In Vivo Models of Cardiovascular Disease: Drosophila melanogaster as a Genetic Model of Congenital Heart Disease
Abstract
1. Introduction
2. Drosophila melanogaster
2.1. Arthropod Cardiovascular Systems
2.2. Anatomy and Histology of the Dorsal Vessel
2.3. Growth of the Dorsal Vessel During Development

3. Cardiac Gene Regulatory Networks During Development
4. Drosophila melanogaster and Congenital Heart Disease
4.1. Evolution of the Heart
4.2. Homology Between Drosophila melanogaster and Homo sapiens
4.3. Drosophila melanogaster and Models of Congenital Heart Disease
4.3.1. Methods for the Evaluation of Gene Function in D. melanogaster
4.3.2. Genes Involved in the Cardiac Gene Regulatory Networks: Mutations and Phenotypes
| Gene | Ortholog | DIOPT Score | Congenital Heart Defect | Reference |
|---|---|---|---|---|
| msh-2 | MSX1 | 16 | VSD | [222] |
| MSX2 | Dextrocardia, dextroversion, and PFO; radial agenesis with Hunter McAlpine syndrome (mental retardation, craniofacial and skeletal abnormalities, characteristic facial attributes) | [223] | ||
| tin | NKX2.5 | 5 | VSD, ASD, HLHS | [10,187] |
| tup | ISL1, ISL2 | 16 | DORV in combination with VSD (heterozygous mutations) | [197] |
| H15 | TBX20 | 11 | DORV, VSD, ASD, TOF, PTA, PFO, BAV, MVP/MR, total anomalous pulmonary venous connection, congenital atrioventricular block, HLHS | [203,205] |
| mid | 13 | |||
| doc1, doc2, doc3 | TBX6 | 10 | Pulmonary atresia with VSD (severe form of TOF) | [210] |
| TBX2 | TOF, single ventricle, single atrium | [212] | ||
| TBX3 | TOF, transposition of the great arteries | [212] | ||
| svp | NR2F2 | 13 | DORV, VSD, ASD, TOF, PDA, BAV | [217] |
| Eve | EVX1 | 10 | Defects in limb development | [213,214] |
| EVX2 | EVX1 and EVX2 have not yet been associated with congenital heart defects in H. sapiens | |||
| Hand | HAND2 | 15 | DORV, VSD, pulmonary stenosis, outflow tract malformations | [214,224] |
| D-mef2 | MEF2A, MEF2C | 13 | DORV, VSD, PDA, pulmonary atresia with VSD | [225,226,227,228] |
4.3.3. Genes Involved in Cellular Metabolism and Protein Synthesis/Trafficking: Mutations and Phenotypes
4.3.4. Genes Involved in Cardiac Progenitor Migration, Alignment, and Dorsal Vessel Assembly During Drosophila melanogaster Embryonic Development: Mutations and Phenotypes
4.3.5. Genes Involved in the Establishment of Segmentation and Polarity During Drosophila melanogaster Embryonic Development: Mutations and Phenotypes
4.3.6. Genes Involved in the Formation of the Animal Body Plan During Drosophila melanogaster Embryonic Development: Mutations and Phenotypes
4.3.7. Genes Involved in Histone Modification During Drosophila melanogaster Embryonic Development: Mutations and Phenotypes
| Gene | D. melanogaster Model Defect | Ortholog | DIOPT Score | Vertebrate Model Defect/ Congenital Heart Defect | Study (Reference) |
|---|---|---|---|---|---|
| Abd-A | Abd-A deficiency associated with loss of heart chamber and cardiac cardioblast identity, reduction in posterior dorsal vessel (heart chamber) diameter now similar to the anterior dorsal vessel (aorta), absence of cellular dimorphism between anterior (aorta) and posterior dorsal vessel (heart chamber) with smaller volume cells present throughout | HOXB6, HOXC6, HOXA6 | 5 (4.87) | Combined deletions in HOXA, HOXB clusters generally associated with defects in cardiac looping and appearance of primitive/atavistic heart morphologies [110] (mouse) | Lo et al., 2002 [45], Lovato et al., 2002 [47], Ponzielli et al., 2002 [309], Perrin et al., 2004 [308], Ryan et al., 2005 [313], Monier et al., 2005 [46], LaBeau et al., 2009 [311] |
| HOXB6 variants associated with thoracic aortic dissection; HOXA5, HOXB6, HOXC6 may correlate with vascular smooth muscle cell de-differentiation in these cases [336] (human) | |||||
| Abd-A overexpression/ectopic expression induces a cardiac identity in the anterior dorsal vessel | HOXA6 has not yet been specifically associated with cardiac development or congenital heart defects [337] | ||||
| Abd-B | Abd-B deficiency associated with increase in posterior dorsal vessel (heart chamber) diameter; increase in cardioblast number with disorganization in their arrangement; and dilation of heart terminus (A6-A8); Abd-B deficiency also rescues the Nacα KD-induced “No-heart” phenotype | HOXA10 | 6 (6.01) | Combined deletions in HOXA, HOXB clusters generally associated with defects in cardiac looping and appearance of primitive/atavistic heart morphologies [110] (Mouse) | Lo et al., 2002 [45], Lovato et al., 2002 [47], Perrin et al., 2004 [308], Schroeder et al., 2022 [312] |
| HOXA10 misexpression/overexpression early during embryoid body development restricts specification to a cardiac lineage and impairs differentiation of NKX2.5 expressing progenitor cells into differentiated cardiomyocytes [338] (in vitro models) | |||||
| Abd-B overexpression/ectopic expression associated with suppression of cardiac morphogenesis and myogenesis and defects in somatic muscle formation | HOXA10 has not yet been specifically associated with congenital heart defects [337] | ||||
| Antp | Antp deficiency associated with mild defects in cardioblast differentiation in segment A1 | HOXA7, HOXA1, HOXA3 | 9 (8.94) | Combined deletions in HOXA, HOXB clusters generally associated with defects in cardiac looping and appearance of primitive/atavistic heart morphologies [110] (mouse) | Lo et al., 2002 [45], Perrin et al., 2004 [308] |
| HOXA1 mutations associated with defects in brainstem, ventilation, inner ear, and craniofacial morphology, along with cardiac malformations, including TOF, interrupted aortic arch, and aberrant subclavian artery [316] (mouse) | |||||
| HOXB1 mutations associated with VSD, shorter outflow tract, upregulation of FGF/ERK, BMP/SMAD in the pharyngeal region, premature myocardial differentiation [318] (mouse) | |||||
| HOXA3 mutations associated with defects in the 3rd pharyngeal artery (carotid artery system), thyroid and parathyroid glands, and carotid body morphology [339] (mouse) | |||||
| HOXA1 (homozygous mutations) associated with Athabascan Brainstem Dysgenesis, Bosley–Salih–Alorainy Syndrome (defects in brainstem, inner ear, cognitive function, and cardiac malformations) [316,317] (human) | |||||
| HOXA3 loss due to 5.6 Mb deletion at chromosome 7p15.1–p15.3 associated with defects in facial, hand–foot morphology, supernumerary nipples, hypospadias, and hearing defects; hand–foot and genital defects associated with HOXA13 deletion in the same locus [340] (human) | |||||
| HOXA7 has not yet been specifically associated with cardiac development or congenital heart defects [337] | |||||
| apoLpp | apoLpp absence associated with cardiac arrhythmia | APOB, LOC400499 | 3 (2.88, 2.82) | APOB mutations reduce cardiomyocyte proliferation due to an upregulation of cell cycle inhibitors and pro-apoptotic factors and downregulation of cell cycle genes (in vitro models) | Theis et al., 2020 [230] |
| APOB mutation associated with a case presenting with cleft lip and palate, DORV, dextrocardia, transposition of the great arteries and hypoplastic right ventricle, along with multisystem defects in the thyroid, nervous system, and eyes though direct association with a causative pathway has been made [341]; maternal dysregulation in lipid profiles; APOB expression associated with higher rates of congenital heart defects in offspring (VSD, TOF, pulmonary valve stenosis) [342] (human) | |||||
| Apt | Apt mutations associated with late embryonic/early larval stage lethality, abnormal dorsal vessel morphology with absent cardioblast/pericardial cells | N/A | N/A | N/A | Su et al., 1999 [343], Liu et al., 2014 [344] |
| Ash1 | Ash1 mutations associated with disruption in physiologic heart function, with disruption in actin filament arrangement; reduction in cardiac myofibril density; increase in pericardin (cardiac fibrosis); increase in systolic diameter and heart period; adult lethality (Adult) | ASH1L | 14 (13.69) | AH1L knockdown associated with reduced expression of genes such as HOXA6, HOXA10 [345] (in vitro models) | J. Zhu et al., 2023 [327] |
| ASH1L variants associated with defects in coronary vascular branching and single left coronary arteries [346,347,348] (human) | |||||
| Ash2 | Ash2 mutations associated with disruption in physiologic heart function, with disruption in actin filament arrangement; reduction in cardiac myofibril density, cardioblast numbers, and increase in pericardin (cardiac fibrosis); increase in systolic diameter and heart period; adult lethality (adult) | ASH2L | 17 (16.75) | Absence of ASH2L leads to early embryonic lethality; interaction with TBX1 may act as a modulating factor for DiGeorge-like syndrome phenotypes (craniofacial defects, immune dysfunction and cardiac defects) [349] (mouse) | Zhu et al., 2024 [328] |
| ASH2L has not yet been directly associated with congenital heart defects in H. sapiens (human) | |||||
| bab2 | Bab2 mutations associated with disruption in the localization of eve+ pericardial cell groups | BTBD18 | 4 (3.91) | BTBD18 has not yet been associated with congenital heart defects in H. sapiens [214] (human) | Junion et al., 2007 [130], Couderc et al., 2002 [128] |
| bic | bic knockdown throughout development associated with reduction in systolic and diastolic diameter (Embryo), ectopic Abd-B expression during metamorphosis leading to aberrant histolysis, leading to ‘No-heart’ phenotype with absent pericardin, cardiac cell dispersal, and fat cell accumulation (Pupa, Adult); bicaudal phenotype with embryo developing with a mirror image duplication of the posterior axis (embryo) | BTF3, BTF3L4 | 15 (14.87, 14.80) | BTF3, BTF3L4 have not yet been associated with cardiac development or congenital heart defects | Schroeder et al., 2022 [312] |
| bifid (also known as omb) | bifid mutations associated with embryonic lethality; human TBX2, TBX2-R20Q, TBX2-R305H variants cannot rescue bifid mutation phenotypes in D. melanogaster | TBX2, TBX3 | 12 (11.89, 11.85) | Mutations associated with postnatal lethality with craniofacial defects (double heterozygous loss for TBX2, TBX3); lack of constriction between left atrium and left ventricle (atrioventricular canal) [211]; atrioventricular canal defects; pericardial edema, defects in palate and limb development (mouse) | Liu et al., 2018 [127] |
| TBX2 variants associated with TOF, single ventricle, single atrium (human) | |||||
| TBX3 variants associated with TOF and transposition of the great arteries [212] (human) | |||||
| Bre1 | Bre1 mutations associated with reduction in cardiac myofibril density and adult lethality (adult) | RNF40 | 16 (15.74) | RNF20, RNF40 knockdown results in defects in ciliogenesis at the left–right organizer and as a result in left–right patterning; defects in cardiac looping [350] (frog) | Zhu et al., 2017 [35] |
| RNF20, RNF40 deletion (mosaic deletion) results in defects in cardiomyocyte maturation [351] (mouse) | |||||
| RNF40 variants associated with HLHS [352] (human) | |||||
| Cdc42 | Cdc42 mutations associated with disruption in myofibril arrangement; disruption in physiologic heart function with increase in diastolic interval; cardiac arrhythmia (adult) | CDC42 | 12 (12) | CDC42 mutations/loss associated with embryonic lethality, reduced cardiac growth with small ventricles (including right ventricle hypoplasia [353]), and enlarged right atrium; deep apical cleft between adjacent ventricular walls; thin ventricular walls with VSD; reduction in the thickness of compact myocardium; reduced cardiomyocyte proliferation throughout; defects in cardiomyocyte cell-to-cell adhesion; disruption in N-cadherin and β-catenin localization within cardiomyocytes [354]; defects in outflow tract septation and aortic arch patterning; craniofacial defects and thymus aplasia; impairment of normal cardiac neural crest cell migration (regulated by BMP2) [355] (mouse) | Qian et al., 2011 [260], Voglet et al., 2014 [251] |
| CDC42 variants/mutations associated with multisystem congenital defects, including cardiac defects such as VSD, ASD, PDA, and PFO; total anomalous pulmonary venous return; coarctation of the aorta; and pulmonary stenosis [356] (human) | |||||
| CG10585 | CG10585 mutations associated with disruption in physiologic heart function with increase in systolic and diastolic diameter | PDSS2 | 16 (15.8) | PDSS2 mutations associated with coQ10 deficiency and defects in the mitochondrial respiratory chains; increase in reactive oxygen species; oxidative stress in some tissues, such as the kidneys, leading to renal failure [357] (mouse) | Schroeder et al., 2019 [233] |
| PDSS2 variants associated with nephrotic syndrome and hypertrophic cardiomyopathy in infants [358]; may contribute to more severe phenotypes in congenital heart defects (human) | |||||
| CG10984 | CG10984 mutations associated with disruption in myofibril arrangement | ANKRD12 | 9 (8.85) | ANKRD12 overexpression associated with defects in the sinus venosus; defects in cardiac rotation; anomalous communications between venous and arterial circulations; defects in the fossa ovalis [359] (mouse) | Schroeder et al., 2019 [233] |
| CG2658 | CG2658 mutations associated with disruption in actin filament and myofibril arrangement | SPG7 | 12 (12.06) | Constitutive activation of SPG7 associated with constitutive activation of a mitochondrial mAAA protease; upregulating ATP and reactive oxygen species production and eventually upregulating cell proliferation [360] (in vitro models) | Schroeder et al., 2019 [233] |
| SPG7 variants associated with atrioventricular canal defects (human) | |||||
| D-mef2 | D-mef2 loss causes absence of cardiac, somatic, and visceral muscle differentiation | MEF2C, MEF2A | 13 (12.96, 12.86) | MEF2C, MEF2A mutations (homozygous loss) associated with failure of cardiac looping, failure of right ventricle development (mouse) | Lilly et al., 1995 [239], Hu et al., 2011 [113], Lin et al., 1997 [361] |
| DORV [225], VSD [226], PDA [227], pulmonary atresia with VSD [228] (human) | |||||
| Dap160 | Dap160 mutations associated with minimal effects on actin filament arrangement | ITSN1 | 16 (15.8) | ITSN1 mutations associated with ASD, 21q deletion syndrome (craniofacial dysmorphias, developmental delay, behavior abnormalities, and various systemic manifestations) [362]; congenital heart defects associated with Down syndrome (partial Trisomy 21 phenotype) [363] (Human) | Schroeder et al., 2019 [233] |
| dChchd3/6 | dChchd3/6 mutations associated with disruption in physiologic heart function with increase in systolic diameter and systolic dysfunction; cardiac arrhythmia; disruption in cell energy production | CHCHD3, CHCHD6 | 11 (6) | CHCHD3, CHCHD6 mutations reduce cardiomyocyte proliferation; rate of oxygen consumption after oligomycin-induced inhibition of ATP synthase; levels of sarcomeric F-actin (in vitro models) | Birker et al., 2023 [237] |
