The Role of mTOR Signaling in Tumor-Induced Alterations to Neuronal Function in Diffusely Infiltrating Glioma
Abstract
1. Introduction
2. mTOR Signaling Network: Structure, Activators, and Downstream Mediators

3. mTOR Signaling in Neuronal Function and Development
| Disorder/Pathology | Gene/Pathway Alteration | mTOR Signaling State | Key Features |
|---|---|---|---|
| Tuberous Sclerosis Complex | TSC1, TSC2 mutations | Hyperactivated | Cortical tubers, epilepsy, ASD, cognitive deficits, SEGA [7,16,22,42,43,46,61] |
| PTEN Hamartoma Tumor Syndrome | PTEN mutation/deletion | Hyperactivated | Macrocephaly, epilepsy, neuronal hypertrophy, ASD-like behavior [40,43,62,63,64,65,66,67] |
| Hemimegaloencephaly | Somatic mTOR/PI3K/AKT3 mut. | Hyperactivated | Unilateral brain overgrowth, seizures, cortical dysplasia [48,49,68,69,70] |
| Focal Cortical Dysplasia | Somatic mTOR pathway mut. | Hyperactivated | Cortical thickening, abnormal lamination, epilepsy [47,48,49,68,69,70,71] |
| Age-associated cognitive decline | Decreased mTOR signaling | Hypoactivated | Impaired neurogenesis, reduced NSC proliferation, memory deficits [40,72,73,74,75,76] |
| Neurodegeneration (various forms) | mTOR dysregulation | Variable | Inclusion bodies, synaptic loss, microglial activation [40,72,73,74,75,76] |
4. Pathologies of Altered mTOR Signaling in the Brain
5. mTOR in Gliomas
6. mTOR-Dependent Mechanisms Underlying Tumor-Induced Neurological Dysfunction in Glioma
7. Clinical Applications of mTOR Inhibitors
8. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ASD | autism spectrum disorder |
| ATG13 | autophagy-related protein 13 |
| BDNF | brain-derived neurotrophic factor |
| Deptor | DEP-domain containing mTOR-interacting protein |
| EGFR | epidermal growth factor receptor |
| EMA | European Medicines Agency |
| FCD | focal cortical dysplasia |
| FDA | U.S. Food and Drug Administration |
| GBM | glioblastoma |
| glioma | diffusely infiltrating gliomas |
| HME | hemimegaloencephaly |
| IGF1 | insulin-like growth factor 1 |
| mLST8 | mammalian lethal with SEC13 protein 8 |
| mSIN | mammalian stress-activated protein kinase-interacting protein 1 |
| mTOR | mammalian/mechanistic target of rapamycin |
| mTORC1/2 | mechanistic target of rapamycin complex 1/2 |
| NSC | neural stem cell |
| PI3K | phosphoinositide 3-kinase |
| PKC | protein kinase C |
| PRAS40 | proline-rich Akt substrate of 40 kDa |
| Protor | protein observed with Rictor |
| pS6 | phosphorylated ribosomal protein S6 |
| Rag | Ras-related GTP-binding protein |
| rapalogs | Rapamycin analogs |
| Raptor | Regulatory-associated protein of mTOR |
| REDD1 | regulated in development and DNA damage response 1 |
| Rheb | Ras homolog enriched in brain |
| Rictor | rapamycin-insensitive companion of mTOR |
| SEGA | subependymal giant cell astrocytoma |
| SGK1 | serum/glucocorticoid regulated kinase 1 |
| SREBP1/2 | sterol regulatory element-binding protein 1/2 |
| TSC | tuberous sclerosis complex |
| TSC1/2 | tuberous sclerosis complex 1/2 |
| ULK1 | Unc-51-like autophagy activating kinase 1 |
| 4E-BP1 | eukaryotic translation initiation factor 4E-binding protein 1 |
| p70S6K | ribosomal protein S6 kinase. |
References
- Leung, A.; Rangamani, P. Computational modeling of AMPK and mTOR crosstalk in glutamatergic synapse calcium signaling. NPJ Syst. Biol. Appl. 2023, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Tee, A.R.; Blenis, J.; Proud, C.G. Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1. FEBS Lett. 2005, 579, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.; Colombi, M.; Moroni, C.; Hall, M.N. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011, 10, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Xin-Long, C.; Zhao-Fan, X.; Dao-Feng, B.; Wei, D. mTOR partly mediates insulin resistance by phosphorylation of insulin receptor substrate-1 on serine307 residues after burn. Burns 2011, 37, 86–93. [Google Scholar] [CrossRef]
- Yan, X.; Huang, S.; Li, H.; Feng, Z.; Kong, J.; Liu, J. The causal effect of mTORC1-dependent circulating protein levels on nonalcoholic fatty liver disease: A Mendelian randomization study. Dig. Liver Dis. 2024, 56, 559–564. [Google Scholar] [CrossRef]
- Lipton, J.O.; Sahin, M. The neurology of mTOR. Neuron 2014, 84, 275–291. [Google Scholar] [CrossRef]
- Fan, Q.W.; Weiss, W.A. Inhibition of PI3K-Akt-mTOR signaling in glioblastoma by mTORC1/2 inhibitors. Methods Mol. Biol. 2012, 821, 349–359. [Google Scholar] [CrossRef]
- Galan-Moya, E.M.; Le Guelte, A.; Lima Fernandes, E.; Thirant, C.; Dwyer, J.; Bidere, N.; Couraud, P.O.; Scott, M.G.; Junier, M.P.; Chneiweiss, H.; et al. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Rep. 2011, 12, 470–476. [Google Scholar] [CrossRef]
- Garros-Regulez, L.; Aldaz, P.; Arrizabalaga, O.; Moncho-Amor, V.; Carrasco-Garcia, E.; Manterola, L.; Moreno-Cugnon, L.; Barrena, C.; Villanua, J.; Ruiz, I.; et al. mTOR inhibition decreases SOX2-SOX9 mediated glioma stem cell activity and temozolomide resistance. Expert. Opin. Ther. Targets 2016, 20, 393–405. [Google Scholar] [CrossRef]
