Complementary Yet Distinct Roles of GLP-1 Receptor Agonists and SGLT2 Inhibitors in Cardiovascular Risk Reduction
Abstract
1. Introduction
2. GLP-1 Receptors and GLP-1 Receptor Agonists
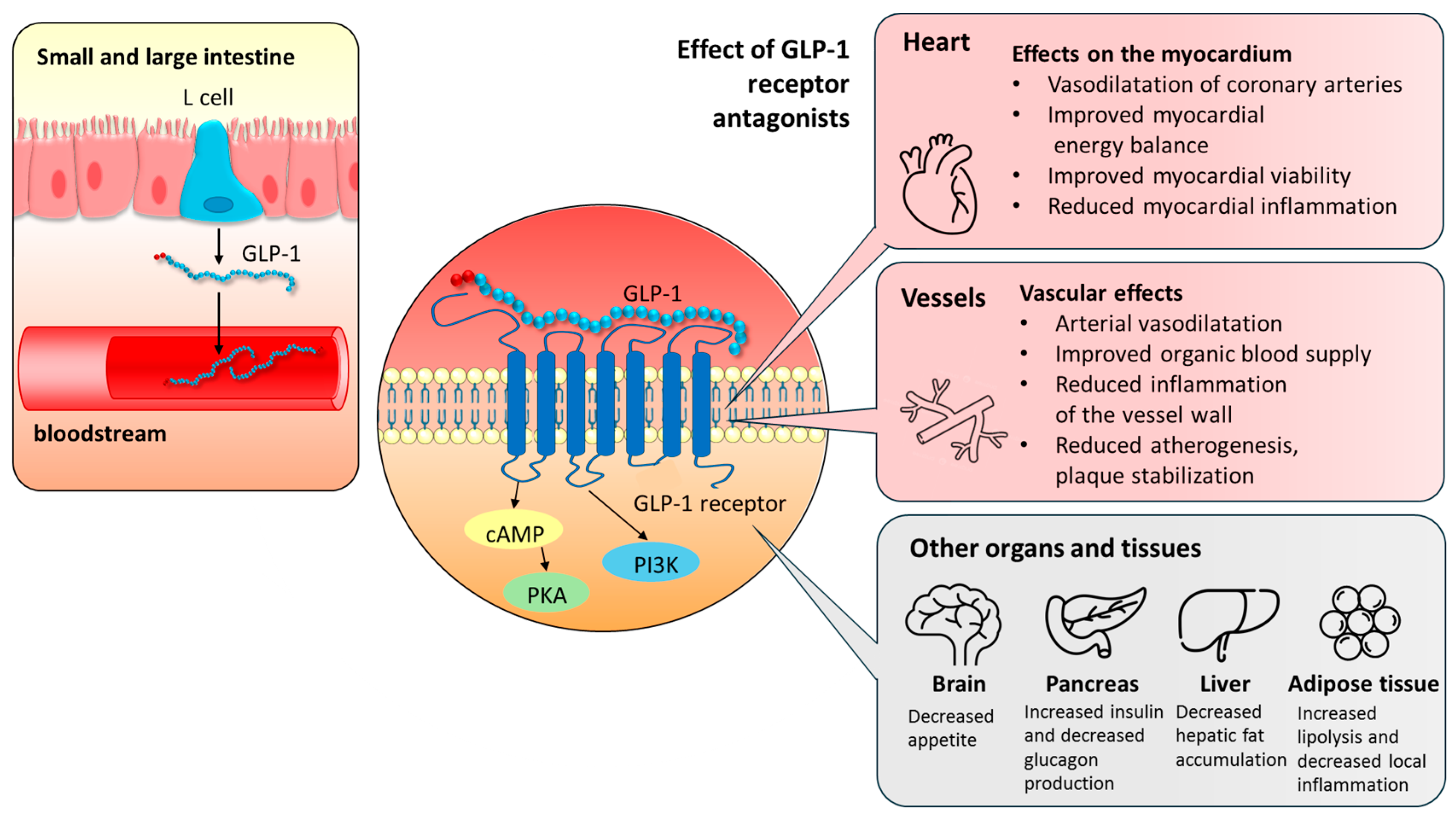
2.1. The Physiological Role of GLP-1 and Its Receptors in the Functioning of the Cardiovascular System
2.2. GLP-1 Receptor Agonists’ Mechanism of Action
3. The SGLT2 Transporter and Its Inhibitors
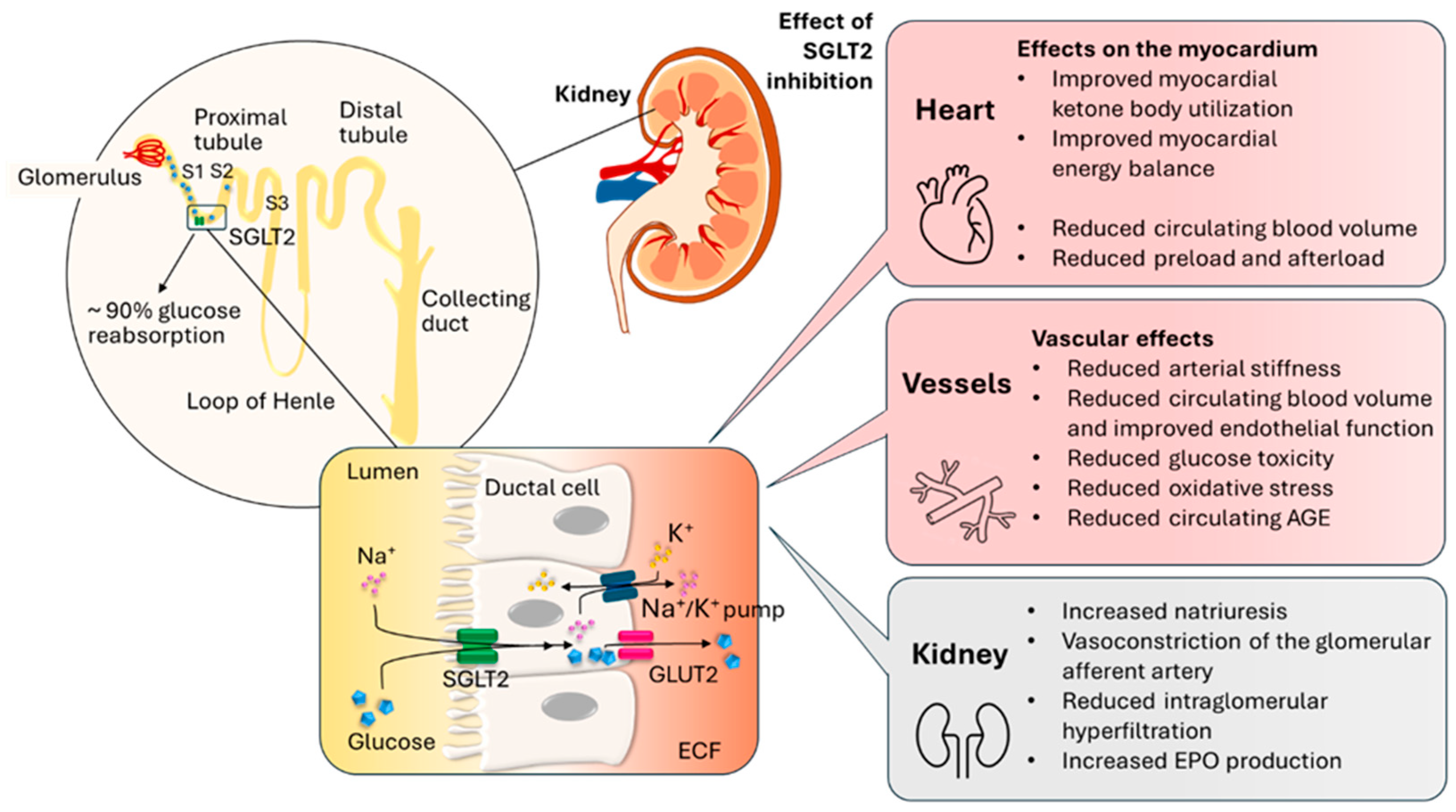
3.1. The Physiological Role of the SGLT2 Transporter in the Function of the Cardiovascular System
3.2. Mechanism of Action of SGLT2 Inhibitors
