Loss of AT8 Nuclear Tau as a Marker of Neuronal Ageing and Alzheimer’s Disease Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Antibodies for Indirect Immunofluorescence
2.3. Immunodetection on the Neuroblastoma Cell Line
2.4. Tissue Sections
2.5. Immunodetection on Human Tissue Sections and CLSM Analyses
2.6. Cell Counting and Statistical Analysis
3. Results
3.1. Tau Epitopes in the Nucleus of Neurons
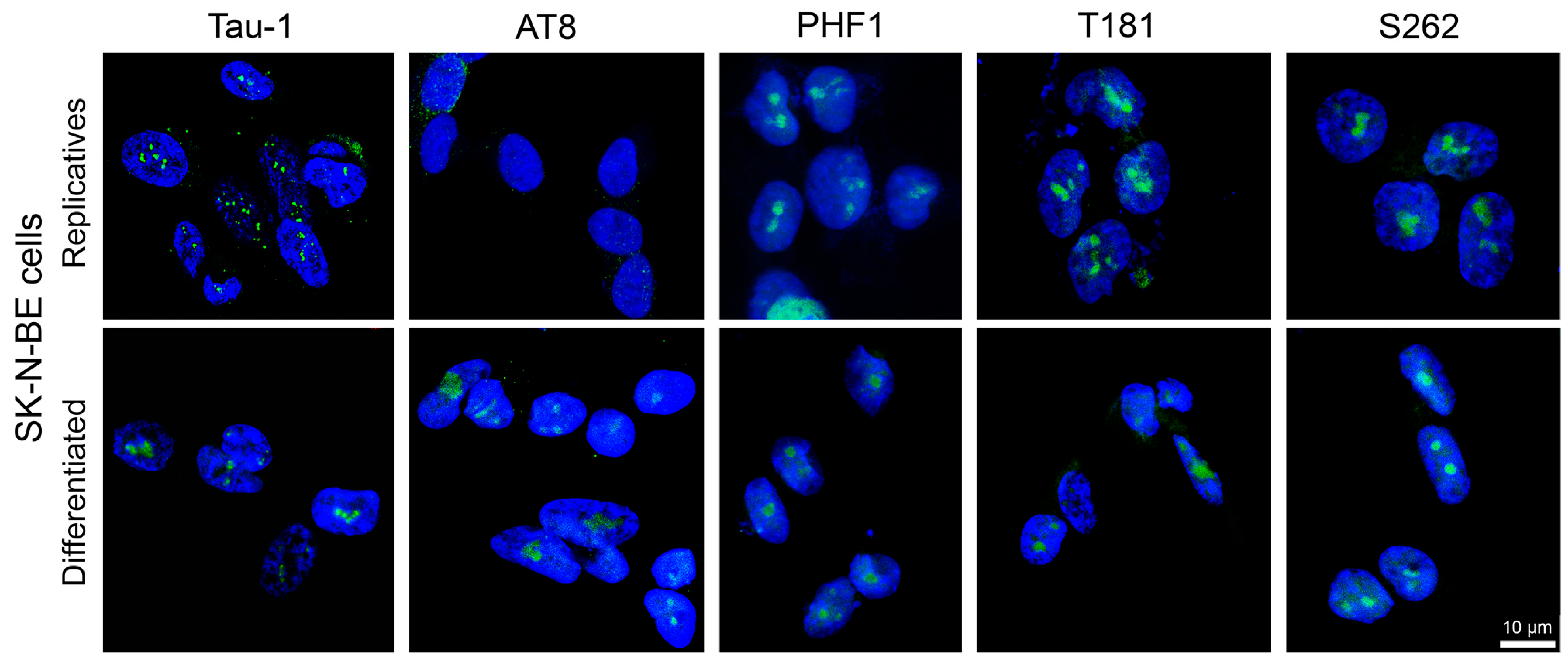
3.1.1. Nuclear Tau Epitopes in SK-N-BE Neuroblastoma Cell Line
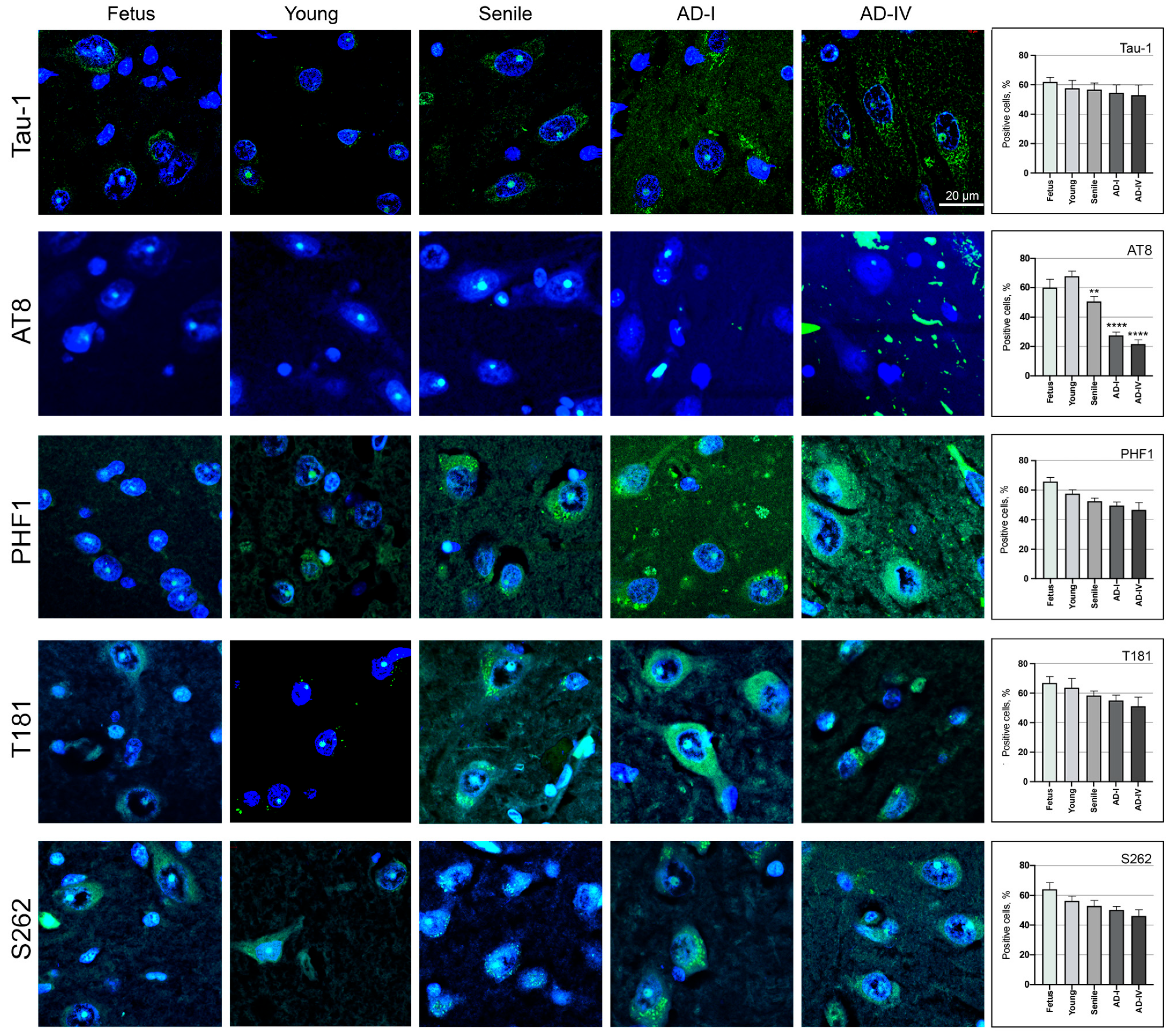
3.1.2. Nuclear Tau Epitopes in Human Neurons from the CA1 Region of the Hippocampus
3.2. AT8 Epitope in Human Neurons from the CA1 Region of the Hippocampus
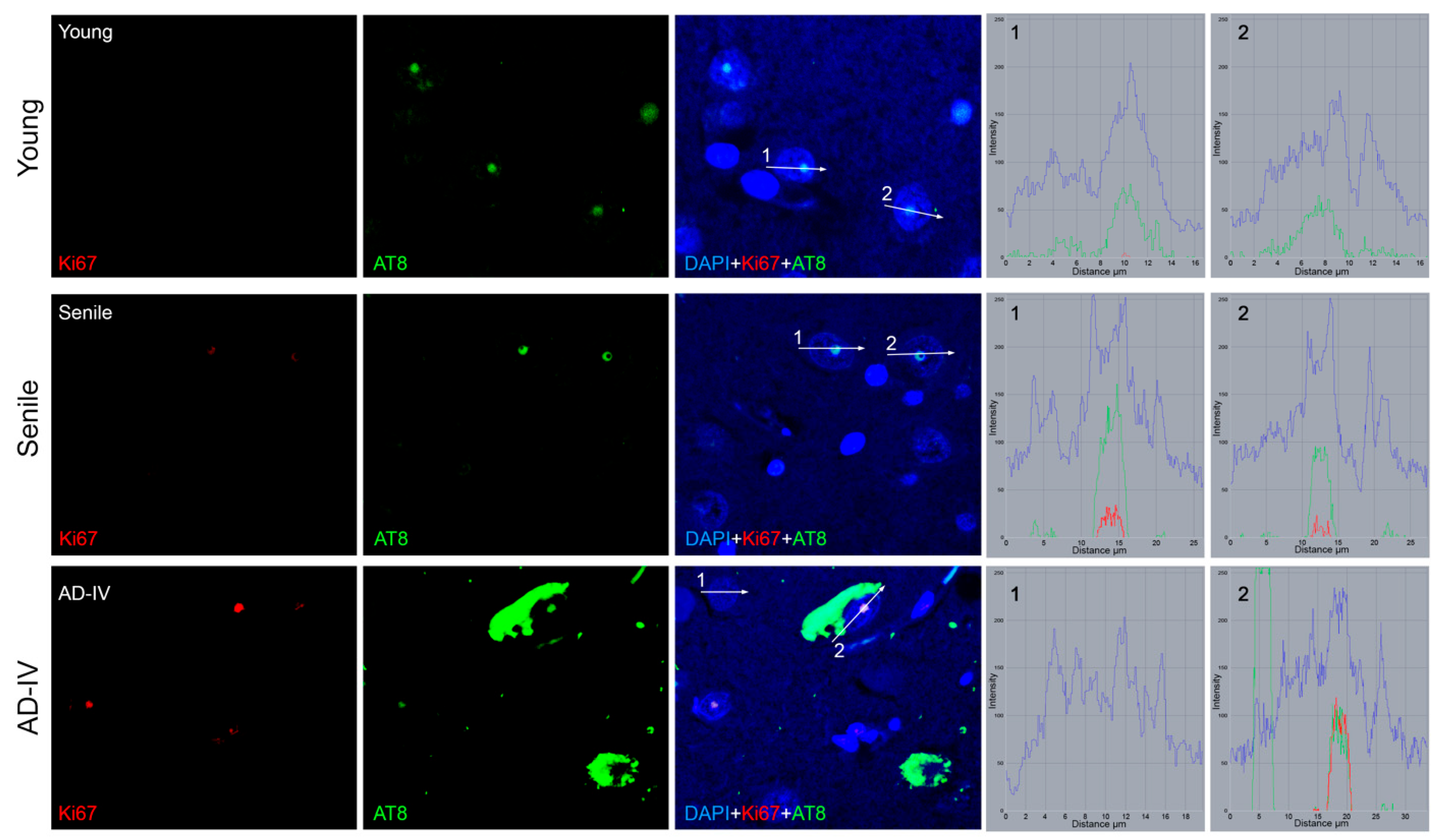
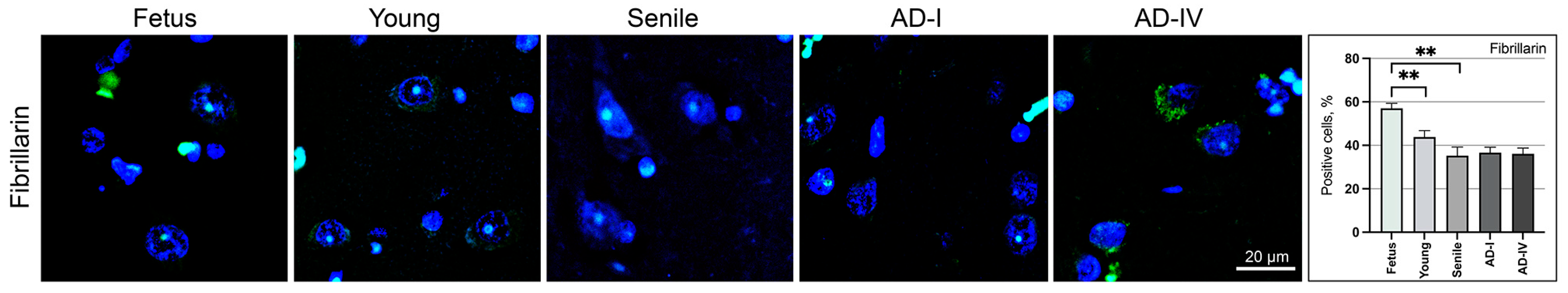
3.3. Transcriptional Status of Neuronal Cells in the CA1 Brain Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Act-D | Actinomycin-D |
| AD | Alzheimer’s disease |
| Aβ | Amyloid-β |
| CA | Cornu Ammonis |
| CLSM | Confocal laser scanning microscopy |
| CNS | Central nervous system |
| FBS | Foetal bovine serum |
| H4Ac | Acetylated histone H4 |
| HUFA | Hospital Universitario Fundación Alcorcón |
| IIF | Indirect immunofluorescence |
| MAPT | Microtubule-associated protein tau |
| NFTs | Neurofibrillary tangles |
| RA | Retinoic acid |
| UBTF | Upstream binding transcription factor |
References
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Zvěřová, M. Clinical Aspects of Alzheimer’s Disease. Clin. Biochem. 2019, 72, 3–6. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s Disease: Inhibition of Amyloid Beta and Tau Tangle Formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s Disease: Insights and New Prospects in Disease Pathophysiology, Biomarkers and Disease-Modifying Drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef]
- Advani, D.; Kumar, P. Uncovering Cell Cycle Dysregulations and Associated Mechanisms in Cancer and Neurodegenerative Disorders: A Glimpse of Hope for Repurposed Drugs. Mol. Neurobiol. 2024, 61, 8600–8630. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Esiri, M.M.; Smith, A.D. Expression of Cell Division Markers in the Hippocampus in Alzheimer’s Disease and Other Neurodegenerative Conditions. Acta Neuropathol. 1997, 93, 294–300. [Google Scholar] [CrossRef]
