Analysis of Immune Cell Infiltration Distribution and Prognostic Value in Obstructive Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Observational Methods
2.1.1. Clinical Data
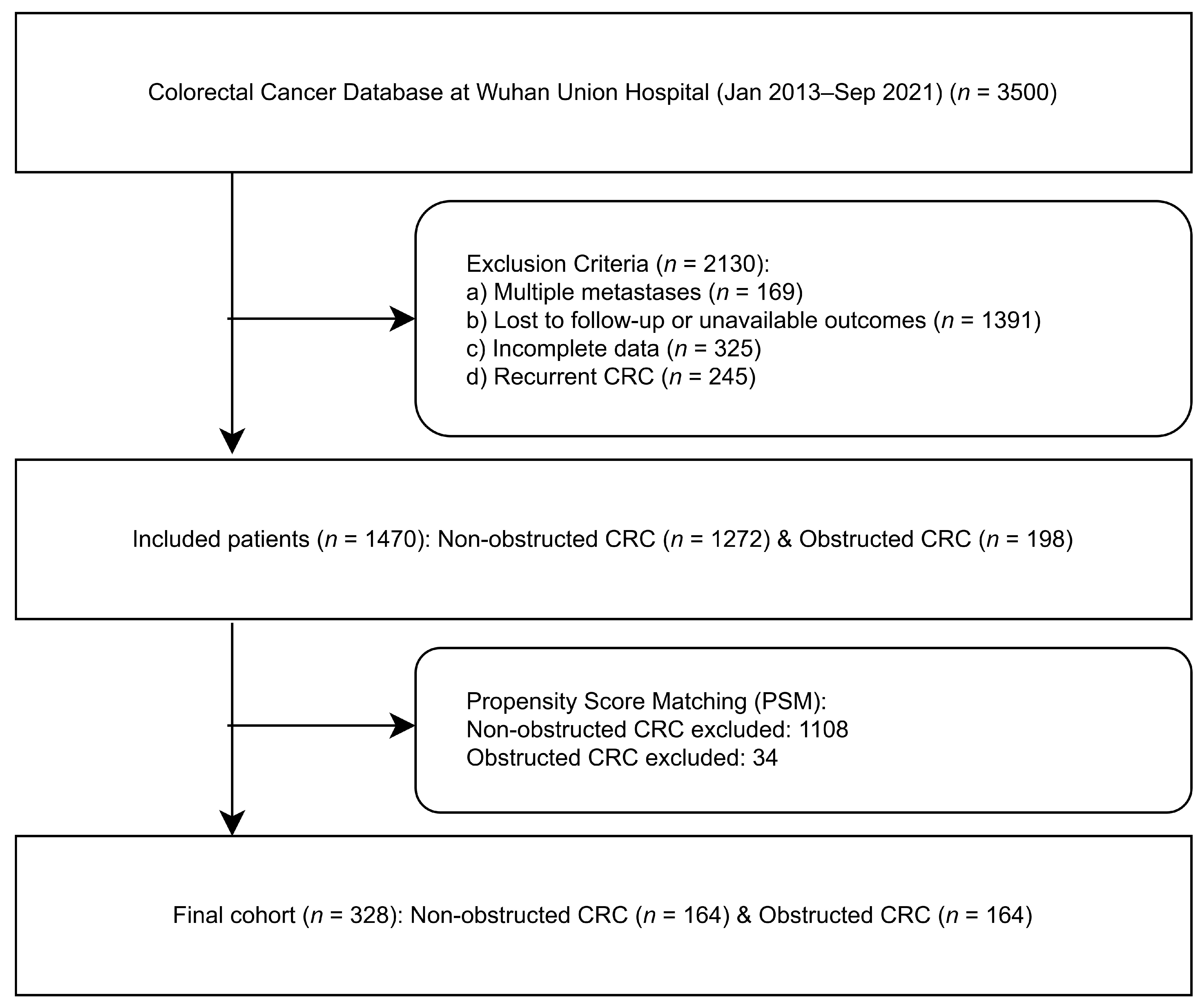
2.1.2. Propensity Score Matching (PSM)
2.2. Experimental Methods
2.2.1. Tissue Analysis
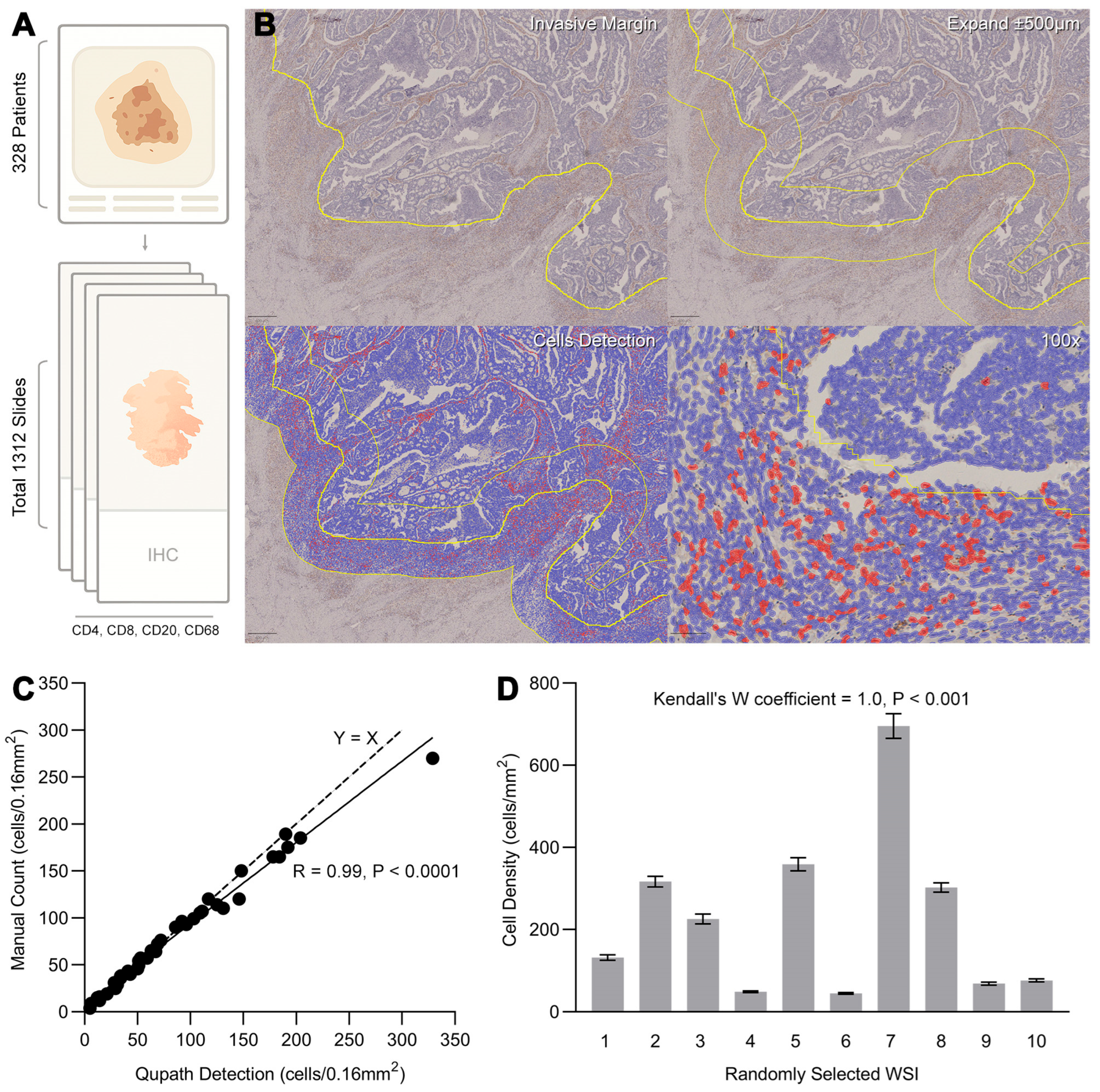
2.2.2. QuPath Digital Image Analysis
2.3. Statistical Analysis
2.3.1. Multivariate Analysis
2.3.2. Survival Analysis
3. Result
3.1. Patient Enrollment Screening and Clinical Baseline Data
3.2. Digital Annotation of Tumor Whole Slide Images and Quantification of Tumor-Infiltrating Immune Cells
3.3. The Impact of Obstruction Status and T Stage on the Patterns of Tumor Immune Cell Infiltration
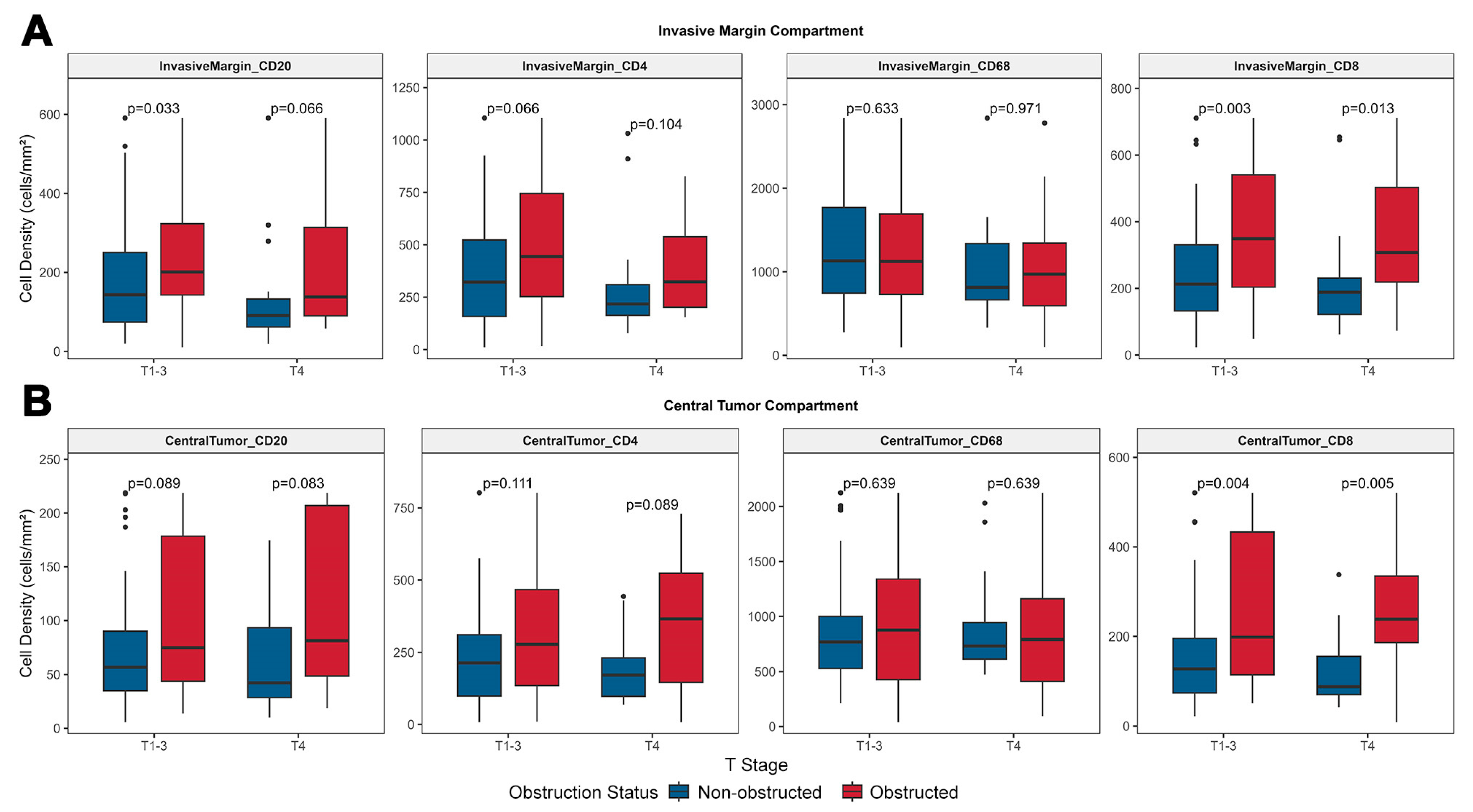
3.3.1. Obstruction May Be Associated with High Lymphocyte Infiltration in Tumors, with T4 Tumors Exhibiting a Lymphocyte Infiltration Tendency Comparable to T1–3 Tumors
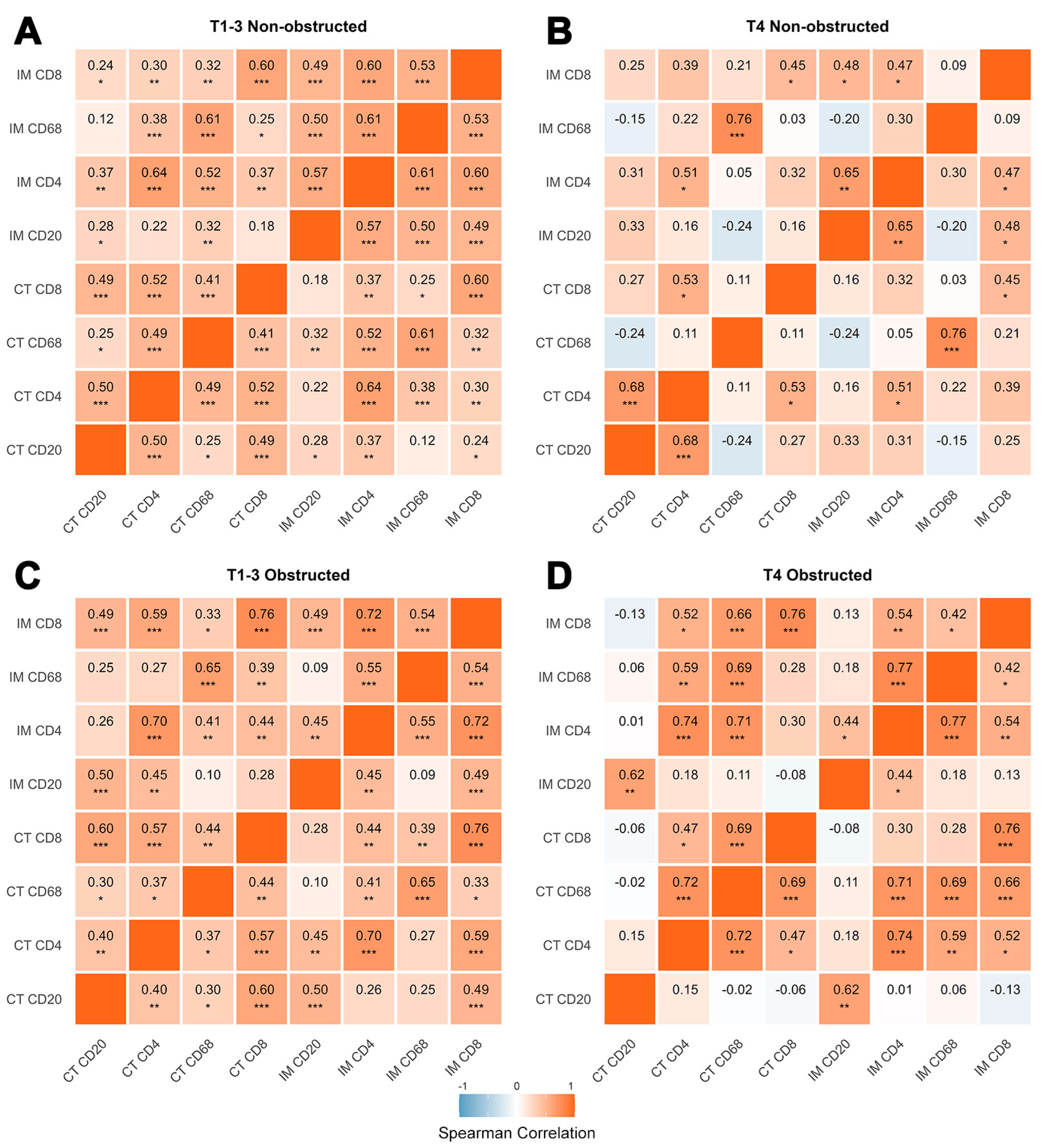
3.3.2. Compartmental and Inter-Subsets Correlations Reveal Distinct Patterns of the Tumor Immune Microenvironment Across Different T Stages and Obstruction Status
3.3.3. Immune Cell CT/IM Ratios Differentially Predict Colorectal Tumor Obstruction Status Across T Stages
3.4. Peripheral Blood Immune Cell Profiles Are Compared Between OCRC and NOCRC Cases
3.5. Impact of Obstruction and Tumor Immune Cell Infiltration on Patient Survival
3.5.1. Prognostic Factors in the Full Cohort
3.5.2. NOCRC Subgroup Analysis
3.5.3. Dichotomous Role of CD8+ T Cells by Obstruction Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal Cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Weng, J.; Li, S.; Zhu, Z.; Liu, Q.; Zhang, R.; Yang, Y.; Li, X. Exploring Immunotherapy in Colorectal Cancer. J. Hematol. Oncol. 2022, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F. Inflammatory Pathways in the Early Steps of Colorectal Cancer Development. World J. Gastroenterol. 2014, 20, 9716. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Wang, J.; Zou, F.; Cheng, D.; Chen, M.; Gu, J.; Shi, J.; Yang, J.; Xue, Y.; Jiang, Z.; et al. Palmitic Acid Accumulation Activates Fibroblasts and Promotes Matrix Stiffness in Colorectal Cancer. Cancer Res. 2025, 85, 1784–1802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, D.; Wu, D.; Gao, X.; Shao, F.; Zhao, M.; Wang, J.; Ma, J.; Wang, W.; Qin, X.; et al. Tissue-Resident Lachnospiraceae Family Bacteria Protect against Colorectal Carcinogenesis by Promoting Tumor Immune Surveillance. Cell Host Microbe 2023, 31, 418–432.e8. [Google Scholar] [CrossRef]