4. Effect of GLP-1 Receptor Agonists on the Cardiovascular System
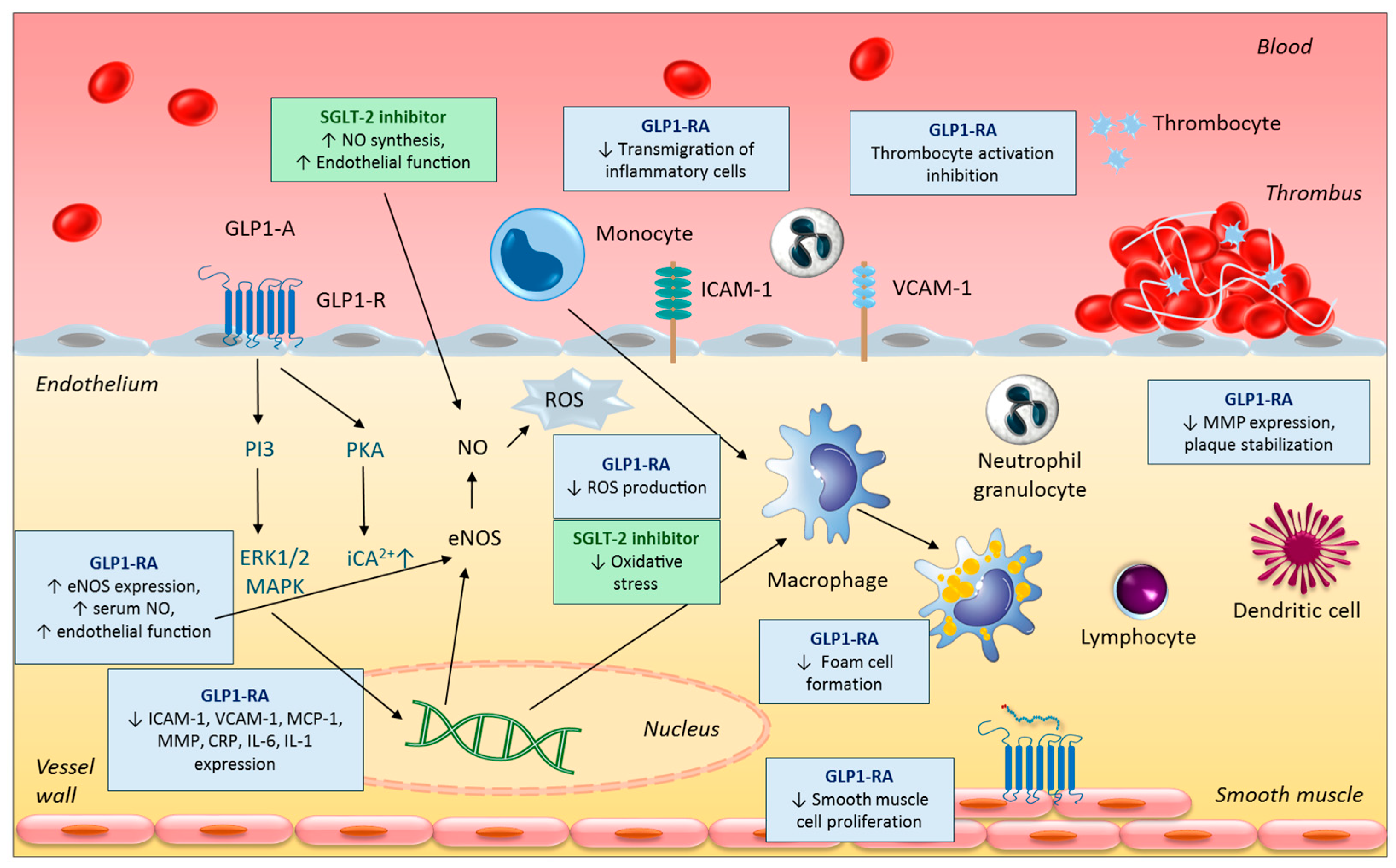
4.1. Anti-Inflammatory and Anti-Atherogenic Effects
4.2. Effects on Lipid Parameters
4.3. Effects on Body Weight
4.4. Further Vasculoprotective Effects
5. Effects of SGLT2 Inhibitors on the Cardiovascular System
5.1. Body Weight
5.2. Blood Pressure
5.3. Cardiac Metabolism/Metabolic Effects
6. Therapeutic Inertia and the Rationale Behind Concerns Regarding Potential Adverse Effects
- 1.
- Fear of Adverse Effects:
- 2.
- Evolving Guidelines:
- 3.
- Prescribing Restrictions:
- 4.