| CHCHD, CHCHD6 variants enriched in HLHS (human) | |||||
| dMnM | dMnM mutations associated with variable effects on heart structure with cardiac dilation (mild knockdown) and cardiac constriction (strong knockdown) if knockdown cardiac-specific; reduction in survival of adult animals with defects in locomotion if knockdown muscle-specific | TTN | 4 (3.81) | MYOM2, TTN variants associated with TOF (human) | Auxerre-Plantié et al., 2020 [152] |
| doc1 doc2 doc3 | doc mutations/loss associated with early embryonic lethality | TBX6, TBX2, TBX3 | 10 (9.88) | TBX6 associated with defects in mesoderm development, including defects in somite development and skeletal muscle formation [364]; TBX6 is involved in the pathological cardiac hypertrophy response in adult individuals [365] (mouse) | Han and Olson, 2005 [78] |
| Deletion in the genomic locus containing TBX6 associated with pulmonary atresia with ventricular septal defect, a severe form of TOF [210] | |||||
| Mutations associated with postnatal lethality with craniofacial defects (double heterozygous loss for TBX2 and TBX3); TBX2 mutations associated with lack of constriction between left atrium and left ventricle (atrioventricular canal) [211]; atrioventricular canal defects and defects in outflow tract septation [208]; pericardial edema; defects in palate and limb development (mouse) | |||||
| TBX2 variants associated with TOF, single ventricle, single atrium [212] (human) | |||||
| TBX3 variants associated with TOF and transposition of the great arteries [212] (human) | |||||
| Dpp | Dpp mutations/overexpression associated with expansion of pericardial cells into the ventral region of the dorsal mesoderm with disruption of normal gene marker expression in cardioblast/pericardial cell groups | BMP2 | 12 (11.84) | Loss of BMP2 leads to reduced cardiac jelly tissue, defects in atrioventricular canal morphogenesis, and loss of atrioventricular canal endocardial cushion cellularization (absent epithelial-to-mesenchymal transition) [366]; DORV; VSD; atrioventricular canal defects [367] (mouse) | Lockwood and Bodmer, 2002 [268], Johnson et al., 2007 [267] |
| VSD, ASD, TOF [367] (human) | |||||
| Dpy-30L1 | Dpy-30L1 mutations associated with disruption in physiologic heart function, with disruption in actin filament arrangement; reduction in cardiac myofibril density, cardioblast numbers, and increase in pericardin (cardiac fibrosis); increase in systolic diameter and heart period; adult lethality (adult) | DPY30 | 10 (9.95) | DPY30 has not yet been directly associated with congenital heart defects | Zhu et al., 2024 [328] |
| Dscam | Dscam associated with variable defects in leading-edge, ranging from reduction in migration velocity and reduction in filopodia per segment to reduction in leading-edge lamellipodial activity (Embryo); overexpression associated with an increase in heart failure rate after electrical-pacing-induced stress (Adult) | DSCAM | 12 (12.01, 11.84) | DSCAM mutations/overexpression due to increased gene dose associated with septal defects in both the perimembranous regions and the muscular regions; defects in the outflow tracts, including failure of outflow tract septation into pulmonary arterial and aortic trunks, DORV, and defects in atrioventricular canal morphogenesis and atrioventricular canal defects; atrial and atrioventricular canal defects may be due to defects in the myocardial tissue that contributes to their development, along with loss of WNT signaling that downregulates cardiac mesoderm progenitor proliferation in the inflow tract [368] (mouse) | Grossman et al., 2011 [369], Raza and Jacobs, 2016 [370] |
| DSCAM variants/overexpression due to increased gene dose associated with the emergence of congenital heart defects associated with Down syndrome (VSD, ASD, atrioventricular canal defects, TOF, PDA) [371] (human) | |||||
| EcR | EcR mutations associated with inhibition of cardiac remodeling in the posterior dorsal vessel (heart chamber); Dorsal vessel maintains larval morphology with absence of histolysis in segments A6-A7; and absence of remodeling in Abd-A+ cardioblasts | NR1H2, NR1H3 | 12 (11.88, 11.7) | NR1H2, NR1H3 have not yet been associated with cardiac development or congenital heart defects | Monier et al., 2005 [46] |
| Egfr | Egfr mutations associated with disruption of relative cardioblast/pericardial cell subpopulations with reduction in generic cardioblast populations and increase in ostial cardioblast populations | ERBB4 | 13 (12.87) | ERBB4 mutations associated with embryonic lethality; cardiac defects including reduced trabeculation (hypotrabeculation) with thin myocardial walls and defects in endocardial cushion formation [372]; dysregulation of valve mesenchyme proliferation [373] (Mouse) | Schwarz et al., 2018 [86] |
| ERBB4 variants associated with defects in the development of the left ventricular outflow tract, including aortic stenosis, HLHS [374], and HRHS [375]; coarctation of the aorta [374]; increased rate of bioprosthetic aortic valve stenosis associated with local foreign tissue reaction [376] (human) | |||||
| Eve | Eve mutations associated with reduction in pericardial cell populations | EVX2 | 10 (10.04) | EVX2 mutations associated with defects in limb development, although they have not yet been associated with congenital heart defects [213,214] (human) | Fujioka et al., 2005 [189] |
| fz | fz mutations associated with defects in endoderm (midgut), mesoderm, and ectoderm (cuticle, wings, wing imaginal disks); absence of cardiac development if both fz and Dfz2 | FZD1, FZD2 | 15 (14.87) | FZD mutations associated with multiple effects during development, including neural tube defects [377] (frog) | Bhanot et al., 1999 [275], Chen and Struhl, 1999 [276] |
| FZD1, FZD2 mutations associated with defects in palate closure, ventricular septum, correct position of the outflow tract, neural tube defects, and inner ear defects [378] (mouse) | |||||
| FZD1, FZD2 have not yet been directly associated with congenital heart defects in H. sapiens (human) | |||||
| Gart | Gart mutations associated with minimal effects on actin filament arrangement, with disruption in myofibril arrangement | GART | 17 (16.75) | GART mutations associated with ASD, 21q deletion syndrome (craniofacial dysmorphias, developmental delay, behavior abnormalities, and various systemic manifestations) [362]; congenital heart defects associated with Down syndrome [379] (human) | Schroeder et al., 2019 [233] |
| GGPPS | GGPPS mutations and relevant pathway protein mutations associated with “Broken-hearted” phenotype with dissociation of cardioblast/pericardial cell adhesion; embryonic lethality | GGPS1 | 16 (15.72) | GGPS1 mutations may be a cause of reduction in GGPP, in turn leading to reduced binding affinity of Rho GTPases for GTP, disrupt their localization below the plasma membrane, leading to vascular destabilization and the progressive dilatation and rupture of cerebral vessels [380] (zebrafish) | Yi et al., 2006 [229] |
| Infantile hemangioma [381]; cerebral cavernous malformations [382] due to disruption in the mevalonate pathway (human) | |||||
| Gia | Gia mutations associated with “Broken-hearted” phenotype with dissociation between cardioblast/pericardial cells, disruption in cell-to-cell adhesion protein distribution, and disruption in cardioblast alignment; late embryonic/early larval stage lethality | ADGRF3, ADGRF4, ADGRD1, ADGRE2, ADGRG3, ADGRG6, ADGRL1, ADGRG7, ADGRF5, ADGRD2, ADGRG2, ADGRE1, ADGRE5, ADGRG4, ADGRL4, ADGRL2, ADGRE3, ADGRL3 | 2 (1.81) | ADGRG6 mutations secondary to placental defects; global inactivation of ADGRG6 associated with embryonic lethality and ventricular myocardium thinning, with no effect on heart patterning or myocardium maturation [383] (mouse) | Patel et al., 2016 [384] |
| ADGRG6 mutations secondary to placental defects; mutations in ADGRG6 have no effect on cardiac development [383] (zebrafish) | |||||
| Combined ADGRF5, ADGRL4 mutations associated with DORV; outflow tract malformations; and aortic arch artery defects, including double aortic arch, embryonic lethality, postnatal renal thrombotic microangiopathy, hemolysis, and splenomegaly [385] (mouse) | |||||
| ADGRL2 mutations/loss associated with defects in vascular remodeling [386] (zebrafish) (mouse) | |||||
| ADGRF3, ADGRF4, ADGRD1, ADGRE2, ADGRG3, ADGRL1, ADGRG7, ADGRD2, ADGRG2, ADGRE1, ADGRE5, ADGRG4, ADGRL4, ADGRE3, and ADGRL3 have not yet been associated with cardiac development or congenital heart defects; ADGRF4 associated with enamel mineralization [387]; ADGRL1 implicated in neurodevelopmental disorders [388]; ADGRG7 implicated in familial endometriosis [389]; ADGRG2 implicated in congenital bilateral absence of the vas deferens [390]; ADGRL4 involved in vascular remodeling during development [385]; ADGRL3 involved in neurogenesis [391] | |||||
| H15 (nmr1) | H15 mutations associated with disruption in cardioblast/pericardial cell diversification divisions; mild cardiac defects | TBX20 | 11 (10.73) | TBX20 mutations associated with hypoplasia in the outflow tract and right ventricle (complete knockdown), lack of septation in the outflow tract with PTA, right ventricle hypoplasia, valve defects [204] (mouse) | Reim et al., 2005 [198], Hu et al., 2011 [113] |
| TBX20 mutations associated with DORV, VSD, ASD, TOF, PTA, PFO, BAV [205], MVP/MR, total anomalous pulmonary venous connection, and congenital atrioventricular block [203]; HLHS [205] (human) | |||||
| Hand | Hand mutations/knockout associated with hypoplastic dorsal vessel with reduction in wall thickness; late embryonic/early larval lethality | HAND2 | 15 (14.74) | HAND2 mutations/absence associated with early embryonic lethality; valve defects, such as tricuspid atresia; double inlet left ventricle; hypoplastic myocardial tissue; rightward shift of the interventricular septum with larger left and smaller right ventricle; hypotrabeculated myocardial tissue with multiple interventricular septa; hypervascularization with multiple coronary arteries [219] (mouse) | Han et al., 2006 [191], Lo et al., 2007 [190] |
| HAND2 variants associated with DORV, VSD, and outflow tract malformations [214]; pulmonary stenosis [224] (human) | |||||
| Hcf | Hcf mutations associated with disruption in physiologic heart function, with disruption in actin filament arrangement; reduction in diastolic diameter and heart rate; adult lethality (adult) | HCFC1, HCFC2 | 8 (8.14, 7.98) | HCFC1 mutations lead to defects in craniofacial development; no evidence of a pathologic cardiac phenotype [392] (zebrafish) | Huang et al., 2022 [330] |
| HCFC1 mutations associated with X-linked form of combined methylmalonic acidemia and hyperhomocysteinemia [393]; HCFC1 has not yet been associated with congenital heart defects | |||||
| HCFC2 has not yet been associated with cardiac development or congenital heart defects | |||||
| Hd | Hd mutations associated with disruption in actin filament and myofibril arrangement | DONSON | 15 (14.77) | DONSON mutations associated with ASD, 21q deletion syndrome (craniofacial dysmorphias, developmental delay, behavior abnormalities, and various systemic manifestations) [233,362]; microcephaly; and short stature [394] (human) | Schroeder et al., 2019 [233] |
| Hh | Hh mutations associated with variable effects on cardiac development ranging from reduction in cardiac cell numbers and no dorsal vessel formation to no effect on dorsal vessel formation, depending on timing of gene mutation | SHH | 15 (14.79) | SHH protein mutations/SHH-related signaling pathway mutations associated with heart defects related to the establishment of left–right asymmetry due to dysfunction of midline structures [286], including situs inversus, dextrocardia, defects in pharyngeal arch patterning, atrioventricular septal defects, transposition of the great arteries, and DORV [287] (mouse) | Park et al., 1996 [176], Liu et al., 2006 [284] |
| Possible association with TOF and 22q11.2DS deletion syndromes [395] (human) | |||||
| HMGCR | HMGCR mutations associated with “Broken-hearted” phenotype with dissociation of cardioblast/pericardial cell adhesion; embryonic lethality | HMGCR | 15 (14.77) | Inhibition of the HMGCR pathway leads to vascular destabilization and the progressive dilatation and rupture of cerebral vessels [380] (zebrafish) | Yi et al., 2006 [229] |
| HMGCR mutations associated with infantile hemangioma [381] and cerebral cavernous malformations [382] due to disruption in the mevalonate pathway (human) | |||||
| Htl | Htl mutations associated with defects in mesoderm migration alongside ectoderm with absence of visceral mesoderm (embryo) | FGFR3 | 15 (14.72) | FGFR3 deficiency affects bone development during postnatal growth [396]; disrupts FGF8-mediated migration of cardiac Neural crest cells (mouse) | Kadam et al., 2009 [261], Dorey and Amaya, 2010 [262] |
| FGFR3 mutations associated with achondroplasia with associated cardiovascular defects in 20% of patients from a patient cohort of 37, including VSD, ASD, pulmonary stenosis, and coarctation of the aorta [397] (human) | |||||
| FGFR2B mutations associated with ventricular septal defects, disruption in outflow tract alignment, poor ventricular trabeculation [398], and fewer epicardial-derived cells in the compact myocardium due to impaired movement of cardiac fibroblasts within the myocardium during development [399] (mouse) | |||||
| FGFR2B mutations/variants have not yet been directly associated with congenital heart defects in H. sapiens (human) | |||||
| Jarid2 | Jarid2 mutations associated with increased levels of pericardin (cardiac fibrosis); embryonic lethality | JARID2 | 14 (13.79) | JARID2 deficiency associated with increased ventricular trabeculation and non-compaction of the ventricular wall [400] (mouse) | Basu et al., 2017 [232] |
| JARID2 mutations have not yet been directly associated with congenital heart defects; JARID2 variants associated with a distinct neurodevelopmental syndrome [401] (human) | |||||
| Kif1A | Kif1A deficiency shows no effect on dorsal vessel structure or function | KIF1A | 15 (14.79) | KIF1A variants identified in left-sided heart defects, HLHS (human) | Akasaka et al., 2020 [236] |
| Kif1A overexpression associated with disruption in myofibrillar arrangement, fewer valves, and increased collagen deposition (cardiac fibrosis) | |||||
