Interaction of Clinical Factors Modestly Predict Anti-TNF-Alpha Antibody Formation in a Real-World Cohort of Inflammatory Bowel Disease Patients
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients’ Enrollment
2.2. Ethical Statement
2.3. Methods
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Clinical Data
3.2. Model Fit and Hierarchical Structure
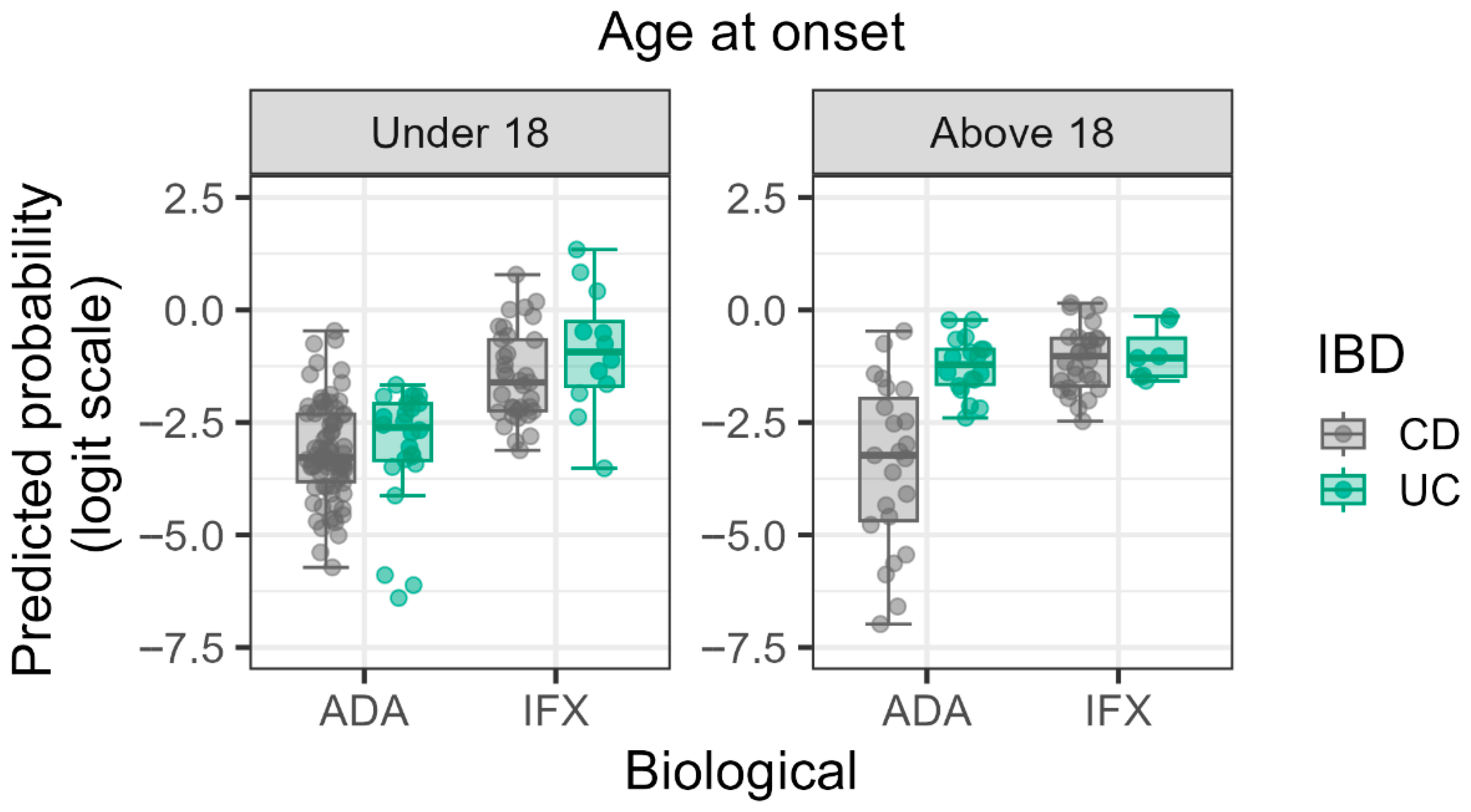
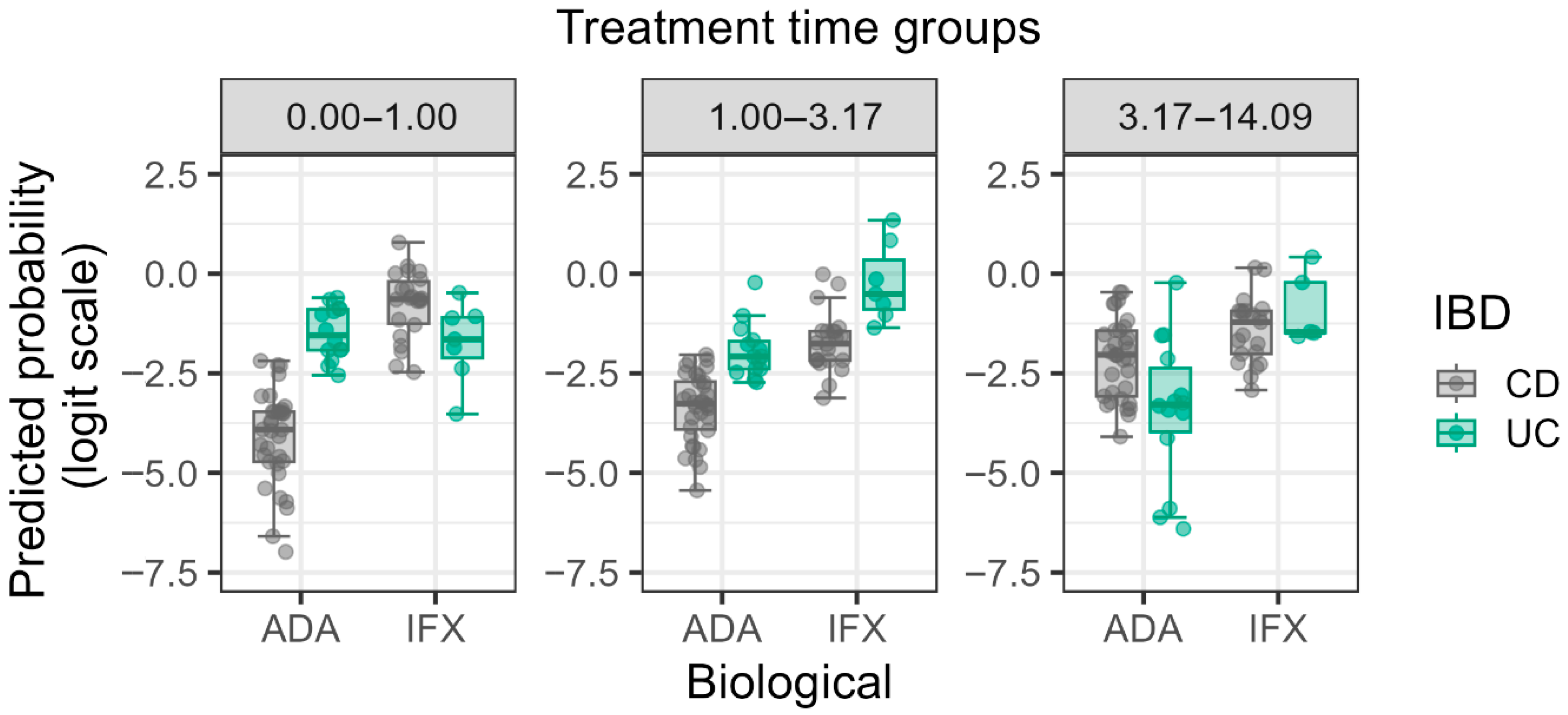
3.3. Significant Main Effects and Interactions
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AADA | Anti-Adalimumab Antibody |
| ADA | Adalimumab |
| AIFX | Anti-Infliximab Antibody |
| AUC | Area Under Curve |
| CD | Crohn’s Disease |
| ELISA | Enzyme-linked Immunosorbent Assay |
| ESPGHAN | European Society for Paediatric Gastroenterology Hepatology and Nutrition |
| IBD | Inflammatory Bowel Disease |
| IFX | Infliximab |
| LoD | Limit of Detection |
| LOR | Loss of Response |
| ORs | Odds Ratios |
| TDM | Therapeutic Drug Monitoring |
| TNF-α | Tumor Necrosis Factor Alpha |
| TRL | Trough Level |
| UC | Ulcerative Colitis |
References
- Long, D. Crohn’s Disease and Ulcerative Colitis: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2024, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Zitomersky, N.; Chi, L.; Liu, E.; Bray, K.R.; Papamichael, K.; Cheifetz, A.S.; Snapper, S.B.; Bousvaros, A.; Silvester, J.A. Anti-infliximab antibodies and low infliximab levels correlate with drug discontinuation in pediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2024, 2, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.W.; Sanderson, J.D.; Irving, P.M. Anti-infliximab antibodies in inflammatory bowel disease: Prevalence, infusion reactions, immunosuppression and response, a meta-analysis. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Weisshof, R.; Ungar, B.; Blatt, A.; Dahan, A.; Pressman, S.; Waterman, M.; Kopylov, U.; Ben-Horin, S.; Chowers, Y. Anti-infliximab Antibodies with Neutralizing Capacity in Patients with Inflammatory Bowel Disease: Distinct Clinical Implications Revealed by a Novel Assay. Inflamm. Bowel Dis. 2016, 22, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Portocarrero-Castillo, A.; Chona, D.; Hellmann, J.; Minar, P.; Rosen, M.J. Favorable Outcomes and Anti-TNF Durability After Addition of an Immunomodulator for Anti-Drug Antibodies in Pediatric IBD Patients. Inflamm. Bowel Dis. 2021, 27, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Marinari, B.; Botti, E.; Bavetta, M.; Spallone, G.; Zangrilli, A.; Talamonti, M.; Richetta, A.; Chimenti, S.; Costanzo, A. Detection of adalimumab and anti-adalimumab levels by ELISA: Clinical considerations. Drug Dev. Res. 2014, 75, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Hendy, P. Infliximab and anti-infliximab antibody levels in Crohn’s disease. Frontline Gastroenterol. 2014, 5, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Lucafò, M.; Curci, D.; Bramuzzo, M.; Alvisi, P.; Martelossi, S.; Silvestri, T.; Guastalla, V.; Labriola, F.; Stocco, G.; Decorti, G. Serum Adalimumab Levels After Induction Are Associated with Long-Term Remission in Children With Inflammatory Bowel Disease. Front. Pediatr. 2021, 9, 646671. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Motoya, S.; Matsumoto, T.; Watanabe, K.; Hisamatsu, T.; Yoshimura, N.; Ishida, T.; Kato, S.; Nakagawa, T.; Esaki, M.; et al. Significance of measurement of serum trough level and anti-drug antibody of adalimumab as personalised pharmacokinetics in patients with Crohn’s disease: A subanalysis of the DIAMOND trial. Aliment. Pharmacol. Ther. 2017, 46, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Nice, R.; Chanchlani, N.; Green, H.; Bewshea, C.; Ahmad, T.; Goodhand, J.R.; McDonald, T.J.; Perry, M.H.; Kenned, N.A. Validating the positivity thresholds of drug-tolerant anti-infliximab and anti-adalimumab antibody assays. Aliment. Pharmacol. Ther. 2021, 53, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Taylor, S.; Allocca, M.; Burisch, J.; Ellul, P.; Iacucci, M.; Maaser, C.; Zilli, A.; Zidar, N.; Wilkens, R.; et al. ECCO-ESGAR-ESP-IBUS Guideline on Diagnostics and Monitoring of Patients with Inflammatory Bowel Disease: Part 1. J. Crohns Colitis 2025, 19, jjaf106. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.theradiag.com/theranostic/tracker/lisa-tracker/ (accessed on 26 June 2025).
- Pais, I.P.; Espinheira, M.C.; Trindale, E.; Dias, J.A. Optimizing antitumor necrosis factor treatment in pediatric inflammatory bowel disease with therapeutic drug monitoring. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Murugesan, S.; Ibrahim, N.; Elawad, M.; Khodor, S.A. Predictive biomarkers for anti-TNF alpha therapy in IBD patients. J. Transl. Med. 2024, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Kennedy, N.A. Immunomodulator and Biologic Combination Therapy in IBD: The Debate That Just Won’t Go Away? J. Crohns Colitis. 