- El Sissy, C.; Kirilovsky, A.; Lagorce Pagès, C.; Marliot, F.; Custers, P.A.; Dizdarevic, E.; Sroussi, M.; Castillo-Martin, M.; Haicheur, N.; Dermani, M.; et al. International Validation of the Immunoscore Biopsy in Patients with Rectal Cancer Managed by a Watch-and-Wait Strategy. J. Clin. Oncol. 2024, 42, 70–80. [Google Scholar] [CrossRef]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef]
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus Molecular Subtypes and the Evolution of Precision Medicine in Colorectal Cancer. Nat. Rev. Cancer 2017, 17, 79–92. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Overman, M.J.; Gelsomino, F.; Aglietta, M.; Wong, M.; Limon Miron, M.L.; Leonard, G.; García-Alfonso, P.; Hill, A.G.; Cubillo Gracian, A.; Van Cutsem, E.; et al. Nivolumab plus Relatlimab in Patients with Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Immunother. Cancer 2024, 12, e008689. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Liu, W.; Kuang, T.; Liu, L.; Deng, W. The Role of Innate Immune Cells in the Colorectal Cancer Tumor Microenvironment and Advances in Anti-Tumor Therapy Research. Front. Immunol. 2024, 15, 1407449. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Rouzaut, A.; Motz, G.T.; Coukos, G. T-Cell and NK-Cell Infiltration into Solid Tumors: A Key Limiting Factor for Efficacious Cancer Immunotherapy. Cancer Discov. 2014, 4, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Lamplugh, Z.; Fan, Y. Vascular Microenvironment, Tumor Immunity and Immunotherapy. Front. Immunol. 2021, 12, 811485. [Google Scholar] [CrossRef]
- Dai, Q.; Wu, W.; Amei, A.; Yan, X.; Lu, L.; Wang, Z. Regulation and Characterization of Tumor-Infiltrating Immune Cells in Breast Cancer. Int. Immunopharmacol. 2021, 90, 107167. [Google Scholar] [CrossRef]
- Decock, J.; Comito, G.; Zaravinos, A. Editorial: Tumor Microenvironment, Inflammation, and Resistance to Immunotherapies. Front. Oncol. 2023, 13, 1215332. [Google Scholar] [CrossRef]
- Mezheyeuski, A.; Micke, P.; Martín-Bernabé, A.; Backman, M.; Hrynchyk, I.; Hammarström, K.; Ström, S.; Ekström, J.; Edqvist, P.-H.; Sundström, M.; et al. The Immune Landscape of Colorectal Cancer. Cancers 2021, 13, 5545. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, G.; Tian, C.; Du, L.; Prochownik, E.V.; Li, Y. Inhibition of DUSP18 Impairs Cholesterol Biosynthesis and Promotes Anti-Tumor Immunity in Colorectal Cancer. Nat. Commun. 2024, 15, 5851. [Google Scholar] [CrossRef]