- Patient-Specific Factors:
6.1. Concerns Regarding GLP-1 RA Therapy
6.2. Concerns Regarding SGLT2 Inhibitor Therapy
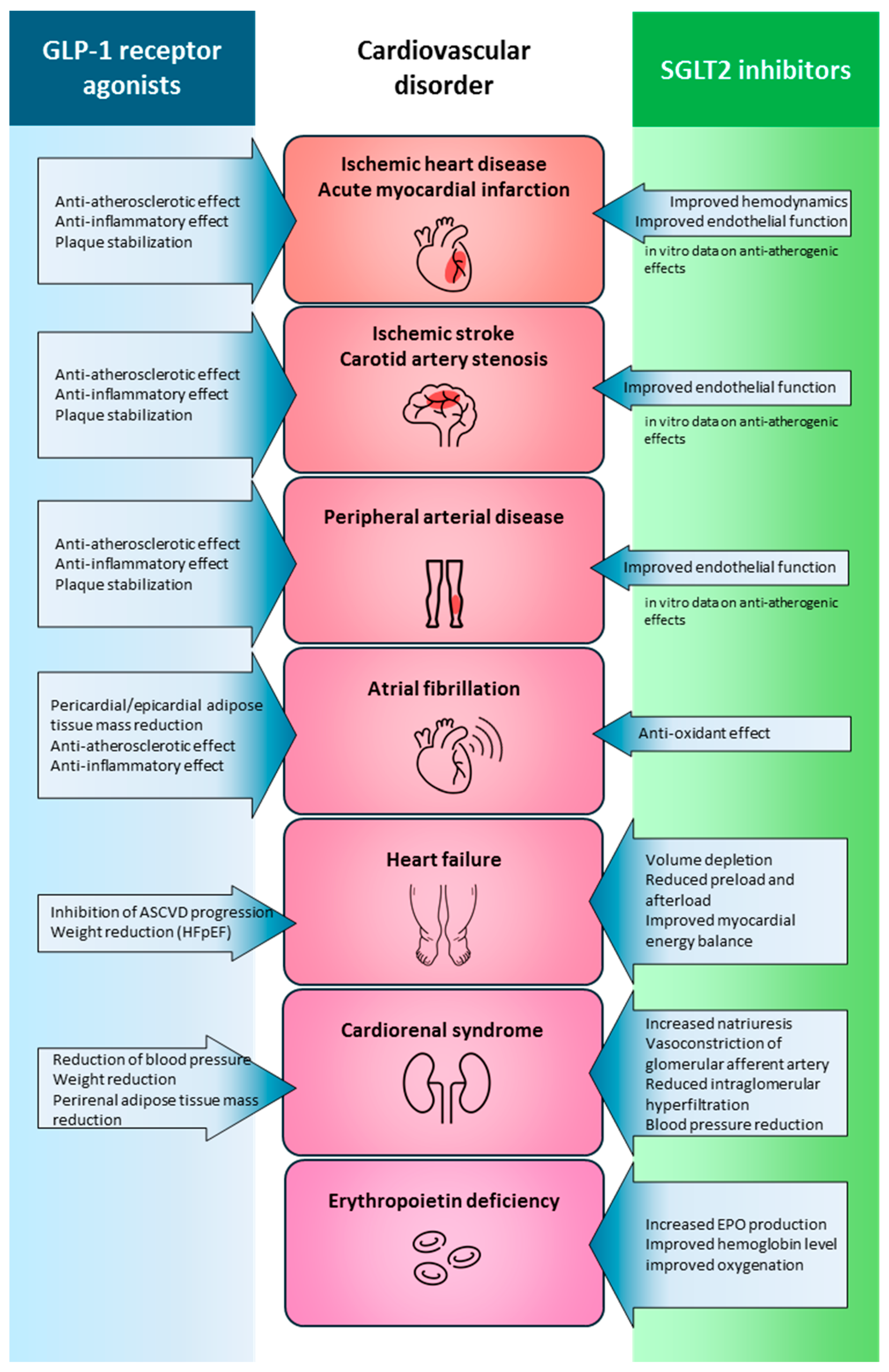
7. Integrated Perspective
7.1. Criteria for Decision-Making in Clinical Practice
7.2. Benefits of Synergy in Metabolic Control and Cardiovascular Prevention
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl-coenzyme A carboxylase |
| AGEs | Advanced glycation end products |
| ApoB48 | Apolipoprotein B48 |
| CKM | Cardio-reno-metabolic |
| CRP | C-reactive protein |
| CVDs | Cardiovascular diseases |
| DPP4 | Dipeptidyl peptidase-4 |
| eNOS | Endothelial nitric oxide synthase |
| ESC | European Society of Cardiology |
| FAS | Fatty acid synthase |
| FGF21 | Fibroblast growth factor 21 |
| GFR | Glomerular filtration rate |
| GIP | Glucose-dependent insulinotropic peptide |
| GLP-1 RA | Glucagon-like peptide-1 receptor agonists |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT | Glucose transporter proteins |
| HbA1c | Hemoglobin A1c |
| HDL-C | High-density lipoprotein cholesterol |
| HF | Heart failure |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| LDL | Low-density lipoprotein |
| LDL-C | LDL cholesterol |
| MACE | Major cardiovascular event |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MMP | Matrix metalloproteinase |
| MTP | Microsomal triglyceride transfer protein |
| Na+ | Sodium |
| NHE1 | Sodium–hydrogen exchanger 1 |
| NO | Nitric oxide |
| PCSK9 | Proprotein convertase subtilisin kexin 9 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator-1 alpha |
| PI3K | Phosphoinositide 3-kinase |
| RAAS | Renin–angiotensin–aldosterone system |
| SCD1 | Stearoyl-CoA desaturase 1 |
| SGLT2 | Sodium–glucose cotransporter 2 |
| SIRT1 | Silent information regulator 1 |
| SREBP-1c | Sterol regulatory element-binding protein-1c |
| T2DM | Type 2 diabetes mellitus |
| TNF-α | Tumor necrosis factor-alpha |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VLDL | Very low-density lipoprotein |
References
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global burden of cardiovascular diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2025, 32, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Ojeda, F.M.; Leong, D.P.; Alegre-Diaz, J.; Amouyel, P.; Aviles-Santa, L.; De Bacquer, D.; Ballantyne, C.M.; Bernabé-Ortiz, A.; Bobak, M.; et al. Global Effect of Modifiable Risk Factors on Cardiovascular Disease and Mortality. N. Engl. J. Med. 2023, 389, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, D.S.; Sandesara, P.B.; Shapiro, M.D.; Wong, N.D. The Evolving Understanding and Approach to Residual Cardiovascular Risk Management. Front. Cardiovasc. Med. 2020, 7, 88. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Sattar, N.; Rosenstock, J.; Ramasundarahettige, C.; Pratley, R.; Lopes, R.D.; Lam, C.S.P.; Khurmi, N.S.; Heenan, L.; Del Prato, S.; et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 896–907. [Google Scholar] [CrossRef]
- Scheen, A.J. GLP-1 Receptor Agonists and SGLT2 Inhibitors in Type 2 Diabetes: Pleiotropic Cardiometabolic Effects and Add-on Value of a Combined Therapy. Drugs 2024, 84, 1347–1364. [Google Scholar] [CrossRef]
- Gajjar, A.; Raju, A.K.; Menon, M.; Shah, S.A.Y.; Dani, S.; Weinberg, A. SGLT2 Inhibitors and GLP-1 Receptor Agonists in Cardiovascular-Kidney-Metabolic Syndrome. Biomedicines 2025, 13, 1924. [Google Scholar] [CrossRef]
- Collaborators, G.D. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Scappaticcio, L.; Longo, M.; Caruso, P.; Maiorino, M.I.; Bellastella, G.; Ceriello, A.; Chiodini, P.; Esposito, K. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: An updated meta-analysis of eight CVOTs. Cardiovasc. Diabetol. 2021, 20, 189. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S181–S206. [Google Scholar] [CrossRef] [PubMed]
- Mojsov, S.; Weir, G.C.; Habener, J.F. Insulinotropin: Glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J. Clin. Investig. 1987, 79, 616–619. [Google Scholar] [CrossRef]
- Holst, J.J.; Orskov, C.; Nielsen, O.V.; Schwartz, T.W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987, 211, 169–174. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Astrup, A.; Holst, J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Investig. 1998, 101, 515–520. [Google Scholar] [CrossRef]
- Huber, H.; Schieren, A.; Holst, J.J.; Simon, M.C. Dietary impact on fasting and stimulated GLP-1 secretion in different metabolic conditions—A narrative review. Am. J. Clin. Nutr. 2024, 119, 599–627. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Wong, O.; Synnott, E.L.; Piyaratna, N.; Douglas, A.; Gribble, F.M.; Holst, J.J.; Chisholm, D.J.; Greenfield, J.R. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J. Nutr. 2011, 141, 1233–1238. [Google Scholar] [CrossRef]
- Roberge, J.N.; Brubaker, P.L. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 1993, 133, 233–240. [Google Scholar] [CrossRef]
- Roberge, J.N.; Gronau, K.A.; Brubaker, P.L. Gastrin-releasing peptide is a novel mediator of proximal nutrient-induced proglucagon-derived peptide secretion from the distal gut. Endocrinology 1996, 137, 2383–2388. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- McGuire, D.K.; Busui, R.P.; Deanfield, J.; Inzucchi, S.E.; Mann, J.F.E.; Marx, N.; Mulvagh, S.L.; Poulter, N.; Engelmann, M.D.M.; Hovingh, G.K.; et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes. Metab. 2023, 25, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Pieber, T.R.; Hartoft-Nielsen, M.L.; Hansen, O.K.H.; Jabbour, S.; Rosenstock, J. Effect of Oral Semaglutide Compared with Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients with Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2017, 318, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Sztanek, F.; Tóth, L.I.; Pető, A.; Hernyák, M.; Diószegi, Á.; Harangi, M. New Developments in Pharmacological Treatment of Obesity and Type 2 Diabetes-Beyond and within GLP-1 Receptor Agonists. Biomedicines 2024, 12, 1320. [Google Scholar] [CrossRef]