- Goldberg, A.R.; Dovas, A.; Torres, D.; Pereira, B.; Viswanathan, A.; Das Sharma, S.; Mela, A.; Merricks, E.M.; Megino-Luque, C.; McInvale, J.J.; et al. Glioma-induced alterations in excitatory neurons are reversed by mTOR inhibition. Neuron 2025, 113, 858–875.e810. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, C.; Salinas, M.; Alcázar, A. Regulation of cap-dependent translation by insulin-like growth factor-1 in neuronal cells. Biochem. Biophys. Res. Commun. 2002, 291, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, L.J.; Gao, X.; Chiarelli, D.A.; Li, L.; Pan, D.; Edgar, B.A. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 2003, 5, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Inamura, N.; Kawamura, M.; Namba, H.; Hara, K.; Yonezawa, K.; Nawa, H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J. Neurosci. 2004, 24, 9760–9769. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.-L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Menon, S.; Dibble, C.C.; Talbott, G.; Hoxhaj, G.; Valvezan, A.J.; Takahashi, H.; Cantley, L.C.; Manning, B.D. Spatial Control of the TSC Complex Integrates Insulin and Nutrient Regulation of mTORC1 at the Lysosome. Cell 2014, 156, 771–785. [Google Scholar] [CrossRef]
- Hara, K.; Yonezawa, K.; Weng, Q.P.; Kozlowski, M.T.; Belham, C.; Avruch, J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998, 273, 14484–14494. [Google Scholar] [CrossRef]
- Long, X.; Ortiz-Vega, S.; Lin, Y.; Avruch, J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J. Biol. Chem. 2005, 280, 23433–23436. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- DeYoung, M.P.; Horak, P.; Sofer, A.; Sgroi, D.; Ellisen, L.W. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes. Dev. 2008, 22, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.C.; Antonia, R.J.; Baldwin, A.S. PI3K/Akt promotes feedforward mTORC2 activation through IKKα. Oncotarget 2016, 7, 21064–21075. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lai, E.; Liu, J.; Lin, J.; Yang, C.; Jia, C.; Li, Y.; Bai, X.; Li, M. IKK interacts with rictor and regulates mTORC2. Cell. Signal. 2013, 25, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Hall, M.N. Regulation of mTORC2 Signaling. Genes 2020, 11, 1045. [Google Scholar] [CrossRef]
- Musa, J.; Orth, M.F.; Dallmayer, M.; Baldauf, M.; Pardo, C.; Rotblat, B.; Kirchner, T.; Leprivier, G.; Grünewald, T.G.P. Eukaryotic initiation factor 4E-binding protein 1 (4E-BP1): A master regulator of mRNA translation involved in tumorigenesis. Oncogene 2016, 35, 4675–4688. [Google Scholar] [CrossRef]
- Roux, P.P.; Topisirovic, I. Regulation of mRNA translation by signaling pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a012252. [Google Scholar] [CrossRef]
- Wu, Z.R.; Yan, L.; Liu, Y.T.; Cao, L.; Guo, Y.H.; Zhang, Y.; Yao, H.; Cai, L.; Shang, H.B.; Rui, W.W.; et al. Inhibition of mTORC1 by lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary tumours. Nat. Commun. 2018, 9, 4624. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a Metabolic Gene Regulatory Network Downstream of mTOR Complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef]
- Li, S.; Brown, M.S.; Goldstein, J.L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3441–3446. [Google Scholar] [CrossRef] [PubMed]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.-L.; Schulze, A. SREBP Activity Is Regulated by mTORC1 and Contributes to Akt-Dependent Cell Growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.T.; Ducker, G.S.; Barczak, A.J.; Barbeau, R.; Erle, D.J.; Shokat, K.M. The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proc. Natl. Acad. Sci. USA 2011, 108, 15201–15206. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, N.; Sasaki, T.; Iemura, S.; Natsume, T.; Hara, T.; Mizushima, N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy 2009, 5, 973–979. [Google Scholar] [CrossRef]
- Hosokawa, N.; Hara, T.; Kaizuka, T.; Kishi, C.; Takamura, A.; Miura, Y.; Iemura, S.; Natsume, T.; Takehana, K.; Yamada, N.; et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 2009, 20, 1981–1991. [Google Scholar] [CrossRef]
- Facchinetti, V.; Ouyang, W.; Wei, H.; Soto, N.; Lazorchak, A.; Gould, C.; Lowry, C.; Newton, A.C.; Mao, Y.; Miao, R.Q.; et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. Embo J. 2008, 27, 1932–1943. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Alessi, D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 2008, 416, 375–385. [Google Scholar] [CrossRef]
- Ikenoue, T.; Inoki, K.; Yang, Q.; Zhou, X.; Guan, K.L. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. Embo J. 2008, 27, 1919–1931. [Google Scholar] [CrossRef]
- Gill, B. Figure 1: mTOR Signaling Pathways. Created in BioRender. 2025. Available online: https://BioRender.com/hdocabj (accessed on 20 October 2025).