- Yoon, P.S.; Del Piccolo, N.; Shirure, V.S.; Peng, Y.; Kirane, A.; Canter, R.J.; Fields, R.C.; George, S.C.; Gholami, S. Advances in Modeling the Immune Microenvironment of Colorectal Cancer. Front. Immunol. 2021, 11, 614300. [Google Scholar] [CrossRef]
- Xu, X.; Ma, J.; Yu, G.; Qiu, Q.; Zhang, W.; Cao, F. Effective Predictor of Colorectal Cancer Survival Based on Exclusive Expression Pattern Among Different Immune Cell Infiltration. J. Histochem. Cytochem. 2021, 69, 271–286. [Google Scholar] [CrossRef]
- Subtil, B.; Cambi, A.; Tauriello, D.V.F.; De Vries, I.J.M. The Therapeutic Potential of Tackling Tumor-Induced Dendritic Cell Dysfunction in Colorectal Cancer. Front. Immunol. 2021, 12, 724883. [Google Scholar] [CrossRef]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic Inflammation in Colorectal Cancer: Underlying Factors, Effects, and Prognostic Significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef]
- De La Cruz-Merino, L.; Henao Carrasco, F.; Vicente Baz, D.; Nogales Fernández, E.; Reina Zoilo, J.J.; Codes Manuel De Villena, M.; Pulido, E.G. Immune Microenvironment in Colorectal Cancer: A New Hallmark to Change Old Paradigms. Clin. Dev. Immunol. 2011, 2011, 174149. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.F.; Bhat, G.A.; Wani, M.A.; Malik, A.A.; Ul Haq, M.I.; Ul Haq, M.E. Outcome of Obstructing vs Nonobstructing Colorectal Carcinomas: Comparative Study at Tertiary Care Hospital in Kashmir. Euroasian J. Hepato-Gastroenterol. 2024, 14, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459.e29. [Google Scholar] [CrossRef] [PubMed]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-Like Receptor 2 Controls Mucosal Inflammation by Regulating Epithelial Barrier Function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef]



| Study Cohort | Statistical Analysis | ||||
|---|---|---|---|---|---|
| Variable | Total (n = 328) | Non-Obstructed (n = 164) | Obstructed (n = 164) | p Value | |
| Demographics | Age (years) | 62.0 ± 12.6 | 62.2 ± 11.8 | 61.7 ± 13.3 | 0.74 |
| BMI (kg/m2) | 21.8 ± 2.9 | 21.6 ± 3.0 | 22.0 ± 2.8 | 0.21 | |
| Male | 183 (55.8) | 91 (55.5) | 92 (56.1) | 1 | |
| Clinical Characteristics | Smoking | 70 (21.3) | 30 (18.3) | 40 (24.4) | 0.23 |
| Comorbidity | 107 (32.6) | 54 (32.9) | 53 (32.3) | 1 | |
| Prior abdominal surgery | 77 (23.5) | 41 (25.0) | 36 (22.0) | 0.6 | |
