: A systematic review. J. Endometr. Uterine Disord. 2024, 7, 100077. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Zhang, L. The Impact of Follicular Fluid Oxidative Stress Levels on the Outcomes of Assisted Reproductive Therapy. Antioxidants 2023, 12, 2117. [Google Scholar] [CrossRef]
- Zhang, C.-M.; Zhao, Y.; Li, R.; Yu, Y.; Yan, L.-Y.; Li, L.; Liu, N.-N.; Liu, P.; Qiao, J. Metabolic Heterogeneity of Follicular Amino Acids in Polycystic Ovary Syndrome Is Affected by Obesity and Related to Pregnancy Outcome. 2014. Available online: http://www.biomedcentral.com/1471-2393/14/11 (accessed on 6 June 2025).
- Chiang, S.K.; Chen, S.E.; Chang, L.C. The role of HO-1 and its crosstalk with oxidative stress in cancer cell survival. Cells 2021, 10, 2401. [Google Scholar] [CrossRef]
- Izumi, Y.; Kataoka, H.; Koshiba, A.; Ito, F.; Tanaka, Y.; Takaoka, O.; Maeda, E.; Okimura, H.; Sugahara, T.; Tarumi, Y.; et al. Hepcidin as a key regulator of iron homeostasis triggers inflammatory features in the normal endometrium. Free Radic. Biol. Med. 2023, 209, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Dai, Y.; Lin, X.; Liu, N.; Shi, L.; Zhuo, F.; Huang, Q.; Gu, W.; Zhao, F.; Zhang, Y.; Zhang, Y.; et al. Integrative analysis of transcriptomic and metabolomic profiles reveals abnormal phosphatidylinositol metabolism in follicles from endometriosis-associated infertility patients. J. Pathol. 2023, 260, 248–260. [Google Scholar] [CrossRef]
- Qin, R.; Tian, G.; Liu, J.; Cao, L. The gut microbiota and endometriosis: From pathogenesis to diagnosis and treatment. Front. Cell. Infect. Microbiol. 2022, 12, 1069557. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, K.; Ding, J.; Sun, S.; Ni, Z.; Yu, C. Influence of the gut microbiota on endometriosis: Potential role of chenodeoxycholic acid and its derivatives. Front. Pharmacol. 2022, 13, 954684. [Google Scholar] [CrossRef]
- Zhang, Y.; Shaukat, A.; Zhang, H.; Yang, Y.-F.; Li, H.-X.; Li, G.-Y.; Liu, Y.-N.; Liang, C.; Kang, J.-W.; Li, S.-C.; et al. Thymol Impacts the Progression of Endometriosis by Disrupting Estrogen Signaling Pathways and Inflammatory Responses. Int. J. Mol. Sci. 2024, 25, 13150. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Xu, H.; Lei, S.; Zhao, D. Overexpressed nicotinamide N-methyltransferase in endometrial stromal cells induced by macrophages and estradiol contributes to cell proliferation in endometriosis. Cell Death Discov. 2024, 10, 463. [Google Scholar] [CrossRef]
- Murgia, F.; Angioni, S.; D’aLterio, M.N.; Pirarba, S.; Noto, A.; Santoru, M.L.; Tronci, L.; Fanos, V.; Atzori, L.; Congiu, F. Metabolic Profile of Patients with Severe Endometriosis: A Prospective Experimental Study. Reprod. Sci. 2020, 28, 728–735. [Google Scholar] [CrossRef]
