Mitral Annular Disjunction and Arrhythmic Risk: Case Series and State of the Art
Abstract
1. Introduction and Definition
2. Case Series
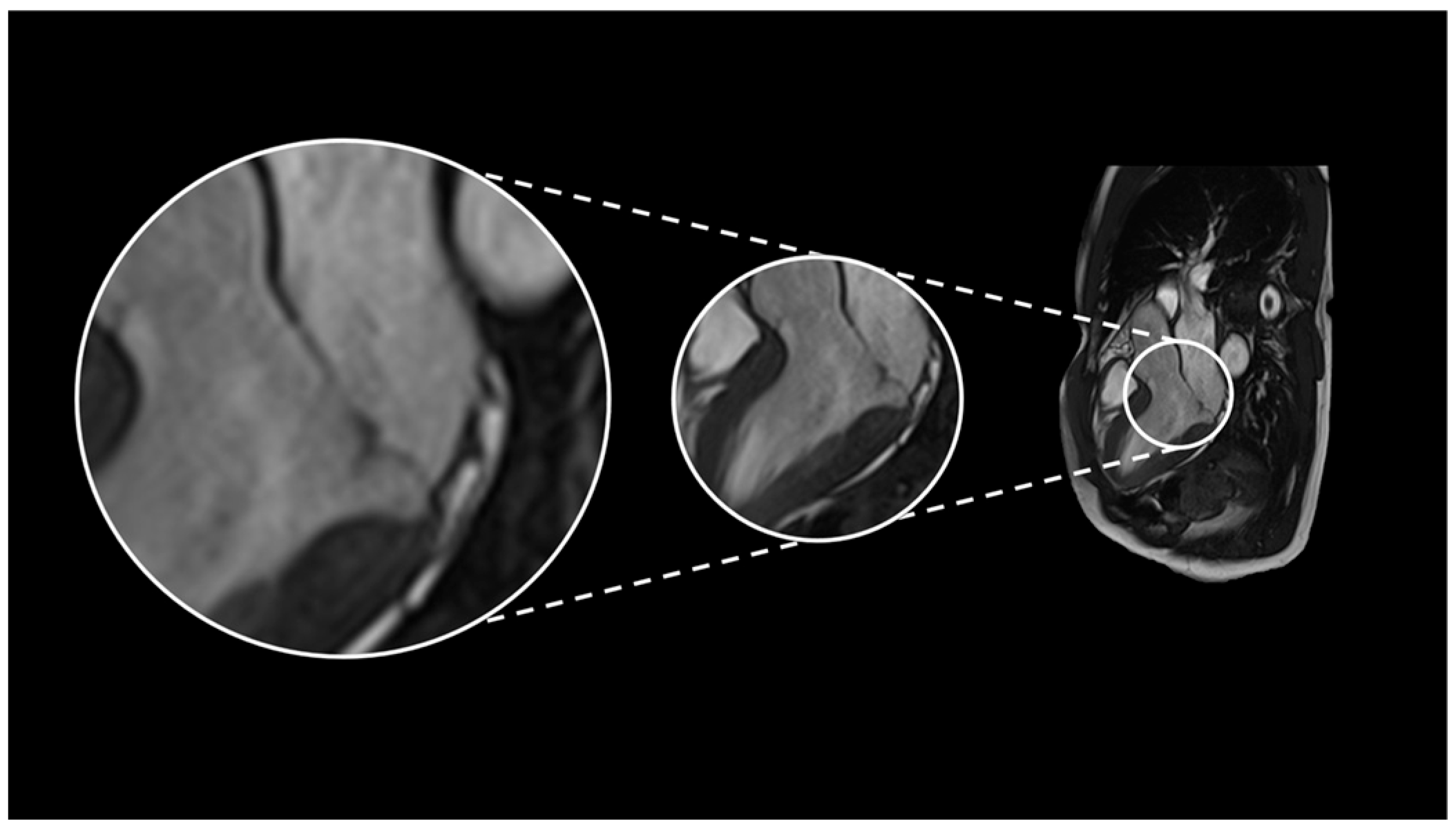
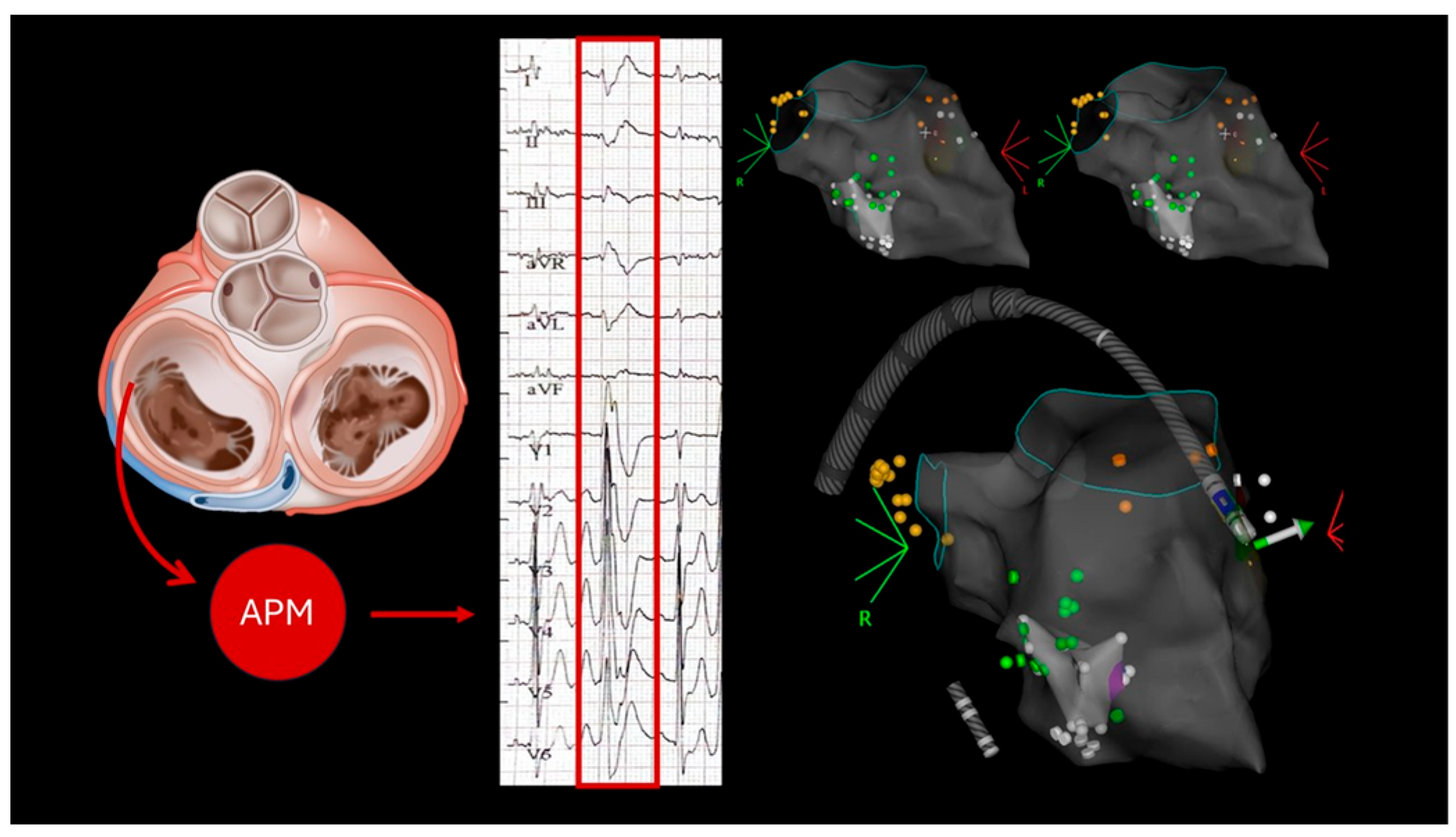
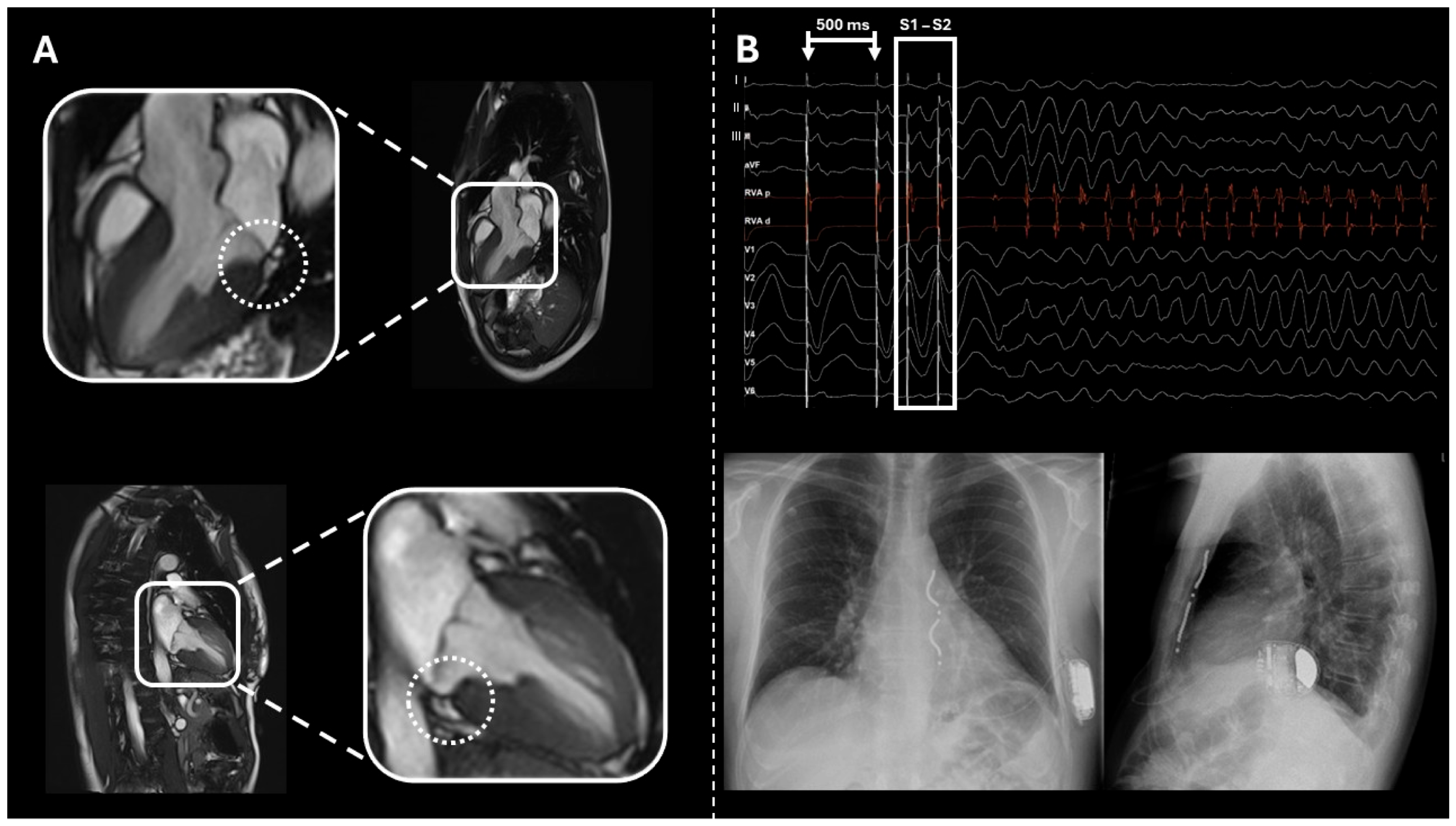