| Cardiovascular disease | 95 (29.0) | 45 (27.4) | 50 (30.5) | 0.63 | |
| COPD | 14 (4.3) | 9 (5.5) | 5 (3.0) | 0.41 | |
| Diabetes mellitus | 27 (8.2) | 13 (7.9) | 14 (8.5) | 1 | |
| Cerebrovascular disease | 7 (2.1) | 1 (0.6) | 6 (3.7) | 0.12 | |
| Hematological disorders | 2 (0.6) | 1 (0.6) | 1 (0.6) | 1 | |
| Tumor Profile | T stage | 0.14 | |||
| T1 | 9 (2.7) | 4 (2.4) | 5 (3.0) | ||
| T2 | 47 (14.3) | 25 (15.2) | 22 (13.4) | ||
| T3 | 182 (55.5) | 99 (60.4) | 83 (50.6) | ||
| T4 | 90 (27.4) | 36 (22.0) | 54 (32.9) | ||
| N stage | 0.74 | ||||
| N0 | 147 (44.8) | 70 (42.7) | 77 (47.0) | ||
| N1 | 123 (37.5) | 64 (39.0) | 59 (36.0) | ||
| N2 | 58 (17.7) | 30 (18.3) | 28 (17.1) | ||
| M stage | 0.72 | ||||
| M0 | 320 (97.6) | 161 (98.2) | 159 (97.0) | ||
| M1 | 8 (2.4) | 3 (1.8) | 5 (3.0) | ||
| TNM | 0.002 | ||||
| I | 21 (6.4) | 18 (11.0) | 3 (1.8) | ||
| II | 118 (36.0) | 50 (30.5) | 68 (41.5) | ||
| III | 173 (52.7) | 92 (56.1) | 81 (49.4) | ||
| IV | 16 (4.9) | 4 (2.4) | 12 (7.3) | ||
| Differentiation | 0.94 | ||||
| Low | 24 (14.5) | 26 (15.8) | |||
| Middle | 117 (70.9) | 114 (69.1) | |||
| High | 23 (13.9) | 24 (14.5) | |||
| Other | 1 (0.6) | 1 (0.6) | |||
| Perineural invasion | 88 (26.8) | 35 (21.3) | 53 (32.3) | 0.034 | |
| Lymphovascular invasion | 65 (19.8) | 28 (17.1) | 37 (22.6) | 0.27 | |
| Tumor Size | 0.54 | ||||
| <2 cm | 13 (4.0) | 5 (3.0) | 8 (4.9) | ||
| 2–5 cm | 222 (67.7) | 115 (70.1) | 107 (65.2) | ||
| ≥5 cm | 93 (28.4) | 44 (26.8) | 49 (29.9) | ||
| Tumor Location | 0.79 | ||||
| Right | 112 (34.1) | 58 (35.4) | 54 (32.9) | ||
| Left | 132 (40.2) | 63 (38.4) | 69 (42.1) | ||
| Rectum | 84 (25.6) | 43 (26.2) | 41 (25.0) | ||
| ASA | 0.16 | ||||
| I | 3 (0.9) | 2 (1.2) | 1 (0.6) | ||
| II | 214 (65.2) | 100 (61.0) | 114 (69.5) | ||
| III | 65 (19.8) | 40 (24.4) | 25 (15.2) | ||
| IV | 46 (14.0) | 22 (13.4) | 24 (14.6) | ||
| Medical History | History of malignancy | 26 (7.9) | 18 (11.0) | 8 (4.9) | 0.066 |
| Neoadjuvant Therapy | 11 (3.4) | 6 (3.7) | 5 (3.0) | 1 | |
| T1–3 | T4 | |||||
|---|---|---|---|---|---|---|
| Immune Cells | No Obstruction n = 127 | with Obstruction n = 105 | p-Value 1 | No Obstruction n = 37 | with Obstruction n = 59 | p-Value 1 |
| WBC (×109/L) | 0.569 | 0.757 | ||||
| Mean ± SD | 6.88 ± 3.49 | 6.37 ± 2.50 | 6.40 ± 2.76 | 6.39 ± 2.76 | ||
| Neutrophils (×109/L) | 0.994 | 0.433 | ||||
| Mean ± SD | 4.87 ± 3.30 | 4.49 ± 2.55 | 4.23 ± 3.02 | 4.48 ± 2.55 | ||
| Lymphocytes (×109/L) | 0.119 | <0.001 | ||||