| Kismet | Kismet mutations associated with disruption in physiologic heart function with reduction in cardiac myofibril density and increase in Prc (cardiac fibrosis) (larva); reduction in cardiac myofibril, cardioblast numbers, and increase in pericardin (cardiac fibrosis); adult lethality (adult) | CHD7 | 12 (11.85) | CHD7 mutations associated with defects in truncus arteriosus and outflow tract positioning due to defects in cardiac neural crest cell function [402] (frog) | Zhu et al., 2017 [35] |
| CHD7 mutations associated with CHARGE-like syndrome phenotype (vestibular dysfunction, heart defects) and hypoplastic pharyngeal arch arteries [403] (heterozygous loss of CHD7) (mouse) | |||||
| CHD7 variants associated with ASD [214] and CHARGE syndrome (otolith defects, coloboma; craniofacial malformations; and heart defects, such as VSD, ASD, conotruncal defects, and defects in endocardial cushion development) [403] (human) | |||||
| Kuz | Kuz mutations associated with variable defects, ranging from rudimentary/missing heart, disruption in cardioblast alignment, and disorganized heart with disruption in cardioblast alignment to hyperplastic heart with increase in all cardioblast populations and reduction in some pericardial cell groups and lymph gland cells | ADAM10, ADAM17 | 14 (13.89) | ADAM10 disruption in endothelial cells associated with early embryonic death, impaired SNAIL, BMP2 expression in cardiac tissues, and NOTCH1-like phenotype, including impaired vascular morphogenesis with reduction in aortic and cardinal vein size, impaired epithelial-to-mesenchymal transition, and defects in ventricular trabeculation [404]; defects in differentiation of coronary artery endothelial cells with enlarged heart and defects in myocardial compaction, upregulation of venous, and immature endothelial markers [405] (mouse) | Albrecht et al., 2006 [406] |
| ADAM17 variants associated with the right ventricular hypertrophy in TOF due to possible effects on HB-EGF/ErbB signaling [407] (human) | |||||
| ADAM10 has not yet been associated with congenital heart defects, possibly due to the embryonic lethality of ADAM10 mutations [407] | |||||
| lanA | lanA mutations associated with complete cardioblast/pericardial cell dissociation with random migration patterns in animals with scb, lanA mutations | LAMA5 | 14 (13.87) | LAMA5 has not yet been directly associated with congenital heart defects; LAMA5 variants associated with a systemic developmental syndrome characterized by glomerulopathy [408] | Stark et al., 1997 [409], Nishiyama et al., 2005 [410] |
| Lid | Lid mutations associated with adult lethality (Adult) | KDM5A | 17 (16.75) | Inhibition of KDM5A shifts cardiac progenitors toward the mature stage via upregulation of genes associated with oxidative phosphorylation, fatty acid oxidation, and sarcomere organization [411] (in vitro models) | Zhu et al., 2017 [35] |
| KDM5A variants associated with VSD, TOF, and patent foramen ovale [412] (human) | |||||
| Lpt | Lpt mutations associated with “Broken-hearted” phenotype (embryo); disruption in physiologic heart function, with disruption in actin filament arrangement; reduction in cardiac myofibril density, cardioblast numbers, and increase in pericardin (cardiac fibrosis); reduction in diastolic diameter and heart rate (adult); late embryonic/early larval stage lethality; adult lethality | KMT2D, KMT2C | 8 (7.89) | KMT2D mutations associated with mild aortic narrowing (heterozygous loss), embryonic lethality, absence of somites, headfolds (homozygous loss), embryonic lethality, disorganized interventricular septum, and absence of outflow tract septation into aorta/pulmonary artery (conditional deletion in cardiac tissues only) [333] (mouse) | Huang et al., 2022 [330] |
| KMT2D variants associated with VSD, ASD, obstructive lesions [214], Kabuki Syndrome [413], and HLHS [414] (human) | |||||
| KMT2C has not yet been associated with cardiac development or congenital heart defects; KMT2C variants/deletion associated with Kleefstra 2 syndrome [415] and a neurodevelopmental syndrome distinct from Kleefstra and Kabuki syndrome [416] | |||||
| mgl | mgl mutations associated with cardiac dilation with increased end diastolic diameter and cardiac arrhythmia | LRP2 | 14 (14.01) | LRP2 mutations reduce cardiomyocyte proliferation due to an upregulation of cell cycle inhibitors, pro-apoptotic factors, and downregulation of cell cycle genes (in vitro models) | Theis et al., 2020 [230], Riedel et al., 2011 [231] |
| LRP2 mutations associated with hypoplastic heart phenotype with reduction in ventricular cardiomyocyte numbers and reduced ventricular dimensions, with an associated reduction in contractility and bradycardia (zebrafish) | |||||
| LRP2 variants enriched 3-fold in patients with HLHS compared to healthy controls (10% compared to 3.4%) (human) | |||||
| mid (nmr2) | mid mutations associated with disruption in physiological cardiac function | TBX20 | 13 (12.78) | TBX20 mutations associated with hypoplasia in the outflow tract, right ventricle (complete knockdown), lack of septation in the outflow tract (PTA), right ventricle hypoplasia, and valve defects [204] (mouse) | Reim et al., 2005 [198], Hu et al., 2011 [113] |
| TBX20 variants associated with DORV, VSD, ASD, TOF, PTA, PFO, BAV [205], MVP/MR, total anomalous pulmonary venous connection, congenital atrioventricular block [203], and HLHS [205] (human) | |||||
| mmp1 | mmp1 mutations associated with disruption in cardioblast arrangement, cardiac lumen formation with reduced diameter, or absence of cardiac lumen formation; absence of cardioblast shape changes/filopodia and variable defects in leading-edge ranging from reduction in migration velocity and number of filopodia per segment to leading-edge lamellipodial activity (embryo) | MMP14, MMP2 | 12 (11.9) | MMP2 mutations between the primitive streak stage and the 14 somite stages associated with failure of heart tube formation, variations of the “cardia–bifida” phenotype, alterations in looping direction within cells proliferating in the dorsal mesocardium and anterior heart field, and failure of heart tube bending in later stages [417] (chicken) | Raza et al., 2017 [253], Hughes et al., 2020 [254] |
| MMP14 mutations associated with death in the early postnatal period and defects in skeleton, skeletal muscle, and lung development [418] (mouse) | |||||
| mmp1 overexpression associated with “cardia–bifida” phenotype with disruption in adhesion junction and myofibril arrangement; incomplete dorsal vessel; luminal and abluminal Viking plaques (Embryo) | MMP2, MMP9, MMP14 associated with unicommissural aortic valves characterized by congenital fusion of adjacent cusps of two commissures [419] (human) | ||||
| mmp2 | mmp2 mutations associated with disruption in cardioblast arrangement, cardiac lumen formation with absence of cardiac lumen formation, absence of cardioblast shape changes/filopodia, and variable defects in leading-edge ranging from reduction in migration velocity, and number of filopodia per segment and leading-edge lamellipodial activity (embryo) | MMP15, MMP9 | 10 (10) | Snail1 mutations reduce/downregulate levels of MMP15; reduce cell migration; and, due to Snail1 deficiency, cellularity in atrioventricular endocardial cushions [420] (mouse) | Raza et al., 2017 [253], Hughes et al., 2020 [254] |
| MMP15 variants associated with congenital heart defects, cholestasis, and dysmorphism [421] (human) | |||||
| mmp2 overexpression associated with “Cardia-Bifida” phenotype with midline tearing, incomplete dorsal vessel, and luminal and abluminal Viking plaques (Embryo) | Elevated MMP9 expression contributes to extracellular matrix degradation, activates a proteinase-activated receptor-1 signaling cascade, and contributes to cardiomyocyte dysfunction and heart failure in single ventricle cases [422]; MMP2, MMP9, MMP14 variants associated with unicommissural aortic valves characterized by congenital fusion of adjacent cusps of two commissures [419]; MMP9 variants associated with ascending aortic aneurysm, thoracic aortic dissection [423] and aortic stenosis [424] (human) | ||||
| Mnn1 | Mnn1 mutations associated with disruption in physiologic heart function, with disruption in actin filament arrangement; reduction in cardiac myofibril density and cardioblast numbers and increase in pericardin (cardiac fibrosis); increase in systolic diameter and reduction in diastolic diameter; adult lethality (adult) | MEN1 | 14 (13.86) | MEN1 mutations associated with reduced growth during embryonic development with body hemorrhages; defects in neural tube development [425] (mouse) | Zhu et al., 2024 [328] |
| MEN1 has not yet been directly associated with congenital heart defects in H. sapiens (human) | |||||
| msh-2 | msh-2 knockout associated with absence of visceral muscle and absence of dorsal vessel | MSX2, MSX1 | 16 (15.8) | MSX2 mutations/knockout associated with reduced accumulation of second heart field (SHF) precursors to the developing outflow tract; increased accumulation of mesenchymal precursors in the conotruncal endocardial cushions disrupts rotation of the truncus arteriosus and leads to alignment defects in the outflow tract [180]; MSX2/MSX1 mutations associated with defects in cardiac neural crest cell development and associated structures [426] (mouse) | Bodmer et al., 2011 [179], Hu et al.,2011 [113] |
| MSX1 variants associated with VSD [222] (human) | |||||
| MSX2 mutations associated with craniosynostosis [427], complex heart defect (dextrocardia, dextroversion, PFO) cases with radial agenesis, along with other characteristics of Hunter–McAlpine syndrome (intellectual disability, craniofacial and skeletal abnormalities, and characteristic facial attributes) [223] (human) | |||||
| Nacα | Nacα knockdown throughout development associated with reduction in systolic and diastolic diameter (embryo), ectopic Abd-B expression during metamorphosis leading to aberrant histolysis, leading to “No-heart” phenotype with absent pericardin, cardiac cell dispersal, and fat cell accumulation (pupa, adult); bicaudal phenotype with embryo developing with a mirror image duplication of the posterior axis (embryo) | NACA | Loss of NACA disrupts skeletal muscle development, including myofibrillar organization, paralysis with little muscle contraction, disorganization in thick, and thin myosin filaments [428]; disruption in hematopoietic niche function with defects in hematopoiesis [429] (zebrafish) | Schroeder et al., 2022 [312] | |
| NACA variants associated with TOF [430] (human) | |||||
| Netrin (netA/netB) | Netrin mutations associated with variable defects in leading-edge, ranging from reduction in migration velocity and reduction in filopodia per segment to reduction in leading-edge lamellipodial activity | NTN1 | 14 (13.77) | NTN1 mutations/loss associated with defects in aortic arch artery formation and defects in guidance in developing vasculature abnormal thyroid morphogenesis due to defects in vascular development [431] (zebrafish) | Raza and Jacobs, 2016 [370] |
| NTN1 mutations associated with embryonic lethality (global loss) and increase in interventricular septum thickness with no overt cardiac phenotype (cardiomyocyte-specific loss) [432] (mouse) | |||||
| NTN1 variants associated with a case presenting with VSD, ASD, and PDA and congenital hypothyroidism due to thyroid dysgenesis [431] (human) | |||||
| Notch | Notch mutations associated with increased levels of pericardin (cardiac fibrosis), reduced levels of cell actin, and embryonic lethality | NOTCH1, NOTCH2, NOTCH3 | 12 (11.91, 11.77, 11.67) | NOTCH1 variants (heterozygous mutations) associated with progressive aortic valve calcification due to release of inhibition in osteogenic and pro-inflammatory pathways due to differential histone acetylation at H3K27 NOTCH1 enhancers [433] (in vitro models) | Basu et al., 2017 [232] |
| NOTCH3 mutations lead to mild defects only, while combined NOTCH2/NOTCH3 mutations lead to severe vascular defects and embryonic lethality [434] (mouse) | |||||
| NOTCH1 mutations associated with VSD [214], TOF, BAV, HLHS, various septal defects, and functional single ventricles [214]; Adams–Oliver syndrome (scalp defects and vascular abnormalities) [435]; obstructive lesions [214] (human) | |||||
| NOTCH2 mutations associated with ASD, malformation of the outflow tracts, obstructive lesions [214], and Alagille syndrome (multisystem disorder with heart defects) (human) | |||||
| NOTCH3 mutations associated with cerebral arteriopathy with subcortical infarcts and leukoencephalopathy [436] (human) | |||||
| Numb | Numb mutations associated with disruption in myofibril arrangement; reduced levels of cell actin; disruption in diversification of cardioblast cell groups and tin+ cardioblast alignment; embryonic lethality | NUMB | 12 (11.93) | NUMB mutations associated with defects in differentiation of second heart field (SHF) progenitors, upregulation of Notch signaling, defects in cardiomyocyte proliferation, outflow tract and atrioventricular canal septation, and embryonic lethality (loss of both NUMB and NUMBL) [437] (mouse) | Basu et al., 2017 [232], Gajewski et al., 2000 [296] |
| NUMB variants associated with cases of heterotaxy/dextrocardia and additional congenital heart defects, including DORV, VSD, pulmonary stenosis, superior–inferior ventricle, left superior vena cava [438] (human) | |||||
| Org-1 | Org-1 mutations/knockouts associated with severe defects/absence of Alary muscles, thoracic alary-related muscles, and ventral longitudinal muscle | TBX1 | 12 (11.8) | TBX1 mutations associated with PTA and reduced ability to form brachiomeric muscles (homozygous loss) [439] (mouse) | Schaub et al., 2012 [241], Boukhatmi et al., 2014 [238] |
| TBX1 mutations associated with phenocopy of the 22q11.2DS deletion syndrome with cardiac outflow tract defects (DiGeorge syndrome) (craniofacial defects, immune dysfunction, and cardiac defects) with cardiac outflow tract defects, reduced proliferation of second heart field progenitors (SHF), and aortic arch patterning defects [439] (human) | |||||
| pnr | pnr mutations associated with disruption in specification of cardioblast cell groups | GATA4 | 12 (11.8) | GATA4 mutations associated with early embryonic lethality due to defects in the extraembryonic endoderm, cardiac bifida, and absence of fusion in the midline; absence of proepicardium; hypoplastic ventricular tissue [440]; valve defects (mouse) | Han and Olson, 2005 [78] |
| GATA4 variants associated with DORV, double-inlet left ventricle, VSD, ASD, atrioventricular septal defect, TOF, and BAV [440]; defects in outflow tract alignment, dextrocardia, and pulmonary stenosis [195] (human) | |||||
| Ptip | Ptip mutations associated with disruption in physiologic heart function, with disruption in actin filament arrangement, reduction in cardiac myofibril density, and cardioblast numbers and increase in pericardin (cardiac fibrosis); increase in systolic diameter and reduction in diastolic diameter; adult lethality (adult) | PAXIP1 | 11 (10.8) | PAXIP1 mutations associated with early embryonic lethality (mouse) | Zhu et al., 2024 [328] |