- Zhu, X.; Lee, H.; Perry, G.; Smith, M.A. Alzheimer Disease, the Two-Hit Hypothesis: An Update. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2007, 1772, 494–502. [Google Scholar] [CrossRef]
- Bonda, D.J.; Evans, T.A.; Santocanale, C.; Llosá, J.C.; Viňa, J.; Bajic, V.P.; Castellani, R.J.; Siedlak, S.L.; Perry, G.; Smith, M.A.; et al. Evidence for the Progression through S-Phase in the Ectopic Cell Cycle Re-Entry of Neurons in Alzheimer Disease. Aging 2009, 1, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Bajić, V.P.; Spremo-Potparevic, B.; Casadesus, G.; Zhu, X.; Smith, M.A.; Lee, H.-G. Review: Cell Cycle Aberrations and Neurodegeneration. Neuropathol. Appl. Neurobiol. 2010, 36, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Koseoglu, M.M.; Norambuena, A.; Sharlow, E.R.; Lazo, J.S.; Bloom, G.S. Aberrant Neuronal Cell Cycle Re-Entry: The Pathological Confluence of Alzheimer’s Disease and Brain Insulin Resistance, and Its Relation to Cancer. J. Alzheimers Dis. 2019, 67, 1–11. [Google Scholar] [CrossRef]
- Nyhus, C.; Pihl, M.; Hyttel, P.; Hall, V.J. Evidence for Nucleolar Dysfunction in Alzheimer’s Disease. Rev. Neurosci. 2019, 30, 685–700. [Google Scholar] [CrossRef]
- Malhotra, N.; Gupta, R.; Kumar, P. Pharmacological Relevance of CDK Inhibitors in Alzheimer’s Disease. Neurochem. Int. 2021, 148, 105115. [Google Scholar] [CrossRef]
- Zhang, X.; Song, S.; Peng, W. Cell Cycle Deregulation in Neurodegenerative Diseases. Int. J. Neurosci. 2023, 133, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Hemberg, M.; Lewis, J.; Feany, M.B. Tau Promotes Neurodegeneration through Global Chromatin Relaxation. Nat. Neurosci. 2014, 17, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Denechaud, M.; Geurs, S.; Comptdaer, T.; Bégard, S.; Garcia-Núñez, A.; Pechereau, L.-A.; Bouillet, T.; Vermeiren, Y.; De Deyn, P.P.; Perbet, R.; et al. Tau Promotes Oxidative Stress-Associated Cycling Neurons in S Phase as a pro-Survival Mechanism: Possible Implication for Alzheimer’s Disease. Prog. Neurobiol. 2023, 223, 102386. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, L.A.G.; Hoozemans, J.J.M. Physiological and Pathophysiological Functions of Cell Cycle Proteins in Post-Mitotic Neurons: Implications for Alzheimer’s Disease. Acta Neuropathol. 2015, 129, 511–525. [Google Scholar] [CrossRef]