- Bakris, G.L.; Fonseca, V.A.; Sharma, K.; Wright, E.M. Renal sodium-glucose transport: Role in diabetes mellitus and potential clinical implications. Kidney Int. 2009, 75, 1272–1277. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Wang, X.X.; Levi, J.; Luo, Y.; Myakala, K.; Herman-Edelstein, M.; Qiu, L.; Wang, D.; Peng, Y.; Grenz, A.; Lucia, S.; et al. SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: SGLT2 Protein Inhibition Decreases Renal Lipid Accumulation, Inflammation, and the Development of Nephropathy in Diabetic Mice. J. Biol. Chem. 2017, 292, 5335–5348. [Google Scholar] [CrossRef]
- Rahmoune, H.; Thompson, P.W.; Ward, J.M.; Smith, C.D.; Hong, G.; Brown, J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005, 54, 3427–3434. [Google Scholar] [CrossRef]
- Rieg, T.; Masuda, T.; Gerasimova, M.; Mayoux, E.; Platt, K.; Powell, D.R.; Thomson, S.C.; Koepsell, H.; Vallon, V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am. J. Physiol.-Ren. Physiol. 2014, 306, F188–F193. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 2017, 13, 11–26. [Google Scholar] [CrossRef]
- Xu, J.; Hirai, T.; Koya, D.; Kitada, M. Effects of SGLT2 Inhibitors on Atherosclerosis: Lessons from Cardiovascular Clinical Outcomes in Type 2 Diabetic Patients and Basic Researches. J. Clin. Med. 2021, 11, 137. [Google Scholar] [CrossRef]
- Dai, Z.C.; Chen, J.X.; Zou, R.; Liang, X.B.; Tang, J.X.; Yao, C.W. Role and mechanisms of SGLT-2 inhibitors in the treatment of diabetic kidney disease. Front. Immunol. 2023, 14, 1213473. [Google Scholar] [CrossRef]
- Panico, C.; Bonora, B.; Camera, A.; Chilelli, N.C.; Prato, G.D.; Favacchio, G.; Grancini, V.; Resi, V.; Rondinelli, M.; Zarra, E.; et al. Pathophysiological basis of the cardiological benefits of SGLT-2 inhibitors: A narrative review. Cardiovasc. Diabetol. 2023, 22, 164. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef]
- Helmstädter, J.; Keppeler, K.; Küster, L.; Münzel, T.; Daiber, A.; Steven, S. Glucagon-like peptide-1 (GLP-1) receptor agonists and their cardiovascular benefits-The role of the GLP-1 receptor. Br. J. Pharmacol. 2022, 179, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Alexiadou, K.; Hartley, A.; Tan, T.M.; Khamis, R. The cardiovascular effects of GLP-1 receptor agonists beyond obesity and type 2 diabetes: An anti-atherosclerotic action. Trends Cardiovasc. Med. 2024, 34, 552–557. [Google Scholar] [CrossRef]
- Zhang, H.; Lui, K.O.; Zhou, B. Endocardial Cell Plasticity in Cardiac Development, Diseases and Regeneration. Circ. Res. 2018, 122, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef]
- Al-Noshokaty, T.M.; Abdelhamid, R.; Abdelmaksoud, N.M.; Khaled, A.; Hossam, M.; Ahmed, R.; Saber, T.; Khaled, S.; Elshaer, S.S.; Abulsoud, A.I. Unlocking the multifaceted roles of GLP-1: Physiological functions and therapeutic potential. Toxicol. Rep. 2025, 14, 101895. [Google Scholar] [CrossRef]
- Park, B.; Bakbak, E.; Teoh, H.; Krishnaraj, A.; Dennis, F.; Quan, A.; Rotstein, O.D.; Butler, J.; Hess, D.A.; Verma, S. GLP-1 receptor agonists and atherosclerosis protection: The vascular endothelium takes center stage. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H1159–H1176. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef]
- Skrobucha, A.; Pindlowski, P.; Krajewska, N.; Grabowski, M.; Jonik, S. Anti-inflammatory effects of glucagon-like peptide-1 (GLP-1) in coronary artery disease: A comprehensive review. Front. Cardiovasc. Med. 2024, 11, 1446468. [Google Scholar] [CrossRef]
- Ravassa, S.; Zudaire, A.; Carr, R.D.; Díez, J. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1361–H1372. [Google Scholar] [CrossRef] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE−/− and LDLr−/− Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic. Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Yusta, B.; Baggio, L.L.; Koehler, J.; Holland, D.; Cao, X.; Pinnell, L.J.; Johnson-Henry, K.C.; Yeung, W.; Surette, M.G.; Bang, K.W.; et al. GLP-1R Agonists Modulate Enteric Immune Responses Through the Intestinal Intraepithelial Lymphocyte GLP-1R. Diabetes 2015, 64, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Crosstalk between glucagon-like peptide 1 and gut microbiota in metabolic diseases. mBio 2024, 15, e0203223. [Google Scholar] [CrossRef]
- Wong, C.K.; Yusta, B.; Koehler, J.A.; Baggio, L.L.; McLean, B.A.; Matthews, D.; Seeley, R.J.; Drucker, D.J. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. 2022, 34, 1514–1531.e1517. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef]
- Bahiru, E.; Hsiao, R.; Phillipson, D.; Watson, K.E. Mechanisms and Treatment of Dyslipidemia in Diabetes. Curr. Cardiol. Rep. 2021, 23, 26. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Hoffman, S.; Adeli, K. Glucagon-like peptide (GLP)-1 regulation of lipid and lipoprotein metabolism. Med. Rev. 2024, 4, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Khound, R.; Taher, J.; Baker, C.; Adeli, K.; Su, Q. GLP-1 Elicits an Intrinsic Gut-Liver Metabolic Signal to Ameliorate Diet-Induced VLDL Overproduction and Insulin Resistance. Arter. Thromb. Vasc. Biol. 2017, 37, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.; Baker, C.; Naples, M.; Taher, J.; Iqbal, J.; Hussain, M.; Adeli, K. Central Nervous System Regulation of Intestinal Lipoprotein Metabolism by Glucagon-like Peptide-1 via a Brain-Gut Axis. Arter. Thromb. Vasc. Biol. 2015, 35, 1092–1100. [Google Scholar] [CrossRef]