| PAXIP1 variants associated with BAV [441] (human) | |||||
| pygo | pygo mutations associated with absence of cardiac valve cell differentiation with lack of high-density myofibrils; absence of physiological posterior dorsal vessel (heart chamber) wall thickening in the valve region; loss of normal heart chamber constriction at valve site (valve site dilation) | PYGO2 | 7 (7.01) | Combined loss of PYGO1, PYGO2 leads to defects in cardiac development after gastrulation including cardiac edema, craniofacial defects, and defects/dysregulation in swimbladder inflation [442] (zebrafish) | Tang et al., 2014 [55] |
| Combined loss of PYGO1, PYGO2 leads to embryonic lethality between E13.5 and E4.5, severe defects between E10.5 and E14.5 with hypoplastic ventricular myocardial tissue, atrial dilation, smaller and thinner atrioventricular valves, defects in chamber septation, and defects in outflow tract development, including transposition of the great arteries, hypoplastic aorta, hypoplastic pulmonary artery [442] (mouse) | |||||
| PYGO1, PYGO2 have not yet been specifically associated with congenital heart defects in H. Sapiens [442] (Human) | |||||
| pyr | pyr mutations/absence associated with defects in mesoderm migration alongside ectoderm, mesoderm aberrant with multilayer formation, severe defects in dorsal mesoderm specification, reduction/absence of eve+ groups (embryo) | FGF8 | -- | FGF8 mutations associated with absence of endoderm and embryonic mesoderm, embryonic lethality during gastrulation; defects in cardiac looping, development of the outflow tract, anterior heart field and survival of cardiac neural crest cells as they migrate toward the outflow tract leading to outflow tract septation defects [443] (mouse) | Kadam et al., 2009 [261], Dorey and Amaya, 2010 [262] |
| FGF8 mutations associated with 22q11.2DS deletion syndrome (craniofacial defects, immune dysfunction, and cardiac defects) phenotypes [444] (human) | |||||
| Rbbp5 | Rbbp4 mutations associated with disruption in physiologic heart function, with disruption in actin filament arrangement; reduction in cardiac myofibril density and cardioblast numbers and increase in pericardin (cardiac fibrosis); increase in systolic diameter and heart period, adult lethality (adult) | RBBP5 | 16 (15.8) | Increased RBBP4 expression due to loss of c-Jun regulation increases H3K4 methylation at cardiogenic genes, upregulates cardiomyocyte generation [445] (in vitro models) | Zhu et al., 2024 [328] |
| RBBP4 variants in the 1p35 locus associated with ASD, characterized as a risk modifier for Down syndrome [446] (human) | |||||
| Robo | Robo mutations associated with varying effects ranging from no effect on cardioblast migration with defects ranging and mild effects on midline cardioblast alignment to severe effects (gaps, intercalation, and double rows) with Robo/Robo2 mutations | ROBO3, ROBO1, ROBO2 | 10 (9.68, 9.67, 9.62) | ROBO1/ROBO2 mutations/loss associated with defects in the membranous ventricular septum, thickened and immature semilunar and atrioventricular valves, bicuspid aortic cushions with BAV, downregulation of NOTCH and HEY/HES downstream effectors leading to downregulation in NOTCH signaling [447], partial absence of the pericardium with severe reduction in sinus horn myocardium, hypoplastic caval veins, persistent left inferior caval vein [448], and complete absence of SLIT2 and SLIT3 binding (mouse) | Qian et al., 2005b [77], MacMullin and Jacobs, 2006 [449], Medioni et al., 2008 [246], Santiago-Martínez et al., 2008 [249], Zmojdzian et al., 2008 [57], Zmojdzian et al., 2018 [56], Raza and Jacobs, 2016 [370] |
| ROBO1 mutations/loss associated with defects in the membranous ventricular septum, downregulation of NOTCH and HEY/HES downstream effectors leading to downregulation in NOTCH signaling [447], partial absence of the pericardium [448], and absence of SLIT3 binding [448] (mouse) | |||||
| ROBO2 mutations alone are not associated with defects in a murine cardiac development model of SLIT/ROBO signaling [448] (mouse) | |||||
| ROBO1 variants associated with VSD (both in the membranous and muscular septum), ASD [450], malformation of the outflow tracts [214], TOF [451], BAV [452], overriding aorta, defects in canal veins [450], ascending aortic aneurysm [453] (human) | |||||
| ROBO2 variants associated with cardiac malformations in a case presenting with neurodevelopmental delay and multisystem defects due to del(3)(p12.3p14.1) (3p interstitial deletion) encompassing 31 open reading frames [454], BAV [453] (human) | |||||
| Robo2 | Robo2 mutations associated with variable defects in dorsal closure and dorsal vessel (delayed migration, gaps, blisters, twists, and midline crossing of cardiac progenitors); highest phenotype severity with sli/scb, Robo2 mutations | ROBO1, ROBO3 | 9 (8.77, 8.68) | ROBO3 variants associated with TOF, BAV, and coarctation of the aorta [453] (human) | |
| RpL13 | RpL13 mutations associated with “No-heart” phenotype with complete absence of dorsal vessel and constrictions in posterior dorsal vessel remnants | RPL13 | 16 (15.8) | RPL13 mutations associated with downregulation of genes related to cell cycle progression (particularly during the S and G2 phases) and cardiac progenitor, cardiomyocyte proliferation; disproportionate increase in fibroblasts compared to cardiomyocytes (in vitro models) | Schroeder et al., 2019 [233] |
| RPL13 variants associated with complete atrioventricular canal defect [455] (human) | |||||
| RpL14 | RpL14 mutations associated with “Minute” syndrome with impaired development, fertility, and cardiac function; partial dorsal vessel atrophy with reduced levels of pericardin | RPL14 | 16 (15.8) | RPL14 mutations associated with “Minute”-like phenotype (impaired development, fertility, and cardiac function) (zebrafish) | Nim et al., 2021 [151] |
| RPL14 has not yet been associated with congenital heart defects in H. sapiens [151] (human) | |||||
| Rpn8 | Rpn8 mutations associated with partial dorsal vessel atrophy | PSMD7 | 16 (15.79) | PSMD12 variants associated with Stankiewicz–Isidor syndrome (neurodevelopmental defects, cardiac defects) (human) | Nim et al., 2021 [151] |
| RpS24 | RpS24 mutations associated with “Minute” Syndrome with impaired development, fertility and cardiac function; complete dorsal vessel atrophy with increased levels of pericardin (cardiac fibrosis), and visible breaks in dorsal vessel structure | RPS24 | 16 (15.8) | RPS24 mutations associated with the congenital heart defects presenting with Diamond Blackfan Anemia (”Minute”-like phenotype with impaired growth, bone marrow function, and congenital heart defects) [151] (human) | Nim et al., 2021 [151] |
| Scny | Scny mutations associated with reduction in cardiac myofibril density and adult lethality (adult) | USP36 | 11 (10.89) | USP36 variants associated with coronary artery structural variants and an increased risk of coronary artery disease [456] (human) | Zhu et al., 2017 [35] |
| Set1 | Set1 mutations associated with disruption in physiologic heart function, with disruption in actin filament arrangement; reduction in cardiac myofibril density and cardioblast numbers and increase in pericardin (cardiac fibrosis); increase in heart period; increased lethality; metabolic dysregulation (upregulation of carbohydrate metabolism genes, downregulation of lipid metabolism genes) (adult) | SETD1A | 12 (11.85) | SETD1A associated with a case of airway defects, characteristic facies and body features, along with congenital heart defects, including ASD and pulmonary hypertension [457] (human) | J. Zhu et al., 2023 [327] |
| Set2 | Set1 mutations associated with disruption in physiologic heart function, with disruption in actin filament arrangement; reduction in cardiac myofibril density; increase in pericardin (cardiac fibrosis); increase in heart period; adult lethality (adult) | SETD2 | 13 (12.64) | SETD2 mutations associated with defects in coronary vascular development with greater effects on left ventricular coronary vasculature, ventricular non-compaction, and embryonic lethality mid-gestation; no effects on other peripheral vasculature [458] (mouse) | J. Zhu et al., 2023 [327] |
| SETD2 has not yet been associated with congenital heart defects in H. sapiens [413] (human) | |||||
| Shg | Shg mutations/loss associated with absence of cardiac lumen formation with extracellular space accumulating between contralateral cardioblasts | CELSR1, CELSR3, CELSR2 | 2 (2.01, 2.01, 2.01) | CELSR1 mutations associated with anteroposterior axis shortening due to defects in convergence and extension during zebrafish embryonic development, neural tube defects, enlarged pericardium [459] (zebrafish) | Santiago-Martínez et al., 2008 [249], Zmojdzian et al., 2008 [57], Zmojdzian et al., 2018 [56] |
| CELSR1, CELSR2, CELSR3 variants associated with neural tube defects and congenital heart defects, including DORV, VSD, ASD, PDA, and pulmonary stenosis, and aortic stenosis [459] (human) | |||||
| Sli | Sli mutations associated with variable defects in dorsal closure and dorsal vessel (delayed migration, gaps, blisters, twists, and midline crossing of cardiac progenitors), with highest phenotype severity with sli/scb, robo2 mutations | SLIT1, SLIT2, SLIT3 | 15 (14.87, 14.87, 14.82) | SLIT1 mutations associated with dysregulation in axonal guidance during development of the optic chiasm [460] (mouse) | Qian et al., 2005b [77], MacMullin and Jacobs, 2006 [449], Medioni et al., 2008 [246], Santiago-Martínez et al., 2008 [249], Zmojdzian et al., 2008 [57], Zmojdzian et al., 2018 [56], Raza and Jacobs, 2016 [370] |
| SLIT2 mutations/loss associated with thickened and immature semilunar valves [447] (mouse) | |||||
| SLIT3 mutations/loss associated with defects in the membranous ventricular septum, thickened and immature atrioventricular valves [447], severe reduction in sinus horn myocardium, hypoplastic caval veins, persistent left inferior caval vein [448], and enlarged right ventricle [461] (mouse) | |||||
| SLIT1, ROBO4 variants associated with a case presenting with BAV, ascending aorta aneurysm, and BAV [453] (human) | |||||
| SLIT2 variants associated with BAV [453] (human) | |||||
| SLIT3 variants associated with congenital heart defects in a case presenting with cardiac and renal malformation [462] and BAV with mitral regurgitation [453] (human) | |||||
| Smox | Smox mutations associated with adult lethality (adult) | SMAD3 | 14 (13.87) | SMAD3 has not yet been specifically associated with cardiac development or congenital heart defects | Zhu et al., 2017 [35] |
| Son | Son mutations associated with disruption in actin filament and myofibril arrangement | SON | 9 (8.77) | SON mutations associated with downregulation of genes related to cell cycle progression (particularly during the S, G2 phases) and cardiac progenitor, cardiomyocyte proliferation, disproportionate increase in fibroblasts compared to cardiomyocytes, and loss of embryonic stem cell pluripotency (in vitro models) | Schroeder et al., 2019 [233] |
| SON variants associated with VSD and ASD, along with intellectual disability and developmental delay, 21q deletion syndrome (craniofacial dysmorphias, developmental delay, behavior abnormalities, and various systemic manifestations) [362] (Human) | |||||
| Src42A | Src42A mutations associated with “Open heart” phenotype with absence of cardioblast migration in the posterior dorsal vessel; absence of cardiac leading-edge activity; persistence of the Amnioserosa near the midline | FRK | 13 (12.95) | FRK has not yet been specifically associated with cardiac development or congenital heart defects | Vanderploeg and Jacobs, 2017 [248] |
| svp | svp mutations/knockout associated with disruption in cardioblast phenotype and loss of svp+ cardioblast groups | NR2F2 | 13 (12.76) | NR2F2 mutations associated with early embryonic lethality (homozygous loss) or lethality during puberty (heterozygous loss) [463] (mouse) | Lo and Frasch, 2001 [188], Hu et al., 2011 [113] |
| NR2F2 variants associated with DORV, VSD, ASD, TOF, PDA, BAV [217] (human) | |||||
| ths | ths mutations associated with defects in mesoderm migration, alongside ectoderm, mesoderm aberrant with multilayer formation, and subtle effects on eve+ groups (embryo) | FGF8 | 1 (0.9) | FGF8 mutations/knockout associated with absence of endoderm and embryonic mesoderm, embryonic lethality during gastrulation; defects involving cardiac looping, development of the outflow tract, anterior heart field, and survival of cardiac neural crest cells as they migrate toward the outflow tract, leading to outflow tract septation defects [443] (mouse) | Kadam et al., 2009 [261], Dorey and Amaya, 2010 [262] |
| FGF8 mutations contribute to 22q11.2DS deletion syndrome (craniofacial defects, immune dysfunction, and cardiac defects) [444] (human) | |||||
| timp | timp mutations associated with “Ectopic ECM” phenotype with longitudinal alary muscle arrangement along the dorsal vessel; disruption in pericardin arrangement with ectopic pericardin; and disruption in somatic muscle alignment (embryo) | TIMP3 | 15 (14.8) | TIMP3, TIMP4 expression increased in embryonic cardiac tissues during episodes of maternal hypoxia, leading to inhibition of cardiomyocyte proliferation and maternal hypoxia associated with reduction in ventricular wall thickness [464] (rat) | Hughes et al., 2020 [254] |
| TIMP1 haploinsufficiency combined with TIMP3 variants associated with BAV, aortopathy/aortic aneurysm in Turner syndrome [465,466] (human) | |||||
| tin | tin knockout associated with “No-heart” phenotype with absence of cardiac and dorsal somatic muscle | NKX2–5 | 5 (4.87) | NKX2–5 mutations knockout associated with embryonic lethality, defects in cardiac morphology, and conduction with thin ventricular walls and septum defects (VSD), disruption in acetylcholine-based ventricular conduction, and cardiac arrhythmia (mouse) [185] | Bodmer et al., 1992 [181], Hu et al., 2011 [113], Yin and Frasch, 1998 [266] |
| VSD, ASD, HLHS [10] (human) | |||||
| Tkv | Tkv overexpression associated with ectopic heart tissue formation in the ventral visceral mesoderm | BMPR1B | 14 (13.74) | BMP1RB has not yet been associated with congenital heart defects in H. sapiens (human) | Yin and Frasch, 1998 [266] |