- Regalado-Reyes, M.; Furcila, D.; Hernández, F.; Ávila, J.; DeFelipe, J.; León-Espinosa, G. Phospho-Tau Changes in the Human CA1 During Alzheimer’s Disease Progression. J. Alzheimers Dis. 2019, 69, 277–288. [Google Scholar] [CrossRef]
- Maina, M.B.; Bailey, L.J.; Wagih, S.; Biasetti, L.; Pollack, S.J.; Quinn, J.P.; Thorpe, J.R.; Doherty, A.J.; Serpell, L.C. The Involvement of Tau in Nucleolar Transcription and the Stress Response. Acta Neuropathol. Commun. 2018, 6, 70. [Google Scholar] [CrossRef]
- Sultan, A.; Nesslany, F.; Violet, M.; Bégard, S.; Loyens, A.; Talahari, S.; Mansuroglu, Z.; Marzin, D.; Sergeant, N.; Humez, S.; et al. Nuclear Tau, a Key Player in Neuronal DNA Protection. J. Biol. Chem. 2011, 286, 4566–4575. [Google Scholar] [CrossRef]
- Bukar Maina, M.; Al-Hilaly, Y.; Serpell, L. Nuclear Tau and Its Potential Role in Alzheimer’s Disease. Biomolecules 2016, 6, 9. [Google Scholar] [CrossRef]
- Joseph, C.; Mangani, A.S.; Gupta, V.; Chitranshi, N.; Shen, T.; Dheer, Y.; Kb, D.; Mirzaei, M.; You, Y.; Graham, S.L.; et al. Cell Cycle Deficits in Neurodegenerative Disorders: Uncovering Molecular Mechanisms to Drive Innovative Therapeutic Development. Aging Dis. 2020, 11, 946–966. [Google Scholar] [CrossRef]
- Busser, J.; Geldmacher, D.S.; Herrup, K. Ectopic Cell Cycle Proteins Predict the Sites of Neuronal Cell Death in Alzheimer’s Disease Brain. J. Neurosci. 1998, 18, 2801–2807. [Google Scholar] [CrossRef]
- McShea, A.; Lee, H.; Petersen, R.B.; Casadesus, G.; Vincent, I.; Linford, N.J.; Funk, J.-O.; Shapiro, R.A.; Smith, M.A. Neuronal Cell Cycle Re-Entry Mediates Alzheimer Disease-Type Changes. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2007, 1772, 467–472. [Google Scholar] [CrossRef]
- Yang, Y.; Mufson, E.J.; Herrup, K. Neuronal Cell Death Is Preceded by Cell Cycle Events at All Stages of Alzheimer’s Disease. J. Neurosci. 2003, 23, 2557–2563. [Google Scholar] [CrossRef]
