Fetal Sex Modulates Hofbauer Cells’ Response to Diabetes in Human Placenta
Abstract
1. Introduction
2. Material and Methods
2.1. Tissue Sample Characteristics
2.2. Immunohistochemical (IHC) Staining
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
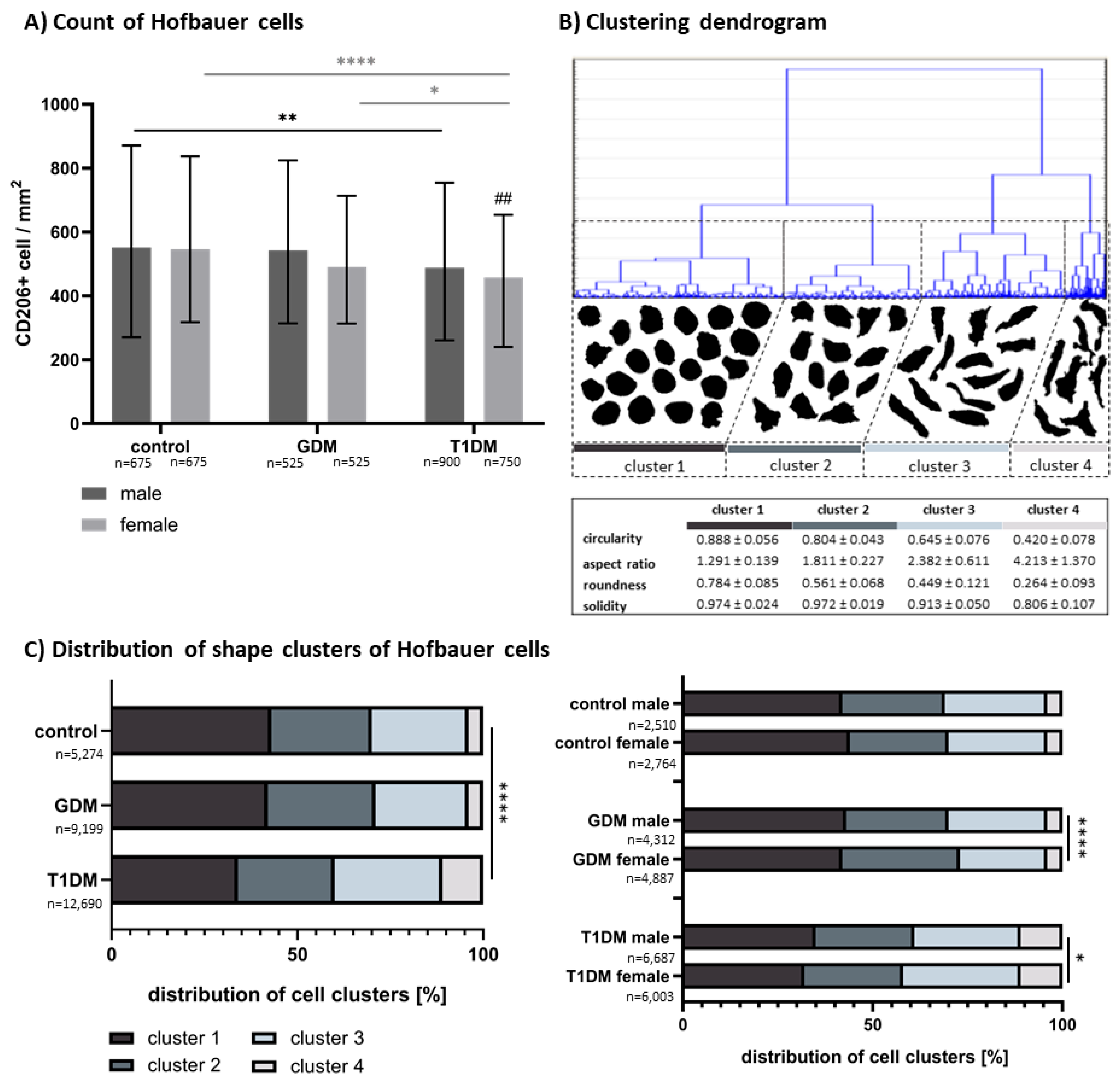
3.1. Morphometric Analysis of CD206+ HBCs
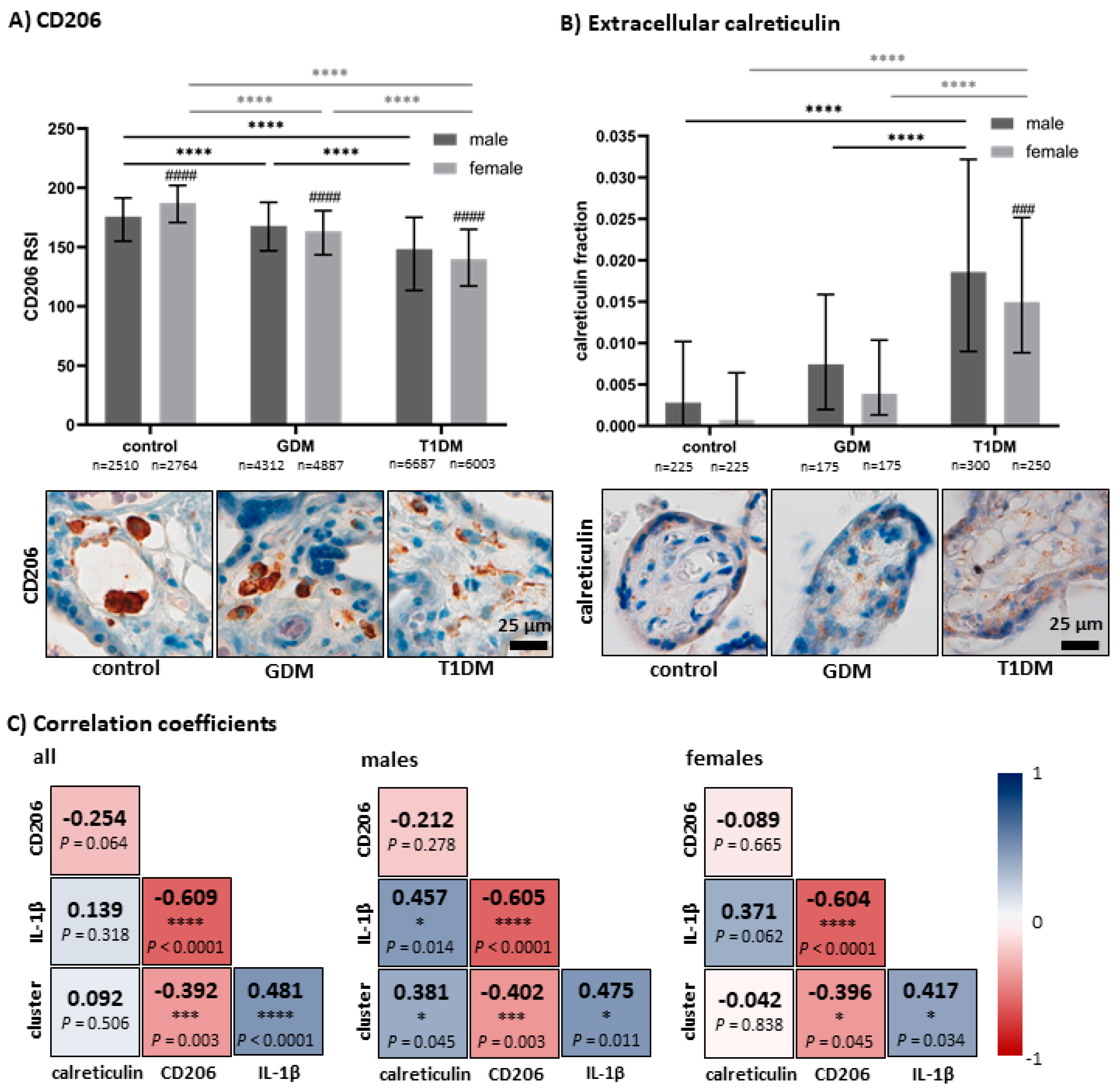
3.2. Quantitative Analysis of CD206 and Accumulation of Apoptotic Bodies in Villous Stroma
3.3. Correlation Between Measured Parameters and IL-1β
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingman, K.; Cookson, V.J.; Jones, C.J.; Aplin, J.D. Characterisation of Hofbauer cells in first and second trimester placenta: Incidence, phenotype, survival in vitro and motility. Placenta 2010, 31, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Loegl, J.; Hiden, U.; Nussbaumer, E.; Schliefsteiner, C.; Cvitic, S.; Lang, I.; Wadsack, C.; Huppertz, B.; Desoye, G. Hofbauer cells of M2a, M2b and M2c polarization may regulate feto-placental angiogenesis. Reproduction 2016, 152, 447–455. [Google Scholar] [CrossRef]
- Tang, Z.; Buhimschi, I.A.; Buhimschi, C.S.; Tadesse, S.; Norwitz, E.; Niven-Fairchild, T.; Huang, S.T.; Guller, S. Decreased levels of folate receptor-β and reduced numbers of fetal macrophages (Hofbauer cells) in placentas from pregnancies with severe pre-eclampsia. Am. J. Reprod. Immunol. 2013, 70, 104–115. [Google Scholar] [CrossRef]
- Schliefsteiner, C.; Peinhaupt, M.; Kopp, S.; Lögl, J.; Lang-Olip, I.; Hiden, U.; Heinemann, A.; Desoye, G.; Wadsack, C. Human Placental Hofbauer Cells Maintain an Anti-inflammatory M2 Phenotype despite the Presence of Gestational Diabetes Mellitus. Front. Immunol. 2017, 8, 888. [Google Scholar] [CrossRef]
- Swieboda, D.; Johnson, E.L.; Beaver, J.; Haddad, L.; Enninga, E.A.L.; Hathcock, M.; Cordes, S.; Jean, V.; Lane, I.; Skountzou, I.; et al. Baby’s First Macrophage: Temporal Regulation of Hofbauer Cell Phenotype Influences Ligand-Mediated Innate Immune Responses across Gestation. J. Immunol. 2020, 204, 2380–2391. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Appios, A.; Zhao, X.; Dutkiewicz, R.; Donde, M.; Lee, C.Y.C.; Naidu, P.; Lee, C.; Cerveira, J.; Liu, B.; et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J. Exp. Med. 2021, 218, e20200891. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Andersen, M.N.; Grønbæk, H.; Damgaard Sandahl, T.; Møller, H.J. Extracellular vesicle-associated soluble CD163 and CD206 in patients with acute and chronic inflammatory liver disease. Scand. J. Gastroenterol. 2020, 55, 588–596. [Google Scholar] [CrossRef]