| Mean ± SD | 1.37 ± 0.56 | 1.29 ± 0.59 | 1.55 ± 0.48 | 1.22 ± 0.42 | ||
| Monocytes (×109/L) | 0.873 | 0.057 | ||||
| Mean ± SD | 0.49 ± 0.24 | 0.46 ± 0.18 | 0.42 ± 0.10 | 0.53 ± 0.28 | ||
| Basophils (×109/L) | 0.395 | 0.991 | ||||
| Mean ± SD | 0.02 ± 0.02 | 0.03 ± 0.04 | 0.03 ± 0.01 | 0.03 ± 0.02 | ||
| Eosinophils (×109/L) | 0.005 | 0.212 | ||||
| Mean ± SD | 0.12 ± 0.09 | 0.11 ± 0.14 | 0.17 ± 0.16 | 0.14 ± 0.14 | ||
| Disease-Free Survival (DFS) Analysis | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Variable | Univariate HR (95% CI) | Univariate p | Multivariate HR (95% CI) | Multivariate p |
| Obstruction | 1.25 (0.86–1.83) | 0.2 | ||
| Age | 1.02 (1.00–1.04) | 0.016 | 1.01 (1.00–1.03) | 0.2 |
| Gender | 1.02 (0.70–1.49) | >0.9 | ||
| Tumor location | - | 0.5 | ||
| Colon | - | - | ||
| Rectum | 0.85 (0.55–1.32) | - | ||
| Differentiation | - | 0.8 | ||
| High/Other | - | - | ||
| Moderate | 1.00 (0.57–1.73) | - | ||
| Low | 1.20 (0.60–2.38) | - | ||
| T stage | - | <0.001 | - | - |
| I-II | - | - | - | - |
| III | 1.98 (1.01–3.89) | - | 2.02 (1.01–4.04) | 0.047 |
| IV | 4.11 (2.06–8.22) | - | 3.39 (1.61–7.13) | 0.001 |
| N stage | - | 0.039 | ||
| N0 | - | - | ||
| N1–2 | 1.50 (1.01–2.21) | - | 1.31 (0.81–2.11) | 0.3 |
| M stage | - | 0.031 | ||
| M0 | - | - | ||
| M1 | 3.22 (1.31–7.94) | - | 1.52 (0.54–4.27) | 0.4 |
| Postoperative radiotherapy | 0.74 (0.18–3.02) | 0.7 | ||
| Postoperative chemotherapy | 0.85 (0.58–1.24) | 0.4 | ||
| Surgical approach | - | 0.034 | ||
| Laparoscope | - | - | ||
| Open | 1.50 (1.03–2.20) | - | 1.08 (0.71–1.64) | 0.7 |
| Blood transfusion | 1.67 (1.13–2.45) | 0.011 | 1.65 (1.08–2.54) | 0.022 |
| Primary anastomosis | 0.42 (0.28–0.63) | <0.001 | 0.53 (0.34–0.84) | 0.007 |
| Vascular embolus | 1.54 (0.98–2.42) | 0.069 | 0.83 (0.47–1.43) | 0.5 |
| Nerve invasion | 1.77 (1.18–2.66) | 0.008 | 1.51 (0.94–2.42) | 0.087 |
| Total lymph nodes | 0.98 (0.96–1.00) | 0.036 | 0.98 (0.96–1.00) | 0.082 |
| Positive lymph nodes | 1.05 (1.00–1.09) | 0.065 | 1.00 (0.94–1.06) | >0.9 |
| CD4+ (Invasive margin) | 0.79 (0.63–0.99) | 0.029 | 0.94 (0.73–1.20) | 0.6 |
| CD8+ (Invasive margin) | 1.04 (0.90–1.20) | 0.6 | ||
| CD20+ (Invasive margin) | 1.01 (0.84–1.22) | >0.9 | ||
| CD68+ (Invasive margin) | 0.56 (0.43–0.73) | <0.001 | 0.59 (0.40–0.87) | 0.008 |
| CD4+ (Central tumor) | 0.94 (0.73–1.20) | 0.6 | ||
| CD8+ (Central tumor) | 1.10 (0.97–1.26) | 0.2 | ||
| CD20+ (Central tumor) | 1.11 (0.99–1.24) | 0.14 | ||