- Hudson, H.R.; Riessland, M.; Orr, M.E. Defining and Characterizing Neuronal Senescence, ‘Neurescence’, as GX Arrested Cells. Trends Neurosci. 2024, 47, 971–984. [Google Scholar] [CrossRef]
- Liu, D.X.; Greene, L.A. Neuronal Apoptosis at the G1/S Cell Cycle Checkpoint. Cell Tissue Res. 2001, 305, 217–228. [Google Scholar] [CrossRef]
- Kuan, C.-Y.; Schloemer, A.J.; Lu, A.; Burns, K.A.; Weng, W.-L.; Williams, M.T.; Strauss, K.I.; Vorhees, C.V.; Flavell, R.A.; Davis, R.J.; et al. Hypoxia-Ischemia Induces DNA Synthesis without Cell Proliferation in Dying Neurons in Adult Rodent Brain. J. Neurosci. 2004, 24, 10763–10772. [Google Scholar] [CrossRef]
- Gupta, R.; Jha, A.; Ambasta, R.K.; Kumar, P. Regulatory Mechanism of Cyclins and Cyclin-Dependent Kinases in Post-Mitotic Neuronal Cell Division. Life Sci. 2021, 285, 120006. [Google Scholar] [CrossRef]
- Asada-Utsugi, M.; Urushitani, M. Tau beyond Tangles: DNA Damage Response and Cytoskeletal Protein Crosstalk on Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 7906. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ortega, K.; Garcia-Esparcia, P.; Gil, L.; Lucas, J.J.; Ferrer, I. Altered Machinery of Protein Synthesis in Alzheimer’s: From the Nucleolus to the Ribosome. Brain Pathol. 2016, 26, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Méndez-López, I.; Blanco-Luquin, I.; Sánchez-Ruiz De Gordoa, J.; Urdánoz-Casado, A.; Roldán, M.; Acha, B.; Echavarri, C.; Zelaya, V.; Jericó, I.; Mendioroz, M. Hippocampal LMNA Gene Expression Is Increased in Late-Stage Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 878. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Niño, S.A.; Chi-Ahumada, E.; Rodríguez-Leyva, I.; Guerrero, C.; Rebolledo, A.B.; Arias, J.A.; Jiménez-Capdeville, M.E. Perinuclear Lamin A and Nucleoplasmic Lamin B2 Characterize Two Types of Hippocampal Neurons through Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2020, 21, 1841. [Google Scholar] [CrossRef]
- Gil, L.; Chi-Ahumada, E.; Niño, S.A.; Capdeville, G.; Méndez-Torres, A.M.; Guerrero, C.; Rebolledo, A.B.; Olazabal, I.M.; Jiménez-Capdeville, M.E. Pathological Nuclear Hallmarks in Dentate Granule Cells of Alzheimer’s Patients: A Biphasic Regulation of Neurogenesis. Int. J. Mol. Sci. 2022, 23, 12873. [Google Scholar] [CrossRef]