2.1. Case 1
2.2. Case 2
2.3. Case 3
2.4. Case 4
2.5. Case 5
3. Discussion
3.1. Mechanisms of Arrhythmias
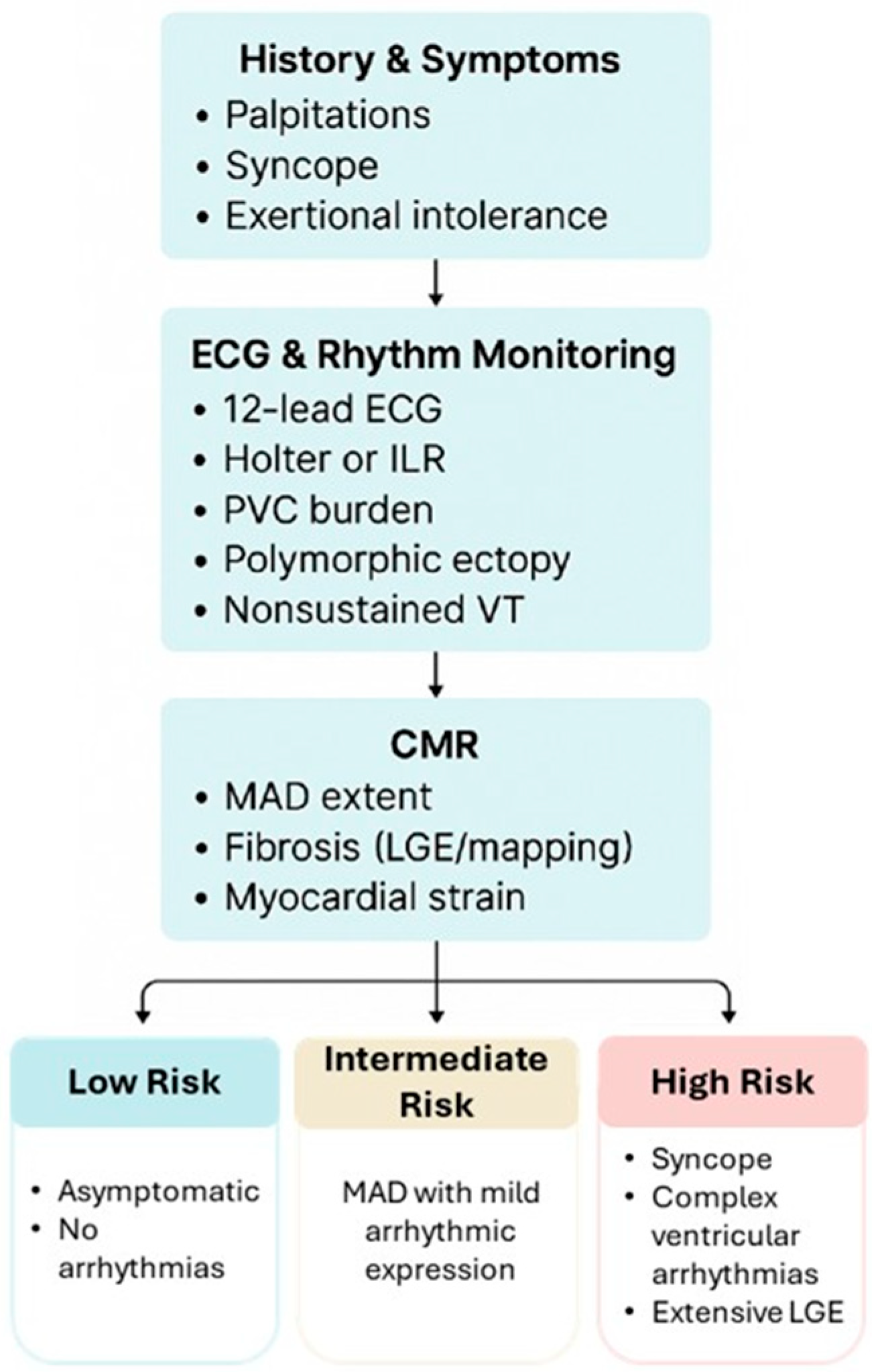
3.2. Arrhythmic Risk Stratification
- -
- Low risk: asymptomatic, short MAD segments, no arrhythmia.