- Taylor, P.R.; Gordon, S.; Martinez-Pomares, L. The mannose receptor: Linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005, 26, 104–110. [Google Scholar] [CrossRef]
- Sturge, J.; Todd, S.K.; Kogianni, G.; McCarthy, A.; Isacke, C.M. Mannose receptor regulation of macrophage cell migration. J. Leukoc. Biol. 2007, 82, 585–593. [Google Scholar] [CrossRef]
- Jones, G.E. Cellular signaling in macrophage migration and chemotaxis. J. Leukoc. Biol. 2000, 68, 593–602. [Google Scholar] [CrossRef]
- Qaseem, M.; Ara, N.; Farooq, K.; Idrees, S.A. Histological Changes in the Extracellular Matrix of the Human Placenta Complicated by Diabetes. Med. Forum Mon. 2024, 35, 57–61. [Google Scholar]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Cabero Roura, L.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. 3), S173–S211. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, T.O.; Zillikens, M.C.; Stančákova, A.; Finucane, F.M.; Ried, J.S.; Langenberg, C.; Zhang, W.; Beckmann, J.S.; Luan, J.; Vandenput, L.; et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat. Genet. 2011, 43, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, T.; Varastehpour, A.; Catalano, P.; Hauguel-de Mouzon, S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 2003, 52, 2951–2958. [Google Scholar] [CrossRef]
- Cano-Cano, F.; Gómez-Jaramillo, L.; Ramos-García, P.; Arroba, A.I.; Aguilar-Diosdado, M. IL-1β Implications in Type 1 Diabetes Mellitus Progression: Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1303. [Google Scholar] [CrossRef]
- Wen, Y.; Gu, J.; Li, S.-L.; Reddy, M.A.; Natarajan, R.; Nadler, J.L. Elevated Glucose and Diabetes Promote Interleukin-12 Cytokine Gene Expression in Mouse Macrophages. Endocrinology 2006, 147, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Sisino, G.; Bouckenooghe, T.; Aurientis, S.; Fontaine, P.; Storme, L.; Vambergue, A. Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype. Biochim. Biophys. Acta 2013, 1832, 1959–1968. [Google Scholar] [CrossRef]
- Dai, M.; Wu, L.; Wang, P.; Wen, Z.; Xu, X.; Wang, D.W. CYP2J2 and Its Metabolites EETs Attenuate Insulin Resistance via Regulating Macrophage Polarization in Adipose Tissue. Sci. Rep. 2017, 7, 46743. [Google Scholar] [CrossRef]
- Orliaguet, L.; Ejlalmanesh, T.; Alzaid, F. Metabolic and Molecular Mechanisms of Macrophage Polarisation and Adipose Tissue Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 5731. [Google Scholar] [CrossRef]