- Antón-Fernández, A.; Vallés-Saiz, L.; Avila, J.; Hernández, F. Neuronal Nuclear Tau and Neurodegeneration. Neuroscience 2023, 518, 178–184. [Google Scholar] [CrossRef]
- Xue, H.; Gate, S.; Gentry, E.; Losert, W.; Cao, K. Development of an Accelerated Cellular Model for Early Changes in Alzheimer’s Disease. Sci. Rep. 2023, 13, 18384. [Google Scholar] [CrossRef]
- Frost, B. Alzheimer’s Disease and Related Tauopathies: Disorders of Disrupted Neuronal Identity. Trends Neurosci. 2023, 46, 797–813. [Google Scholar] [CrossRef]
- Yin, X.; Qiu, Y.; Zhao, C.; Zhou, Z.; Bao, J.; Qian, W. The Role of Amyloid-Beta and Tau in the Early Pathogenesis of Alzheimer’s Disease. Med. Sci. Monit. 2021, 27, e933084. [Google Scholar] [CrossRef]
- Merino-Serrais, P.; Soria, J.M.; Arrabal, C.A.; Ortigado-López, A.; Esparza, M.Á.G.; Muñoz, A.; Hernández, F.; Ávila, J.; DeFelipe, J.; León-Espinosa, G. Protein Tau Phosphorylation in the Proline Rich Region and Its Implication in the Progression of Alzheimer’s Disease. Exp. Neurol. 2025, 383, 115049. [Google Scholar] [CrossRef] [PubMed]
- De Flores, R.; La Joie, R.; Chételat, G. Structural Imaging of Hippocampal Subfields in Healthy Aging and Alzheimer’s Disease. Neuroscience 2015, 309, 29–50. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and Its Involvement in Alzheimer’s Disease: A Review. 3 Biotech 2022, 12, 55. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Terro, F. Post-Translational Modifications of Tau Protein: Implications for Alzheimer’s Disease. Neurochem. Int. 2011, 58, 458–471. [Google Scholar] [CrossRef]
- Alonso, A.D.; Cohen, L.S.; Corbo, C.; Morozova, V.; ElIdrissi, A.; Phillips, G.; Kleiman, F.E. Hyperphosphorylation of Tau Associates With Changes in Its Function Beyond Microtubule Stability. Front. Cell. Neurosci. 2018, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E.; Bohl, J. Staging of Alzheimer-Related Cortical Destruction. Eur. Neurol. 1993, 33, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Delatour, B.; Potier, M.-C. Classification and Basic Pathology of Alzheimer Disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Ohkawa, N.; Saitoh, Y.; Ghandour, K.; Murayama, E.; Nishizono, H.; Matsuo, M.; Hirayama, T.; Kaneko, R.; Muramatsu, S.; et al. Pcdhβ Deficiency Affects Hippocampal CA1 Ensemble Activity and Contextual Fear Discrimination. Mol. Brain 2020, 13, 7. [Google Scholar] [CrossRef]