- Yang, S.H.; Xu, R.X.; Cui, C.J.; Wang, Y.; Du, Y.; Chen, Z.G.; Yao, Y.H.; Ma, C.Y.; Zhu, C.G.; Guo, Y.L.; et al. Liraglutide downregulates hepatic LDL receptor and PCSK9 expression in HepG2 cells and db/db mice through a HNF-1a dependent mechanism. Cardiovasc. Diabetol. 2018, 17, 48. [Google Scholar] [CrossRef]
- Hoffman, S.; Alvares, D.; Adeli, K. GLP-1 attenuates intestinal fat absorption and chylomicron production via vagal afferent nerves originating in the portal vein. Mol. Metab. 2022, 65, 101590. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, A.; Li, D.; Wu, Y.; Wang, C.Z.; Wan, J.Y.; Yuan, C.S. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: Systematic review and network meta-analysis. BMJ 2024, 384, e076410. [Google Scholar] [CrossRef]
- Tóth, L.I.; Harsányi, A.; Csiha, S.; Molnár, Á.; Lőrincz, H.; Nagy, A.C.; Paragh, G.; Harangi, M.; Sztanek, F. Semaglutide Improves Lipid Subfraction Profiles in Type 2 Diabetes: Insights from a One-Year Follow-Up Study. Int. J. Mol. Sci. 2025, 26, 5951. [Google Scholar] [CrossRef]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am. J. Med. 2025, 138, 934–940. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Ten Kulve, J.S.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of glucagon-like peptide 1 on appetite and body weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1–T16. [Google Scholar] [CrossRef]
- Maselli, D.B.; Camilleri, M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv. Exp. Med. Biol. 2021, 1307, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Ronveaux, C.C.; Tomé, D.; Raybould, H.E. Glucagon-like peptide 1 interacts with ghrelin and leptin to regulate glucose metabolism and food intake through vagal afferent neuron signaling. J. Nutr. 2015, 145, 672–680. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, I.H.R.; da Silva, R.S.; Mendonça, I.P.; de Souza, J.R.B.; Peixoto, C.A. Semaglutide Attenuates Anxious and Depressive-like Behaviors and Reverses the Cognitive Impairment in a Type 2 Diabetes Mellitus Mouse Model via the Microbiota-Gut-Brain Axis. J. Neuroimmune Pharmacol. 2024, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, C.; Lu, M.; Chen, C.; Nie, X.; Abudukerimu, B.; Zhang, K.; Ning, Z.; Chen, Y.; Cheng, J.; et al. The key role of a glucagon-like peptide-1 receptor agonist in body fat redistribution. J. Endocrinol. 2019, 240, 271–286. [Google Scholar] [CrossRef]
- Buse, J.B.; Bain, S.C.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Pratley, R.E.; Linder, M.; Monk Fries, T.; et al. Cardiovascular Risk Reduction with Liraglutide: An Exploratory Mediation Analysis of the LEADER Trial. Diabetes Care 2020, 43, 1546–1552. [Google Scholar] [CrossRef]
- Braunwald, E. SGLT2 inhibitors: The statins of the 21st century. Eur. Heart J. 2022, 43, 1029–1030. [Google Scholar] [CrossRef]
- Alvarado, F.; Crane, R.K. Phlorizin as a competitive inhibitor of the active transport of sugars by hamster small intestine, in vitro. Biochim. Biophys. Acta 1962, 56, 170–172. [Google Scholar] [CrossRef]
- Vasilakou, D.; Karagiannis, T.; Athanasiadou, E.; Mainou, M.; Liakos, A.; Bekiari, E.; Sarigianni, M.; Matthews, D.R.; Tsapas, A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 262–274. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Kosiborod, M.N. Chapter 3: Clinical Trials of Sodium-Glucose Co-Transporter-2 Inhibitors for Treatment of Heart Failure. Am. J. Med. 2024, 137, S25–S34. [Google Scholar] [CrossRef]
- Brown, E.; Wilding, J.P.H.; Barber, T.M.; Alam, U.; Cuthbertson, D.J. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes. Rev. 2019, 20, 816–828. [Google Scholar] [CrossRef]
- Lee, P.C.; Ganguly, S.; Goh, S.Y. Weight loss associated with sodium-glucose cotransporter-2 inhibition: A review of evidence and underlying mechanisms. Obes. Rev. 2018, 19, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.N.; Ersbøll, M.K.; Gustafsson, F. Haemodynamic Effects of Sodium-Glucose Cotransporter 2 Inhibitor Treatment in Chronic Heart Failure Patients. Card. Fail. Rev. 2024, 10, e09. [Google Scholar] [CrossRef] [PubMed]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Herat, L.Y.; Matthews, J.; Azzam, O.; Schlaich, M.P.; Matthews, V.B. Targeting Features of the Metabolic Syndrome Through Sympatholytic Effects of SGLT2 Inhibition. Curr. Hypertens. Rep. 2022, 24, 67–74. [Google Scholar] [CrossRef]
- Packer, M. Cardioprotective Effects of Sirtuin-1 and Its Downstream Effectors: Potential Role in Mediating the Heart Failure Benefits of SGLT2 (Sodium-Glucose Cotransporter 2) Inhibitors. Circ. Heart Fail. 2020, 13, e007197. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: Inhibition of Na. Diabetologia 2018, 61, 722–726. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Simental-Mendía, M.; Millán-Alanís, J.M.; Simental-Mendía, L.E. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: A systematic review and meta-analysis of 48 randomized controlled trials. Pharmacol. Res. 2020, 160, 105068. [Google Scholar] [CrossRef]
- Yahaya, J.J.; Doya, I.F.; Morgan, E.D.; Ngaiza, A.I.; Bintabara, D. Poor glycemic control and associated factors among patients with type 2 diabetes mellitus: A cross-sectional study. Sci. Rep. 2023, 13, 9673. [Google Scholar] [CrossRef]
- Phillips, L.S.; Branch, W.T.; Cook, C.B.; Doyle, J.P.; El-Kebbi, I.M.; Gallina, D.L.; Miller, C.D.; Ziemer, D.C.; Barnes, C.S. Clinical inertia. Ann. Intern. Med. 2001, 135, 825–834. [Google Scholar] [CrossRef]