2020, 14, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; Resche-Rigon, M.; Laharie, D.; Satsangi, J.; Ding, N.; Siegmund, B.; D’Haens, G.; Picon, L.; Bossuyt, P.; Vuitton, L.; et al. Withdrawal of infliximab or concomitant immunosuppressant therapy in patients with Crohn’s disease on combination therapy (SPARE): A multicentre, open-label, randomised controlled trial. Lancet Gastro. Hepatol. 2023, 8, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Gonczi, L.; Kurti, Z.; Rutka, M.; Vegh, Z.; Farkas, K.; Lovasz, B.D.; Golovics, P.A.; Gecse, K.B.; Szalay, B.; Molnar, T.; et al. Drug persistence and need for dose intensification to adalimumab therapy; the importance of therapeutic drug monitoring in inflammatory bowel diseases. BMC Gastroenterol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Click, B.; Barnes, E.L.; Cohen, B.L.; Sands, B.E.; Hanson, J.S.; Rubin, D.T.; Dubinsky, M.C.; Regueiro, M.; Gazis, D.; Crawford, J.M.; et al. Objective disease activity assessment and therapeutic drug monitoring prior to biologic therapy changes in routine inflammatory bowel disease clinical practice: TARGET-IBD. BMC Gastroenterol. 2022, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Colman, R.J.; Dotson, J.L.; Mock, M.; Sandberg, K.; Saeed, S.; Margolis, P.; Dhaliwal, J. Therapeutic Drug Monitoring in Pediatric Inflammatory Bowel Disease: A Nationwide Survey of Anti-TNF Therapy Practices, Attitudes, and Barriers. Crohns Colitis 360 2025, 7, otaf050. [Google Scholar] [CrossRef] [PubMed]
- Felipez, L.M.; Ali, S.; de Zoeten, E.F.; Griffiths, A.M.; Kim, S.C.; Patel, A.S.; Rosh, J.R.; Adler, J. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition position paper on the therapeutic drug monitoring in pediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2025, 81, 1100–1117. [Google Scholar] [CrossRef] [PubMed]
| Limit of detection (LoD) | Adalimumab/Infliximab 0.3 mg/L (>95th percentile) | anti-Adalimumab/anti-Infliximab 10 µg/L (>95th percentile) |
| Assay range | Adalimumab/Infliximab 0.3 mg/L–20 mg/L | anti-Adalimumab/anti-Infliximab 10 µg/L–160 µg/L/10 µg/L–200 µg/L |
| Diagnostic Test | Value | Interpretation |
|---|---|---|
| Hosmer–Lemeshow Goodness-of-Fit Test (p-value) | 0.518 | Confirms adequate model calibration (p > 0.05). |