| CD68+ (Central tumor) | 0.77 (0.62–0.95) | 0.010 | 1.13 (0.83–1.54) | 0.4 |
| Overall Survival (OS) Analysis | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Variable | Univariate HR (95% CI) | Univariate p | Multivariate HR (95% CI) | Multivariate p |
| Obstruction | 1.60 (1.07–2.40) | 0.021 | 1.26 (0.80–1.99) | 0.3 |
| Age | 1.03 (1.01–1.05) | 0.001 | 1.02 (1.00–1.04) | 0.090 |
| Gender | 0.95 (0.64–1.42) | 0.8 | ||
| Tumor location | - | 0.5 | ||
| Colon | - | - | ||
| Rectum | 0.86 (0.54–1.37) | - | ||
| Differentiation | - | 0.3 | ||
| High/Other | - | - | ||
| Moderate | 0.83 (0.47–1.46) | - | ||
| Low | 1.24 (0.62–2.46) | - | ||
| T stage | - | <0.001 | - | - |
| I-II | - | - | - | - |
| III | 2.07 (0.98–4.38) | - | 2.01 (0.93–4.33) | 0.074 |
| IV | 4.34 (2.03–9.28) | - | 3.29 (1.46–7.39) | 0.004 |
| N stage | - | 0.001 | - | - |
| N0 | - | - | - | - |
| N1–2 | 1.98 (1.29–3.04) | - | 1.93 (1.15–3.25) | 0.013 |
| M stage | - | 0.15 | ||
| M0 | - | - | ||
| M1 | 2.32 (0.85–6.32) | - | ||
| Postoperative radiotherapy | 0.41 (0.06–2.97) | 0.3 | ||
| Postoperative chemotherapy | 0.66 (0.44–0.99) | 0.043 | 0.72 (0.45–1.15) | 0.2 |
| Surgical approach | - | <0.001 | - | - |
| Laparoscope | - | - | - | - |
| Open | 2.11 (1.39–3.21) | - | 1.55 (0.98–2.44) | 0.058 |
| Blood transfusion | 1.99 (1.33–2.98) | 0.001 | 1.92 (1.22–3.02) | 0.005 |
| Primary anastomosis | 0.34 (0.22–0.51) | <0.001 | 0.54 (0.34–0.86) | 0.010 |
| Vascular embolus | 1.64 (1.03–2.63) | 0.048 | 1.03 (0.57–1.84) | >0.9 |
| Nerve invasion | 1.59 (1.03–2.45) | 0.043 | 1.29 (0.78–2.16) | 0.3 |
| Total lymph nodes | 0.97 (0.95–1.00) | 0.011 | 0.97 (0.94–1.00) | 0.024 |
| Positive lymph nodes | 1.07 (1.02–1.12) | 0.007 | 1.01 (0.95–1.07) | 0.8 |
| CD4+ (Invasive margin) | 0.81 (0.64–1.02) | 0.064 | 1.04 (0.78–1.38) | 0.8 |
| CD8+ (Invasive margin) | 1.04 (0.90–1.21) | 0.6 | ||
| CD20+ (Invasive margin) | 0.98 (0.78–1.22) | 0.8 | ||
| CD68+ (Invasive margin) | 0.49 (0.36–0.66) | <0.001 | 0.60 (0.39–0.94) | 0.024 |
| CD4+ (Central tumor) | 0.97 (0.76–1.24) | 0.8 | ||
| CD8+ (Central tumor) | 0.99 (0.81–1.20) | >0.9 | ||
| CD20+ (Central tumor) | 1.13 (1.01–1.27) | 0.077 | 1.02 (0.89–1.16) | 0.8 |
| CD68+ (Central tumor) | 0.66 (0.51–0.84) | <0.001 | 0.93 (0.66–1.31) | 0.7 |
| Disease-Free Survival (DFS) Analysis | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Variable | Univariate HR (95% CI) | Univariate p | Multivariate HR (95% CI) | Multivariate p |