- Lee, D.Y. Roles of mTOR Signaling in Brain Development. Exp. Neurobiol. 2015, 24, 177–185. [Google Scholar] [CrossRef]
- Hentges, K.E.; Sirry, B.; Gingeras, A.C.; Sarbassov, D.; Sonenberg, N.; Sabatini, D.; Peterson, A.S. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc. Natl. Acad. Sci. USA 2001, 98, 13796–13801. [Google Scholar] [CrossRef]
- Feliciano, D.M.; Su, T.; Lopez, J.; Platel, J.C.; Bordey, A. Single-cell Tsc1 knockout during corticogenesis generates tuber-like lesions and reduces seizure threshold in mice. J. Clin. Investig. 2011, 121, 1596–1607. [Google Scholar] [CrossRef]
- Orlova, K.A.; Crino, P.B. The tuberous sclerosis complex. Ann. N. Y. Acad. Sci. 2010, 1184, 87–105. [Google Scholar] [CrossRef]
- Poduri, A.; Evrony, G.D.; Cai, X.; Elhosary, P.C.; Beroukhim, R.; Lehtinen, M.K.; Hills, L.B.; Heinzen, E.L.; Hill, A.; Hill, R.S.; et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron 2012, 74, 41–48. [Google Scholar] [CrossRef]
- Kassai, H.; Sugaya, Y.; Noda, S.; Nakao, K.; Maeda, T.; Kano, M.; Aiba, A. Selective activation of mTORC1 signaling recapitulates microcephaly, tuberous sclerosis, and neurodegenerative diseases. Cell Rep. 2014, 7, 1626–1639. [Google Scholar] [CrossRef] [PubMed]
- Rennebeck, G.; Kleymenova, E.V.; Anderson, R.; Yeung, R.S.; Artzt, K.; Walker, C.L. Loss of function of the tuberous sclerosis 2 tumor suppressor gene results in embryonic lethality characterized by disrupted neuroepithelial growth and development. Proc. Natl. Acad. Sci. USA 1998, 95, 15629–15634. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.B. mTOR signaling in epilepsy: Insights from malformations of cortical development. Cold Spring Harb. Perspect. Med. 2015, 5, a022442. [Google Scholar] [CrossRef] [PubMed]
- D’Gama, A.M.; Geng, Y.; Couto, J.A.; Martin, B.; Boyle, E.A.; LaCoursiere, C.M.; Hossain, A.; Hatem, N.E.; Barry, B.J.; Kwiatkowski, D.J.; et al. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann. Neurol. 2015, 77, 720–725. [Google Scholar] [CrossRef]
- Mirzaa, G.M.; Poduri, A. Megalencephaly and hemimegalencephaly: Breakthroughs in molecular etiology. Am. J. Med. Genet. C Semin. Med. Genet. 2014, 166C, 156–172. [Google Scholar] [CrossRef]
- Swiech, L.; Perycz, M.; Malik, A.; Jaworski, J. Role of mTOR in physiology and pathology of the nervous system. Biochim. Biophys. Acta 2008, 1784, 116–132. [Google Scholar] [CrossRef]
- Liang, L.; Tao, B.; Fan, L.; Yaster, M.; Zhang, Y.; Tao, Y.X. mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Res. 2013, 1513, 17–25. [Google Scholar] [CrossRef]
- Tavazoie, S.F.; Alvarez, V.A.; Ridenour, D.A.; Kwiatkowski, D.J.; Sabatini, B.L. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 2005, 8, 1727–1734. [Google Scholar] [CrossRef]
- Urbanska, M.; Gozdz, A.; Swiech, L.J.; Jaworski, J. Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons. J. Biol. Chem. 2012, 287, 30240–30256. [Google Scholar] [CrossRef]
- Jaworski, J.; Sheng, M. The growing role of mTOR in neuronal development and plasticity. Mol. Neurobiol. 2006, 34, 205–219. [Google Scholar] [CrossRef]
- Choi, Y.J.; Di Nardo, A.; Kramvis, I.; Meikle, L.; Kwiatkowski, D.J.; Sahin, M.; He, X. Tuberous sclerosis complex proteins control axon formation. Genes. Dev. 2008, 22, 2485–2495. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.C.; Zivraj, K.H.; Holt, C.E. Local translation and mRNA trafficking in axon pathfinding. Results Probl. Cell Differ. 2009, 48, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Angliker, N.; Ruegg, M.A. In vivo evidence for mTORC2-mediated actin cytoskeleton rearrangement in neurons. Bioarchitecture 2013, 3, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, R. Actin Cytoskeleton Role in the Maintenance of Neuronal Morphology and Long-Term Memory. Cells 2021, 10, 1795. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, P.J.; Zhang, S.; Zhou, H.; Stoica, L.; Galiano, M.; Krnjevic, K.; Roman, G.; Costa-Mattioli, M. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat. Neurosci. 2013, 16, 441–448. [Google Scholar] [CrossRef]
- Stoica, L.; Zhu, P.J.; Huang, W.; Zhou, H.; Kozma, S.C.; Costa-Mattioli, M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc. Natl. Acad. Sci. USA 2011, 108, 3791–3796. [Google Scholar] [CrossRef]