| Trr | Trr mutations associated with “Broken-hearted” phenotype (Embryo), disruption in physiologic heart function, with disruption in actin filament arrangement, reduction in cardiac myofibril density and cardioblast numbers, and increase in pericardin (cardiac fibrosis); reduction in diastolic/systolic diameter and heart rate (adult); late embryonic/early larval stage lethality; adult lethality; metabolic dysregulation (downregulation of muscle development genes; downregulation of ion transport genes) (Adult) | KMT2C | 12 (11.73) | KMT2C mutations increase risk for the emergence of conotruncal defects in 22q11.2DS deletion syndrome (craniofacial defects, immune dysfunction, and cardiac defects) [467]; Kleefstra Syndrome (intellectual disability, autism spectrum disorder, and craniofacial defects) [416] (human) | J. Zhu et al., 2023 [327], Huang et al., 2022 [330] |
| Trx | Trx c in physiologic heart function, with disruption in actin filament arrangement, reduction in cardiac myofibril density and cardioblast numbers and increase in pericardin (cardiac fibrosis); increase in heart period; metabolic dysregulation (downregulation of muscle development genes; upregulation of ion transport genes) (adult) | KMT2A | 13 (12.84) | KMT2A mutations associated with defects in the axial skeleton, hematopoiesis (Heterozygous loss), Embryonic lethality (Homozygous loss) [468] (mouse) | J. Zhu et al., 2023 [327] |
| KMT2A variants associated with Wiedeman–Steiner Syndrome (excessive hair growth, short stature, distinct facial features, and heart defects) [469] (human) | |||||
| tup | tup mutations/loss associated with hypoplastic dorsal vessel with reduction in all cardioblast populations, disruption in pericardial cell alignment, and disruption in valve myofibril arrangement | ISL1, ISL2 | 16 (15.8, 15.75) | Deficiency of ISL1 leads to complete absence of most of the atrial tissue, the right ventricle, and the outflow tract [193] (mouse) | Tao et al., 2007 [80] |
| Deficiency of ISL2a leads to defects in cardiac looping, and deficiency of ISL2b is associated with defects in development of the arterial pole [196] (zebrafish) | |||||
| ISL1 variant associated with DORV in combination with VSD (heterozygous mutations) [197] (human) | |||||
| ISL2 has not yet been associated with congenital heart defects in H. sapiens (human) | |||||
| UbcD6 | UbcD6 mutations associated with disruption in physiologic heart function, with reduction in cardiac myofibril density and increase in pericardin (cardiac fibrosis) (larva); reduction in cardiac myofibril density and cardioblast numbers and increase in Prc (cardiac fibrosis); adult lethality (adult) | UBE2B | 15 (14.8) | Absence of monoubiquitylation at H2Bub1 (RNF20 mutations), carried out by a complex involving RNF20, RNF40, UBE2B, associated with ventricular septum and ventricular compact myocardium thinning and abnormal sarcomere structure [350] (mouse) | Zhu et al., 2017 [35] |
| UBE2B variants associated with TOF and right aortic arch [352] (human) | |||||
| Ubx | Ubx deficiency associated with disruption in anterior dorsal vessel structure; pericardial cell arrangement and cardioblast differentiation in segments T3-A1 and A2; absence of alary muscle formation in the anterior dorsal vessel with loss of the anterior 3 alary muscle pairs | HOXB6, HOXC6, HOXC5, HOXA7, HOXB7, HOXB5, HOXA5, HOXD4, HOXA4, HOXB4, HOXC4 | 4 (3.91, 3.91, 3.91, 3.91, 3.91, 3.91, 3.91, 3.81, 3.81, 3.81, 3.81) | Combined deletions in HOXA, HOXB clusters generally associated with defects in cardiac looping and appearance of primitive/atavistic heart morphologies [110] (mouse) | Lo et al., 2002 [45], Lovato et al., 2002 [47], Ponzielli et al., 2002 [309], Perrin et al., 2004 [308], Monier et al., 2005 [46], Ryan et al., 2005 [313], LaBeau et al., 2009 [311] |
| Ubx overexpression/ectopic expression represses Antp expression and induces A5 segment identity in A1–A4 tin+ cardioblasts | HOXB7, HOXD8 cardiac expression altered in embryos after maternal alcohol consumption, via RNA-sequencing data [470]; HOXB7 gain-of-function mutation associated with VSD, along with other congenital defects (cleft palate, renal anomalies, skeletal abnormalities [craniocervical, costosternal regions]) [471] (mouse) | ||||
| HOXB5 mutations associated with PDA [472] (animal models) | |||||
| HOXA1, HOXA2, HOXA3, HOXA4, HOXA13 mutations associated with 7p15 deletion syndrome (defects in facial, hand-foot morphology, supernumerary nipples, hypospadias, and hearing defects) [473] (human) | |||||
| HOXB6 variants associated with thoracic aortic dissection; HOX genes (HOXA5, HOXB6, HOXC6) may correlate with vascular smooth muscle cell de-differentiation in these cases [336] (human) | |||||
| HOXC4, HOXC5, HOXC6 variants associated with increased risk for simple congenital heart disease (human) [474] | |||||
| HOXA7, HOXB4, HOXD4 have not yet been specifically associated with cardiac development or congenital heart defects [337] | |||||
| Vegf | Vegf mutations associated with disruption in physiologic heart function with reduction in systolic motion (embryo) and cardiac output (larva), as well as disruption in ostial and aortic valve function | PDGFA | 9 (8.83) | Loss of PDGFA leads to atrial and ventricular myocardial hypertrophy, defects in epicardial and endocardial cell groups, and aortic dilatation [475] (mouse) | Wu and Sato, 2008 [149] |
| Increased maternal levels of PDGFAA associated with HLHS in the fetus [476] (human) | |||||
| Wdr82 | Wdr82 mutations associated with disruption in physiologic heart function with, disruption in actin filament arrangement; reduction in cardiac myofibril density and cardioblast numbers and increase in pericardin (cardiac fibrosis); increase in systolic diameter and reduction in diastolic diameter; adult lethality (adult) | WDR82 | 16 (15.8) | WDR82 has not yet been directly associated with congenital heart defects in H. sapiens (human) | Zhu et al., 2024 [328] |
| Wds | Wds mutations associated with disruption in physiologic heart function, reduction in cardiac myofibril density and increase in pericardin (larva); reduction in cardiac myofibril density, pericardin, and cardioblast numbers; adult lethality (adult) | WDR5 | 16 (15.75) | WDR5 mutations associated with defects in cilia formation and left–right patterning [477] (frog) | Zhu et al., 2017 [35], Zhao et al., 2023 [478] |
| WDR5 variants associated with conotruncal defects with right aortic arch and mild heterotaxy phenotype [477] (human) | |||||
| Wg | Wg mutations associated with variable effects on cardiac development, ranging from no dorsal vessel formation, severe effects with reduction in cardioblast/pericardial cell numbers to no effects on dorsal vessel formation, depending on timing of gene mutation | WNT1, WNT7A, WNT5A | 15 (14.7) | There are 19 Wnt proteins in mammalian vertebrates, many of which are implicated in cardiac development and associated with cardiac defects, including outflow tract defects and vascular smooth muscle defects [271]; WNT1 implicated in neural crest development [479] | Wu et al., 1995 [265], Lockwood and Bodmer, 2002 [268] |
| WNT1 possibly implicated in HLHS (human) [479] | |||||
| WNT5A mutations associated with disruption of second heart field (SHF) progenitor migration to the outflow tract, outflow tract defects, including PTA [279] | |||||
| WNT5A variants associated with conotruncal defects [480], BAV [481] (human) | |||||
| WNT7A cardiac expression altered in embryos after maternal alcohol consumption, based on RNA-sequencing data [470] (mouse) | |||||
| Levels of DNA methylation in various genes, including WNT7A, may be associated with TOF [482] (human) | |||||
| WNT11 mutations associated with defects in ventricle and outflow tract formation [280] (mouse) | |||||
| WNT11 variants/mutations associated with VSD, TOF [281] (human) | |||||
| DWnt4, Wnt4 | DWnt4, Wnt4 mutations, overall, not as severe as Wg mutations with disruption of normal gene marker expression in pericardial cell groups; disruption in the expression of pericardin and Dmef2; defects in cardioblast alignment with absence of unique morphology (constricted, elongated) of ostia progenitor cells in the posterior dorsal vessel; absence of ostia formation (embryo) | WNT9B | 8 (7.76) | WNT9B mutations associated with enlargement of endocardial cushions, with septal cushion defects, valve defects and death in utero while endocardial-specific WNT9B deficiency does not affect valve development or survival [483] (zebrafish) | Tauc et al., 2012 [171], Graba et al., 1995 [170], Chen et al., 2016 [277] |
| WNT9B variants associated with Alagille syndrome (multisystem disorder with heart defects) [484]; complex risk locus on chromosome 17 interacting with WNT9B, among others, associated with septal defects (VSD, ASD) and left-side congenital heart defects [485] (human) | |||||
| wun2, wun | Wun2/wun mutations associated with variable defects in dorsal closure and dorsal vessel structure ranging from delayed ectoderm leading-edge migration, gaps, multiple lumens, and loose cardioblast/pericardial cell attachment to luminal ectoderm/Amnioserosa remnants with disruption in midline cardioblast assembly (embryo) | PLPP3, PLPP1 | 15 (14.8) | PLPP3 associated with extraembryonic vascular defects and early embryonic lethality [486] (mouse) | Haack et al., 2014 [84] |
| PLPP1 has not yet been associated with cardiac development or congenital heart defects | |||||
| αPS3(scb) | αPS3 mutations associated with variable defects ranging from reduction/disruption of pericardial cell arrangement to complete cardioblast/pericardial cell dissociation with random migration patterns; absence of cardiac lumen formation | ITGA4, ITGA5 | 7 (6.76) | ITGA4 mutations associated with defects in vascular development, absence of epicardium leading to embryonic lethality due to cardiac hemorrhage, defects in pericyte and presumptive vascular smooth muscle cell motility [487], and endocardial extrusions [488] (mouse) | Stark et al., 1997 [409], Moreira et al., 2013 [489], Vanderploeg et al., 2012 [83] |
| ITGA5 mutations associated with defects in endocardial morphology, endocardial differentiation with delayed formation of the endocardial sheet, pericardial edema, defects in cardiac looping, and defects in valve development; combined ITGA4, ITGA5 mutations lead to severe defects in endocardial and myocardial migration, cardia–bifida possibly due to defects in anterior endodermal sheet formation; single ITGA4 mutations show no cardiac defects in zebrafish [490] (zebrafish) | |||||
| ITGA5 mutations associated with defects in cardiac morphology, including defects in endocardial and myocardial migration, although less severe than fibronectin 1 mutations, resulting in cardia–bifida [490] (mouse) | |||||
| ITGA4 mutations possibly associated with a case presenting with DOLV, outlet VSD, large coronary arterio-ventricular fistula, hypertrabeculation, and poor compaction of the right ventricle [488]; ITGA4 variants also associated with aortic stenosis [424] (human) | |||||
| βPS (mys) | βPS mutations associated with variable defects, including cardioblast displacement (most severe with mys); reduction in leading-edge activity | ITGB1 | 16 (15.82) | ITGB1 mutation associated with expansion in endoderm formation in iPSC cultures [491] (in vitro models) | Stark et al., 1997 [409], Moreira et al., 2013 [489], Vanderploeg et al., 2012 [83] |
| FLNC mutations/loss lead to disruption of the ITGB1-mediated interaction between FLNC and other factors, disrupting the interactions between actin filaments and extracellular matrix in cardiomyocytes during cardiac development; this leads to embryonic lethality and cardiac defects such as ventricular wall malformations and reduced cardiomyocyte proliferation [492] (mouse) | |||||
| ITGB1 has not yet been specifically associated with congenital heart defects in H. sapiens (human) |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A1 | Abdominal segment A1 |
| A2 | Abdominal segment A2 |
| A3 | Abdominal segment A3 |
| A4 | Abdominal segment A4 |
| A5 | Abdominal segment A5 |
| A6 | Abdominal segment A6 |
| A7 | Abdominal segment A7 |
| A8 | Abdominal segment A8 |
| Abd-A | Abdominal-A |
| Abd-B | Abdominal-B |
| ANT-C | Antennapedia (ANTP) complex |
| Antp | Antennapedia |
| ANTP | Named after Antennapedia (Antp) gene in D. melanogaster |
| Ash2 | Absent, small, or homeotic disks 2 |
| Bab1/2 | Bric-à-brac |
| Bag | Bagpipe |
| bHLH | Basic helix–loop–helix transcription factor |
| BMP2/4 | Bone morphogenetic protein 2/4 |
| BX-C | Bithorax Complex |
| Cas9 | Clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 |
| Cas9n | Clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 nickase |
| Cdc42 | Cell division control protein 42 |
| CERS | Ceramide synthase |
| Ci | Cubitus interruptus |
| COMPASS | Complex of proteins associated with SET-containing domain 1 (Set1) |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CUT | Named after the cut gene in D. melanogaster |
| D-mef2 | Drosophila myocyte enhancer factor 2 |
| D. melanogaster | Drosophila melanogaster |
| dDAAM | Dishevelled-associated activator of morphogenesis |
| DE-Cadherin | Drosophila epithelial cadherin |
| Dg | Dystroglycan |
| DIOPT | Drosophila RNAi Screening Center Integrative ortholog prediction tool |
| Dlg | Disks-large |
| DNA | Deoxyribonucleic acid |
| DNMT1 | DNA methyltransferase 1 |
| DNMT3 | DNA methyltransferase 3 |
| Doc1/2/3 | Dorsocross 1/2/3 |
| Dpp | Decapentaplegic |
| Dpy-30L1 | Dpy-30-like 1 |
| DRF | Diaphanous related formin |
| EGFR | Epidermal growth factor receptor |
| ELPC | End-of-the-line pericardial cells |
| Ena | Enabled |
| EPC | Even-skipped (Eve)+ tinman+ (tin)+ pericardial cell |
| ERBB | Erb-B2 Receptor Tyrosine Kinase 2 |
| Eve | Even-skipped |
| EVX1/2 | Even-Skipped Homeobox ½ |
| FGF | Fibroblast growth factor |
| FGF8/10 | Fibroblast growth factor 8/10 |
| FGFR | Fibroblast growth factor receptor |
| FGFR2B | Fibroblast growth factor receptor 2 |
| GAL4 | Transcription factor GAL4 |
| Gart | Phosphoribosylglycinamide formyltransferase, phosphoribosylglycinamide synthetase, phosphoribosylaminoimidazole synthetase |
| GGPPS/qm | Geranylgeranyl pyrophosphate synthase |
| GTPase | Guanosine triphosphatase |
| Gγ1 | G protein gamma (γ) subunit 1 |
| H3K27ac | Lysine 27 of Histone 3 acetylation |
| H3K36 | Lysine 36 of Histone 3 |
| H3K4 | Lysine 4 of Histone 3 |
| H3K4me | Lysine 4 of Histone 3 methylation |
| Hand | Heart and neural crest derivatives |
| HAND1/2 | Heart and neural crest derivatives expressed 1/2 |
| HMGCR | Hydroxymethyl-glutaryl (HMG) CoA reductase |
| HNF | Named after Hnf1 (mammalian) |
| HOM-C | Homeotic Complex |
| Hox | Homeobox gene |
| Hox1–13 | Homeobox 1–13 |
| HoxA/B/C/D | Homeobox A/B/C/D |
| If | Inflated |
| ISL1/2 | ISL LIM Homeobox 1/2 |
| K+ | Potassium |
| KMT2C | Lysine methyltransferase 2C |
| KMT2D | Lysine methyltransferase 2D |
| L1 | Larval stage L1 |