- Tauber, Z.; Burianova, A.; Koubova, K.; Mrstik, M.; Jirkovska, M.; Cizkova, K. The interplay of inflammation and placenta in maternal diabetes: Insights into Hofbauer cell expression patterns. Front. Immunol. 2024, 15, 1386528. [Google Scholar] [CrossRef]
- Baines, K.J.; West, R.C. Sex differences in innate and adaptive immunity impact fetal, placental, and maternal health. Biol. Reprod. 2023, 109, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, P.; Kaforou, M.; Tang, Z.; Abrahams, V.M.; McArdle, A.; Guller, S.; Holder, B. Placental macrophage responses to viral and bacterial ligands and the influence of fetal sex. iScience 2022, 25, 105653. [Google Scholar] [CrossRef] [PubMed]
- Batorsky, R.; Ceasrine, A.M.; Shook, L.L.; Kislal, S.; Bordt, E.A.; Devlin, B.A.; Perlis, R.H.; Slonim, D.K.; Bilbo, S.D.; Edlow, A.G. Hofbauer cells and fetal brain microglia share transcriptional profiles and responses to maternal diet-induced obesity. Cell Rep. 2024, 43, 114326. [Google Scholar] [CrossRef] [PubMed]
- Landini, G.; Martinelli, G.; Piccinini, F. Colour deconvolution: Stain unmixing in histological imaging. Bioinformatics 2020, 37, 1485–1487. [Google Scholar] [CrossRef]
- Nguyen, D. Quantifying Chromogen Intensity in Immunohistochemistry via Reciprocal Intensity. 2013. Available online: https://www.protocols.io/view/quantifying-chromogen-intensity-in-immunohistochem-261gerbp7l47/v1/references (accessed on 9 September 2024).
- Zhang, M.; Cui, D.; Yang, H. The Distributional Characteristics of M2 Macrophages in the Placental Chorionic Villi are Altered Among the Term Pregnant Women with Uncontrolled Type 2 Diabetes Mellitus. Front. Immunol. 2022, 13, 837391. [Google Scholar] [CrossRef]
- Gosain, R.; Motwani, R.; Anupama, H. CD68 expression in the placenta of gestational diabetic mothers: A case-control study. Indian J. Pathol. Microbiol. 2023, 66, 727–731. [Google Scholar] [CrossRef]
- Kerby, A.; Shingleton, D.; Batra, G.; Sharps, M.C.; Baker, B.C.; Heazell, A.E.P. Placental Morphology and Cellular Characteristics in Stillbirths in Women with Diabetes and Unexplained Stillbirths. Arch. Pathol. Lab. Med. 2020, 145, 82–89. [Google Scholar] [CrossRef]
- Bari, M.F.; Weickert, M.O.; Sivakumar, K.; James, S.G.; Snead, D.R.; Tan, B.K.; Randeva, H.S.; Bastie, C.C.; Vatish, M. Elevated soluble CD163 in gestational diabetes mellitus: Secretion from human placenta and adipose tissue. PLoS ONE 2014, 9, e101327. [Google Scholar] [CrossRef]