- -
- Intermediate risk: MAD with mild arrhythmic expression.
- -
- High risk: syncope, complex ventricular arrhythmias, or extensive LGE.
3.3. Therapeutic Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMR | Cardiac Magnetic Resonance |
| EPS | Electrophysiological Study |
| LGE | Late Gadolinium Enhancement |
| MAD | Mitral Annular Disjunction |
| MVP | Mitral valve prolapse |
| NSVT | Non-Sustained Ventricular Tachycardia |
| SVT | Sustained Ventricular Tachycardia |
| VF | Ventricular Fibrillation |
| TAD | Tricuspid Annular Disjunction |
References
- Bharati, S.; Granston, A.S.; Liebson, P.R.; Loeb, H.S.; Rosen, K.M.; Lev, M. The conduction system in mitral valve prolapse syndrome with sudden death. Am. Heart J. 1981, 101, 667–670. [Google Scholar] [CrossRef]
- Hutchins, G.M.; Moore, G.W.; Skoog, D.K. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N. Engl. J. Med. 1986, 314, 535–540. [Google Scholar] [CrossRef]
- Toh, H.; Mori, S.; Izawa, Y.; Fujita, H.; Miwa, K.; Suzuki, M.; Takahashi, Y.; Toba, T.; Watanabe, Y.; Kono, A.K.; et al. Prevalence and extent of mitral annular disjunction in structurally normal hearts: Comprehensive 3D analysis using cardiac computed tomography. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 614–622. [Google Scholar] [CrossRef]
- Zugwitz, D.; Fung, K.; Aung, N.; Rauseo, E.; McCracken, C.; Cooper, J.; El Messaoudi, S.; Anderson, R.H.; Piechnik, S.K.; Neubauer, S.; et al. Mitral Annular Disjunction Assessed Using CMR Imaging: Insights From the UK Biobank Population Study. JACC Cardiovasc. Imaging 2022, 15, 1856–1866. [Google Scholar] [CrossRef]
- Melamed, T.; Badiani, S.; Harlow, S.; Laskar, N.; A Treibel, T.; Aung, N.; Bhattacharyya, S.; Lloyd, G. Prevalence, Progression and Clinical Outcomes of Mitral Valve Prolapse: A Systematic Review and Meta-Analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2025, 11, 631–641. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Mahajan, R.; Elliott, A.D.; Haqqani, H.; Lau, D.H.; Vohra, J.K.; Morton, J.B.; Semsarian, C.; Marwick, T.; Kalman, J.M.; et al. Mitral valve prolapse and sudden cardiac death: A systematic review and meta-analysis. Heart 2019, 105, 144–151. [Google Scholar] [CrossRef]
- Aabel, E.W.; Chivulescu, M.; Lie, Ø.H.; Hopp, E.; Gjertsen, E.; Ribe, M.; Helle-Valle, T.M.; Edvardsen, T.; Hegbom, F.; Dejgaard, L.A.; et al. Ventricular arrhythmias in arrhythmic mitral valve syndrome-a prospective continuous long-term cardiac monitoring study. Europace 2023, 25, 506–516. [Google Scholar] [CrossRef]
- DeMaria, A.N.; Amsterdam, E.A.; Vismara, L.A.; Neumann, A.; Mason, D.T. Arrhythmias in the mitral valve prolapse syndrome. Prevalence, nature, and frequency. Ann. Intern. Med. 1976, 84, 656–660. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Yang, L.-T.; Maalouf, J.; Asirvatham, S.; Michelena, H.; Enriquez-Sarano, M. Presentation and Outcome of Arrhythmic Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef]
- Figliozzi, S.; Georgiopoulos, G.; Lopes, P.M.; Bauer, K.B.; Moura-Ferreira, S.; Tondi, L.; Mushtaq, S.; Censi, S.; Pavon, A.G.; Bassi, I.; et al. Myocardial Fibrosis at Cardiac MRI Helps Predict Adverse Clinical Outcome in Patients with Mitral Valve Prolapse. Radiology 2023, 306, 112–121. [Google Scholar] [CrossRef]
