Ethylbenzene Exposure and Bronchoalveolar CD4/CD8 T Cells in Hypersensitivity Pneumonitis Development and Clinical Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Calculation of Personal Exposure to Air Pollutants
2.3. Flow Cytometry Immunophenotypic Studies
2.4. Statistical Analysis
3. Results
3.1. Clinical and Biological Characteristics of the Study Groups
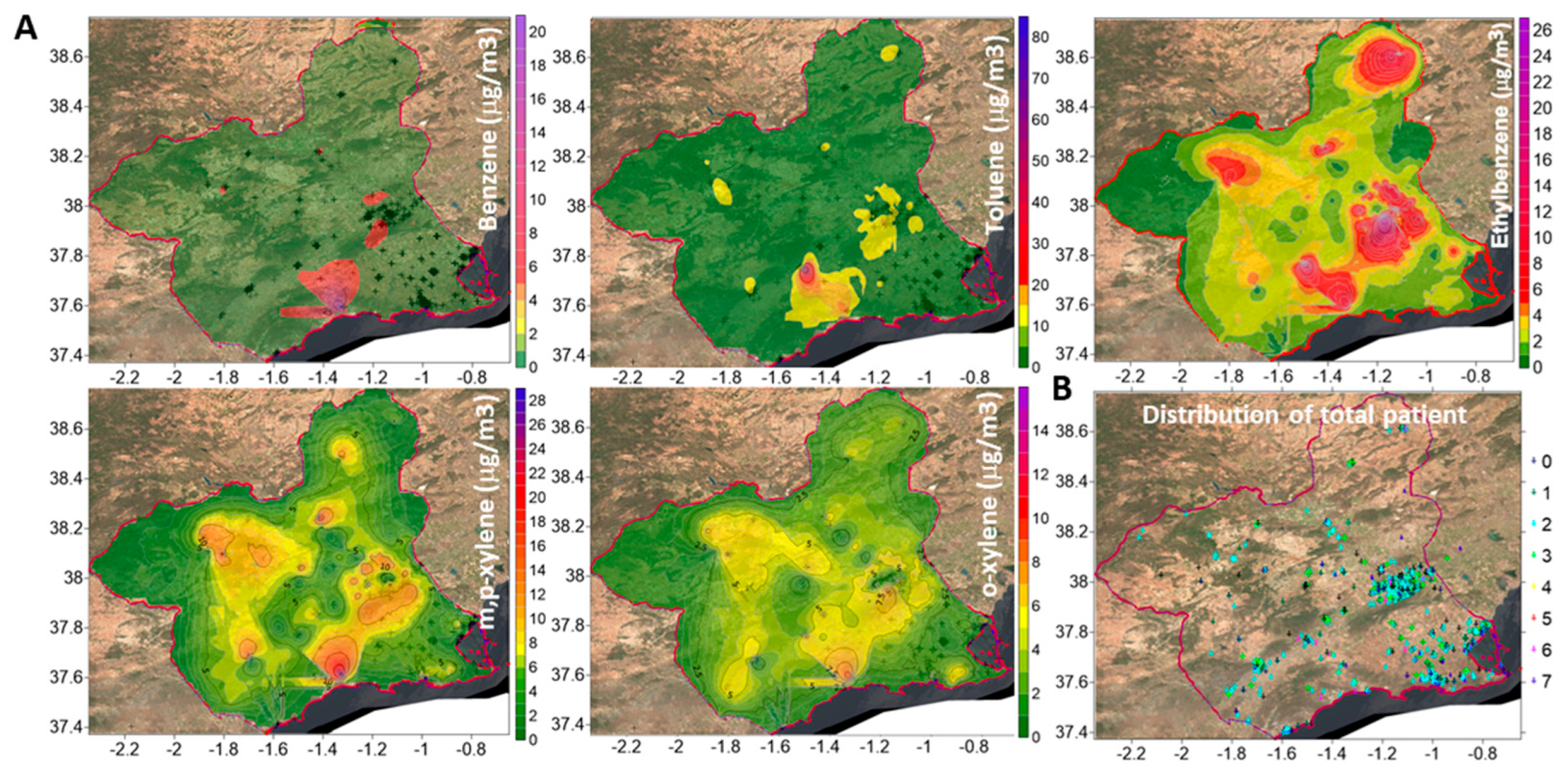
3.2. Geospatial Relationships of Air Pollutants and Lung Diseases
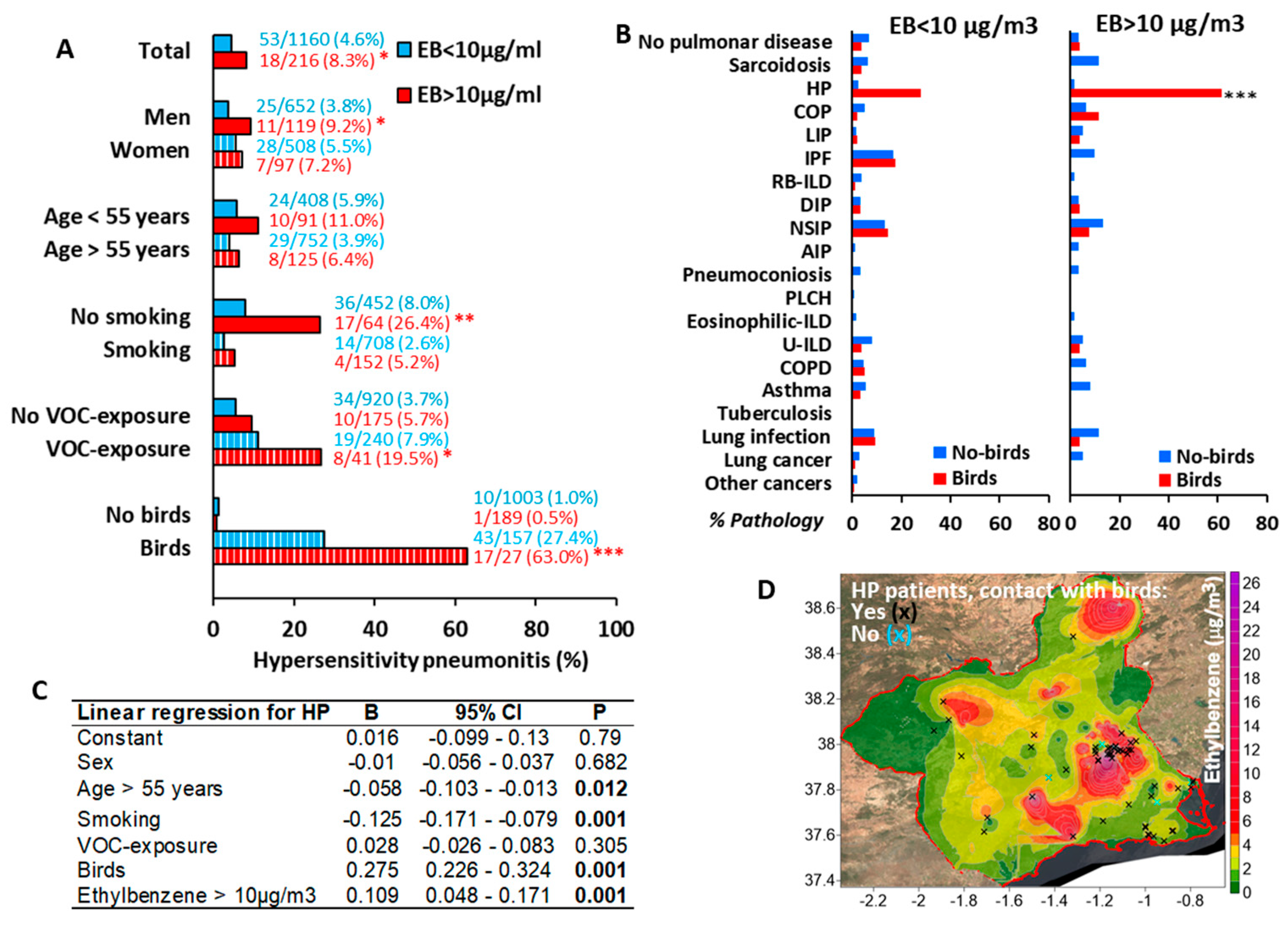
3.3. Environmental Ethylbenzene Concentrations Are Associated with HP in Bird Keepers and Workers Exposed to Chemical Compounds
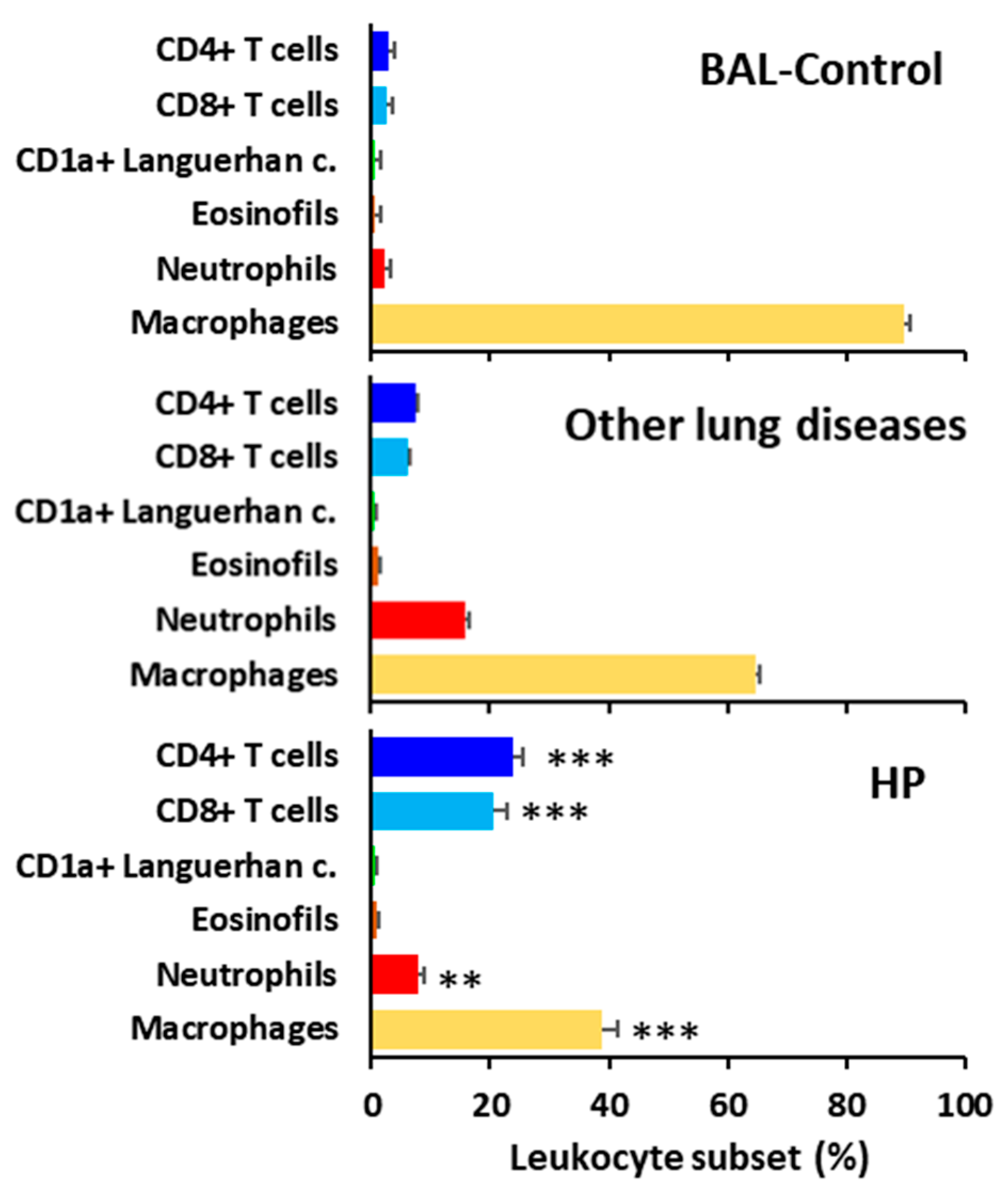
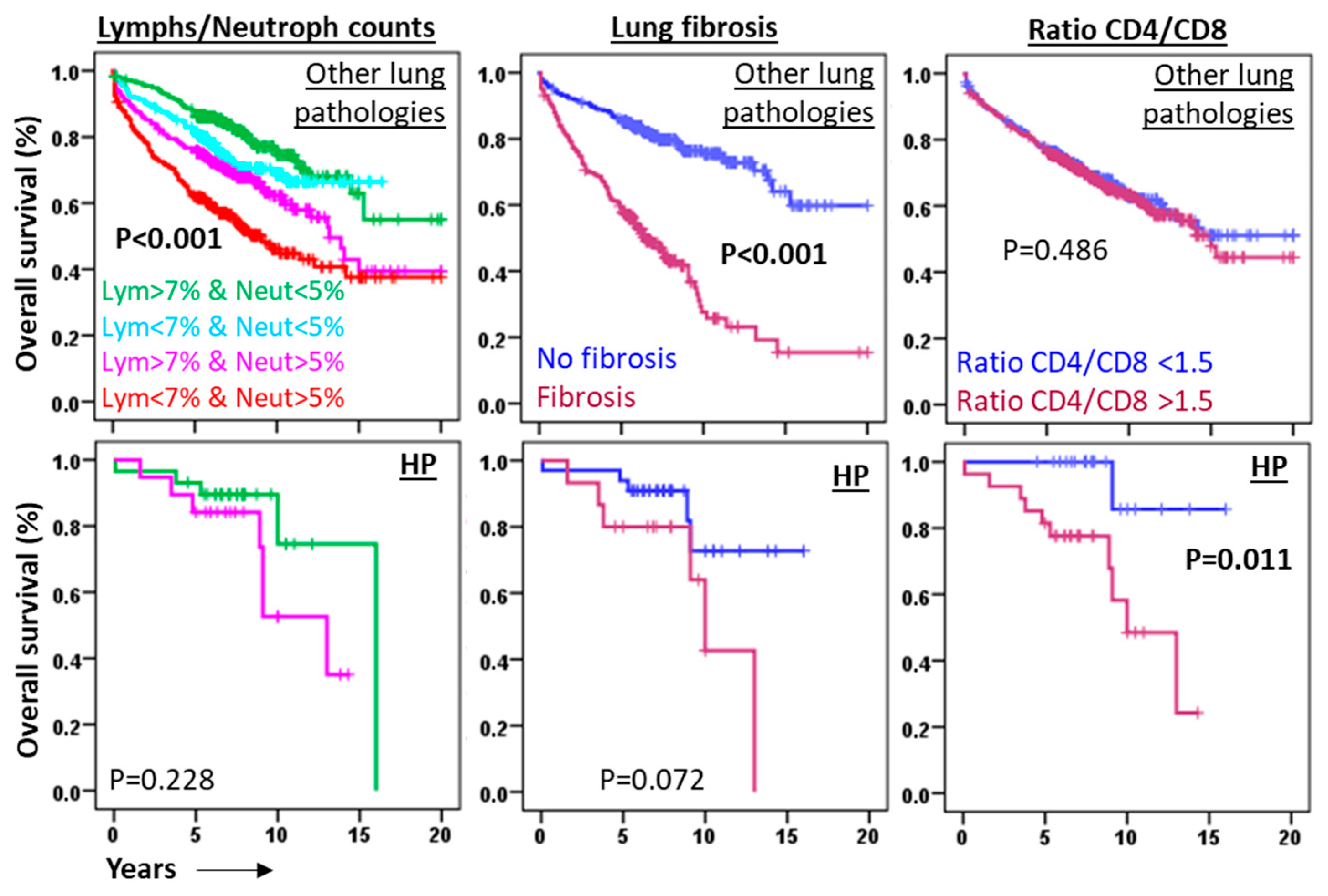
3.4. HP Is Characterized by BAL T-Lymphocytosis