- Alkadhi, K.A. Cellular and Molecular Differences Between Area CA1 and the Dentate Gyrus of the Hippocampus. Mol. Neurobiol. 2019, 56, 6566–6580. [Google Scholar] [CrossRef]
- Mufson, E.J.; Mahady, L.; Waters, D.; Counts, S.E.; Perez, S.E.; DeKosky, S.T.; Ginsberg, S.D.; Ikonomovic, M.D.; Scheff, S.W.; Binder, L.I. Hippocampal Plasticity during the Progression of Alzheimer’s Disease. Neuroscience 2015, 309, 51–67. [Google Scholar] [CrossRef]
- Loomis, P.A.; Howard, T.H.; Castleberry, R.P.; Binder, L.I. Identification of Nuclear Tau Isoforms in Human Neuroblastoma Cells. Proc. Natl. Acad. Sci. USA 1990, 87, 8422–8426. [Google Scholar] [CrossRef]
- Sjöberg, M.K.; Shestakova, E.; Mansuroglu, Z.; Maccioni, R.B.; Bonnefoy, E. Tau Protein Binds to Pericentromeric DNA: A Putative Role for Nuclear Tau in Nucleolar Organization. J. Cell Sci. 2006, 119, 2025–2034. [Google Scholar] [CrossRef]
- Mansuroglu, Z.; Benhelli-Mokrani, H.; Marcato, V.; Sultan, A.; Violet, M.; Chauderlier, A.; Delattre, L.; Loyens, A.; Talahari, S.; Bégard, S.; et al. Loss of Tau Protein Affects the Structure, Transcription and Repair of Neuronal Pericentromeric Heterochromatin. Sci. Rep. 2016, 6, 33047. [Google Scholar] [CrossRef]
- Diez, L.; Wegmann, S. Nuclear Transport Deficits in Tau-Related Neurodegenerative Diseases. Front. Neurol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Violet, M.; Delattre, L.; Tardivel, M.; Sultan, A.; Chauderlier, A.; Caillierez, R.; Talahari, S.; Nesslany, F.; Lefebvre, B.; Bonnefoy, E.; et al. A Major Role for Tau in Neuronal DNA and RNA Protection in Vivo under Physiological and Hyperthermic Conditions. Front. Cell. Neurosci. 2014, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Federico, C.; Gil, L.; Bruno, F.; D’Amico, A.G.; D’Agata, V.; Saccone, S. Phosphorylated Nucleolar Tau Protein Is Related to the Neuronal in Vitro Differentiation. Gene 2018, 664, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Biedler, J.L.; Roffler-Tarlov, S.; Schachner, M.; Freedman, L.S. Multiple Neurotransmitter Synthesis by Human Neuroblastoma Cell Lines and Clones. Cancer Res. 1978, 38, 3751–3757. [Google Scholar] [PubMed]