- Aşır, F.; Nergiz, Y.; Nergiz Öztürk, Ş.; Şahin, A.; Ağaçayak, E. Investigation of placental Hofbauer cells by immunohistochemistry method in complicated pregnancies. Middle East J. Sci. 2021, 7, 150–159. [Google Scholar] [CrossRef]
- Dairi, A.S.; Himayda, A.S.A.; Moulana, A.A.R.; Bukhari, H.S.H.; Hakeem, I.M.; Elbarrany, W. The Effect of Gestational Diabetes Mellitus on the Chorionic Villi of Human Placenta Among Saudi Arabian Mothers: A Quantitative and Comparative Study. Cureus 2020, 12, e11130. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, M.; Cui, D.; Yang, H. The pathologic changes of human placental macrophages in women with hyperglycemia in pregnancy. Placenta 2022, 130, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Paparini, D.E.; Grasso, E.; Aguilera, F.; Arslanian, M.A.; Lella, V.; Lara, B.; Schafir, A.; Gori, S.; Merech, F.; Hauk, V.; et al. Sex-specific phenotypical, functional and metabolic profiles of human term placenta macrophages. Biol. Sex Differ. 2024, 15, 80. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef]
- Eligini, S.; Crisci, M.; Bono, E.; Songia, P.; Tremoli, E.; Colombo, G.I.; Colli, S. Human monocyte-derived macrophages spontaneously differentiated in vitro show distinct phenotypes. J. Cell. Physiol. 2013, 228, 1464–1472. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Glim, J.E.; Stavenuiter, A.W.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Tedesco, S.; Bolego, C.; Toniolo, A.; Nassi, A.; Fadini, G.P.; Locati, M.; Cignarella, A. Phenotypic activation and pharmacological outcomes of spontaneously differentiated human monocyte-derived macrophages. Immunobiology 2015, 220, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- van der Zande, H.J.P.; Nitsche, D.; Schlautmann, L.; Guigas, B.; Burgdorf, S. The Mannose Receptor: From Endocytic Receptor and Biomarker to Regulator of (Meta)Inflammation. Front. Immunol. 2021, 12, 765034. [Google Scholar] [CrossRef]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci. Transl. Med. 2020, 12, eaax6337. [Google Scholar] [CrossRef]
- Franco-Barraza, J.; Valdivia-Silva, J.E.; Zamudio-Meza, H.; Castillo, A.; García-Zepeda, E.A.; Benítez-Bribiesca, L.; Meza, I. Actin cytoskeleton participation in the onset of IL-1beta induction of an invasive mesenchymal-like phenotype in epithelial MCF-7 cells. Arch. Med. Res. 2010, 41, 170–181. [Google Scholar] [CrossRef]
- Kesapragada, M.; Sun, Y.H.; Zhu, K.; Recendez, C.; Fregoso, D.; Yang, H.Y.; Rolandi, M.; Isseroff, R.; Zhao, M.; Gomez, M. A data-driven approach to establishing cell motility patterns as predictors of macrophage subtypes and their relation to cell morphology. PLoS ONE 2024, 19, e0315023. [Google Scholar] [CrossRef]