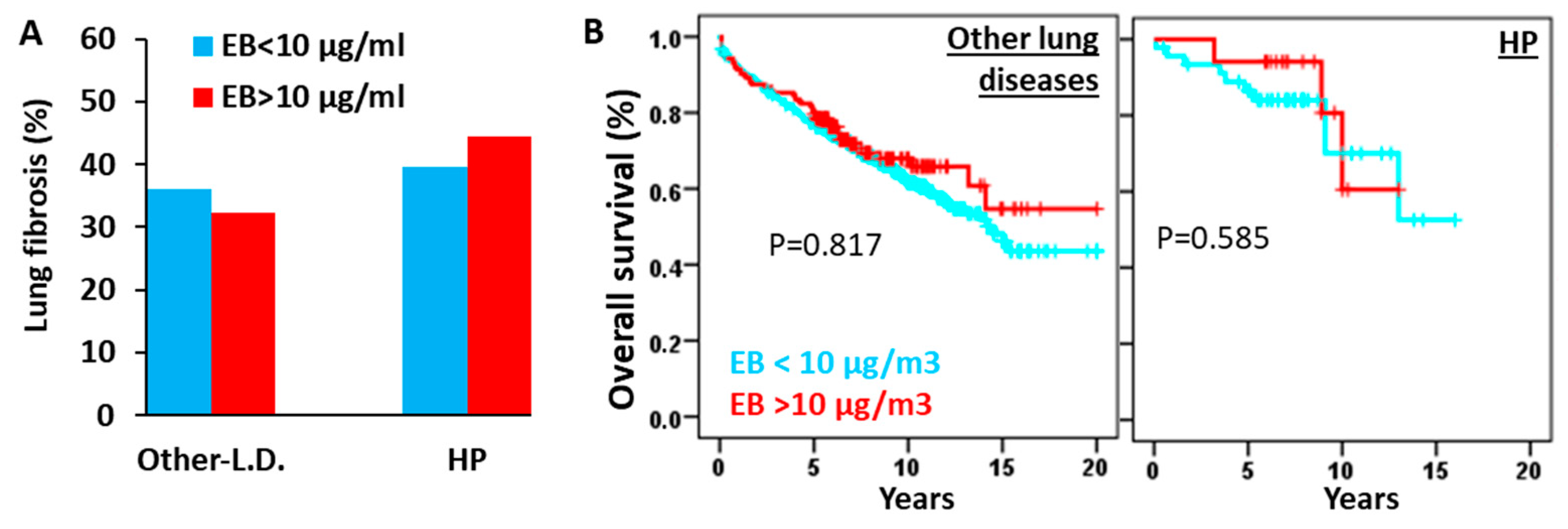
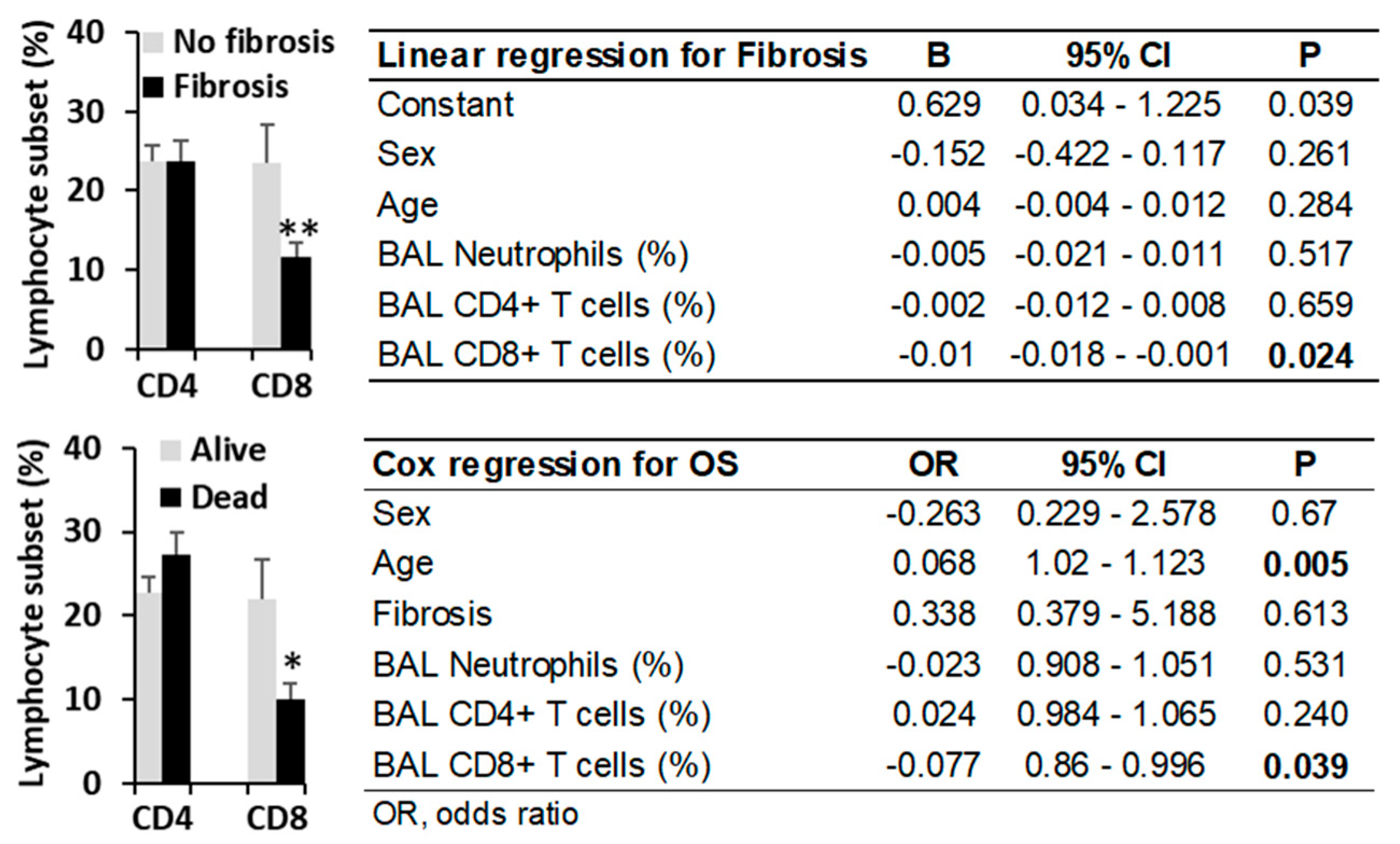
3.5. High Environmental Ethylbenzene Concentrations Do Not Significantly Affect Fibrosis Incidence or Overall Survival in Patients with HP or Other Pulmonary Diseases
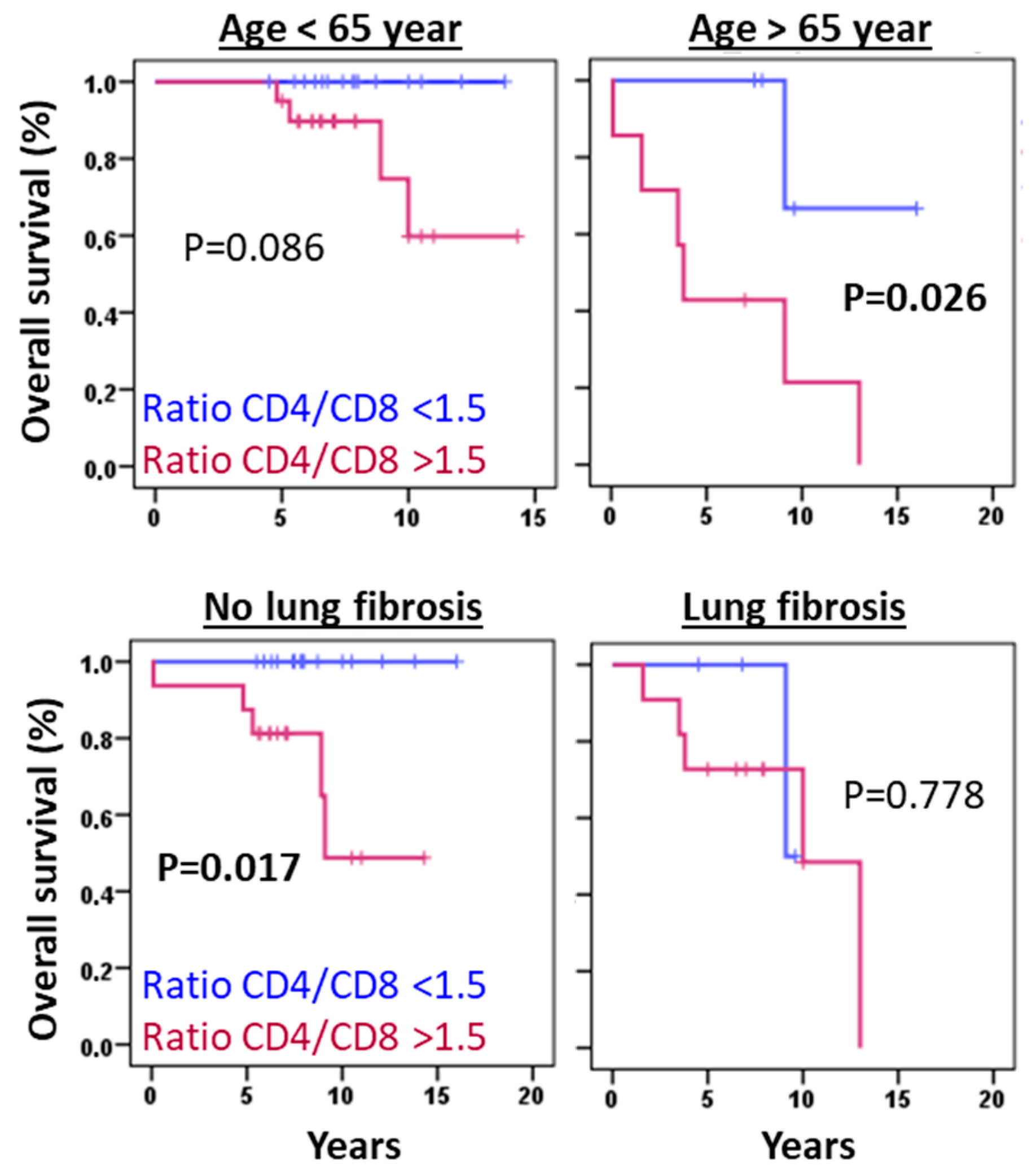
3.6. The CD4/CD8 Lymphocyte Ratio Serves as a Predictive Marker in HP at Diagnosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calaras, D.; David, A.; Vasarmidi, E.; Antoniou, K.; Corlateanu, A. Hypersensitivity Pneumonitis: Challenges of a Complex Disease. Can. Respir. J. 2024, 2024, 4919951. [Google Scholar] [CrossRef]
- Alberti, M.L.; Rincon-Alvarez, E.; Buendia-Roldan, I.; Selman, M. Hypersensitivity Pneumonitis: Diagnostic and Therapeutic Challenges. Front. Med. 2021, 8, 718299. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.; Troy, L.; Lee, C.T.; Sperling, A.; Strek, M.; Glaspole, I. Hypersensitivity Pneumonitis: Current Concepts in Pathogenesis, Diagnosis, and Treatment. Allergy 2022, 77, 442–453. [Google Scholar] [CrossRef]
- Sánchez-Díez, S.; Cruz, M.J.; de Homdedeu, M.; Ojanguren, I.; Romero-Mesones, C.; Sansano, I.; Muñoz, X. Immunopathological Mechanisms of Bird-Related Hypersensitivity Pneumonitis. Int. J. Mol. Sci. 2023, 24, 2884. [Google Scholar] [CrossRef]
- BAUR, X. Hypersensitivity Pneumonitis (Extrinsic Allergic Alveolitis) Induced by Isocyanates. J. Allergy Clin. Immunol. 1995, 95, 1004–1010. [Google Scholar] [CrossRef]
- Vasakova, M.; Selman, M.; Morell, F.; Sterclova, M.; Molina-Molina, M.; Raghu, G. Hypersensitivity Pneumonitis: Current Concepts of Pathogenesis and Potential Targets for Treatment. Am. J. Respir. Crit. Care Med. 2019, 200, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults: An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Pardo, A.; King, T.E. Hypersensitivity Pneumonitis. Am. J. Respir. Crit. Care Med. 2012, 186, 314–324. [Google Scholar] [CrossRef]
- Johansson, E.; Boivin, G.P.; Yadav, J.S. Early Immunopathological Events in Acute Model of Mycobacterial Hypersensitivity Pneumonitis in Mice. J. Immunotoxicol. 2017, 14, 77–88. [Google Scholar] [CrossRef]
- Angerer, J.; Wulf, H. Occupational Chronic Exposure to Organic Solvents XI. Alkylbenzene Exposure of Varnish Workers: Effects on Hematopoetic System. Int. Arch. Occup. Environ. Health 1985, 56, 307–321. [Google Scholar] [CrossRef]
- Dorfmüller, P.; Cavazza, A. Lung Pathology for the Clinician: A Comprehensive Approach. Eur. Respir. Rev. 2017, 26, 170041. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, L.; Luppi, F.; Ferrara, G.; Mura, M. Immunomodulatory Treatment of Interstitial Lung Disease. Ther. Adv. Respir. Dis. 2022, 16, 17534666221117002. [Google Scholar] [CrossRef]
- Cocheo, V.; Boaretto, C.; Sacco, P. High Uptake Rate Radial Diffusive Sampler Suitable for Both Solvent and Thermal Desorption. Am. Ind. Hyg. Assoc. J. 1996, 57, 897–904. [Google Scholar] [CrossRef]
- EN 14662-2:2005; Ambient Air Quality—Standard Method for Measurement of Benzene Concentrations—Part 2: Pumped Sampling Followed by Solvent Desorption and Gas Chromatography. CEN: Brussels, Belgium, 2005.