- Akyea, R.K.; Figliozzi, S.; Lopes, P.M.; Moura-Ferreira, S.; Tondi, L.; Mushtaq, S.; Censi, S.; Pavon, A.G.; Bassi, I.; Galian, L.; et al. Arrhythmic Mitral Valve Prolapse Phenotype: An Unsupervised Machine Learning Analysis Using a Multicenter Cardiac MRI Registry. Radiol. Cardiothorac. Imaging 2024, 6, e230247. [Google Scholar] [CrossRef]
- Konda, T.; Tani, T.; Suganuma, N.; Nakamura, H.; Sumida, T.; Fujii, Y.; Kawai, J.; Kitai, T.; Kim, K.; Kaji, S.; et al. The analysis of mitral annular disjunction detected by echocardiography and comparison with previously reported pathological data. J. Echocardiogr. 2017, 15, 176–185. [Google Scholar] [CrossRef]
- Bennett, S.; Thamman, R.; Griffiths, T.; Oxley, C.; Khan, J.N.; Phan, T.; Patwala, A.; Heatlie, G.; Kwok, C.S. Mitral annular disjunction: A systematic review of the literature. Echocardiography 2019, 36, 1549–1558. [Google Scholar] [CrossRef]
- Mantegazza, V.; Tamborini, G.; Muratori, M.; Gripari, P.; Fusini, L.; Italiano, G.; Volpato, V.; Sassi, V.; Pepi, M. Mitral Annular Disjunction in a Large Cohort of Patients With Mitral Valve Prolapse and Significant Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 2278–2280. [Google Scholar] [CrossRef]
- Putnam, A.J.; Kebed, K.; Mor-Avi, V.; Rashedi, N.; Sun, D.; Patel, B.; Balkhy, H.; Lang, R.M.; Patel, A.R. Prevalence of mitral annular disjunction in patients with mitral valve prolapse and severe regurgitation. Int. J. Cardiovasc. Imaging 2020, 36, 1363–1370. [Google Scholar] [CrossRef]
- Dejgaard, L.A.; Skjølsvik, E.T.; Lie, Ø.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The Mitral Annulus Disjunction Arrhythmic Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef]
- Verheul, L.M.; Guglielmo, M.; Groeneveld, S.A.; Kirkels, F.P.; Scrocco, C.; Cramer, M.J.; Bootsma, M.; Kapel, G.F.L.; Alings, M.; Evertz, R.; et al. Mitral annular disjunction in idiopathic ventricular fibrillation patients: Just a bystander or a potential cause? Eur. Heart J. Cardiovasc. Imaging 2024, 25, 764–770. [Google Scholar] [CrossRef]
- Gilbert, B.W.; Schatz, R.A.; VonRamm, O.T.; Behar, V.S.; Kisslo, J.A. Mitral valve prolapse. Two-dimensional echocardiographic and angiographic correlation. Circulation 1976, 54, 716–723. [Google Scholar] [CrossRef]
- Marra, M.P.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef]
- Basso, C.; Marra, M.P.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Haikal, M.; Alpert, M.A.; Whiting, R.B.; Kelly, D. Increased left ventricular mass in idiopathic mitral valve prolapse. Chest 1982, 82, 329–333. [Google Scholar] [CrossRef]
- Muthukumar, L.; Jahangir, A.; Jan, M.F.; Perez Moreno, A.C.; Khandheria, B.K.; Tajik, A.J. Association Between Malignant Mitral Valve Prolapse and Sudden Cardiac Death: A Review. JAMA Cardiol. 2020, 5, 1053–1061. [Google Scholar] [CrossRef]
- Buonocunto, M.; Lyon, A.; Delhaas, T.; Heijman, J.; Lumens, J. Electrophysiological effects of stretch-activated ion channels: A systematic computational characterization. J. Physiol. 2024, 602, 4585–4604. [Google Scholar] [CrossRef]