- Stinson, M.W.; Liu, S.; Laurenson, A.J.; Rotty, J.D. Macrophage migration is differentially regulated by fibronectin and laminin through altered adhesion and myosin II localization. Mol. Biol. Cell 2024, 35, ar22. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.R.; Hu, W.T.; Wei, C.Y.; Tang, L.L.; Liu, Y.K.; Liu, Y.Y.; Qiu, J.P.; Li, D.J.; Zhu, X.Y. Insights of efferocytosis in normal and pathological pregnancy. Am. J. Reprod. Immunol. 2019, 82, e13088. [Google Scholar] [CrossRef]
- Tao, H.; Ma, R.; Cui, J.; Yang, Z.; He, W.; Li, Y.; Zhao, Y. Immunomodulatory effect of efferocytosis at the maternal-fetal interface. Cell Commun. Signal. 2025, 23, 49. [Google Scholar] [CrossRef]
- Yu, J.; Su, X.-L.; Jia, J.; Zeng, Y.; Zhang, J.-Y.; Wang, S.-S.; Feng, L.; Pan, Y.; Shi, D.-D. The Relationship Between the Expression of Resistin and Apoptosis Factors in Placenta and the Pathogenesis of Gestational Diabetes Mellitus. Matern. Fetal Med. 2020, 2, 80–83. [Google Scholar] [CrossRef]
- Sgarbosa, F.; Barbisan, L.F.; Brasil, M.A.; Costa, E.; Calderon, I.M.; Gonçalves, C.R.; Bevilacqua, E.; Rudge, M.V. Changes in apoptosis and Bcl-2 expression in human hyperglycemic, term placental trophoblast. Diabetes Res. Clin. Pract. 2006, 73, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Magee, T.R.; Ross, M.G.; Wedekind, L.; Desai, M.; Kjos, S.; Belkacemi, L. Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta. J. Diabetes Complicat. 2014, 28, 448–459. [Google Scholar] [CrossRef]
- Hung, T.-H.; Huang, S.-Y.; Chen, S.-F.; Wu, C.-P.; Hsieh, T.S.-T.A. Decreased placental apoptosis and autophagy in pregnancies complicated by gestational diabetes with large-for-gestational age fetuses. Placenta 2020, 90, 27–36. [Google Scholar] [CrossRef]
- Chakraborty, B.; Chakraborty, P.; Brown, M.C.; Crowder, D.; Goyal, A.; Safi, R.; Artham, S.; Kirkland, M.; Racioppi, A.; Juras, P.; et al. Estrogens increase cancer cell efferocytosis to establish an immunosuppressive tumor microenvironment. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, Y.; Huang, S.; He, H.; Hu, N.; Lin, S.; You, Z. The reduction of microglial efferocytosis is concomitant with depressive-like behavior in CUMS-treated mice. J. Affect. Disord. 2024, 352, 76–86. [Google Scholar] [CrossRef]