- Ballesta, P.P.; Field, R.A.; Saeger, E. De Population Exposure to Air Pollutants in Europe (People). In Communicating European Research 2005; Springer: Dordrecht, The Netherlands, 2007; pp. 211–217. [Google Scholar]
- Jerrett, M.; Arain, A.; Kanaroglou, P.; Beckerman, B.; Potoglou, D.; Sahsuvaroglu, T.; Morrison, J.; Giovis, C. A Review and Evaluation of Intraurban Air Pollution Exposure Models. J. Expo. Sci. Environ. Epidemiol. 2005, 15, 185–204. [Google Scholar] [CrossRef]
- Xie, X.; Semanjski, I.; Gautama, S.; Tsiligianni, E.; Deligiannis, N.; Rajan, R.; Pasveer, F.; Philips, W. A Review of Urban Air Pollution Monitoring and Exposure Assessment Methods. ISPRS Int. J. Geoinf. 2017, 6, 389. [Google Scholar] [CrossRef]
- Novoa-Bolivar, E.M.; Ros, J.A.; Pérez-Fernández, S.; Campillo, J.A.; López-Hernández, R.; González-López, R.; Otalora-Alcaraz, A.; Ortuño-Hernández, C.; Gimeno, L.; Ruiz-Lorente, I.; et al. Neutrophils and Lymphocytes: Yin and Yang of Lung Fibrosis and Patient Outcome in Diffuse Interstitial Lung Diseases. Biomedicines 2024, 12, 2439. [Google Scholar] [CrossRef] [PubMed]
- Novoa-Bolivar, E.M.; Ros, J.A.; Pérez-Fernández, S.; Campillo, J.A.; López-Hernández, R.; González-López, R.; Otálora-Alcaraz, A.; Ortuño-Hernández, C.; Gimeno, L.; Ruiz-Lorente, I.; et al. Predictive Value of Flow Cytometry Quantification of BAL Lymphocytes and Neutrophils in ILD. Cells 2024, 13, 2066. [Google Scholar] [CrossRef]
- Novoa-Bolivar, E.M.; Ros, J.A.; Pérez-Fernández, S.; Campillo, J.A.; López-Hernández, R.; González-López, R.; Ruiz-Lorente, I.; Otálora-Alcaraz, A.; Ortuño-Hernández, C.; Gimeno, L.; et al. Diagnostic Utility of Bronchoalveolar Lavage Flow Cytometric Leukocyte Profiling in Interstitial Lung Disease and Infection. Biomolecules 2025, 15, 597. [Google Scholar] [CrossRef]
- Lv, J.; Li, X.; Shen, Y.; You, J.; Wen, M.; Wang, J.; Yang, X. Assessing Volatile Organic Compounds Exposure and Chronic Obstructive Pulmonary Diseases in US Adults. Front. Public Health 2023, 11, 1210136. [Google Scholar] [CrossRef]
- Blanchet, M.-R.; Israël-Assayag, E.; Cormier, Y. Inhibitory Effect of Nicotine on Experimental Hypersensitivity Pneumonitis In Vivo and In Vitro. Am. J. Respir. Crit. Care Med. 2004, 169, 903–909. [Google Scholar] [CrossRef]
- Hamblin, M.; Prosch, H.; Vašáková, M. Diagnosis, Course and Management of Hypersensitivity Pneumonitis. Eur. Respir. Rev. 2022, 31, 210169. [Google Scholar] [CrossRef]
- Zakharova, M.Y.; Belyanina, T.A.; Sokolov, A.V.; Kiselev, I.S.; Mamedov, A.E. The Contribution of Major Histocompatibility Complex Class II Genes to an Association with Autoimmune Diseases. Acta Naturae 2019, 11, 4–12. [Google Scholar] [CrossRef]
- Falfán-Valencia, R.; Camarena, Á.; Pineda, C.L.; Montaño, M.; Juárez, A.; Buendía-Roldán, I.; Pérez-Rubio, G.; Reséndiz-Hernández, J.M.; Páramo, I.; Vega, A.; et al. Genetic Susceptibility to Multicase Hypersensitivity Pneumonitis Is Associated with the TNF-238 GG Genotype of the Promoter Region and HLA-DRB1*04 Bearing HLA Haplotypes. Respir. Med. 2014, 108, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Collins, B.F.; Bairwa, M.; Joshi, J.M.; Talwar, D.; Singh, N.; Samaria, J.K.; Mangal, D.K.; Singh, V.; Raghu, G. Hypersensitivity Pneumonitis and Its Correlation with Ambient Air Pollution in Urban India. Eur. Respir. J. 2019, 53, 1801563. [Google Scholar] [CrossRef] [PubMed]
- Bals, R.; Boyd, J.; Esposito, S.; Foronjy, R.; Hiemstra, P.S.; Jiménez-Ruiz, C.A.; Katsaounou, P.; Lindberg, A.; Metz, C.; Schober, W.; et al. Electronic Cigarettes: A Task Force Report from the European Respiratory Society. Eur. Respir. J. 2019, 53, 1801151. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Kouadio, K.; Tuekpe, M.K.N.; Ariizumi, M. Eosinophil Infiltration in Airways of Mice Exposed to Toluene Diisocyanate. J. Occup. Health 2004, 46, 299–302. [Google Scholar] [CrossRef]