| Age | 1.02 (1.00–1.05) | 0.09 | 1.04 (1.01–1.07) | 0.013 |
| Gender | 1.00 (0.57–1.73) | >0.9 | ||
| Tumor location | - | 0.8 | ||
| Colon | - | - | ||
| Rectum | 0.93 (0.50–1.75) | - | ||
| Differentiation | - | 0.6 | ||
| High/Other | - | - | ||
| Moderate | 1.16 (0.49–2.75) | - | ||
| Low | 1.64 (0.58–4.61) | - | ||
| T stage | - | <0.001 | - | - |
| I–II | - | - | - | - |
| III | 5.65 (1.35–23.6) | - | 6.59 (1.51–28.7) | 0.012 |
| IV | 10.2 (2.35–44.3) | - | 10.1 (2.21–46.0) | 0.003 |
| N stage | - | 0.027 | - | - |
| N0 | - | - | - | - |
| N1–2 | 1.91 (1.06–3.46) | - | 1.78 (0.83–3.81) | 0.14 |
| M stage | - | 0.082 | - | - |
| M0 | - | - | - | - |
| M1 | 4.78 (1.14–20.0) | - | 6.70 (1.39–32.2) | 0.018 |
| Postoperative radiotherapy | 0.88 (0.12–6.42) | >0.9 | ||
| Postoperative chemotherapy | 0.88 (0.51–1.53) | 0.6 | ||
| Surgical approach | - | 0.025 | - | - |
| Laparoscope | - | - | - | - |
| Open | 1.91 (1.07–3.42) | - | 1.36 (0.73–2.55) | 0.3 |
| Blood transfusion | 1.43 (0.82–2.51) | 0.2 | ||
| Primary anastomosis | 0.46 (0.25–0.86) | 0.021 | 0.50 (0.24–1.05) | 0.068 |
| Vascular embolus | 1.63 (0.83–3.19) | 0.2 | ||
| Nerve invasion | 2.01 (1.09–3.71) | 0.034 | 1.51 (0.77–2.96) | 0.2 |
| Total lymph nodes | 0.98 (0.96–1.01) | 0.3 | ||
| Positive lymph nodes | 1.13 (1.05–1.21) | 0.003 | 1.07 (0.97–1.18) | 0.2 |
| CD4+ (Invasive margin) | 0.62 (0.41–0.93) | 0.008 | 1.18 (0.58–2.43) | 0.6 |
| CD8+ (Invasive margin) | 1.05 (0.86–1.29) | 0.6 | ||
| CD20+ (Invasive margin) | 0.64 (0.46–0.89) | 0.005 | 0.95 (0.65–1.41) | 0.8 |
| CD68+ (Invasive margin) | 0.53 (0.36–0.79) | <0.001 | 0.68 (0.40–1.16) | 0.2 |
| CD4+ (Central tumor) | 0.69 (0.48–1.01) | 0.033 | 0.75 (0.43–1.32) | 0.3 |
| CD8+ (Central tumor) | 1.15 (0.95–1.39) | 0.2 | ||
| CD20+ (Central tumor) | 0.84 (0.62–1.15) | 0.3 | ||
| CD68+ (Central tumor) | 0.58 (0.39–0.84) | 0.001 | 0.79 (0.48–1.31) | 0.4 |
| Overall Survival (OS) Analysis | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Variable | Univariate HR (95% CI) | Univariate p | Multivariate HR (95% CI) | Multivariate p |
| Age | 1.04 (1.01–1.07) | 0.015 | 1.05 (1.01–1.09) | 0.017 |
| Gender | 0.84 (0.45–1.57) | 0.6 | ||
| Tumor location | - | >0.9 | ||
| Colon | - | - | ||
| Rectum | 0.97 (0.49–1.95) | - | ||
| Differentiation | - | 0.3 | ||
| High/Other | - | - | ||
| Moderate | 0.78 (0.32–1.89) | - | ||
| Low | 1.52 (0.54–4.29) | - | ||
| T stage | - | 0.003 | - | - |
| I–II | - | - | - | - |
| III | 4.21 (1.00–17.8) | - | 4.60 (1.04–20.3) | 0.044 |