- Jabeen, S.; Landazuri, J.; Nagvenkar, S.; Czuj, B.; Maghsoudi, A.; Javdan, M.; Entezari, M.; Lockshin, R.A.; Zakeri, Z. TLR4 sex dimorphism correlates with sex dimorphic phagocytosis in primary macrophages. Ital. J. Gend. Specif. Med. 2020, 6, 100–106. [Google Scholar] [CrossRef]
- Igarashi, Y.; Nawaz, A.; Kado, T.; Bilal, M.; Kuwano, T.; Yamamoto, S.; Sasahara, M.; Jiuxiang, X.; Inujima, A.; Koizumi, K.; et al. Partial depletion of CD206-positive M2-like macrophages induces proliferation of beige progenitors and enhances browning after cold stimulation. Sci. Rep. 2018, 8, 14567. [Google Scholar] [CrossRef]
- Ray, A.; Hu, K.H.; Kersten, K.; Courau, T.; Kuhn, N.F.; Zaleta-Linares, I.; Samad, B.; Combes, A.J.; Krummel, M.F. Targeting CD206+ macrophages disrupts the establishment of a key antitumor immune axis. J. Exp. Med. 2025, 222, e20240957. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.M.; Frost, L.I.; Anumba, D.O. Impaired autophagy with augmented apoptosis in a Th1/Th2-imbalanced placental micromilieu is associated with spontaneous preterm birth. Front. Mol. Biosci. 2022, 9, 897228. [Google Scholar] [CrossRef]
- Peelen, M.J.; Kazemier, B.M.; Ravelli, A.C.; De Groot, C.J.; Van Der Post, J.A.; Mol, B.W.; Hajenius, P.J.; Kok, M. Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstet. Gynecol. Scand. 2016, 95, 1034–1041. [Google Scholar] [CrossRef]
- Zhou, J.; Teng, Y.; Zhang, F.; Ru, X.; Li, P.; Wang, J.; Yan, S.; Zhu, P.; Tao, F.; Huang, K. Sex-specific association between placental inflammatory cytokine mRNA expression and preschoolers’ behavioral development: The Ma’anshan birth cohort study. Brain Behav. Immun. 2022, 104, 110–121. [Google Scholar] [CrossRef]
- Fitzgerald, E.; Shen, M.; Yong, H.E.J.; Wang, Z.; Pokhvisneva, I.; Patel, S.; O’Toole, N.; Chan, S.-Y.; Chong, Y.S.; Chen, H.; et al. Hofbauer cell function in the term placenta associates with adult cardiovascular and depressive outcomes. Nat. Commun. 2023, 14, 7120. [Google Scholar] [CrossRef]
- Tauber, Z.; Stetkova, I.; Cizkova, K. Influence of fixation method and duration of archiving on immunohistochemical staining intensity in embryonic and fetal tissues. Ann. Anat. Anat. Anz. 2019, 224, 55–61. [Google Scholar] [CrossRef] [PubMed]
| Control | GDM | T1DM | |
|---|---|---|---|
| mother age | |||
| mean ± SD | 30.1 ± 4.2 | 30.4 ± 2.8 | 31.7 ± 5.5 |
| median (IQR) | 30.0 (26.75–31.50) | 30.0 (28.0–32.25) | 33.0 (28.75–36.0) |
| duration of diabetes | |||
| mean ± SD | - | - | 12.8 ± 6.8 |
| median (IQR) | - | - | 13.5 (6.75–17.75) |
| gestational age | |||
| mean ± SD | 39.1 ± 0.9 | 38.9 ± 1.1 | 37.4 ± 2.3 |