| L2 | Larval stage L2 |
| L3 | Larval stage L3 |
| lb | ladybird |
| LIM | Named after Lin-11 (nematodes) ISL1 (mammalian) Mec-3 (nematodes) |
| MAPK | Mitogen-associated protein kinase |
| Mef-2 | Myocyte enhancer factor-2 |
| MEF2A-2D | Myocyte enhancer factor 2A-2D |
| Mesp1 | Mesoderm posterior basic helix–loop–helix transcription factor (BHLH) transcription factor 1 |
| Mew | Multiple edematous wings |
| Mid | Midline |
| Mnn1 | Menin 1 |
| Msh-2 | MutS Homolog 2 |
| MSX-2 | Msh Homeobox 2 |
| Mys | Myospheroid |
| NK | NK Homeobox |
| NK2 | NK2 Homeobox |
| NKX2.1–2.6 | NK2 Homeobox 1–6 |
| Nmr1 | Neuromancer 1 |
| Nmr2 | Neuromancer 2 |
| Nos3 | Nitric oxide synthase 3 |
| NR2F2 | Even-skipped homeobox 1 |
| Odd | Odd-skipped |
| Omb | Optomotor blind |
| OPC | Odd-skipped (Odd) pericardial cells |
| Org-1 | Optomotor blind-related gene 1 |
| PcG | Polycomb group |
| PCP | Planar cell polarity |
| pERK1/2 | Protein kinase R-like endoplasmic reticulum kinase |
| POU | Named after POU1F1 (mammalian) OCT1, OCT2 (mammalian) Unc-86 (nematodes) |
| PRD | Named after Paired (prd) gene in D. melanogaster |
| PROS | Named after the pros gene in D. melanogaster |
| Ptip | PAX transcription activation domain-interacting protein |
| RAS | Rat sarcoma virus |
| Rbbp5 | Retinoblastoma binding protein 5 |
| RNA | Ribonucleic acid |
| RNAi | Ribonucleic acid interference |
| Robo/2 | Roundabout |
| RTK | Receptor Tyrosine Kinase |
| Scb | Scab |
| Scro | Scarecrow |
| Set1 | SET-containing domain 1 |
| Shg | Shotgun |
| SINE | Named after co: sine oculis gene in D. melanogaster |
| Svp | Sevenup |
| T1 | Thoracic segment T1 |
| T2 | Thoracic segment T2 |
| T3 | Thoracic segment T3 |
| TALE | Three-amino acid loop extension |
| TALEN | Transcription activator-like effector nuclease |
| TBX2–6, 20 | T-Box Transcription Factor 2–6, 20 |
| tin | tinman |
| Trr | Trithorax-related |
| Trx | Trithorax |
| TrxG | Trithorax group |
| Tup | Tailup |
| UASG | Upstream activator sequence |
| Ubx | Ultrabithorax |
| Vnd | Ventral nervous system defective |
| Wdr82 | WD repeat domain 82 |
| Wds | Will die slowly |
| Wg | Wingless |
| WHPCs | Wing heart pericardial cells |
| Wnt2a/2b/5a/8a/11 | Wingless (Wg)-related integration site 2a/2b/5a/8a/11 |
| ZF | Zinc finger |
| ZFN | Zinc-finger nuclease |
| αPS1–3 | alpha subunit integrin chain 1–3 |
| βPS | Beta subunit integrin chain |
References
- Majumdar, U.; Yasuhara, J.; Garg, V. In Vivo and In Vitro Genetic Models of Congenital Heart Disease. Cold Spring Harb. Perspect. Biol. 2019, 13, a036764. [Google Scholar] [CrossRef]
- Hasan, A.A.; Abu Lehyah, N.A.A.; Al Tarawneh, M.K.; Abbad, M.Y.; Fraijat, A.G.; Al-Jammal, R.A.; Moamar, D.M.; Shersheer, Q.A.; Guthrie, S.O.; Starnes, J.R. Incidence and Types of Congenital Heart Disease at a Referral Hospital in Jordan: Retrospective Study from a Tertiary Center. Front. Pediatr. 2023, 11, 1261130. [Google Scholar] [CrossRef]
- Walsh, E.P.; Gonzales, C.; Atallah, J. Multicenter Case-Control Study of Ventricular Arrhythmia in Tetralogy of Fallot. Can. J. Cardiol. 2013, 29, S92–S93. [Google Scholar] [CrossRef]
- Blue, G.M.; Mekel, M.; Das, D.; Troup, M.; Rath, E.; Ip, E.; Gudkov, M.; Perumal, G.; Harvey, R.P.; Sholler, G.F.; et al. Whole Genome Sequencing in Transposition of the Great Arteries and Associations with Clinically Relevant Heart, Brain and Laterality Genes. Am. Heart J. 2022, 244, 1–13. [Google Scholar] [CrossRef]
- Shi, G.; Zhu, F.; Wen, C.; Yan, Y.; Zhang, H.; Zhu, Z.; Chen, H. Cardiac-Type Total Anomalous Pulmonary Venous Return Is Not Benign. J. Thorac. Cardiovasc. Surg. 2023, 165, 449–459.e4. [Google Scholar] [CrossRef]
- Aldweib, N.; Broberg, C. Failing with Cyanosis-Heart Failure in End-Stage Unrepaired or Partially Palliated Congenital Heart Disease. Heart Fail. Clin. 2024, 20, 223–236. [Google Scholar] [CrossRef]
- Kido, T.; Guariento, A.; Doulamis, I.P.; Porras, D.; Baird, C.W.; del Nido, P.J.; Nathan, M. Aortic Valve Surgery After Neonatal Balloon Aortic Valvuloplasty in Congenital Aortic Stenosis. Circ. Cardiovasc. Interv. 2021, 14, e009933. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cao, H.; Liu, J.; Hong, L.; Ma, J.; Zhu, Y.; Xie, Y.; Zhang, Z.; Shi, J.; Cui, L.; et al. A Novel Diagnostic Model for Fetal Coarctation of the Aorta with Ventricular Septal Defect. Int. J. Cardiol. 2025, 422, 132927. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Woo, M.H.; Kim, G.B.; Song, M.K.; Lee, S.Y.; Bae, E.J.; Choi, M.; Kim, Y.-S. A Family with NKX2.5 Gene Mutations Presenting as Familial Atrial Septal Defect and Atrioventricular Block: A Case Report. J. Genet. Med. 2018, 15, 20–23. [Google Scholar] [CrossRef]
- Perrot, A.; Rickert-Sperling, S. Human Genetics of Ventricular Septal Defect. Adv. Exp. Med. Biol. 2024, 1441, 505–534. [Google Scholar] [CrossRef]
- Gupta, S.; Donn, S.M. Management of Patent Ductus Arteriosus–Evidence to Practice. Semin. Fetal Neonatal Med. 2024, 29, 101565. [Google Scholar] [CrossRef] [PubMed]
- Belhadjer, Z.; Pontailler, M.; Hily, M.; Gaudin, R.; Raisky, O.; Bonnet, D.; Houyel, L. The Particular Anatomy of Atrioventricular Septal Defect with a Common Valvar Orifice in Patients with Down Syndrome: An Echocardiographic Study. Int. J. Cardiol. 2025, 423, 133003. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.T.; Henmi, S.; Buratto, E.; Haverty, M.C.; Yerebakan, C.; Fricke, T.; Brizard, C.P.; d’Udekem, Y.; Konstantinov, I.E. Young Infants with Symptomatic Tetralogy of Fallot: Shunt or Primary Repair? JTCVS Open 2024, 19, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Buja, L.M. Chapter 6-Congenital Heart Disease: Pathology, Natural History, and Interventions. In Cardiovascular Pathology, 5th ed.; Buja, L.M., Butany, J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 223–264. ISBN 978-0-12-822224-9. [Google Scholar]
- Griffith, E.G.; Musaalo, K.; Jackson, S.H.; Ribeiro, E.R. Cardiovascular Disease Associated with Genetic Defects. Prog. Pediatr. Cardiol. 2024, 75, 101765. [Google Scholar] [CrossRef]
- Spendlove, S.J.; Bondhus, L.; Lluri, G.; Sul, J.H.; Arboleda, V.A. Polygenic Risk Scores of Endo-Phenotypes Identify the Effect of Genetic Background in Congenital Heart Disease. Hum. Genet. Genom. Adv. 2022, 3, 100112. [Google Scholar] [CrossRef]
- Ehrlich, L.; Prakash, S.K. Copy-Number Variation in Congenital Heart Disease. Curr. Opin. Genet. Dev. 2022, 77, 101986. [Google Scholar] [CrossRef]
- Behiry, E.G.; Al-Azzouny, M.A.; Sabry, D.; Behairy, O.G.; Salem, N.E. Association of NKX2-5, GATA4, and TBX5 Polymorphisms with Congenital Heart Disease in Egyptian Children. Mol. Genet. Genom. Med. 2019, 7, e612. [Google Scholar] [CrossRef]
- Mustafa, H.J.; Jacobs, K.M.; Tessier, K.M.; Narasimhan, S.L.; Tofte, A.N.; McCarter, A.R.; Cross, S.N. Chromosomal Microarray Analysis in the Investigation of Prenatally Diagnosed Congenital Heart Disease. Am. J. Obstet. Gynecol. MFM 2020, 2, 100078. [Google Scholar] [CrossRef]
- El-Ella, S.S.A.; El Gendy, F.; Tawfik, M.A.M.; El Sobky, E.; Khattab, A.; El-mekkawy, M. Chromosome 22 Microdeletion in Children with Syndromic Congenital Heart Disease by Fluorescent in Situ Hybridization (FISH). Egypt. J. Med. Hum. Genet. 2012, 13, 313–322. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Y.; Meng, M.; Hou, Z.; Meng, P.; Zhao, Y.; Gao, H.; Tang, J.; Liu, Z.; Yang, L.; et al. Generation of Human iPSC Line from a Patient with Tetralogy of Fallot, YAHKMUi001-A, Carrying a Mutation in TBX1 Gene. Stem Cell Res. 2020, 42, 101687. [Google Scholar] [CrossRef]
- Lin, A.E.; Santoro, S.; High, F.A.; Goldenberg, P.; Gutmark-Little, I. Congenital Heart Defects Associated with Aneuploidy Syndromes: New Insights into Familiar Associations. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 53–63. [Google Scholar] [CrossRef]
- Choudhury, T.Z.; Garg, V. Molecular Genetic Mechanisms of Congenital Heart Disease. Curr. Opin. Genet. Dev. 2022, 75, 101949. [Google Scholar] [CrossRef] [PubMed]
- Albar, R.F.; Alghamdi, M.S.; Almasrahi, A.M.; Aldawsari, M.K.; Aljahdali, F.F.; Alhwaity, A.S. A Six-Year-Old Child With Mosaic Trisomy 13. Cureus 2021, 13, e18346. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, V.; Meroni, A.; Leoni, C.; Sirchia, F.; Politano, D.; Fiandrino, G.; Giorgio, V.; Rigante, D.; Limongelli, D.; Perri, L.; et al. Trisomy 22 Mosaicism from Prenatal to Postnatal Findings: A Case Series and Systematic Review of the Literature. Genes 2024, 15, 346. [Google Scholar] [CrossRef] [PubMed]
- Milani, D.A.Q.; Chauhan, P.R. Genetics, Mosaicism. In StatPearls [Internet]; StatPearls Publishing: Petersburg, FL, USA, 2023. [Google Scholar]
- Phung, V.; Singh, K.E.; Danon, S.; Tan, C.A.; Dabagh, S. Non-Mosaic Trisomy 22 and Congenital Heart Surgery Using the Shared Decision Making Model: A Case Report. BMC Pediatr. 2023, 23, 122. [Google Scholar] [CrossRef]
- Agarwal, M.; Kumar, V.; Dwivedi, A. Diagnosis of 22q11.2 Deletion Syndrome in Children with Congenital Heart Diseases and Facial Dysmorphisms. Med. J. Armed Forces India 2023, 79, S196–S201. [Google Scholar] [CrossRef]
- Simmons, M.A.; Brueckner, M. The Genetics of Congenital Heart Disease…Understanding and Improving Long Term Outcomes in Congenital Heart Disease: A Review for the General Cardiologist and Primary Care Physician. Curr. Opin. Pediatr. 2017, 29, 520–528. [Google Scholar] [CrossRef]
- Zhao, Y.; van de Leemput, J.; Han, Z. The Opportunities and Challenges of Using Drosophila to Model Human Cardiac Diseases. Front. Physiol. 2023, 14, 1182610. [Google Scholar] [CrossRef]
- Beller, M.; Oliver, B. One Hundred Years of High-Throughput Drosophila Research. Chromosome Res. 2006, 14, 349–362. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Pass, G. The Insect Circulatory System: Structure, Function, and Evolution. Annu. Rev. Entomol. 2020, 65, 121–143. [Google Scholar] [CrossRef]
- Vivien, C.J.; Hudson, J.E.; Porrello, E.R. Evolution, Comparative Biology and Ontogeny of Vertebrate Heart Regeneration. NPJ Regen. Med. 2016, 1, 16012. [Google Scholar] [CrossRef]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The Genome Sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, Y.; Nettleton, M.; Richman, A.; Han, Z. High Throughput in Vivo Functional Validation of Candidate Congenital Heart Disease Genes in Drosophila. eLife 2017, 6, e22617. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N. Gene Regulatory Networks in the Evolution and Development of the Heart. Science 2006, 313, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Peel, A.D.; Akam, M. Arthropod Segmentation. Development 2019, 146, dev170480. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J.; Palli, S.R. Chapter 7-Circulatory Systems. In Physiological Systems in Insects, 4th ed.; Klowden, M.J., Palli, S.R., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 359–407. ISBN 978-0-12-820359-0. [Google Scholar]
- Hillyer, J.F.; Strand, M.R. Mosquito Hemocyte-Mediated Immune Responses. Curr. Opin. Insect Sci. 2014, 3, 14–21. [Google Scholar] [CrossRef]
- Wasserthal, L.T. Drosophila Flies Combine Periodic Heartbeat Reversal with a Circulation in the Anterior Body Mediated by a Newly Discovered Anterior Pair of Ostial Valves and `venous’ Channels. J. Exp. Biol. 2007, 210, 3707–3719. [Google Scholar] [CrossRef]
- Rotstein, B.; Paululat, A. On the Morphology of the Drosophila Heart. J. Cardiovasc. Dev. Dis. 2016, 3, 15. [Google Scholar] [CrossRef]
- Monahan-Earley, R.; Dvorak, A.M.; Aird, W.C. Evolutionary Origins of the Blood Vascular System and Endothelium. J. Thromb. Haemost. 2013, 11, 46–66. [Google Scholar] [CrossRef]
- Farmer, C.G. Evolution of the vertebrate cardio-pulmonary system. Annu. Rev. Physiol. 1999, 61, 573–592. [Google Scholar] [CrossRef]
- Stephenson, A.; Adams, J.W.; Vaccarezza, M. The Vertebrate Heart: An Evolutionary Perspective. J. Anat. 2017, 231, 787–797. [Google Scholar] [CrossRef]
- Lo, P.C.H.; Skeath, J.B.; Gajewski, K.; Schulz, R.A.; Frasch, M. Homeotic Genes Autonomously Specify the Anteroposterior Subdivision of the Drosophila Dorsal Vessel into Aorta and Heart. Dev. Biol. 2002, 251, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Monier, B.; Astier, M.; Sémériva, M.; Perrin, L. Steroid-Dependent Modification of Hox Function Drives Myocyte Reprogramming in the Drosophila Heart. Development 2005, 132, 5283–5293. [Google Scholar] [CrossRef] [PubMed]
- Lovato, T.L.; Nguyen, T.P.; Molina, M.R.; Cripps, R.M. The Hox Gene Abdominal-A Specifies Heart Cell Fate in the Drosophila Dorsal Vessel. Development 2002, 129, 5019–5027. [Google Scholar] [CrossRef] [PubMed]
- Lehmacher, C.; Abeln, B.; Paululat, A. The Ultrastructure of Drosophila Heart Cells. Arthropod Struct. Dev. 2012, 41, 459–474. [Google Scholar] [CrossRef]
- Ward, E.J.; Coulter, D.E. Odd-Skipped Is Expressed in Multiple Tissues during Drosophila Embryogenesis. Mech. Dev. 2000, 96, 233–236. [Google Scholar] [CrossRef]
- Ward, E.J.; Skeath, J.B. Characterization of a Novel Subset of Cardiac Cells and Their Progenitors in the Drosophila Embryo. Development 2000, 127, 4959–4969. [Google Scholar] [CrossRef]
- Huang, X.; Fu, Y.; Lee, H.; Zhao, Y.; Yang, W.; van de Leemput, J.; Han, Z. Single-Cell Profiling of the Developing Embryonic Heart in Drosophila. Development 2023, 150, dev201936. [Google Scholar] [CrossRef]
- Pass, G.; Tögel, M.; Krenn, H.; Paululat, A. The Circulatory Organs of Insect Wings: Prime Examples for the Origin of Evolutionary Novelties. Zool. Anz. J. Comp. Zool. 2015, 256, 82–95. [Google Scholar] [CrossRef]
- Jürgens, K.J.; Drechsler, M.; Paululat, A. An Anatomical Atlas of Drosophila melanogaster—The Wild-Type. Genetics 2024, 228, iyae129. [Google Scholar] [CrossRef]
- Lammers, K.; Abeln, B.; Hüsken, M.; Lehmacher, C.; Psathaki, O.E.; Alcorta, E.; Meyer, H.; Paululat, A. Formation and Function of Intracardiac Valve Cells in the Drosophila Heart. J. Exp. Biol. 2017, 220, 1852–1863. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Yuan, W.; Bodmer, R.; Wu, X.; Ocorr, K. The Role of Pygopus in the Differentiation of Intra-Cardiac Valves in Drosophila. Genesis 2014, 52, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zmojdzian, M.; de Joussineau, S.; Da Ponte, J.P.; Jagla, K. Distinct Subsets of Eve-Positive Pericardial Cells Stabilise Cardiac Outflow and Contribute to Hox Gene-Triggered Heart Morphogenesis in Drosophila. Development 2018, 145, dev158717. [Google Scholar] [CrossRef] [PubMed]
- Zmojdzian, M.; Da Ponte, J.P.; Jagla, K. Cellular Components and Signals Required for the Cardiac Outflow Tract Assembly in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 2475–2480. [Google Scholar] [CrossRef]