- Leotta, C.G.; Federico, C.; Brundo, M.V.; Tosi, S.; Saccone, S. HLXB9 Gene Expression, and Nuclear Location during in Vitro Neuronal Differentiation in the SK-N-BE Neuroblastoma Cell Line. PLoS ONE 2014, 9, e105481. [Google Scholar] [CrossRef]
- Andres, D.; Keyser, B.M.; Petrali, J.; Benton, B.; Hubbard, K.S.; McNutt, P.M.; Ray, R. Morphological and Functional Differentiation in BE(2)-M17 Human Neuroblastoma Cells by Treatment with Trans-Retinoic Acid. BMC Neurosci. 2013, 14, 49. [Google Scholar] [CrossRef]
- Petry, F.R.; Pelletier, J.; Bretteville, A.; Morin, F.; Calon, F.; Hébert, S.S.; Whittington, R.A.; Planel, E. Specificity of Anti-Tau Antibodies When Analyzing Mice Models of Alzheimer’s Disease: Problems and Solutions. PLoS ONE 2014, 9, e94251. [Google Scholar] [CrossRef]
- Gil, L.; Federico, C.; Pinedo, F.; Bruno, F.; Rebolledo, A.B.; Montoya, J.J.; Olazabal, I.M.; Ferrer, I.; Saccone, S. Aging Dependent Effect of Nuclear Tau. Brain Res. 2017, 1677, 129–137. [Google Scholar] [CrossRef]
- Linhoff, M.W.; Garg, S.K.; Mandel, G. A High-Resolution Imaging Approach to Investigate Chromatin Architecture in Complex Tissues. Cell 2015, 163, 246–255. [Google Scholar] [CrossRef]
- Scuderi, S.; D’Amico, A.G.; Castorina, A.; Federico, C.; Marrazzo, G.; Drago, F.; Bucolo, C.; D’Agata, V. Davunetide (NAP) Protects the Retina Against Early Diabetic Injury by Reducing Apoptotic Death. J. Mol. Neurosci. 2014, 54, 395–404. [Google Scholar] [CrossRef]
- Oliveira, V.C.; Carrara, R.C.V.; Simoes, D.L.C.; Saggioro, F.P.; Carlotti, C.G.; Covas, D.T.; Neder, L. Sudan Black B Treatment Reduces Autofluorescence and Improves Resolution of in Situ Hybridization Specific Fluorescent Signals of Brain Sections. Histol. Histopathol. 2010, 25, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Sturiale, V.; Bruno, F.; Brancato, D.; D’Amico, A.G.; Maugeri, G.; D’Agata, V.; Saccone, S.; Federico, C. Cell Cycle Reactivation, at the Start of Neurodegeneration, Induced by Forskolin and Aniline in Differentiated Neuroblastoma Cells. Int. J. Mol. Sci. 2023, 24, 14373. [Google Scholar] [CrossRef] [PubMed]