- Naksuk, N.; Syed, F.F.; Krittanawong, C.; Anderson, M.J.; Ebrille, E.; DeSimone, C.V.; Vaidya, V.R.; Ponamgi, S.P.; Suri, R.M.; Ackerman, M.J.; et al. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol. J. 2016, 16, 187–191. [Google Scholar] [CrossRef]
- Hourdain, J.; Clavel, M.A.; Deharo, J.-C.; Asirvatham, S.; Avierinos, J.F.; Habib, G.; Franceschi, F.; Probst, V.; Sadoul, N.; Martins, R.; et al. Common Phenotype in Patients With Mitral Valve Prolapse Who Experienced Sudden Cardiac Death. Circulation 2018, 138, 1067–1069. [Google Scholar] [CrossRef]
- Kaya, Ü.; Eren, H. Fragmented QRS may be associated with complex ventricular arrhythmias in mitral valve prolapse. Minerva Cardioangiol. 2020, 68, 577–585. [Google Scholar] [CrossRef]
- Sabbag, A.; Essayagh, B.; Barrera, J.D.R.; Basso, C.; Berni, A.; Cosyns, B.; Deharo, J.-C.; Deneke, T.; Di Biase, L.; Enriquez-Sarano, M.; et al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed cby the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace 2022, 24, 1981–2003. [Google Scholar]
- Groeneveld, S.A.; Kirkels, F.P.; Cramer, M.J.; Evertz, R.; Haugaa, K.H.; Postema, P.G.; Prakken, N.H.J.; Teske, A.J.; Wilde, A.A.M.; Velthuis, B.K.; et al. Prevalence of Mitral Annulus Disjunction and Mitral Valve Prolapse in Patients With Idiopathic Ventricular Fibrillation. J. Am. Heart Assoc. 2022, 11, e025364. [Google Scholar] [CrossRef]
- Carmo, P.; Andrade, M.J.; Aguiar, C.; Rodrigues, R.; Gouveia, R.; Silva, J.A. Mitral annular disjunction in myxomatous mitral valve disease: A relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc. Ultrasound 2010, 8, 53. [Google Scholar] [CrossRef]
- Bui, A.H.; Roujol, S.; Foppa, M.; Kissinger, K.V.; Goddu, B.; Hauser, T.H.; Zimetbaum, P.J.; Ngo, L.H.; Manning, W.J.; Nezafat, R.; et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017, 103, 204–209. [Google Scholar] [CrossRef]
- Daza, A.R.; Chokshi, A.; Pardo, P.; Maneiro, N.; Contreras, A.G.; Larrañaga-Moreira, J.M.; Ibañez, B.; Fuster, V.; Friera, L.F.; Solís, J.; et al. Mitral valve prolapse morphofunctional features by cardiovascular magnetic resonance: More than just a valvular disease. J. Cardiovasc. Magn. Reson. 2021, 23, 107. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Sherrid, M.V.; Haas, T.S.; Lindberg, J.; Kitner, C.; Lesser, J.R. Novel hypertrophic cardiomyopathy phenotype: Segmental hypertrophy isolated to the posterobasal left ventricular free wall. Am. J. Cardiol. 2010, 106, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Nerlekar, N.; Haugaa, K.H.; Edvardsen, T.; Marwick, T.H. Prediction of Ventricular Arrhythmias With Left Ventricular Mechanical Dispersion: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2020, 13, 562–572. [Google Scholar] [CrossRef]
- Ermakov, S.; Gulhar, R.; Lim, L.; Bibby, D.; Fang, Q.; Nah, G.; Abraham, T.P.; Schiller, N.B.; Delling, F.N. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart 2019, 105, 1063–1069. [Google Scholar] [CrossRef]