- Crino, P.B.; Nathanson, K.L.; Henske, E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006, 355, 1345–1356. [Google Scholar] [CrossRef]
- Pilarski, R.; Burt, R.; Kohlman, W.; Pho, L.; Shannon, K.M.; Swisher, E. Cowden syndrome and the PTEN hamartoma tumor syndrome: Systematic review and revised diagnostic criteria. J. Natl. Cancer Inst. 2013, 105, 1607–1616. [Google Scholar] [CrossRef]
- Butler, M.G.; Dasouki, M.J.; Zhou, X.P.; Talebizadeh, Z.; Brown, M.; Takahashi, T.N.; Miles, J.H.; Wang, C.H.; Stratton, R.; Pilarski, R.; et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 2005, 42, 318–321. [Google Scholar] [CrossRef]
- Kwon, C.H.; Luikart, B.W.; Powell, C.M.; Zhou, J.; Matheny, S.A.; Zhang, W.; Li, Y.; Baker, S.J.; Parada, L.F. Pten regulates neuronal arborization and social interaction in mice. Neuron 2006, 50, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.H.; Zhu, X.; Zhang, J.; Knoop, L.L.; Tharp, R.; Smeyne, R.J.; Eberhart, C.G.; Burger, P.C.; Baker, S.J. Pten regulates neuronal soma size: A mouse model of Lhermitte-Duclos disease. Nat. Genet. 2001, 29, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Cullen, E.; Getz, S.A.; Conching, A.K.S.; Goyette, A.R.; Prina, M.L.; Wang, W.; Li, M.; Weston, M.C.; Luikart, B.W. Disruption of mTORC1 rescues neuronal overgrowth and synapse function dysregulated by Pten loss. Cell Rep. 2022, 41, 111574. [Google Scholar] [CrossRef] [PubMed]
- Skelton, P.D.; Frazel, P.W.; Lee, D.; Suh, H.; Luikart, B.W. Pten loss results in inappropriate excitatory connectivity. Mol. Psychiatry 2019, 24, 1627–1640. [Google Scholar] [CrossRef]
- Lee, J.H.; Huynh, M.; Silhavy, J.L.; Kim, S.; Dixon-Salazar, T.; Heiberg, A.; Scott, E.; Bafna, V.; Hill, K.J.; Collazo, A.; et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat. Genet. 2012, 44, 941–945. [Google Scholar] [CrossRef]
- Pirozzi, F.; Berkseth, M.; Shear, R.; Gonzalez, L.; Timms, A.E.; Sulc, J.; Pao, E.; Oyama, N.; Forzano, F.; Conti, V.; et al. Profiling PI3K-AKT-MTOR variants in focal brain malformations reveals new insights for diagnostic care. Brain 2022, 145, 925–938. [Google Scholar] [CrossRef]
- Szczaluba, K.; Rydzanicz, M.; Walczak, A.; Kosinska, J.; Koppolu, A.; Biernacka, A.; Iwanicka-Pronicka, K.; Grajkowska, W.; Jurkiewicz, E.; Kowalczyk, P.; et al. Brain Tissue Low-Level Mosaicism for MTOR Mutation Causes Smith-Kingsmore Phenotype with Recurrent Hypoglycemia-A Novel Phenotype and a Further Proof for Testing of an Affected Tissue. Diagnostics 2021, 11, 1269. [Google Scholar] [CrossRef]
- Wong, M. Animal models of focal cortical dysplasia and tuberous sclerosis complex: Recent progress toward clinical applications. Epilepsia 2009, 50 (Suppl. 9), 34–44. [Google Scholar] [CrossRef]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Van Skike, C.E.; Lin, A.L.; Roberts Burbank, R.; Halloran, J.J.; Hernandez, S.F.; Cuvillier, J.; Soto, V.Y.; Hussong, S.A.; Jahrling, J.B.; Javors, M.A.; et al. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell 2020, 19, e13057. [Google Scholar] [CrossRef]
- Wilkinson, J.E.; Burmeister, L.; Brooks, S.V.; Chan, C.C.; Friedline, S.; Harrison, D.E.; Hejtmancik, J.F.; Nadon, N.; Strong, R.; Wood, L.K.; et al. Rapamycin slows aging in mice. Aging Cell 2012, 11, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.J.; Reis, G.; Kang, H.; Gingras, A.C.; Sonenberg, N.; Schuman, E.M. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. USA 2002, 99, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Romine, J.; Gao, X.; Xu, X.M.; So, K.F.; Chen, J. The proliferation of amplifying neural progenitor cells is impaired in the aging brain and restored by the mTOR pathway activation. Neurobiol. Aging 2015, 36, 1716–1726. [Google Scholar] [CrossRef]
- Nie, D.; Di Nardo, A.; Han, J.M.; Baharanyi, H.; Kramvis, I.; Huynh, T.; Dabora, S.; Codeluppi, S.; Pandolfi, P.P.; Pasquale, E.B.; et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat. Neurosci. 2010, 13, 163–172. [Google Scholar] [CrossRef]
- Abe, N.; Borson, S.H.; Gambello, M.J.; Wang, F.; Cavalli, V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J. Biol. Chem. 2010, 285, 28034–28043. [Google Scholar] [CrossRef]