| IV | 7.95 (1.80–35.1) | - | 7.24 (1.54–34.1) | 0.012 |
| N stage | - | 0.010 | - | - |
| N0 | - | - | - | - |
| N1–2 | 2.38 (1.19–4.75) | - | 2.53 (1.03–6.23) | 0.044 |
| M stage | - | 0.6 | ||
| M0 | - | - | ||
| M1 | 1.95 (0.27–14.3) | - | ||
| Postoperative radiotherapy | 0 (0.00-Inf) | 0.2 | ||
| Postoperative chemotherapy | 0.52 (0.27–1.00) | 0.044 | 0.54 (0.26–1.12) | 0.10 |
| Surgical approach | - | 0.004 | - | - |
| Laparoscope | - | - | - | - |
| Open | 2.62 (1.31–5.22) | - | 2.05 (0.95–4.41) | 0.066 |
| Blood transfusion | 2.28 (1.24–4.21) | 0.010 | 1.27 (0.64–2.52) | 0.5 |
| Primary anastomosis | 0.29 (0.16–0.56) | <0.001 | 0.50 (0.22–1.12) | 0.092 |
| Vascular embolus | 1.44 (0.66–3.12) | 0.4 | ||
| Nerve invasion | 1.47 (0.71–3.01) | 0.3 | ||
| Total lymph nodes | 0.98 (0.95–1.02) | 0.3 | ||
| Positive lymph nodes | 1.17 (1.09–1.25) | <0.001 | 1.11 (0.99–1.25) | 0.066 |
| CD4+ (Invasive margin) | 0.67 (0.44–1.03) | 0.041 | 1.13 (0.71–1.81) | 0.6 |
| CD8+ (Invasive margin) | 1.1 (0.92–1.32) | 0.4 | ||
| CD20+ (Invasive margin) | 0.5 (0.33–0.76) | <0.001 | 0.82 (0.52–1.30) | 0.4 |
| CD68+ (Invasive margin) | 0.51 (0.32–0.80) | <0.001 | 0.73 (0.41–1.29) | 0.3 |
| CD4+ (Central tumor) | 0.83 (0.58–1.19) | 0.3 | ||
| CD8+ (Central tumor) | 0.97 (0.68–1.37) | 0.9 | ||
| CD20+ (Central tumor) | 0.91 (0.66–1.27) | 0.6 | ||
| CD68+ (Central tumor) | 0.59 (0.39–0.90) | 0.005 | 0.70 (0.38–1.27) | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Jiang, Z.; Gu, J.; Deng, S.; Cai, K.; Wu, K. Analysis of Immune Cell Infiltration Distribution and Prognostic Value in Obstructive Colorectal Cancer. Biomedicines 2025, 13, 2596. https://doi.org/10.3390/biomedicines13112596
Xue Y, Jiang Z, Gu J, Deng S, Cai K, Wu K. Analysis of Immune Cell Infiltration Distribution and Prognostic Value in Obstructive Colorectal Cancer. Biomedicines. 2025; 13(11):2596. https://doi.org/10.3390/biomedicines13112596
Chicago/Turabian StyleXue, Yifan, Zhenxing Jiang, Junnan Gu, Shenghe Deng, Kailin Cai, and Ke Wu. 2025. "Analysis of Immune Cell Infiltration Distribution and Prognostic Value in Obstructive Colorectal Cancer" Biomedicines 13, no. 11: 2596. https://doi.org/10.3390/biomedicines13112596
APA StyleXue, Y., Jiang, Z., Gu, J., Deng, S., Cai, K., & Wu, K. (2025). Analysis of Immune Cell Infiltration Distribution and Prognostic Value in Obstructive Colorectal Cancer. Biomedicines, 13(11), 2596. https://doi.org/10.3390/biomedicines13112596