- Atkins, H.M.; Bharadwaj, M.S.; Cox, A.O.; Furdui, C.M.; Appt, S.E.; Caudell, D.L. Endometrium and endometriosis tissue mitochondrial energy metabolism in a nonhuman primate model. Reprod. Biol. Endocrinol. 2019, 17, 70. [Google Scholar] [CrossRef]
- Clower, L.; Fleshman, T.; Geldenhuys, W.J.; Santanam, N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules 2022, 12, 1055. [Google Scholar] [CrossRef]
- Máté, G.; Bernstein, L.R.; Török, A.L. Endometriosis Is a Cause of Infertility. Does Reactive Oxygen Damage to Gametes and Embryos Play a Key Role in the Pathogenesis of Infertility Caused by Endometriosis? Front. Endocrinol. 2018, 9, 725. [Google Scholar] [CrossRef]
- Lu, C.; Xu, J.; Li, K.; Wang, J.; Dai, Y.; Chen, Y.; Chai, R.; Xu, C.; Kang, Y. Chronic Stress Blocks the Endometriosis Immune Response by Metabolic Reprogramming. Int. J. Mol. Sci. 2024, 25, 29. [Google Scholar] [CrossRef]
- Li, X.; Guo, L.; Zhang, W.; He, J.; Ai, L.; Yu, C.; Wang, H.; Liang, W. Identification of Potential Molecular Mechanism Related to Infertile Endometriosis. Front. Vet. Sci. 2022, 9, 845709. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Hoffman, J.M. Serine metabolism in aging and age-related diseases. GeroScience 2025, 47, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, C.-H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic regulation of the immune system in health and diseases: Mechanisms and interventions. Signal Transduct. Target. Ther. 2024, 9, 1–31. [Google Scholar] [CrossRef] [PubMed]



| Endometriosis [Mean ± SD] (n = 17) | Control [Mean ± SD] (n = 39) | Significance (p-Value) | |
|---|---|---|---|
| Age | 35.14 ± 4.34 | 33.26 ± 4.18 | 0.234 |
| BMI | 22.87 ± 5.71 | 25.29 ± 5.66 | 0.025 |
| Number of oocytes retrieved | 8.13 ± 4.49 | 10.49 ± 6.66 | 1.000 |
| Number of fertilized oocytes | 3.18 ± 2.00 | 3.89 ± 3.91 | 0.685 |
| Number of IVF cycles | 1.94 ± 0.93 | 1.86 ± 1.38 | 0.338 |
| Baseline estradiol | 2954.44 ± 749.75 | 2426.51 ± 912.06 | 0.04 |
| FSH dose during stimulation | 1083.94 ± 1430.84 | 1417.31 ± 1382.14 | 0.118 |
| Cause of infertility | |||
| Male factor | - | 26 (66.66%) | |
| Female factor | 15 (88.23%) | 8 (20.51%) | |
| Combined male–female | 2 (11.76%) | 5 (12.82%) |
| Endometriosis [Mean ± SD] µmol/L | Control [Mean ± SD] µmol/L | |
|---|---|---|
| Aspartate | 7.01 ± 4.28 | 8.45 ± 16.49 |
| Glutamate | 63.35 ± 20.85 | 69.67 ± 47.92 |
| Asparagine * | 33.25 ± 6.28 | 28.29 ± 7.12 |
| Serine | 57.31 ± 15.84 | 50.09 ± 18.28 |
| Glutamine | 350.41 ± 72.65 | 346.30 ± 101.53 |
| Histidine * | 58.95 ± 13.26 | 51.71 ± 11.33 |
| Glycine * | 178.58 ± 51.28 | 147.43 ± 49.84 |
| Threonine | 109.51 ± 23.00 | 100.46 ± 29.82 |
| Arginine | 34.85 ± 11.00 | 33.16 ± 13.65 |
| Alanine | 240.32 ± 56.45 | 226.05 ± 60.46 |
| Tyrosine | 30.82 ± 8.45 | 30.75 ± 11.26 |
| Cysteine | 20.09 ± 6.56 | 20.40 ± 6.66 |