- Reith, R.M.; McKenna, J.; Wu, H.; Hashmi, S.S.; Cho, S.H.; Dash, P.K.; Gambello, M.J. Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2013, 51, 93–103. [Google Scholar] [CrossRef]
- Tang, G.; Gudsnuk, K.; Kuo, S.H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Shoorei, H.; Mohaqiq, M.; Majidpoor, J.; Sayad, A.; Taheri, M. Regulatory role of microRNAs on PTEN signaling. Biomed. Pharmacother. 2021, 133, 110986. [Google Scholar] [CrossRef]
- Bermudez Brito, M.; Goulielmaki, E.; Papakonstanti, E.A. Focus on PTEN Regulation. Front. Oncol. 2015, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chow, L.M.; Bayazitov, I.T.; Tong, Y.; Gilbertson, R.J.; Zakharenko, S.S.; Solecki, D.J.; Baker, S.J. Pten deletion causes mTorc1-dependent ectopic neuroblast differentiation without causing uniform migration defects. Development 2012, 139, 3422–3431. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Gravendeel, L.A.M.; Kouwenhoven, M.C.M.; Gevaert, O.; de Rooi, J.J.; Stubbs, A.P.; Duijm, J.E.; Daemen, A.; Bleeker, F.E.; Bralten, L.B.C.; Kloosterhof, N.K.; et al. Intrinsic Gene Expression Profiles of Gliomas Are a Better Predictor of Survival than Histology. Cancer Res. 2009, 69, 9065–9072. [Google Scholar] [CrossRef]
- Labussiere, M.; Sanson, M.; Idbaih, A.; Delattre, J.Y. IDH1 gene mutations: A new paradigm in glioma prognosis and therapy? Oncologist 2010, 15, 196–199. [Google Scholar] [CrossRef]
- Sanson, M.; Marie, Y.; Paris, S.; Idbaih, A.; Laffaire, J.; Ducray, F.; El Hallani, S.; Boisselier, B.; Mokhtari, K.; Hoang-Xuan, K.; et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J. Clin. Oncol. 2009, 27, 4150–4154. [Google Scholar] [CrossRef]
- Kloosterhof, N.K.; Bralten, L.B.; Dubbink, H.J.; French, P.J.; van den Bent, M.J. Isocitrate dehydrogenase-1 mutations: A fundamentally new understanding of diffuse glioma? Lancet Oncol. 2011, 12, 83–91. [Google Scholar] [CrossRef]
- Ghosh, H.S.; Patel, R.V.; Woodward, E.; Greenwald, N.F.; Bhave, V.M.; Maury, E.A.; Cello, G.; Hoffman, S.E.; Li, Y.; Gupta, H.; et al. Contemporary prognostic signatures and refined risk stratification of gliomas: An analysis of 4400 tumors. Neuro-Oncology 2024, 27, 195–208. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro-Oncology 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.A.; Hunn, B.H.M.; Thomas, M.S.C.; Tolmie, A.K. Influences on cognitive outcomes in adult patients with gliomas: A systematic review. Front. Oncol. 2022, 12, 943600. [Google Scholar] [CrossRef]
- Acevedo-Vergara, K.; Perez-Florez, M.; Ramirez, A.; Torres-Bayona, S.; Dau, A.; Salva, S.; Maloof, D.; Garcia, C.; Luque, M.; Guillen-Burgos, H.F. Cognitive deficits in adult patients with high-grade glioma: A systematic review. Clin. Neurol. Neurosurg. 2022, 219, 107296. [Google Scholar] [CrossRef]
- Ijzerman-Korevaar, M.; Snijders, T.J.; de Graeff, A.; Teunissen, S.C.C.M.; de Vos, F.Y.F. Prevalence of symptoms in glioma patients throughout the disease trajectory: A systematic review. J. Neuro-Oncol. 2018, 140, 485–496. [Google Scholar] [CrossRef]
- Hansen, A.L.; Desai, S.M.; Cooper, A.N.; Steinbach, M.A.; Gosselin, K.; Wanebo, J.E. The clinical progression of patients with glioblastoma. Interdiscip. Neurosurg. 2023, 32, 101756. [Google Scholar] [CrossRef]
- Rudà, R.; Bello, L.; Duffau, H.; Soffietti, R. Seizures in low-grade gliomas: Natural history, pathogenesis, and outcome after treatments. Neuro-Oncology 2012, 14 (Suppl. 4), iv55-64. [Google Scholar] [CrossRef]
- Li, X.; Wu, C.; Chen, N.; Gu, H.; Yen, A.; Cao, L.; Wang, E.; Wang, L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 2016, 7, 33440–33450. [Google Scholar] [CrossRef]
- Huang, P.H.; Xu, A.M.; White, F.M. Oncogenic EGFR signaling networks in glioma. Sci. Signal. 2009, 2, re6. [Google Scholar] [CrossRef]
- Dhaliwal, N.K.; Weng, O.Y.; Dong, X.; Bhattacharya, A.; Ahmed, M.; Nishimura, H.; Choi, W.W.Y.; Aggarwal, A.; Luikart, B.W.; Shu, Q.; et al. Synergistic hyperactivation of both mTORC1 and mTORC2 underlies the neural abnormalities of PTEN-deficient human neurons and cortical organoids. Cell Rep. 2024, 43, 114173. [Google Scholar] [CrossRef]
- Akhavan, D.; Cloughesy, T.F.; Mischel, P.S. mTOR signaling in glioblastoma: Lessons learned from bench to bedside. Neuro-Oncology 2010, 12, 882–889. [Google Scholar] [CrossRef]