- Khunti, K.; Gomes, M.B.; Pocock, S.; Shestakova, M.V.; Pintat, S.; Fenici, P.; Hammar, N.; Medina, J. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes. Metab. 2018, 20, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, K.M.; Misra-Hebert, A.D.; Hobbs, T.M.; Ji, X.; Kong, S.X.; Milinovich, A.; Weng, W.; Bauman, J.; Ganguly, R.; Burguera, B.; et al. Clinical Inertia in Type 2 Diabetes Management: Evidence from a Large, Real-World Data Set. Diabetes Care 2018, 41, e113–e114. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, K.M.; Wells, B.J.; Chagin, K.M.; Ejzykowicz, F.; Yu, C.; Milinovich, A.; Bauman, J.M.; Kattan, M.W.; Rajpathak, S.; Zimmerman, R.S. Intensification of Diabetes Therapy and Time Until A1C Goal Attainment Among Patients with Newly Diagnosed Type 2 Diabetes Who Fail Metformin Monotherapy Within a Large Integrated Health System. Diabetes Care 2016, 39, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Esposito, K. Clinical inertia as a clinical safeguard. JAMA 2011, 305, 1591–1592. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.J.; Kansagara, D.; Horwitch, C.; Barry, M.J.; Forciea, M.A.; Fitterman, N.; Balzer, K.; Boyd, C.; Humphrey, L.L.; et al. Hemoglobin A1c Targets for Glycemic Control with Pharmacologic Therapy for Nonpregnant Adults with Type 2 Diabetes Mellitus: A Guidance Statement Update from the American College of Physicians. Ann. Intern. Med. 2018, 168, 569–576. [Google Scholar] [CrossRef]
- Giugliano, D.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Clinical inertia, reverse clinical inertia, and medication non-adherence in type 2 diabetes. J. Endocrinol. Investig. 2019, 42, 495–503. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Csatordai, M.; Benkő, R.; Matuz, M.; Engi, Z.; Csupor, D.; Lengyel, C.; Doró, P. Trends and regional differences in antidiabetic medication use: A nationwide retrospective observational study. Diabetol. Metab. Syndr. 2024, 16, 88. [Google Scholar] [CrossRef]
- Sharma, A.; Aziz, H.; Verma, S.; Abramson, B.L.; Choi, R.; Chua, G.L.; Connelly, K.A.; Honos, G.; Mancini, G.B.J.; Ramer, S.A.; et al. Permission to prescribe: Do cardiologists need permission to prescribe diabetes medications that afford cardiovascular benefit? Curr. Opin. Cardiol. 2021, 36, 672–681. [Google Scholar] [CrossRef]
- Neuen, B.L.; Fletcher, R.A.; Heath, L.; Perkovic, A.; Vaduganathan, M.; Badve, S.V.; Tuttle, K.R.; Pratley, R.; Gerstein, H.C.; Perkovic, V.; et al. Cardiovascular, Kidney, and Safety Outcomes with GLP-1 Receptor Agonists Alone and in Combination with SGLT2 Inhibitors in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Circulation 2024, 150, 1781–1790. [Google Scholar] [CrossRef]
- Luo, J.; Feldman, R.; Callaway Kim, K.; Rothenberger, S.; Korytkowski, M.; Hernandez, I.; Gellad, W.F. Evaluation of Out-of-Pocket Costs and Treatment Intensification with an SGLT2 Inhibitor or GLP-1 RA in Patients with Type 2 Diabetes and Cardiovascular Disease. JAMA Netw. Open 2023, 6, e2317886. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Galiero, R.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Sardu, C.; Vetrano, E.; Monda, M.; et al. Modern Challenges in Type 2 Diabetes: Balancing New Medications with Multifactorial Care. Biomedicines 2024, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Gabriel, N.; Korytkowski, M.; Hernandez, I.; Gellad, W.F. Association of formulary restrictions and initiation of an SGLT2i or GLP1-RA among Medicare beneficiaries with type 2 diabetes. Diabetes Res. Clin. Pract. 2022, 187, 109855. [Google Scholar] [CrossRef]
- Ladebo, L.; Ernst, M.T.; Mailhac, A.; Dirksen, C.; Bojsen-Møller, K.N.; Pottegård, A. Real-World Use of Semaglutide for Weight Management: Patient Characteristics and Dose Titration-A Danish Cohort Study. Diabetes Care 2024, 47, 1834–1837. [Google Scholar] [CrossRef]
- Shetty, R.; Basheer, F.T.; Poojari, P.G.; Thunga, G.; Chandran, V.P.; Acharya, L.D. Adverse drug reactions of GLP-1 agonists: A systematic review of case reports. Diabetes Metab. Syndr. 2022, 16, 102427. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Batlle, J.C.; Ayati, A.; Maqsood, M.H.; Long, C.; Tarabanis, C.; McGowan, N.; Liebers, D.T.; Laynor, G.; Hosseini, K.; et al. Suicide and Self-Harm Events with GLP-1 Receptor Agonists in Adults with Diabetes or Obesity: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2025, 82, 888–895. [Google Scholar] [CrossRef]
- Jalleh, R.J.; Rayner, C.K.; Hausken, T.; Jones, K.L.; Camilleri, M.; Horowitz, M. Gastrointestinal effects of GLP-1 receptor agonists: Mechanisms, management, and future directions. Lancet Gastroenterol. Hepatol. 2024, 9, 957–964. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef]
- Artigas, C.F.; Stokes, V.; Tan, G.D.; Theodorakis, M.J. Insulin dose adjustments with add-on glucagon-like peptide-1 receptor (GLP-1R) agonists in clinical practice. Expert. Opin. Pharmacother. 2015, 16, 1417–1421. [Google Scholar] [CrossRef]
- Bui, V.; Neumiller, J.J. Oral Semaglutide. Clin. Diabetes 2018, 36, 327–329. [Google Scholar] [CrossRef]