- Gu, H.; Itoh, M.; Matsuyama, N.; Hayashi, S.; Iimura, A.; Nakamura, Y.; Miki, T.; Takeuchi, Y. Toluene Diisocyanate Exposure Induces Laryngo-Tracheal Eosinophilia, Which Can Be Ameliorated by Supplementation with Antioxidant Vitamins in Guinea Pigs. Acta Otolaryngol. 2003, 123, 965–971. [Google Scholar] [CrossRef]
- Salonen, J.; Lampela, H.; Keskitalo, E.; Vähänikkilä, H.; Purokivi, M.; Kaarteenaho, R. Bronchoalveolar Lavage Differential Cell Count on Prognostic Assessment of Patients with Stable or Acute Interstitial Lung Disease: A Retrospective Real-Life Study. Clin. Immunol. 2020, 220, 108594. [Google Scholar] [CrossRef]
- Kinder, B.W.; Brown, K.K.; Schwarz, M.I.; Ix, J.H.; Kervitsky, A.; King, T.E. Baseline BAL Neutrophilia Predicts Early Mortality in Idiopathic Pulmonary Fibrosis. Chest 2008, 133, 226–232. [Google Scholar] [CrossRef]
- Hirasawa, Y.; Nakada, T.-A.; Shimazui, T.; Abe, M.; Isaka, Y.; Sakayori, M.; Suzuki, K.; Yoshioka, K.; Kawasaki, T.; Terada, J.; et al. Prognostic Value of Lymphocyte Counts in Bronchoalveolar Lavage Fluid in Patients with Acute Respiratory Failure: A Retrospective Cohort Study. J. Intensive Care 2021, 9, 21. [Google Scholar] [CrossRef]
- Kanaoka, K.; Minami, S.; Ihara, S.; Komuta, K. High Neutrophils and Low Lymphocytes Percentages in Bronchoalveolar Lavage Fluid Are Prognostic Factors of Higher In-Hospital Mortality in Diffuse Alveolar Hemorrhage. BMC Pulm. Med. 2021, 21, 288. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.; Bergantini, L.; Carleo, A.; Cameli, P.; Perrone, A.; Fossi, A.; Sestini, P.; Bargagli, E. Neutrophil-to-Lymphocyte Ratio in Bronchoalveolar Lavage from IPF Patients: A Novel Prognostic Biomarker? Minerva Med. 2022, 113, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Tzilas, V.; Bouros, D. Diagnostic Confidence and Prognosis in Fibrotic Hypersensitivity Pneumonitis: Classification Is Fundamental. Respiration 2021, 100, 937–939. [Google Scholar] [CrossRef]
- De Sadeleer, L.J.; Hermans, F.; De Dycker, E.; Yserbyt, J.; Verschakelen, J.A.; Verbeken, E.K.; Verleden, G.M.; Verleden, S.E.; Wuyts, W.A. Impact of BAL Lymphocytosis and Presence of Honeycombing on Corticosteroid Treatment Effect in Fibrotic Hypersensitivity Pneumonitis: A Retrospective Cohort Study. Eur. Respir. J. 2020, 55, 1901983. [Google Scholar] [CrossRef]
- Feng, X.; Yu, F.; He, X.-L.; Cheng, P.-P.; Niu, Q.; Zhao, L.-Q.; Li, Q.; Cui, X.-L.; Jia, Z.-H.; Ye, S.-Y.; et al. CD8 + Tissue-Resident Memory T Cells Are Essential in Bleomycin-Induced Pulmonary Fibrosis. Am. J. Physiol.-Cell Physiol. 2024, 327, C1178–C1191. [Google Scholar] [CrossRef] [PubMed]
| Patients | Sex | Age | Smoking | Fibrosis * | |
|---|---|---|---|---|---|
| (N) | (% Man) | (Mean ± SD) | (%) | (%) | |
| No pulmonary disease † | 112 | 52.7% | 53.5 ± 16.6 | 72.20% | 0% |
| Interstitial lung diseases (ILD) | |||||
| Sarcoidosis | 81 | 47.6% | 55.6 ± 14.8 | 47.80% | 19.5% |
| Hypersensitivity pneumonitis (HP) | 71 | 50.0% | 54.8 ± 18.1 | 28.20% | 40.80% |
| Organized cryptogenic pneumonia (COP) | 44 | 43.2% | 62.3 ± 17.4 | 52.60% | 18.6% |
| Lymphocytic interstitial pneumonia (LIP) | 37 | 64.9% | 53.2 ± 15.9 | 56.30% | 7.9% |
| Idiopathic pulmonary fibrosis (IPF) | 148 | 76.40% | 66.4 ± 10.9 | 67.20% | 100% |
| Respiratory bronchiolitis ILD (RB-ILD) | 25 | 40.0% | 53.3 ± 22.8 | 52.20% | 8.0% |
| Desquamative interstitial pneumonia (DIP) | 34 | 51.4% | 54.4 ± 20.8 | 56.70% | 5.6% |
| Nonspecific interstitial pneumonia (NSIP) ‡ | 156 | 49.4% | 59.3 ± 15.5 | 60.20% | 33.8% |
| Pneumoconiosis | 27 | 96.3% | 56.5 ± 16.0 | 75.0% | 19.2% |
| Pulmonary Langerhans cell histiocytosis (PLCH) | 9 | 66.7% | 36.7 ± 15.8 | 88.90% | 11.1% |
| Eosinophilic-ILD | 17 | 76.5% | 51.2 ± 22.7 | 50.0% | 5.9% |
| Unclassifiable-ILD (U-ILD) | 81 | 60.0% | 61.3 ± 12.4 | 75.0% | 25% |