| Maximum VIF (Variance Inflation Factor) | 2.85 | Indicates minimal multicollinearity. |
| Model Performance (Nagelkerke R2) | 0.287 | Indicates partial variance explanation. |
| CD | UC | Total | |||
|---|---|---|---|---|---|
| IFX | ADA | IFX | ADA | ||
| Number of patients (m/f) | 50 (27/23) | 59 (23/36) | 13 (6/7) | 31 (14/17) | 153 (70/83) |
| Children | 10 (6%) | 18 (12%) | 5 (3%) | 7 (5%) | 40 (26%) |
| Adult | 40 (26%) | 41 (27%) | 8 (5%) | 24 (16%) | 113 (74%) |
| Childhood onset | 24 (16%) | 37 (24%) | 5 (3%) | 10 (7%) | 76 (50%) |
| Disease initial age (year) | 19.1 [11.3–25] | 15.3 [12.5–21.8] | 22.5 [15.7–32.9] | 24.1 [14.8–40.8] | 18.6 [12.2–27.7] |
| Time from the onset of the disease to biological therapy (year) | 5.09 [3.6–12.9] | 3.92 [1.4–11.5] | 4.16 [1.2–8.9] | 3.59 [1.5–10.4] | 4.42 [1.7–11.3] |
| Antibody against IFX or ADA: | |||||
| Yes | 16 (11%) | 5 (3%) | 5 (3%) | 6 (4%) | 32 (21%) |
| No | 34 (22%) | 54 (36%) | 8 (5%) | 25 (16%) | 121 (79%) |
| Age at sampling (year) | 31.9 [23.4–41] | 26.6 [17.8–38.8] | 32.5 [17.2–45.1] | 33.98 | 30.7 [18–41.7] |
| [18.6–53] | |||||
| Localization: | |||||
| L1/E1 | 7 (4%) | 11 (7%) | 1 (1%) | - | 19 (12%) |
| L2/E2 | 16 (10%) | 14 (9%) | 6(4%) | 12 (8%) | 48 (31%) |
| L3–4/E3–4 | 21 (14%) | 29 (19%) | 5 (3%) | 18 (12%) | 73 (48%) |
| N/A | 6 (4%) | 5 (3%) | 1 (1%) | 1 (1%) | 13 (9%) |
| Attitude: | |||||
| B1/S0 | 26 (17%) | 25 (16%) | 5 (3%) | 14 (9%) | 68 (45%) |
| B2/B3/S1 | 21 (14%) | 30 (19%) | 7 (5%) | 17 (11%) | 75 (49%) |
| N/A | 3 (2%) | 4 (3%) | 1 (1%) | - | 10 (6%) |
| Immunosuppression: | |||||
| Yes | 21 (14%) | 33 (22%) | 6 (4%) | 13 (8%) | 73 (48%) |
| No | 16 (10%) | 20 (13%) | 4 (3%) | 11 (7%) | 51 (33%) |
| N/A | 13 (8%) | 6 (4%) | 3 (2%) | 7 (5%) | 29 (19%) |
| Steroid: | |||||
| Yes | 9 (6%) | 8 (5%) | 3 (2%) | 6 (4%) | 26 (17%) |
| No | 27 (17%) | 44 (29%) | 7 (5%) | 18 (12%) | 96 (63%) |
| N/A | 14 (9%) | 7 (5%) | 3 (1%) | 7 (5%) | 31 (20%) |
| 5-ASA: | |||||
| Yes | 9 (6%) | 21 (14%) | 7 (5%) | 18 (12%) | 55 (36%) |
| No | 7 (5%) | 20 (13%) | 2 (1%) | 3 (2%) | 32 (21%) |
| N/A | 34 (22%) | 18 (12%) | 4 (3%) | 10 (6%) | 66 (43%) |
| Intensification: | |||||
| Yes | 11 (7%) | 13 (8%) | 4 (3%) | 8 (5%) | 36 (23%) |
| No | 35 (23%) | 44 (29%) | 7 (5%) | 22 (14%) | 108 (71%) |
| N/A | 4 (3%) | 2 (1%) | 2 (1%) | 1 (1%) | 9 (6%) |
| Model | p | R2 | Variables |
|---|---|---|---|
| M0 | |||
| M1 | 0.701 | 0.001 | Gender |