- Scheen, A.J. Underuse of GLP-1 receptor agonists in the management of type 2 diabetes despite a favorable benefit-safety profile. Expert. Opin. Drug Saf. 2024, 23, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Winn, A.N.; Skandari, M.R.; Franco, M.I.; Staab, E.M.; Alexander, J.; Wan, W.; Zhu, M.; Huang, E.S.; Philipson, L.; et al. First-Line Therapy for Type 2 Diabetes with Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists: A Cost-Effectiveness Study. Ann. Intern. Med. 2022, 175, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, J.; de Beeck, I.O.; Mamouris, P.; Mathieu, C.; Goderis, G. Knowledge and prescribing behaviour of Flemish general practitioners regarding novel glucose-lowering medications: Online cross-sectional survey. Prim. Care Diabetes 2024, 18, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, B.; Smith, E.; Kaur, J.; Sherling, C.; Vanapalli, S.; Lussier, M. Medicare formulary restrictions for glucagon-like peptide 1 receptor agonists and sodium glucose cotransporter 2 inhibitors used in type 2 diabetes mellitus: 2019–2023. J. Manag. Care Spec. Pharm. 2024, 30, 34–42. [Google Scholar] [CrossRef]
- Pishdad, R.; Auwaerter, P.G.; Kalyani, R.R. Diabetes, SGLT-2 Inhibitors, and Urinary Tract Infection: A Review. Curr. Diabetes Rep. 2024, 24, 108–117. [Google Scholar] [CrossRef]
- Bhanushali, K.B.; Asnani, H.K.; Nair, A.; Ganatra, S.; Dani, S.S. Pharmacovigilance study for SGLT 2 inhibitors- Safety review of real-world data & randomized clinical trials. Curr. Probl. Cardiol. 2024, 49, 102664. [Google Scholar] [CrossRef]
- Arshad, M.; Hoda, F.; Siddiqui, N.A.; Najmi, A.K.; Ahmad, M. Genito Urinary Infection and Urinary Tract Infection in Patients with Type 2 Diabetes Mellitus Receiving SGLT2 Inhibitors: Evidence from a Systematic Literature Review of Landmark Randomized Clinical Trial. Drug Res. 2024, 74, 307–313. [Google Scholar] [CrossRef]
- Chow, E.; Clement, S.; Garg, R. Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors. BMJ Open Diabetes Res. Care 2023, 11, e003666. [Google Scholar] [CrossRef]
- Menghoum, N.; Oriot, P.; Hermans, M.P. Clinical and biochemical characteristics and analysis of risk factors for euglycaemic diabetic ketoacidosis in type 2 diabetic individuals treated with SGLT2 inhibitors: A review of 72 cases over a 4.5-year period. Diabetes Metab. Syndr. 2021, 15, 102275. [Google Scholar] [CrossRef]
- Laffel, L.M.; Danne, T.; Klingensmith, G.J.; Tamborlane, W.V.; Willi, S.; Zeitler, P.; Neubacher, D.; Marquard, J.; Group, D.S. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): A multicentre, randomised, double-blind, parallel group, phase 3 trial. Lancet Diabetes Endocrinol. 2023, 11, 169–181. [Google Scholar] [CrossRef]
- Østergaard, H.B.; Humphreys, V.; Hengeveld, E.M.; Honoré, J.B.; Mach, F.; Visseren, F.L.J.; Westerink, J.; Yadav, G.; Mosenzon, O.; CAPTURE Investigators. Cardiovascular risk and lifetime benefit from preventive treatment in type 2 diabetes: A post hoc analysis of the CAPTURE study. Diabetes Obes. Metab. 2023, 25, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Singh, A.K.; Zargar, A.H.; Almeida, A.; Bhalla, A.K.; Mohan, J.C.; Dalal, J.; Sahay, M.; Mohanan, P.P.; Maitra, S.; et al. Cardiorenal disease management in type 2 diabetes: An expert consensus. Diabetes Metab. Syndr. 2022, 16, 102661. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes. Metab. 2017, 19, 1353–1362. [Google Scholar] [CrossRef]
- Vale, C.; Lourenço, I.M.; Jordan, G.; Golovaty, I.; Torres, H.; Moin, T.; Buysschaert, M.; Neves, J.S.; Bergman, M. Early combination therapy with SGLT2i and GLP-1 RA or dual GIP/GLP-1 RA in type 2 diabetes. Diabetes Obes. Metab. 2025, 27, 468–481. [Google Scholar] [CrossRef]
- Jabbour, S.A.; Frías, J.P.; Hardy, E.; Ahmed, A.; Wang, H.; Öhman, P.; Guja, C. Safety and Efficacy of Exenatide Once Weekly Plus Dapagliflozin Once Daily Versus Exenatide or Dapagliflozin Alone in Patients with Type 2 Diabetes Inadequately Controlled with Metformin Monotherapy: 52-Week Results of the DURATION-8 Randomized Controlled Trial. Diabetes Care 2018, 41, 2136–2146. [Google Scholar] [CrossRef]
- Jabbour, S.A.; Frías, J.P.; Guja, C.; Hardy, E.; Ahmed, A.; Öhman, P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes. Metab. 2018, 20, 1515–1519. [Google Scholar] [CrossRef]
- Jabbour, S.A.; Frías, J.P.; Ahmed, A.; Hardy, E.; Choi, J.; Sjöström, C.D.; Guja, C. Efficacy and Safety Over 2 Years of Exenatide Plus Dapagliflozin in the DURATION-8 Study: A Multicenter, Double-Blind, Phase 3, Randomized Controlled Trial. Diabetes Care 2020, 43, 2528–2536. [Google Scholar] [CrossRef]
- Ludvik, B.; Frías, J.P.; Tinahones, F.J.; Wainstein, J.; Jiang, H.; Robertson, K.E.; García-Pérez, L.E.; Woodward, D.B.; Milicevic, Z. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): A 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 370–381. [Google Scholar] [CrossRef]
- Zinman, B.; Bhosekar, V.; Busch, R.; Holst, I.; Ludvik, B.; Thielke, D.; Thrasher, J.; Woo, V.; Philis-Tsimikas, A. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): A randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 356–367. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; Jiang, M.; Wang, K. The Efficacy and Safety of the Combination Therapy with GLP-1 Receptor Agonists and SGLT-2 Inhibitors in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Front. Pharmacol. 2022, 13, 838277. [Google Scholar] [CrossRef]
- Mantsiou, C.; Karagiannis, T.; Kakotrichi, P.; Malandris, K.; Avgerinos, I.; Liakos, A.; Tsapas, A.; Bekiari, E. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2020, 22, 1857–1868. [Google Scholar] [CrossRef]
- Guo, M.; Gu, J.; Teng, F.; Chen, J.; Ma, X.; Chen, Q.; Pu, Y.; Jiang, Z.; Long, Y.; Xu, Y. The efficacy and safety of combinations of SGLT2 inhibitors and GLP-1 receptor agonists in the treatment of type 2 diabetes or obese adults: A systematic review and meta-analysis. Endocrine 2020, 67, 294–304. [Google Scholar] [CrossRef]
- Harashima, S.I.; Inagaki, N.; Kondo, K.; Maruyama, N.; Otsuka, M.; Kawaguchi, Y.; Watanabe, Y. Efficacy and safety of canagliflozin as add-on therapy to a glucagon-like peptide-1 receptor agonist in Japanese patients with type 2 diabetes mellitus: A 52-week, open-label, phase IV study. Diabetes Obes. Metab. 2018, 20, 1770–1775. [Google Scholar] [CrossRef]