| Acute interstitial pneumonia (AIP) | 25 | 60.4% | 60.4 ± 19.5 | 75.0% | 12.5% |
| Non-ILD pulmonary pathologies | |||||
| Chronic obstructive pulmonary disease (COPD) | 82 | 74.4% | 63.4 ± 12.3 | 79.10% | 36.4% |
| Asthma | 90 | 26.1% | 46.3 ± 21.7 | 47.80% | 11.1% |
| Tuberculosis | 29 | 62.1% | 51.0 ± 17.3 | 50.0% | 0.0% |
| Infectious disease | |||||
| Pulmonary infections | 236 | 58.5% | 62.0 ± 15.7 | 68.70% | 16.1% |
| Cancer | |||||
| Lung cancer | 55 | 60.0% | 60.4 ± 11.1 | 71.40% | 50.0% |
| Other cancers | 156 | 51.9% | 56.7 ± 18.9 | 60.0% | 16.7% |
| N | N * | Benzene > 5 µg/m3 | Ethylbenzene > 10 µg/m3 | Toluene > 80 mg/m3 | m,p-Xylene > 10 µg/m3 | o-Xylene > 10 µg/m3 | |
|---|---|---|---|---|---|---|---|
| No pulmonary disease | 112 | 86 | 10.5% | 15.6% | 1.2% | 9.3% | 4.7% |
| Total patients | 1515 | 1344 | 7.1% | 13.7% | 1.0% | 9.6% | 3.6% |
| Interstitial lung diseases (ILD) | |||||||
| Sarcoidosis (n = 82) | 81 | 75 | 8.2% | 14.5% | 0% | 11% | 5.5% |
| HP | 71 | 71 | 4.6% | 23.9% † | 1.5% | 9.2% | 1.5% |
| COP | 44 | 44 | 11.4% | 22.7% | 0% | 11.4% | 6.8% |
| LIP | 37 | 28 | 7.4% | 21.4% | 0% | 14.8% | 7.4% |
| IPF | 148 | 143 | 6.7% | 10.5% † | 1.5% | 11.1% | 3% |
| RB-ILD | 25 | 25 | 12% | 12% | 0% | 8% | 4% |
| DIP | 34 | 34 | 3.0% | 14.3% | 0% | 6.1% | 3% |
| NSIP | 156 | 146 | 9.9% | 13.7% | 1.4% | 11.3% | 5.6% |
| AIP | 25 | 24 | 4.2% | 20.8% | 0% | 8.3% | 4.2% |
| Pneumoconiosis | 27 | 26 | 0% | 15.4% | 4.2% | 12.5% | 0% |
| PLCH | 9 | 9 | 0% | 0% | 0% | 22.2% | 0% |
| Eosinophilic-ILD | 17 | 15 | 0% | 26.7% | 13.3% † | 13.3% | 0% |
| U-ILD | 81 | 80 | 7.8% | 15% | 1.3% | 10.4% | 2.6% |
| Non-ILD pathologies | |||||||
| COPD | 82 | 71 | 15.4% † | 19.7% | 0% | 13.8% | 10.8% † |
| Asthma | 90 | 80 | 9% | 17.5% | 1.3% | 5.1% | 1.3% |
| Tuberculosis | 29 | 27 | 3.8% | 22.2% | 3.8% | 15.4% | 3.8% |
| Pulmonary infectious diseases | |||||||
| Pulmonary infections | 236 | 197 | 8.8% | 14.2% | 0% | 11.9% | 4.7% |
| Cancer | |||||||
| Lung cancer | 55 | 45 | 7.3% | 17.8% | 4.9% | 9.8% | 0% |
| Other cancers | 156 | 118 | 8.9% | 12.1% | 0.9% | 15.2% | 4.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minguela, A.; Campillo, J.A.; Aguilar Sanchís, M.I.; Baeza Caracena, A.; Esquembre, F.; Novoa-Bolivar, E.M.; González-López, R.; Otalora, A.; Ortuño-Hernández, C.; López-Hernández, R.; et al. Ethylbenzene Exposure and Bronchoalveolar CD4/CD8 T Cells in Hypersensitivity Pneumonitis Development and Clinical Outcome. Biomedicines 2025, 13, 2611. https://doi.org/10.3390/biomedicines13112611
Minguela A, Campillo JA, Aguilar Sanchís MI, Baeza Caracena A, Esquembre F, Novoa-Bolivar EM, González-López R, Otalora A, Ortuño-Hernández C, López-Hernández R, et al. Ethylbenzene Exposure and Bronchoalveolar CD4/CD8 T Cells in Hypersensitivity Pneumonitis Development and Clinical Outcome. Biomedicines. 2025; 13(11):2611. https://doi.org/10.3390/biomedicines13112611
Chicago/Turabian StyleMinguela, Alfredo, José A. Campillo, María Isabel Aguilar Sanchís, Antonia Baeza Caracena, Francisco Esquembre, Erika M. Novoa-Bolivar, Rosana González-López, Almudena Otalora, Cristina Ortuño-Hernández, Ruth López-Hernández, and et al. 2025. "Ethylbenzene Exposure and Bronchoalveolar CD4/CD8 T Cells in Hypersensitivity Pneumonitis Development and Clinical Outcome" Biomedicines 13, no. 11: 2611. https://doi.org/10.3390/biomedicines13112611
APA StyleMinguela, A., Campillo, J. A., Aguilar Sanchís, M. I., Baeza Caracena, A., Esquembre, F., Novoa-Bolivar, E. M., González-López, R., Otalora, A., Ortuño-Hernández, C., López-Hernández, R., Gimeno, L., Ruiz-Lorente, I., Ceballos, D., Solana-Martínez, E., Alcántara-Fructuoso, J., Muro, M., & Ros, J. A. (2025). Ethylbenzene Exposure and Bronchoalveolar CD4/CD8 T Cells in Hypersensitivity Pneumonitis Development and Clinical Outcome. Biomedicines, 13(11), 2611. https://doi.org/10.3390/biomedicines13112611