- Tayal, B.; Delling, F.N.; Malahfji, M.; Shah, D.J. Cardiac Imaging for Risk Assessment of Malignant Ventricular Arrhythmias in Patients With Mitral Valve Prolapse. Front. Cardiovasc. Med. 2021, 8, 574446. [Google Scholar] [CrossRef]
- Disse, S.; Abergel, E.; Berrebi, A.; Houot, A.-M.; Le Heuzey, J.-Y.; Diebold, B.; Guize, L.; Carpentier, A.; Corvol, P.; Jeunemaitre, X. Mapping of a first locus for autosomal dominant myxomatous mitral-valve prolapse to chromosome 16p11.2-p12.1. Am. J. Hum. Genet. 1999, 65, 1242–1251. [Google Scholar] [CrossRef]
- Nesta, F.; Leyne, M.; Yosefy, C.; Simpson, C.; Dai, D.; Marshall, J.E.; Hung, J.; Slaugenhaupt, S.A.; Levine, R.A. New locus for autosomal dominant mitral valve prolapse on chromosome 13, clinical insights from genetic studies. Circulation 2005, 112, 2022–2030. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar]
- Syed, F.F.; Ackerman, M.J.; McLeod, C.J.; Kapa, S.; Mulpuru, S.K.; Sriram, C.S.; Cannon, B.C.; Asirvatham, S.J.; Noseworthy, P.A. Sites of Successful Ventricular Fibrillation Ablation in Bileaflet Mitral Valve Prolapse Syndrome. Circ. Arrhythmia Electrophysiol. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
| Age | Gender | Symptoms | ECG | Arrhythmias | CMR | MVP | Mitral Regurgitation | LVEF | Therapy | EPS |
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | M | Pre-Syncope after exercise | SVT |
| Present | MODERATE | 55% | Bisoprolol | Performed, negative | |
| 58 | M | Syncope | Negative T waves in inferior leads | - NSVT |
| Present | MILD | 55% | No | Not performed |
| 43 | F | - |
| - NSVT |
| Present | MILD | 44% | Bisoprolol | Not performed |
| 34 | F | - | Spontaneous type 1 Brugada pattern | - None |
| Present | MILD | 55% | No | Performed, positive for VF |
| 70 | M | - | - SVT |
| Present | MILD | 50% | No | Performed, Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varrenti, M.; Bonvicini, E.; Milillo, L.F.; Garofani, I.; Gigli, L.; Baroni, M.; Preda, A.; Carbonaro, M.; Menè, R.; Colombo, G.; et al. Mitral Annular Disjunction and Arrhythmic Risk: Case Series and State of the Art. Biomedicines 2025, 13, 2589. https://doi.org/10.3390/biomedicines13112589
Varrenti M, Bonvicini E, Milillo LF, Garofani I, Gigli L, Baroni M, Preda A, Carbonaro M, Menè R, Colombo G, et al. Mitral Annular Disjunction and Arrhythmic Risk: Case Series and State of the Art. Biomedicines. 2025; 13(11):2589. https://doi.org/10.3390/biomedicines13112589
Chicago/Turabian StyleVarrenti, Marisa, Eleonora Bonvicini, Leandro Fabrizio Milillo, Ilaria Garofani, Lorenzo Gigli, Matteo Baroni, Alberto Preda, Marco Carbonaro, Roberto Menè, Giulia Colombo, and et al. 2025. "Mitral Annular Disjunction and Arrhythmic Risk: Case Series and State of the Art" Biomedicines 13, no. 11: 2589. https://doi.org/10.3390/biomedicines13112589
APA StyleVarrenti, M., Bonvicini, E., Milillo, L. F., Garofani, I., Gigli, L., Baroni, M., Preda, A., Carbonaro, M., Menè, R., Colombo, G., Frontera, A., Falco, R., Giordano, F., Vargiu, S., Guarracini, F., Pedrotti, P., Giannattasio, C., & Mazzone, P. (2025). Mitral Annular Disjunction and Arrhythmic Risk: Case Series and State of the Art. Biomedicines, 13(11), 2589. https://doi.org/10.3390/biomedicines13112589