- Ishihara, H.; Yamaguchi, S.; Nakao, I.; Sakatani, T. Ipragliflozin Add-on Therapy to a GLP-1 Receptor Agonist in Japanese Patients with Type 2 Diabetes (AGATE): A 52-Week Open-Label Study. Diabetes Ther. 2018, 9, 1549–1567. [Google Scholar] [CrossRef]
- Blonde, L.; Belousova, L.; Fainberg, U.; Garcia-Hernandez, P.A.; Jain, S.M.; Kaltoft, M.S.; Mosenzon, O.; Nafach, J.; Palle, M.S.; Rea, R. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2020, 22, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.S.; Borges-Canha, M.; Vasques-Nóvoa, F.; Green, J.B.; Leiter, L.A.; Granger, C.B.; Carvalho, D.; Leite-Moreira, A.; Hernandez, A.F.; Del Prato, S.; et al. GLP-1 Receptor Agonist Therapy with and Without SGLT2 Inhibitors in Patients with Type 2 Diabetes. J. Am. Coll. Cardiol. 2023, 82, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Clegg, L.E.; Penland, R.C.; Bachina, S.; Boulton, D.W.; Thuresson, M.; Heerspink, H.J.L.; Gustavson, S.; Sjöström, C.D.; Ruggles, J.A.; Hernandez, A.F.; et al. Effects of exenatide and open-label SGLT2 inhibitor treatment, given in parallel or sequentially, on mortality and cardiovascular and renal outcomes in type 2 diabetes: Insights from the EXSCEL trial. Cardiovasc. Diabetol. 2019, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Apperloo, E.M.; Neuen, B.L.; Fletcher, R.A.; Jongs, N.; Anker, S.D.; Bhatt, D.L.; Butler, J.; Cherney, D.Z.I.; Herrington, W.G.; Inzucchi, S.E.; et al. Efficacy and safety of SGLT2 inhibitors with and without glucagon-like peptide 1 receptor agonists: A SMART-C collaborative meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2024, 12, 545–557. [Google Scholar] [CrossRef]
- Simms-Williams, N.; Treves, N.; Yin, H.; Lu, S.; Yu, O.; Pradhan, R.; Renoux, C.; Suissa, S.; Azoulay, L. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: Population based cohort study. BMJ 2024, 385, e078242. [Google Scholar] [CrossRef]
- Hou, W.; Tuttle, K.R.; Shen, W.; Reikes, A.; Watanabe, J.H. Trends in Pharmacological Treatment of Patients with New Onset Type 2 Diabetes: Usage Patterns in an Evolving Guideline Landscape. J. Diabetes 2025, 17, e70108. [Google Scholar] [CrossRef]
- Lim, C.E.; Pasternak, B.; Eliasson, B.; Danaei, G.; Ueda, P. Use of sodium-glucose co-transporter 2 inhibitors and glucagon-like peptide-1 receptor agonists according to the 2019 ESC guidelines and the 2019 ADA/EASD consensus report in a national population of patients with type 2 diabetes. Eur. J. Prev. Cardiol. 2023, 30, 634–643. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ba-Essa, E.M.; Ahmed, A.; Ahmad, E.; Annabi, F.A.; Chaabane, H.; Tuccinardi, D.; Davies, M.J.; De Domenico, F.; Eckel, R.H.; et al. Recommendations for the Management of Diabetes During Ramadan Applying the Principles of the ADA/ EASD Consensus: Update 2025. Diabetes Metab. Res. Rev. 2025, 41, e70057. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Rossing, P.; Bakris, G.; Belmar, N.; Bosch-Traberg, H.; Busch, R.; Charytan, D.M.; Hadjadj, S.; Gillard, P.; Górriz, J.L.; et al. Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial. Nat. Med. 2024, 30, 2849–2856. [Google Scholar] [CrossRef]
- Schnell, O.; Barnard-Kelly, K.; Battelino, T.; Ceriello, A.; Larsson, H.E.; Fernández-Fernández, B.; Forst, T.; Frias, J.P.; Gavin, J.R.; Giorgino, F.; et al. CVOT Summit Report 2023: New cardiovascular, kidney, and metabolic outcomes. Cardiovasc. Diabetol. 2024, 23, 104. [Google Scholar] [CrossRef]

| EMA-Approved | FDA-Approved | |
|---|---|---|
| GLP-1 receptor agonists | exenatide | exenatide |
| lixisenatide | lixisenatide | |
| semaglutide | semaglutide | |
| dulaglutide | dulaglutide | |
| liraglutide | liraglutide | |
| GLP-1/GIP receptor agonists | tirzepatide | tirzepatide |
| SGLT2 inhibitors | canagliflozin | canagliflozin |
| dapagliflozin | dapagliflozin | |
| empagliflozin | empagliflozin | |
| ertugliflozin | ||
| bexagliflozin | ||
| SGLT1/SGLT2 inhibitor | sotagliflozin |
| When to prioritize GLP-1 RAs |
GLP-1 RAs should be considered the preferred option for patients with
|
| When to prioritize SGLT2 inhibitors |
SGLT2 inhibitors are recommended as the first choice in patients with
|
| When to consider combination therapy (GLP-1 RAs + SGLT2 inhibitors) |
The concomitant use of GLP-1 RAs and SGLT2 inhibitors should be considered in
|
| General considerations |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homoródi, N.; Varga, É.; Szabó, Z.; Sztanek, F.; Harangi, M. Complementary Yet Distinct Roles of GLP-1 Receptor Agonists and SGLT2 Inhibitors in Cardiovascular Risk Reduction. Biomedicines 2025, 13, 2595. https://doi.org/10.3390/biomedicines13112595
Homoródi N, Varga É, Szabó Z, Sztanek F, Harangi M. Complementary Yet Distinct Roles of GLP-1 Receptor Agonists and SGLT2 Inhibitors in Cardiovascular Risk Reduction. Biomedicines. 2025; 13(11):2595. https://doi.org/10.3390/biomedicines13112595
Chicago/Turabian StyleHomoródi, Nóra, Éva Varga, Zoltán Szabó, Ferenc Sztanek, and Mariann Harangi. 2025. "Complementary Yet Distinct Roles of GLP-1 Receptor Agonists and SGLT2 Inhibitors in Cardiovascular Risk Reduction" Biomedicines 13, no. 11: 2595. https://doi.org/10.3390/biomedicines13112595
APA StyleHomoródi, N., Varga, É., Szabó, Z., Sztanek, F., & Harangi, M. (2025). Complementary Yet Distinct Roles of GLP-1 Receptor Agonists and SGLT2 Inhibitors in Cardiovascular Risk Reduction. Biomedicines, 13(11), 2595. https://doi.org/10.3390/biomedicines13112595