- Reim, I.; Frasch, M. Genetic and Genomic Dissection of Cardiogenesis in the Drosophila Model. Pediatr. Cardiol. 2010, 31, 325–334. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Matsumoto, A.; Miyaki, T.; Kinoshita, M.; Kakuta, S.; Sakai, T.; Ichimura, K. Three-Dimensional Architecture of Pericardial Nephrocytes in Drosophila melanogaster Revealed by FIB/SEM Tomography. Cell Tissue Res. 2019, 378, 289–300. [Google Scholar] [CrossRef]
- Lim, H.-Y.; Wang, W.; Chen, J.; Ocorr, K.; Bodmer, R. ROS Regulate Cardiac Function via a Distinct Paracrine Mechanism. Cell Rep. 2014, 7, 35–44. [Google Scholar] [CrossRef]
- Sláma, K. Physiology of Heartbeat Reversal in Adult Drosophila melanogaster (Diptera: Drosophilidae). Eur. J. Entomol. 2013, 107, 13–31. [Google Scholar] [CrossRef]
- Meyer, C.; Drechsler, M.; Meyer, H.; Paululat, A. Differentiation and Function of Cardiac Valves in the Adult Drosophila Heart. J. Exp. Biol. 2023, 226, jeb245839. [Google Scholar] [CrossRef]
- Schaub, C.; März, J.; Reim, I.; Frasch, M. Org-1-Dependent Lineage Reprogramming Generates the Ventral Longitudinal Musculature of the Drosophila Heart. Curr. Biol. 2015, 25, 488–494. [Google Scholar] [CrossRef]
- Drechsler, M.; Schmidt, A.C.; Meyer, H.; Paululat, A. The Conserved ADAMTS-like Protein Lonely Heart Mediates Matrix Formation and Cardiac Tissue Integrity. PLoS Genet. 2013, 9, e1003616. [Google Scholar] [CrossRef]
- Blice-Baum, A.C.; Guida, M.C.; Hartley, P.S.; Adams, P.D.; Bodmer, R.; Cammarato, A. As Time Flies by: Investigating Cardiac Aging in the Short-Lived Drosophila Model. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 1831–1844. [Google Scholar] [CrossRef] [PubMed]
- Bataillé, L.; Lebreton, G.; Boukhatmi, H.; Vincent, A. Insights and Perspectives on the Enigmatic Alary Muscles of Arthropods. Front. Cell Dev. Biol. 2024, 11, 1337708. [Google Scholar] [CrossRef] [PubMed]
- Tögel, M.; Pass, G.; Paululat, A. The Drosophila Wing Hearts Originate from Pericardial Cells and Are Essential for Wing Maturation. Dev. Biol. 2008, 318, 29–37. [Google Scholar] [CrossRef]
- Farmer, A.J.; Katariya, R.; Islam, S.; Rayhan, M.d.S.A.; Inlow, M.H.; Ahmad, S.M.; Schwab, K.R. Trithorax Is an Essential Regulator of Cardiac Hox Gene Expression and Anterior-Posterior Patterning of the Drosophila Embryonic Heart Tube. Biol. Open 2025, 14, bio061919. [Google Scholar] [CrossRef]
- Koehler, S.; Huber, T.B. Insights into Human Kidney Function from the Study of Drosophila. Pediatr. Nephrol. 2023, 38, 3875–3887. [Google Scholar] [CrossRef]
- Molina, M.R.; Cripps, R.M. Ostia, the Inflow Tracts of the Drosophila Heart, Develop from a Genetically Distinct Subset of Cardial Cells. Mech. Dev. 2001, 109, 51–59. [Google Scholar] [CrossRef]
- Gilbert, S.F. An Introduction to Early Developmental Processes. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Jaeger, J.; Manu; Reinitz, J. Drosophila Blastoderm Patterning. Curr. Opin. Genet. Dev. 2012, 22, 533–541. [Google Scholar] [CrossRef]
- Hickman, C.P.; Keen, S.; Eisenhour, D.; Larson, A.; l’Anson, H. Integrated Principles of Zoology, 19th ed.; McGraw-Hil Higher Education: New York, NY, USA, 2023; ISBN 978-1-266-57724-6. [Google Scholar]
- Gomez, J.M.; Bevilacqua, C.; Thayambath, A.; Heriche, J.-K.; Leptin, M.; Belmonte, J.M.; Prevedel, R. Highly Dynamic Mechanical Transitions in Embryonic Cell Populations during Drosophila Gastrulation. Nat. Commun. 2025, 16, 6473. [Google Scholar] [CrossRef]
- Dondi, C.; Bertin, B.; Da Ponte, J.-P.; Wojtowicz, I.; Jagla, K.; Junion, G. A Polarized Nucleus-Cytoskeleton-ECM Connection in Migrating Cardioblasts Controls Heart Tube Formation in Drosophila. Development 2021, 148, dev192146. [Google Scholar] [CrossRef]
- Han, Z.; Bodmer, R. Myogenic Cells Fates Are Antagonized by Notch Only in Asymmetric Lineages of the Drosophila Heart, with or without Cell Division. Development 2003, 130, 3039–3051. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Liu, J.; Bodmer, R. Slit and Robo Control Cardiac Cell Polarity and Morphogenesis. Curr. Biol. 2005, 15, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Olson, E.N. Hand Is a Direct Target of Tinman and GATA Factors during Drosophila Cardiogenesis and Hematopoiesis. Development 2005, 132, 3525–3536. [Google Scholar] [CrossRef]
- Zaffran, S.; Reim, I.; Qian, L.; Lo, P.C.; Bodmer, R.; Frasch, M. Cardioblast-Intrinsic Tinman Activity Controls Proper Diversification and Differentiation of Myocardial Cells in Drosophila. Development 2006, 133, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, J.; Tokusumi, T.; Gajewski, K.; Schulz, R.A. Requirement of the LIM Homeodomain Transcription Factor Tailup for Normal Heart and Hematopoietic Organ Formation in Drosophila melanogaster. Mol. Cell Biol. 2007, 27, 3962–3969. [Google Scholar] [CrossRef]
- Mann, T.; Bodmer, R.; Pandur, P. The Drosophila Homolog of Vertebrate Islet1 Is a Key Component in Early Cardiogenesis. Development 2009, 136, 317–326. [Google Scholar] [CrossRef]
- Bodmer, R.; Frasch, M. Development and Aging of the Drosophila Heart. In Heart Development and Regeneration; Rosenthal, N., Harvey, R.P., Eds.; Academic Press: Boston, MA, USA, 2010; pp. 47–86. ISBN 978-0-12-381332-9. [Google Scholar]
- Vanderploeg, J.; Vazquez Paz, L.L.; MacMullin, A.; Jacobs, J.R. Integrins Are Required for Cardioblast Polarisation in Drosophila. BMC Dev. Biol. 2012, 12, 8. [Google Scholar] [CrossRef]
- Haack, T.; Schneider, M.; Schwendele, B.; Renault, A.D. Drosophila Heart Cell Movement to the Midline Occurs through Both Cell Autonomous Migration and Dorsal Closure. Dev. Biol. 2014, 396, 169–182. [Google Scholar] [CrossRef]
- Ahmad, S.M. Conserved Signaling Mechanisms in Drosophila Heart Development. Dev. Dyn. 2017, 246, 641–656. [Google Scholar] [CrossRef]
- Schwarz, B.; Hollfelder, D.; Scharf, K.; Hartmann, L.; Reim, I. Diversification of Heart Progenitor Cells by EGF Signaling and Differential Modulation of ETS Protein Activity. eLife 2018, 7, e32847. [Google Scholar] [CrossRef]
- Panta, M.; Kump, A.J.; Dalloul, J.M.; Schwab, K.R.; Ahmad, S.M. Three Distinct Mechanisms, Notch Instructive, Permissive, and Independent, Regulate the Expression of Two Different Pericardial Genes to Specify Cardiac Cell Subtypes. PLoS ONE 2020, 15, e0241191. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kim, J.; Wang, Z.; Yan, X.-X.; Dean, A.; Xu, W. Crystal Structure of Human LDB1 in Complex with SSBP2. Proc. Natl. Acad. Sci. USA 2020, 117, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Babaoglan, A.B.; O’Connor-Giles, K.M.; Mistry, H.; Schickedanz, A.; Wilson, B.A.; Skeath, J.B. Sanpodo: A Context-Dependent Activator and Inhibitor of Notch Signaling during Asymmetric Divisions. Development 2009, 136, 4089–4098. [Google Scholar] [CrossRef] [PubMed]
- Bileckyj, C.; Blotz, B.; Cripps, R.M. Drosophila as a Model to Understand Second Heart Field Development. J. Cardiovasc. Dev. Dis. 2023, 10, 494. [Google Scholar] [CrossRef]
- Favarolo, M.B.; López, S.L. Notch Signaling in the Division of Germ Layers in Bilaterian Embryos. Mech. Dev. 2018, 154, 122–144. [Google Scholar] [CrossRef]
- Hariri, F.; Nemer, M.; Nemer, G. T-Box Factors: Insights into the Evolutionary Emergence of the Complex Heart. Ann. Med. 2012, 44, 680–693. [Google Scholar] [CrossRef]
- Spring, J.; Yanze, N.; Jösch, C.; Middel, A.M.; Winninger, B.; Schmid, V. Conservation of Brachyury, Mef2, and Snail in the Myogenic Lineage of Jellyfish: A Connection to the Mesoderm of Bilateria. Dev. Biol. 2002, 244, 372–384. [Google Scholar] [CrossRef]
- Cridge, A.G.; Dearden, P.K.; Brownfield, L.R. Convergent Occurrence of the Developmental Hourglass in Plant and Animal Embryogenesis? Ann. Bot. 2016, 117, 833–843. [Google Scholar] [CrossRef]
- Liu, L.; Yu, L.; Kubatko, L.; Pearl, D.K.; Edwards, S.V. Coalescent Methods for Estimating Phylogenetic Trees. Mol. Phylogenetics Evol. 2009, 53, 320–328. [Google Scholar] [CrossRef]
- Nixon, K.C. Phylogeny. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2001; pp. 16–23. ISBN 978-0-12-384720-1. [Google Scholar]
- Pamilo, P.; Nei, M. Relationships between Gene Trees and Species Trees. Mol. Biol. Evol. 1988, 5, 568–583. [Google Scholar] [CrossRef]
- Blais, C.; Archibald, J.M. The Past, Present and Future of the Tree of Life. Curr. Biol. 2021, 31, R314–R321. [Google Scholar] [CrossRef]
- Soucy, S.M.; Huang, J.; Gogarten, J.P. Horizontal Gene Transfer: Building the Web of Life. Nat. Rev. Genet. 2015, 16, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Mindell, D.P.; Meyer, A. Homology Evolving. Trends Ecol. Evol. 2001, 16, 434–440. [Google Scholar] [CrossRef]
- Mabee, P.M.; Balhoff, J.P.; Dahdul, W.M.; Lapp, H.; Mungall, C.J.; Vision, T.J. A Logical Model of Homology for Comparative Biology. Syst. Biol. 2020, 69, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Ochoterena, H.; Vrijdaghs, A.; Smets, E.; Claßen-Bockhoff, R. The Search for Common Origin: Homology Revisited. Syst. Biol. 2019, 68, 767–780. [Google Scholar] [CrossRef]
- Cerca, J. Understanding Natural Selection and Similarity: Convergent, Parallel and Repeated Evolution. Mol. Ecol. 2023, 32, 5451–5462. [Google Scholar] [CrossRef]
- Gabora, L. Convergent Evolution. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 178–180. ISBN 978-0-08-096156-9. [Google Scholar]
- Webber, C.; Ponting, C.P. Genes and Homology. Curr. Biol. 2004, 14, R332–R333. [Google Scholar] [CrossRef]
- Fitch, W.M. Homology: A Personal View on Some of the Problems. Trends Genet. 2000, 16, 227–231. [Google Scholar] [CrossRef]
- Mallo, D.; de Oliveira Martins, L.; Posada, D. Unsorted Homology within Locus and Species Trees. Syst. Biol. 2014, 63, 988–992. [Google Scholar] [CrossRef]
- Gabaldón, T.; Koonin, E.V. Functional and Evolutionary Implications of Gene Orthology. Nat. Rev. Genet. 2013, 14, 360–366. [Google Scholar] [CrossRef]
- Hardison, R.C. Evolution of Hemoglobin and Its Genes. Cold Spring Harb. Perspect. Med. 2012, 2, a011627. [Google Scholar] [CrossRef] [PubMed]
- Soshnikova, N.; Dewaele, R.; Janvier, P.; Krumlauf, R.; Duboule, D. Duplications of Hox Gene Clusters and the Emergence of Vertebrates. Dev. Biol. 2013, 378, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yang, J.; Zhou, J.; Liu, G.; Shen, L.; Zhou, Z.; Su, Z.; Gu, X. Anciently Duplicated Genes Continuously Recruited to Heart Expression in Vertebrate Evolution Are Associated with Heart Chamber Increase. J. Exp. Zool. Part B: Mol. Dev. Evol. 2024, 342, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Zahn-Zabal, M.; Dessimoz, C.; Glover, N.M. Identifying Orthologs with OMA: A Primer. F1000Research 2020, 9, 27. [Google Scholar] [CrossRef]
- Hu, Y.; Flockhart, I.; Vinayagam, A.; Bergwitz, C.; Berger, B.; Perrimon, N.; Mohr, S.E. An Integrative Approach to Ortholog Prediction for Disease-Focused and Other Functional Studies. BMC Bioinform. 2011, 12, 357. [Google Scholar] [CrossRef]
- Cao, C.; Li, L.; Zhang, Q.; Li, H.; Wang, Z.; Wang, A.; Liu, J. Nkx2.5: A Crucial Regulator of Cardiac Development, Regeneration and Diseases. Front. Cardiovasc. Med. 2023, 10, 1270951. [Google Scholar] [CrossRef]
- Mio, C.; Baldan, F.; Damante, G. NK2 Homeobox Gene Cluster: Functions and Roles in Human Diseases. Genes Dis. 2023, 10, 2038–2048. [Google Scholar] [CrossRef]
- Yoo, S.; Nair, S.; Kim, H.-J.; Kim, Y.; Lee, C.; Lee, G.; Park, J.H. Knock-in Mutations of Scarecrow, a Drosophila Homolog of Mammalian Nkx2.1, Reveal a Novel Function Required for Development of the Optic Lobe in Drosophila melanogaster. Dev. Biol. 2020, 461, 145–159. [Google Scholar] [CrossRef]
- Jiménez, F.; Martin-Morris, L.E.; Velasco, L.; Chu, H.; Sierra, J.; Rosen, D.R.; White, K. Vnd, a Gene Required for Early Neurogenesis of Drosophila, Encodes a Homeodomain Protein. EMBO J. 1995, 14, 3487–3495. [Google Scholar] [CrossRef]
- Chmykhalo, V.K.; Amendola, D.; Shidlovskii, Y.V.; Lebedeva, L.A.; Schedl, P.; Giordano, E. Functional Role of Bap170 Domains in Enhancer-Dependent Gene Activity in Drosophila melanogaster. Dokl. Biochem. Biophys. 2025, 520, 152–155. [Google Scholar] [CrossRef]
- Scott, I.C. Life Before Nkx2.5. In Current Topics in Developmental Biology; Heart Development; Bruneau, B.G., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 100, pp. 1–31. [Google Scholar]
- Lien, C.-L.; Wu, C.; Mercer, B.; Webb, R.; Richardson, J.A.; Olson, E.N. Control of Early Cardiac-Specific Transcription of Nkx2-5 by a GATA-Dependent Enhancer. Development 1999, 126, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yan, W.; Mohun, T.J.; Evans, S.M. Vertebrate Tinman Homologues XNkx2-3 and XNkx2-5 Are Required for Heart Formation in a Functionally Redundant Manner. Development 1998, 125, 4439–4449. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lewis, C.; Turbay, D.; Chung, A.; Chen, J.-N.; Evans, S.; Breitbart, R.E.; Fishman, M.C.; Izumo, S.; Bodmer, R. Differential Rescue of Visceral and Cardiac Defects in Drosophila by Vertebrate Tinman-Related Genes. Proc. Natl. Acad. Sci. USA 1998, 95, 9366–9371. [Google Scholar] [CrossRef] [PubMed]
- Reim, I.; Frasch, M.; Schaub, C. T-Box Genes in Drosophila Mesoderm Development. In Current Topics in Developmental Biology; T-box Genes in Development and Disease; Frasch, M., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 122, pp. 161–193. [Google Scholar]
- Sadahiro, T.; Isomi, M.; Muraoka, N.; Kojima, H.; Haginiwa, S.; Kurotsu, S.; Tamura, F.; Tani, H.; Tohyama, S.; Fujita, J.; et al. Tbx6 Induces Nascent Mesoderm from Pluripotent Stem Cells and Temporally Controls Cardiac versus Somite Lineage Diversification. Cell Stem Cell 2018, 23, 382–395.e5. [Google Scholar] [CrossRef]
- Pflugfelder, G.O.; Roth, H.; Poeck, B.; Kerscher, S.; Schwarz, H.; Jonschker, B.; Heisenberg, M. The Lethal(1)Optomotor-Blind Gene of Drosophila melanogaster Is a Major Organizer of Optic Lobe Development: Isolation and Characterization of the Gene. Proc. Natl. Acad. Sci. USA 1992, 89, 1199–1203. [Google Scholar] [CrossRef]
- Grimm, S.; Pflugfelder, G.O. Control of the Gene Optomotor-Blind in Drosophila Wing Development by Decapentaplegic and Wingless. Science 1996, 271, 1601–1604. [Google Scholar] [CrossRef]
- Liu, N.; Schoch, K.; Luo, X.; Pena, L.D.M.; Bhavana, V.H.; Kukolich, M.K.; Stringer, S.; Powis, Z.; Radtke, K.; Mroske, C.; et al. Functional Variants in TBX2 Are Associated with a Syndromic Cardiovascular and Skeletal Developmental Disorder. Hum. Mol. Genet. 2018, 27, 2454–2465. [Google Scholar] [CrossRef]
- Couderc, J.-L.; Godt, D.; Zollman, S.; Chen, J.; Li, M.; Tiong, S.; Cramton, S.E.; Sahut-Barnola, I.; Laski, F.A. The Bric à Brac Locus Consists of Two Paralogous Genes Encoding BTB/POZ Domain Proteins and Acts as a Homeotic and Morphogenetic Regulator of Imaginal Development in Drosophila. Development 2002, 129, 2419–2433. [Google Scholar] [CrossRef]