- Kim, L.C.; Cook, R.S.; Chen, J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 2017, 36, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gelinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Mead, H.; Zeremski, M.; Guba, M. mTOR Signaling in Angiogenesis. In mTOR Pathway and mTOR Inhibitors in Cancer Therapy; Humana Press: Totowa, NJ, USA, 2009; pp. 49–74. [Google Scholar]
- Eyler, C.E.; Foo, W.-C.; LaFiura, K.M.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. Brain Cancer Stem Cells Display Preferential Sensitivity to Akt Inhibition. Stem Cells 2008, 26, 3027–3036. [Google Scholar] [CrossRef]
- Sandoval, J.A.; Tomilov, A.; Datta, S.; Allen, S.; O’Donnell, R.; Sears, T.; Woolard, K.; Kovalskyy, D.; Angelastro, J.M.; Cortopassi, G. Novel mTORC1 Inhibitors Kill Glioblastoma Stem Cells. Pharmaceuticals 2020, 13, 419. [Google Scholar] [CrossRef]
- Josset, E.; Burckel, H.; Noël, G.; Bischoff, P. The mTOR inhibitor RAD001 potentiates autophagic cell death induced by temozolomide in a glioblastoma cell line. Anticancer Res. 2013, 33, 1845–1851. [Google Scholar]
- Singh, S.; Barik, D.; Lawrie, K.; Mohapatra, I.; Prasad, S.; Naqvi, A.R.; Singh, A.; Singh, G. Unveiling Novel Avenues in mTOR-Targeted Therapeutics: Advancements in Glioblastoma Treatment. Int. J. Mol. Sci. 2023, 24, 14960. [Google Scholar] [CrossRef]
- Zhang, W.B.; Wang, Z.; Shu, F.; Jin, Y.H.; Liu, H.Y.; Wang, Q.J.; Yang, Y. Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J. Biol. Chem. 2010, 285, 40461–40471. [Google Scholar] [CrossRef]
- Jiang, B.H.; Liu, L.Z. Role of mTOR in anticancer drug resistance: Perspectives for improved drug treatment. Drug Resist. Updat. 2008, 11, 63–76. [Google Scholar] [CrossRef]
- Gill, B.J.A.; Khan, F.A.; Goldberg, A.R.; Merricks, E.M.; Wu, X.; Sosunov, A.A.; Sudhakar, T.D.; Dovas, A.; Lado, W.; Michalak, A.J.; et al. Single unit analysis and wide-field imaging reveal alterations in excitatory and inhibitory neurons in glioma. Brain 2022, 145, 3666–3680. [Google Scholar] [CrossRef]
- Pallud, J.; Audureau, E.; Blonski, M.; Sanai, N.; Bauchet, L.; Fontaine, D.; Mandonnet, E.; Dezamis, E.; Psimaras, D.; Guyotat, J.; et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain 2014, 137, 449–462. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Tam, L.T.; Woo, P.J.; Nagaraja, S.; Gillespe, S.M.; Lennon, J.; Ni, J.; Duveau, D.Y.; Morris, P.J.; Zhao, J.J.; et al. Targeting neuronal activity-regulated neuroligin-3 dependency for high-grade glioma therapy. Nature 2017, 549, 553-537. [Google Scholar] [CrossRef]
- Karreman, M.A.; Venkataramani, V. Calm in the chaos: Targeting mTOR to reduce glioma-driven neuronal hyperexcitability. Neuron 2025, 113, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Garzon-Muvdi, T.; Schiapparelli, P.; ap Rhys, C.; Guerrero-Cazares, H.; Smith, C.; Kim, D.H.; Kone, L.; Farber, H.; Lee, D.Y.; An, S.S.; et al. Regulation of brain tumor dispersal by NKCC1 through a novel role in focal adhesion regulation. PLoS Biol. 2012, 10, e1001320. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.T.; Wierenga, C.J.; Bruining, H. Chloride transporters and GABA polarity in developmental, neurological and psychiatric conditions. Neurosci. Biobehav. Rev. 2018, 90, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.T.; Deeb, T.Z.; Puskarjov, M.; Silayeva, L.; Liang, B.; Kaila, K.; Moss, S.J. Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci. 2013, 36, 726–737. [Google Scholar] [CrossRef]
- Ye, Z.Y.; Li, D.P.; Byun, H.S.; Li, L.; Pan, H.L. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J. Neurosci. 2012, 32, 8560–8568. [Google Scholar] [CrossRef][Green Version]
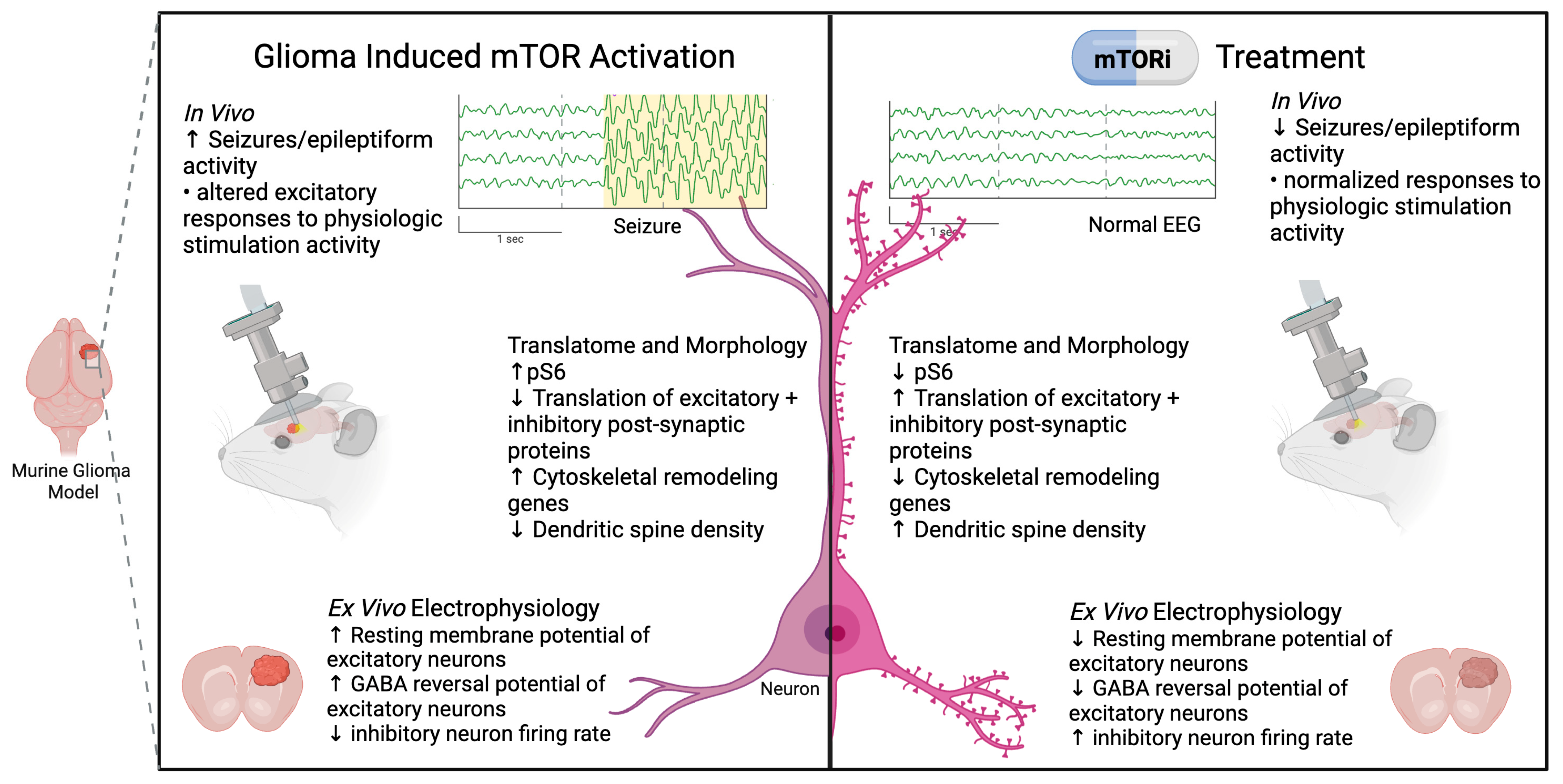
- Gill, B. Figure 2. mTOR-mediated Neuronal Dysfunction in Glioma. Created in BioRender. 2025. Available online: https://BioRender.com/0dhef7k (accessed on 20 October 2025).[Green Version]
- Sami, A.; Karsy, M. Targeting the PI3K/AKT/mTOR signaling pathway in glioblastoma: Novel therapeutic agents and advances in understanding. Tumor Biol. 2013, 34, 1991–2002. [Google Scholar] [CrossRef]
- Faivre, S.; Kroemer, G.; Raymond, E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006, 5, 671–688. [Google Scholar] [CrossRef]