| M2 | 0.132 | 0.025 | M1 + Age at onset, Localization, IBD type |
| M3 | 0.002 | 0.107 | M2 + Treatment Time, Immune suppression therapy, Type of Biological Therapy, Intensification |
| M4 | 0.043 | 0.291 | M3 + Interaction terms |
| Variable | Odds Ratio (OR) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Type of Biological Therapy (IFX vs. ADA) | 6.940 | 1.890–25.467 | 0.004 |
| Treatment Time | 2.505 | 1.107–5.672 | 0.029 |
| Localization | 2.216 | 1.002–4.902 | 0.049 |
| Interaction: Treatment Time × Type of Biological Therapy | 0.220 | 0.076–0.638 | 0.005 |
| Interaction: Age at Onset × IBD Type | 8.023 | 1.222–52.684 | 0.031 |
| Interaction: Age at Onset × IBD Type × Type of Biological Therapy | 0.019 | 0.001–0.725 | 0.042 |
| Interaction: Treatment Time × IBD Type × Type of Biological Therapy | 82.745 | 1.250–5473.497 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, K.; Nagypál, P.; Vásárhelyi, B.; Dezsőfi-Gottl, A.; Béres, N.; Miheller, P.; Iliás, Á.; Balogh, A.; Prehoda, B.; Tóbi, L.; et al. Interaction of Clinical Factors Modestly Predict Anti-TNF-Alpha Antibody Formation in a Real-World Cohort of Inflammatory Bowel Disease Patients. Biomedicines 2025, 13, 2622. https://doi.org/10.3390/biomedicines13112622
Kovács K, Nagypál P, Vásárhelyi B, Dezsőfi-Gottl A, Béres N, Miheller P, Iliás Á, Balogh A, Prehoda B, Tóbi L, et al. Interaction of Clinical Factors Modestly Predict Anti-TNF-Alpha Antibody Formation in a Real-World Cohort of Inflammatory Bowel Disease Patients. Biomedicines. 2025; 13(11):2622. https://doi.org/10.3390/biomedicines13112622
Chicago/Turabian StyleKovács, Krisztián, Petra Nagypál, Barna Vásárhelyi, Antal Dezsőfi-Gottl, Nóra Béres, Pál Miheller, Ákos Iliás, Anna Balogh, Bence Prehoda, Luca Tóbi, and et al. 2025. "Interaction of Clinical Factors Modestly Predict Anti-TNF-Alpha Antibody Formation in a Real-World Cohort of Inflammatory Bowel Disease Patients" Biomedicines 13, no. 11: 2622. https://doi.org/10.3390/biomedicines13112622
APA StyleKovács, K., Nagypál, P., Vásárhelyi, B., Dezsőfi-Gottl, A., Béres, N., Miheller, P., Iliás, Á., Balogh, A., Prehoda, B., Tóbi, L., Szabó, A., & Cseh, Á. (2025). Interaction of Clinical Factors Modestly Predict Anti-TNF-Alpha Antibody Formation in a Real-World Cohort of Inflammatory Bowel Disease Patients. Biomedicines, 13(11), 2622. https://doi.org/10.3390/biomedicines13112622