- Younas, N.; Saleem, T.; Younas, A.; Zerr, I. Nuclear Face of Tau: An inside Player in Neurodegeneration. Acta Neuropathol. Commun. 2023, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Li, H.; Tian, R.; Nie, C.; Liu, Y.; Han, B.; He, R. Neuronal Tau Induces DNA Conformational Changes Observed by Atomic Force Microscopy. Neuroreport 2004, 15, 2723–2727. [Google Scholar]
- Smith, T.W.; Lippa, C.F. Ki-67 Immunoreactivity in Alzheimerʼs Disease and Other Neurodegenerative Disorders. J. Neuropathol. Exp. Neurol. 1995, 54, 297–303. [Google Scholar] [CrossRef]
- El-Khodor, B.F.; Frances Oo, T.; Kholodilov, N.; Burke, R.E. Ectopic Expression of Cell Cycle Markers in Models of Induced Programmed Cell Death in Dopamine Neurons of the Rat Substantia Nigra Pars Compacta. Exp. Neurol. 2003, 179, 17–27. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, F.; Gil, L.; Sturiale, V.; Guerrero, C.; Rebolledo, A.B.; Brancato, D.; Morales, J.; Saccone, S.; Federico, C. Loss of AT8 Nuclear Tau as a Marker of Neuronal Ageing and Alzheimer’s Disease Progression. Biomedicines 2025, 13, 2587. https://doi.org/10.3390/biomedicines13112587
Bruno F, Gil L, Sturiale V, Guerrero C, Rebolledo AB, Brancato D, Morales J, Saccone S, Federico C. Loss of AT8 Nuclear Tau as a Marker of Neuronal Ageing and Alzheimer’s Disease Progression. Biomedicines. 2025; 13(11):2587. https://doi.org/10.3390/biomedicines13112587
Chicago/Turabian StyleBruno, Francesca, Laura Gil, Valentina Sturiale, Carmen Guerrero, Ana Belen Rebolledo, Desiree Brancato, Javier Morales, Salvatore Saccone, and Concetta Federico. 2025. "Loss of AT8 Nuclear Tau as a Marker of Neuronal Ageing and Alzheimer’s Disease Progression" Biomedicines 13, no. 11: 2587. https://doi.org/10.3390/biomedicines13112587
APA StyleBruno, F., Gil, L., Sturiale, V., Guerrero, C., Rebolledo, A. B., Brancato, D., Morales, J., Saccone, S., & Federico, C. (2025). Loss of AT8 Nuclear Tau as a Marker of Neuronal Ageing and Alzheimer’s Disease Progression. Biomedicines, 13(11), 2587. https://doi.org/10.3390/biomedicines13112587