| median (IQR) | 39.0 (38.0–39.25) | 39.0 (38.0–40.0) | 38.0 (36.75–39.0) |
| fetal sex | 9 males, 9 females | 7 males, 7 females | 12 males, 10 females |
| b | std. Err. of b | p-Value | ||
|---|---|---|---|---|
| CD206 intensity | mother age | −1.1433 | 0.8400 | 0.1796 |
| gestational age | 1.1224 | 2.0931 | 0.5942 | |
| delivery | −5.3578 | 7.7942 | 0.4950 | |
| calreticulin fraction | mother age | −1.1494 | 0.8397 | 0.1772 |
| gestational age | 1.1969 | 2.0924 | 0.5699 | |
| delivery | −6.2947 | 7.7918 | 0.4230 | |
| cell size | mother age | −0.3228 | 0.4593 | 0.4854 |
| gestational age | 1.3679 | 1.1445 | 0.2377 | |
| delivery | 9.1660 | 4.2619 | 0.0364 | |
| circularity | mother age | −0.0009 | 0.0012 | 0.4387 |
| gestational age | 0.0011 | 0.0029 | 0.6960 | |
| delivery | −0.0068 | 0.0107 | 0.5285 | |
| aspect ratio | mother age | 0.0102 | 0.0077 | 0.1895 |
| gestational age | −0.0298 | 0.0192 | 0.1266 | |
| delivery | 0.0208 | 0.0714 | 0.7726 | |
| roundness | mother age | −0.0018 | 0.0015 | 0.2449 |
| gestational age | 0.0065 | 0.0039 | 0.1000 | |
| delivery | 0.0022 | 0.0144 | 0.8789 | |
| solidity | mother age | −0.0002 | 0.0005 | 0.7166 |
| gestational age | −0.0007 | 0.0012 | 0.5531 | |
| delivery | −0.0063 | 0.0044 | 0.1611 | |
| cell cluster | mother age | 0.0054 | 0.0073 | 0.4617 |
| gestational age | −0.0182 | 0.0182 | 0.3222 | |
| delivery | 0.0247 | 0.0680 | 0.7180 | |
| CD206+ cells per mm2 | mother age | 0.0623 | 5.4150 | 0.9909 |
| gestational age | 1.1433 | 13.4936 | 0.9328 | |
| delivery | −69.5418 | 50.2477 | 0.1725 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauber, Z.; Mrstik, M.; Burianova, A.; Koubova, K.; Cizkova, K. Fetal Sex Modulates Hofbauer Cells’ Response to Diabetes in Human Placenta. Biomedicines 2025, 13, 2606. https://doi.org/10.3390/biomedicines13112606
Tauber Z, Mrstik M, Burianova A, Koubova K, Cizkova K. Fetal Sex Modulates Hofbauer Cells’ Response to Diabetes in Human Placenta. Biomedicines. 2025; 13(11):2606. https://doi.org/10.3390/biomedicines13112606
Chicago/Turabian StyleTauber, Zdenek, Max Mrstik, Adela Burianova, Katerina Koubova, and Katerina Cizkova. 2025. "Fetal Sex Modulates Hofbauer Cells’ Response to Diabetes in Human Placenta" Biomedicines 13, no. 11: 2606. https://doi.org/10.3390/biomedicines13112606
APA StyleTauber, Z., Mrstik, M., Burianova, A., Koubova, K., & Cizkova, K. (2025). Fetal Sex Modulates Hofbauer Cells’ Response to Diabetes in Human Placenta. Biomedicines, 13(11), 2606. https://doi.org/10.3390/biomedicines13112606