- Chang, S.M.; Wen, P.; Cloughesy, T.; Greenberg, H.; Schiff, D.; Conrad, C.; Fink, K.; Robins, H.I.; De Angelis, L.; Raizer, J.; et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Investig. New Drugs 2005, 23, 357–361. [Google Scholar] [CrossRef]
- Galanis, E.; Buckner, J.C.; Maurer, M.J.; Kreisberg, J.I.; Ballman, K.; Boni, J.; Peralba, J.M.; Jenkins, R.B.; Dakhil, S.R.; Morton, R.F.; et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J. Clin. Oncol. 2005, 23, 5294–5304. [Google Scholar] [CrossRef]
- Ali, E.S.; Mitra, K.; Akter, S.; Ramproshad, S.; Mondal, B.; Khan, I.N.; Islam, M.T.; Sharifi-Rad, J.; Calina, D.; Cho, W.C. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer Cell Int. 2022, 22, 284. [Google Scholar] [CrossRef]
- Colardo, M.; Segatto, M.; Di Bartolomeo, S. Targeting RTK-PI3K-mTOR Axis in Gliomas: An Update. Int. J. Mol. Sci. 2021, 22, 4899. [Google Scholar] [CrossRef] [PubMed]
- Mecca, C.; Giambanco, I.; Donato, R.; Arcuri, C. Targeting mTOR in Glioblastoma: Rationale and Preclinical/Clinical Evidence. Dis. Markers 2018, 2018, 9230479. [Google Scholar] [CrossRef] [PubMed]
- Pournajaf, S.; Pourgholami, M.H. The mTOR pathway in Gliomas: From molecular insights to targeted therapies. Biomed. Pharmacother. 2025, 189, 118237. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Kumar, A.; Zhang, Y.; Wang, A.S.; Chen, K.; Lim, Y.; Shai, A.; Taylor, J.W.; Clarke, J.; Hilz, S.; et al. PI3K/AKT/mTOR signaling pathway activity in IDH-mutant diffuse glioma and clinical implications. Neuro-Oncology 2022, 24, 1471–1481. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Won, M.; Wen, P.Y.; Rojiani, A.M.; Wendland, M.; Dipetrillo, T.A.; Corn, B.W.; Mehta, M.P. RTOG 0913: A phase 1 study of daily everolimus (RAD001) in combination with radiation therapy and temozolomide in patients with newly diagnosed glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 880–884. [Google Scholar] [CrossRef]
- Chinnaiyan, P.; Won, M.; Wen, P.Y.; Rojiani, A.M.; Werner-Wasik, M.; Shih, H.A.; Ashby, L.S.; Michael Yu, H.H.; Stieber, V.W.; Malone, S.C.; et al. A randomized phase II study of everolimus in combination with chemoradiation in newly diagnosed glioblastoma: Results of NRG Oncology RTOG 0913. Neuro-Oncology 2018, 20, 666–673. [Google Scholar] [CrossRef]
- Mason, W.P.; Macneil, M.; Kavan, P.; Easaw, J.; Macdonald, D.; Thiessen, B.; Urva, S.; Lwin, Z.; McIntosh, L.; Eisenhauer, E. A phase I study of temozolomide and everolimus (RAD001) in patients with newly diagnosed and progressive glioblastoma either receiving or not receiving enzyme-inducing anticonvulsants: An NCIC CTG study. Investig. New Drugs 2012, 30, 2344–2351. [Google Scholar] [CrossRef]
- Wick, W.; Gorlia, T.; Bady, P.; Platten, M.; van den Bent, M.J.; Taphoorn, M.J.B.; Steuve, J.; Brandes, A.A.; Hamou, M.-F.; Wick, A.; et al. Phase II Study of Radiotherapy and Temsirolimus versus Radiochemotherapy with Temozolomide in Patients with Newly Diagnosed Glioblastoma without MGMT Promoter Hypermethylation (EORTC 26082). Clin. Cancer Res. 2016, 22, 4797–4806. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, S.; Mason, W.; MacNeil, M.; Harlos, C.; Tsang, R.; Sederias, J.; Luchman, H.A.; Weiss, S.; Rossiter, J.P.; Tu, D.; et al. A phase I study of vistusertib (dual mTORC1/2 inhibitor) in patients with previously treated glioblastoma multiforme: A CCTG study. Investig. New Drugs 2020, 38, 1137–1144. [Google Scholar] [CrossRef]
- Moloney, P.B.; Cavalleri, G.L.; Delanty, N. Epilepsy in the mTORopathies: Opportunities for precision medicine. Brain Commun. 2021, 3, fcab222. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.M.; Rosenow, F.; Schubert-Bast, S.; Kurlemann, G.; Zöllner, J.P.; Bast, T.; Bertsche, A.; Bettendorf, U.; Ebrahimi-Fakhari, D.; Grau, J.; et al. Efficacy, Retention and Tolerability of Everolimus in Patients with Tuberous Sclerosis Complex: A Survey-Based Study on Patients’ Perspectives. CNS Drugs 2021, 35, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.N.; Agricola, K.; Mays, M.; Tudor, C.; Care, M.M.; Holland-Bouley, K.; Berkowitz, N.; Miao, S.; Peyrard, S.; Krueger, D.A. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann. Neurol. 2015, 78, 929–938. [Google Scholar] [CrossRef]