| Valine | 134.37 ± 53.51 | 128.41 ± 34.81 |
| Methionine | 16.81 ± 4.39 | 15.75 ± 4.10 |
| Tryptophan | 47.65 ± 5.92 | 48.44 ± 6.70 |
| Phenylalanine | 38.52 ± 9.65 | 40.04 ± 9.21 |
| Isoleucine | 27.22 ± 9.19 | 29.42 ± 8.82 |
| Leucine | 54.74 ± 20.94 | 54.74 ± 18.48 |
| Lysine | 89.35 ± 21.57 | 81.89 ± 25.42 |
| Proline | 143.05 ± 42.37 | 150.10 ± 54.00 |
| Significance (p) Values | ||||
|---|---|---|---|---|
| EM/CG | BMI | Age | Outcome | |
| Aspartate | 0.515 | 0.177 | 0.967 | 0.314 |
| Glutamate | 0.599 | 0.069 | 0.755 | 0.142 |
| Asparagine | 0.002 | 0.018 | 0.651 | 0.212 |
| Serine | 0.06 | 0.921 | 1 | 0.755 |
| Glutamine | 0.493 | 0.208 | 0.384 | 0.755 |
| Histidine | 0.049 | 0.431 | 0.332 | 0.076 |
| Glycine | 0.025 | 0.42 | 0.033 | 0.350 |
| Threonine | 0.173 | 0.48 | 0.56 | 0.612 |
| Arginine | 0.368 | 0.651 | 0.48 | 0.810 |
| Alanine | 0.25 | 0.273 | 0.393 | 0.838 |
| Tyrosine | 0.493 | 0.135 | 0.663 | 0.482 |
| Cysteine | 0.908 | 0.474 | 0.954 | 0.702 |
| Valine | 0.922 | 0.042 | 0.576 | 0.386 |
| Methionine | 0.465 | 0.758 | 0.712 | 0.402 |
| Tryptophane | 0.782 | 0.556 | 0.576 | 0.281 |
| Phenylalanine | 0.605 | 0.436 | 0.495 | 0.412 |
| Isoleucine | 0.222 | 0.13 | 0.332 | 0.515 |
| Leucine | 0.88 | 0.265 | 0.657 | 0.769 |
| Lysine | 0.151 | 0.863 | 0.818 | 0.587 |
| Proline | 0.838 | 0.25 | 0.44 | 0.551 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurdi, C.; Hesszenberger, D.; Csabai, D.; Lajtai, A.; Lakatos, Á.; Jakabfi-Csepregi, R.; Gödöny, K.; Mauchart, P.; Várnagy, Á.; Kovács, G.L.; et al. Follicular Fluid Amino Acid Alterations in Endometriosis: Evidence for Oxidative Stress and Metabolic Dysregulation. Biomedicines 2025, 13, 2634. https://doi.org/10.3390/biomedicines13112634
Kurdi C, Hesszenberger D, Csabai D, Lajtai A, Lakatos Á, Jakabfi-Csepregi R, Gödöny K, Mauchart P, Várnagy Á, Kovács GL, et al. Follicular Fluid Amino Acid Alterations in Endometriosis: Evidence for Oxidative Stress and Metabolic Dysregulation. Biomedicines. 2025; 13(11):2634. https://doi.org/10.3390/biomedicines13112634
Chicago/Turabian StyleKurdi, Csilla, Dávid Hesszenberger, Dávid Csabai, Anikó Lajtai, Ágnes Lakatos, Rita Jakabfi-Csepregi, Krisztina Gödöny, Péter Mauchart, Ákos Várnagy, Gábor L. Kovács, and et al. 2025. "Follicular Fluid Amino Acid Alterations in Endometriosis: Evidence for Oxidative Stress and Metabolic Dysregulation" Biomedicines 13, no. 11: 2634. https://doi.org/10.3390/biomedicines13112634
APA StyleKurdi, C., Hesszenberger, D., Csabai, D., Lajtai, A., Lakatos, Á., Jakabfi-Csepregi, R., Gödöny, K., Mauchart, P., Várnagy, Á., Kovács, G. L., & Kőszegi, T. (2025). Follicular Fluid Amino Acid Alterations in Endometriosis: Evidence for Oxidative Stress and Metabolic Dysregulation. Biomedicines, 13(11), 2634. https://doi.org/10.3390/biomedicines13112634