- Krueger, D.A.; Care, M.M.; Holland, K.; Agricola, K.; Tudor, C.; Mangeshkar, P.; Wilson, K.A.; Byars, A.; Sahmoud, T.; Franz, D.N. Everolimus for Subependymal Giant-Cell Astrocytomas in Tuberous Sclerosis. N. Engl. J. Med. 2010, 363, 1801–1811. [Google Scholar] [CrossRef]
- Palavra, F.; Robalo, C.; Reis, F. Recent Advances and Challenges of mTOR Inhibitors Use in the Treatment of Patients with Tuberous Sclerosis Complex. Oxidative Med. Cell. Longev. 2017, 2017, 9820181. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, C.; Zhang, X.; Liu, J.; Liu, J.; Xia, Z. Advances in the mTOR signaling pathway and its inhibitor rapamycin in epilepsy. Brain Behav. 2023, 13, e2995. [Google Scholar] [CrossRef]
- Auvin, S.; Baulac, S. mTOR-therapy and targeted treatment opportunities in mTOR-related epilepsies associated with cortical malformations. Rev. Neurol. 2023, 179, 337–344. [Google Scholar] [CrossRef]
- French, J.A.; Lawson, J.A.; Yapici, Z.; Ikeda, H.; Polster, T.; Nabbout, R.; Curatolo, P.; de Vries, P.J.; Dlugos, D.J.; Berkowitz, N.; et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): A phase 3, randomised, double-blind, placebo-controlled study. Lancet 2016, 388, 2153–2163. [Google Scholar] [CrossRef]
- Ravizza, T.; Scheper, M.; Di Sapia, R.; Gorter, J.; Aronica, E.; Vezzani, A. mTOR and neuroinflammation in epilepsy: Implications for disease progression and treatment. Nat. Rev. Neurosci. 2024, 25, 334–350. [Google Scholar] [CrossRef]
- van Kessel, E.; Emons, M.A.C.; Wajer, I.H.; van Baarsen, K.M.; Broekman, M.L.; Robe, P.A.; Snijders, T.J.; Van Zandvoort, M.J.E. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: A retrospective cohort study prior to antitumor treatment. Neuro-Oncol. Pract. 2019, 6, 463–472. [Google Scholar] [CrossRef]
- Soefje, S.A.; Karnad, A.; Brenner, A.J. Common toxicities of mammalian target of rapamycin inhibitors. Target. Oncol. 2011, 6, 125–129. [Google Scholar] [CrossRef]
- Graham-Gurysh, E.G.; Murthy, A.B.; Moore, K.M.; Hingtgen, S.D.; Bachelder, E.M.; Ainslie, K.M. Synergistic drug combinations for a precision medicine approach to interstitial glioblastoma therapy. J. Controll. Release 2020, 323, 282–292. [Google Scholar] [CrossRef]
- Brem, H.; Piantadosi, S.; Burger, P.C.; Walker, M.; Selker, R.; Vick, N.A.; Black, K.; Sisti, M.; Brem, S.; Mohr, G.; et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet 1995, 345, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Kreatsoulas, D.; Damante, M.; Cua, S.; Lonser, R.R. Adjuvant convection-enhanced delivery for the treatment of brain tumors. J. Neuro-Oncol. 2024, 166, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W.F. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert. Rev. Mol. Med. 2011, 13, e17. [Google Scholar] [CrossRef]
- Gill, B. Figure 3. Neuron–Glioma Crosstalk: From Modeling to Therapy. Created in BioRender. 2025. Available online: https://BioRender.com/84igv1s (accessed on 20 October 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haile, H.; Leskinen, S.; Adapa, A.R.; R. Goldberg, A.; Viswanathan, A.; Milligan, C.; Conboy, K.; Schevon, C.; Canoll, P.; Gill, B.J.A. The Role of mTOR Signaling in Tumor-Induced Alterations to Neuronal Function in Diffusely Infiltrating Glioma. Biomedicines 2025, 13, 2593. https://doi.org/10.3390/biomedicines13112593
Haile H, Leskinen S, Adapa AR, R. Goldberg A, Viswanathan A, Milligan C, Conboy K, Schevon C, Canoll P, Gill BJA. The Role of mTOR Signaling in Tumor-Induced Alterations to Neuronal Function in Diffusely Infiltrating Glioma. Biomedicines. 2025; 13(11):2593. https://doi.org/10.3390/biomedicines13112593
Chicago/Turabian StyleHaile, Hannah, Sandra Leskinen, Arjun R. Adapa, Alexander R. Goldberg, Ashwin Viswanathan, Charlotte Milligan, Karen Conboy, Catherine Schevon, Peter Canoll, and Brian J. A. Gill. 2025. "The Role of mTOR Signaling in Tumor-Induced Alterations to Neuronal Function in Diffusely Infiltrating Glioma" Biomedicines 13, no. 11: 2593. https://doi.org/10.3390/biomedicines13112593
APA StyleHaile, H., Leskinen, S., Adapa, A. R., R. Goldberg, A., Viswanathan, A., Milligan, C., Conboy, K., Schevon, C., Canoll, P., & Gill, B. J. A. (2025). The Role of mTOR Signaling in Tumor-Induced Alterations to Neuronal Function in Diffusely Infiltrating Glioma. Biomedicines, 13(11), 2593. https://doi.org/10.3390/biomedicines13112593