- Bourbon, H.-M.G.; Benetah, M.H.; Guillou, E.; Mojica-Vazquez, L.H.; Baanannou, A.; Bernat-Fabre, S.; Loubiere, V.; Bantignies, F.; Cavalli, G.; Boube, M. A Shared Ancient Enhancer Element Differentially Regulates the Bric-a-Brac Tandem Gene Duplicates in the Developing Drosophila Leg. PLoS Genet. 2022, 18, e1010083. [Google Scholar] [CrossRef]
- Junion, G.; Bataillé, L.; Jagla, T.; Ponte, J.P.D.; Tapin, R.; Jagla, K. Genome-Wide View of Cell Fate Specification: Ladybird Acts at Multiple Levels during Diversification of Muscle and Heart Precursors. Genes Dev. 2007, 21, 3163–3180. [Google Scholar] [CrossRef]
- Rauzi, M.; Hočevar Brezavšček, A.; Ziherl, P.; Leptin, M. Physical Models of Mesoderm Invagination in Drosophila Embryo. Biophys. J. 2013, 105, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Saavedra, M.; Rock, A.Q.; Martindale, M.Q. Germ Layer-Specific Regulation of Cell Polarity and Adhesion Gives Insight into the Evolution of Mesoderm. eLife 2018, 7, e36740. [Google Scholar] [CrossRef] [PubMed]
- Nájera, G.S.; Weijer, C.J. The Evolution of Gastrulation Morphologies. Development 2023, 150, dev200885. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Heisenberg, C.-P. Convergent Extension: Using Collective Cell Migration and Cell Intercalation to Shape Embryos. Development 2012, 139, 3897–3904. [Google Scholar] [CrossRef]
- Holland, L.Z. Evolution of Basal Deuterostome Nervous Systems. J. Exp. Biol. 2015, 218, 637–645. [Google Scholar] [CrossRef]
- Martindale, M.Q. Evolution of Development: The Details Are in the Entrails. Curr. Biol. 2013, 23, R25–R28. [Google Scholar] [CrossRef]
- Ruggiero, M.A.; Gordon, D.P.; Orrell, T.M.; Bailly, N.; Bourgoin, T.; Brusca, R.C.; Cavalier-Smith, T.; Guiry, M.D.; Kirk, P.M. A Higher Level Classification of All Living Organisms. PLoS ONE 2015, 10, e0119248. [Google Scholar] [CrossRef]
- Court, R.; Namiki, S.; Armstrong, J.D.; Börner, J.; Card, G.; Costa, M.; Dickinson, M.; Duch, C.; Korff, W.; Mann, R.; et al. A Systematic Nomenclature for the Drosophila Ventral Nerve Cord. Neuron 2020, 107, 1071–1079.e2. [Google Scholar] [CrossRef]
- Arendt, D. Animal Evolution: Convergent Nerve Cords? Curr. Biol. 2018, 28, R225–R227. [Google Scholar] [CrossRef]
- Gerhart, J. Inversion of the Chordate Body Axis: Are There Alternatives? Proc. Natl. Acad. Sci. USA 2000, 97, 4445–4448. [Google Scholar] [CrossRef]
- Arendt, D.; Nübler-Jung, K. Inversion of Dorsoventral Axis? Nature 1994, 371, 26. [Google Scholar] [CrossRef] [PubMed]
- Bier, E.; Bodmer, R. Drosophila, an Emerging Model for Cardiac Disease. Gene 2004, 342, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saijoh, Y.; Hamada, H. Making the Right Loop for the Heart. Dev. Cell 2020, 55, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Dor, Y.; Camenisch, T.D.; Itin, A.; Fishman, G.I.; McDonald, J.A.; Carmeliet, P.; Keshet, E. A Novel Role for VEGF in Endocardial Cushion Formation and Its Potential Contribution to Congenital Heart Defects. Development 2001, 128, 1531–1538. [Google Scholar] [CrossRef]
- Jiao, K.; Langworthy, M.; Batts, L.; Brown, C.B.; Moses, H.L.; Baldwin, H.S. Tgfβ Signaling Is Required for Atrioventricular Cushion Mesenchyme Remodeling during in Vivo Cardiac Development. Development 2006, 133, 4585–4593. [Google Scholar] [CrossRef]
- Jensen, B.; Wang, T.; Moorman, A.F.M. Evolution and Development of the Atrial Septum. Anat. Rec. 2019, 302, 32–48. [Google Scholar] [CrossRef]
- Poelmann, R.E.; Groot, A.C.G.; Vicente-Steijn, R.; Wisse, L.J.; Bartelings, M.M.; Everts, S.; Hoppenbrouwers, T.; Kruithof, B.P.T.; Jensen, B.; de Bruin, P.W.; et al. Evolution and Development of Ventricular Septation in the Amniote Heart. PLoS ONE 2014, 9, e106569. [Google Scholar] [CrossRef]
- Choma, M.A.; Suter, M.J.; Vakoc, B.J.; Bouma, B.E.; Tearney, G.J. Physiological Homology between Drosophila melanogaster and Vertebrate Cardiovascular Systems. Dis. Models Mech. 2011, 4, 411–420. [Google Scholar] [CrossRef]
- Wu, M.; Sato, T.N. On the Mechanics of Cardiac Function of Drosophila Embryo. PLoS ONE 2008, 3, e4045. [Google Scholar] [CrossRef]
- Venken, K.J.T.; Sarrion-Perdigones, A.; Vandeventer, P.J.; Abel, N.S.; Christiansen, A.E.; Hoffman, K.L. Genome Engineering: Drosophila melanogaster and Beyond. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 233–267. [Google Scholar] [CrossRef]
- Nim, H.T.; Dang, L.; Thiyagarajah, H.; Bakopoulos, D.; See, M.; Charitakis, N.; Sibbritt, T.; Eichenlaub, M.P.; Archer, S.K.; Fossat, N.; et al. A Cis-Regulatory-Directed Pipeline for the Identification of Genes Involved in Cardiac Development and Disease. Genome Biol. 2021, 22, 335. [Google Scholar] [CrossRef]
- Auxerre-Plantié, E.; Nielsen, T.; Grunert, M.; Olejniczak, O.; Perrot, A.; Özcelik, C.; Harries, D.; Matinmehr, F.; Dos Remedios, C.; Mühlfeld, C.; et al. Identification of MYOM2 as a Candidate Gene in Hypertrophic Cardiomyopathy and Tetralogy of Fallot, and Its Functional Evaluation in the Drosophila Heart. Dis. Models Mech. 2020, 13, dmm045377. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Kalay, G. Cis-Regulatory Elements: Molecular Mechanisms and Evolutionary Processes Underlying Divergence. Nat. Rev. Genet. 2012, 13, 59–69. [Google Scholar] [CrossRef]
- Szallasi, Z. To Kill Two Birds with One Stone: A General Concept in Gene Regulation? Trends Pharmacol. Sci. 2001, 22, 110. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.M.; Pan, Y.K.; Chandrapalan, T.; Kwong, R.W.M.; Perry, S.F. Loss-of-Function Approaches in Comparative Physiology: Is There a Future for Knockdown Experiments in the Era of Genome Editing? J. Exp. Biol. 2019, 222, jeb175737. [Google Scholar] [CrossRef] [PubMed]
- Haiyong, H. RNA Interference to Knock Down Gene Expression. Methods Mol. Biol. 2018, 1706, 293–302. [Google Scholar] [CrossRef]
- Ghosh, A.; Banerjee, A.; Gupta, S.; Sinha, S. A Unified Phosphoramidite Platform for the Synthesis of Morpholino Oligonucleotides and Diverse Chimeric Backbones. J. Am. Chem. Soc. 2024, 146, 32989–33001. [Google Scholar] [CrossRef]
- Kok, F.O.; Shin, M.; Ni, C.-W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse Genetic Screening Reveals Poor Correlation between Morpholino-Induced and Mutant Phenotypes in Zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef]
- Paschon, D.E.; Lussier, S.; Wangzor, T.; Xia, D.F.; Li, P.W.; Hinkley, S.J.; Scarlott, N.A.; Lam, S.C.; Waite, A.J.; Truong, L.N.; et al. Diversifying the Structure of Zinc Finger Nucleases for High-Precision Genome Editing. Nat. Commun. 2019, 10, 1133. [Google Scholar] [CrossRef]
- Becker, S.; Boch, J. TALE and TALEN Genome Editing Technologies. Gene Genome Ed. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Sloutskin, A.; Itzhak, D.; Vogler, G.; Pozeilov, H.; Ideses, D.; Alter, H.; Adato, O.; Shachar, H.; Doniger, T.; Shohat-Ophir, G.; et al. From Promoter Motif to Cardiac Function: A Single DPE Motif Affects Transcription Regulation and Organ Function in Vivo. Development 2024, 151, dev202355. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Joung, J.K. Defining and Improving the Genome-Wide Specificities of CRISPR–Cas9 Nucleases. Nat. Rev. Genet. 2016, 17, 300–312. [Google Scholar] [CrossRef]
- Fu, Y.; Sander, J.D.; Reyon, D.; Cascio, V.M.; Joung, J.K. Improving CRISPR-Cas Nuclease Specificity Using Truncated Guide RNAs. Nat. Biotechnol. 2014, 32, 279–284. [Google Scholar] [CrossRef]
- Arkin, M. In Vitro Mutagenesis. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 46–50. ISBN 978-0-08-096156-9. [Google Scholar]
- Franke, J.D.; Montague, R.A.; Rickoll, W.L.; Kiehart, D.P. An MYH9 Human Disease Model in Flies: Site-Directed Mutagenesis of the Drosophila Non-Muscle Myosin II Results in Hypomorphic Alleles with Dominant Character. Hum. Mol. Genet. 2007, 16, 3160–3173. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chang, Y.-Y.; Chan, C.-C. Strategies for Gene Disruption in Drosophila. Cell Biosci. 2014, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Roote, J.; Russell, S. Toward a Complete Drosophiladeficiency Kit. Genome Biol. 2012, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Prelich, G. Gene Overexpression: Uses, Mechanisms, and Interpretation. Genetics 2012, 190, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Jagla, K.; Frasch, M.; Jagla, T.; Dretzen, G.; Bellard, F.; Bellard, M. Ladybird, a New Component of the Cardiogenic Pathway in Drosophila Required for Diversification of Heart Precursors. Development 1997, 124, 3471–3479. [Google Scholar] [CrossRef]
- Graba, Y.; Gieseler, K.; Aragnol, D.; Laurenti, P.; Mariol, M.-C.; Berenger, H.; Sagnier, T.; Pradel, J. DWnt-4, a Novel Drosophila Wnt Gene Acts Downstream of Homeotic Complex Genes in the Visceral Mesoderm. Development 1995, 121, 209–218. [Google Scholar] [CrossRef]
- Tauc, H.M.; Mann, T.; Werner, K.; Pandur, P. A Role for Drosophila Wnt-4 in Heart Development. Genesis 2012, 50, 466–481. [Google Scholar] [CrossRef]
- Elliott, D.A.; Brand, A.H. The GAL4 System. In Drosophila: Methods and Protocols; Dahmann, C., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 79–95. ISBN 978-1-59745-583-1. [Google Scholar]
- Xu, Y.; Gan, E.-S.; Ito, T. Misexpression Approaches for the Manipulation of Flower Development. Methods Mol. Biol. 2023, 2686, 429–451. [Google Scholar] [CrossRef]
- Veitia, R.A. Dominant Negative Variants and Cotranslational Assembly of Macromolecular Complexes. BioEssays 2023, 45, 2300105. [Google Scholar] [CrossRef]
- Harrington, S.A.; Backhaus, A.E.; Fox, S.; Rogers, C.; Borrill, P.; Uauy, C.; Richardson, A. A Heat-Shock Inducible System for Flexible Gene Expression in Cereals. Plant Methods 2020, 16, 137. [Google Scholar] [CrossRef]
- Park, M.; Wu, X.; Golden, K.; Axelrod, J.D.; Bodmer, R. The Wingless Signaling Pathway Is Directly Involved in Drosophila Heart Development. Dev. Biol. 1996, 177, 104–116. [Google Scholar] [CrossRef]
- Schramm, T.; Lubrano, P.; Pahl, V.; Stadelmann, A.; Verhülsdonk, A.; Link, H. Mapping Temperature-sensitive Mutations at a Genome Scale to Engineer Growth Switches in Escherichia coli. Mol. Syst. Biol. 2023, 19, e11596. [Google Scholar] [CrossRef]
- Susman, M. Conditional Lethality. In Encyclopedia of Genetics; Brenner, S., Miller, J.H., Eds.; Academic Press: New York, NY, USA, 2001; pp. 441–443. ISBN 978-0-12-227080-2. [Google Scholar]
- Bodmer, R.; Jan, L.Y.; Jan, Y.N. A New Homeobox-Containing Gene, Msh-2, Is Transiently Expressed Early during Mesoderm Formation of Drosophila. Development 1990, 110, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Ishii, M.; Sun, J.; Sucov, H.M.; Maxson, R.E. Msx1 and Msx2 Regulate Survival of Secondary Heart Field Precursors and Post-Migratory Proliferation of Cardiac Neural Crest in the Outflow Tract. Dev. Biol. 2007, 308, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R. The Gene Tinman Is Required for Specification of the Heart and Visceral Muscles in Drosophila. Development 1993, 118, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Reim, I.; Frasch, M. The Dorsocross T-Box Genes Are Key Components of the Regulatory Network Controlling Early Cardiogenesis in Drosophila. Development 2005, 132, 4911–4925. [Google Scholar] [CrossRef]
- Lovato, T.L.; Blotz, B.; Bileckyj, C.; Johnston, C.A.; Cripps, R.M. Modeling a Variant of Unknown Significance in the Drosophila Ortholog of the Human Cardiogenic Gene NKX2.5. DMM Dis. Models Mech. 2023, 16, dmm050059. [Google Scholar] [CrossRef]
- Targoff, K.L.; Schell, T.; Yelon, D. Nkx Genes Regulate Heart Tube Extension and Exert Differential Effects on Ventricular and Atrial Cell Number. Dev. Biol. 2008, 322, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Terada, R.; Warren, S.; Lu, J.T.; Chien, K.R.; Wessels, A.; Kasahara, H. Ablation of Nkx2-5 at Mid-Embryonic Stage Results in Premature Lethality and Cardiac Malformation. Cardiovasc. Res. 2011, 91, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Targoff, K.L.; Colombo, S.; George, V.; Schell, T.; Kim, S.-H.; Solnica-Krezel, L.; Yelon, D. Nkx Genes Are Essential for Maintenance of Ventricular Identity. Development 2013, 140, 4203–4213. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.A.; Kirk, E.P.; Yeoh, T.; Chandar, S.; McKenzie, F.; Taylor, P.; Grossfeld, P.; Fatkin, D.; Jones, O.; Hayes, P.; et al. Cardiac Homeobox Gene NKX2-5 Mutations and Congenital Heart Disease: Associations with Atrial Septal Defect and Hypoplastic Left Heart Syndrome. J. Am. Coll. Cardiol. 2003, 41, 2072–2076. [Google Scholar] [CrossRef]
- Lo, P.C.H.; Frasch, M. A Role for the COUP-TF-Related Gene Seven-up in the Diversification of Cardioblast Identities in the Dorsal Vessel of Drosophila. Mech. Dev. 2001, 104, 49–60. [Google Scholar] [CrossRef]
- Fujioka, M.; Wessells, R.J.; Han, Z.; Liu, J.; Fitzgerald, K.; Yusibova, G.L.; Zamora, M.; Ruiz-Lozano, P.; Bodmer, R.; Jaynes, J.B. Embryonic Even Skipped–Dependent Muscle and Heart Cell Fates Are Required for Normal Adult Activity, Heart Function, and Lifespan. Circ. Res. 2005, 97, 1108–1114. [Google Scholar] [CrossRef]
- Lo, P.C.H.; Zaffran, S.; Sénatore, S.; Frasch, M. The Drosophila Hand Gene Is Required for Remodeling of the Developing Adult Heart and Midgut during Metamorphosis. Dev. Biol. 2007, 311, 287–296. [Google Scholar] [CrossRef]
- Han, Z.; Yi, P.; Li, X.; Olson, E.N. Hand, an Evolutionarily Conserved bHLH Transcription Factor Required for Drosophila Cardiogenesis and Hematopoiesis. Development 2006, 133, 1175–1182. [Google Scholar] [CrossRef]
- Meyer, C.; Bataillé, L.; Drechsler, M.; Paululat, A. Tailup Expression in Larval and Adult Cardiac Valve Cells. Genesis 2023, 61, e23506. [Google Scholar] [CrossRef]
- Cai, C.-L.; Liang, X.; Shi, Y.; Chu, P.-H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 Identifies a Cardiac Progenitor Population That Proliferates Prior to Differentiation and Contributes a Majority of Cells to the Heart. Dev. Cell 2003, 5, 877. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, X.; Najafi, N.; Cass, M.; Lin, L.; Cai, C.-L.; Chen, J.; Evans, S.M. Islet 1 Is Expressed in Distinct Cardiovascular Lineages, Including Pacemaker and Coronary Vascular Cells. Dev. Biol. 2007, 304, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, J.; Deng, S.; Liu, B.; Jing, X.; Yan, Y.; Liu, Y.; Wang, J.; Zhou, X.; She, Q. The Molecular Mechanisms of Cardiac Development and Related Diseases. Signal Transduct. Target. Ther. 2024, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Witzel, H.R.; Cheedipudi, S.; Gao, R.; Stainier, D.Y.R.; Dobreva, G.D. Isl2b Regulates Anterior Second Heart Field Development in Zebrafish. Sci. Rep. 2017, 7, 41043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, H.-M.; Wang, F.; Zhao, C.-M.; Huang, R.-T.; Xue, S.; Li, R.-G.; Qiu, X.-B.; Xu, Y.-J.; Liu, X.-Y.; et al. A New ISL1 Loss-of-Function Mutation Predisposes to Congenital Double Outlet Right Ventricle. Int. Heart J. 2019, 60, 1113–1122. [Google Scholar] [CrossRef]
- Reim, I.; Mohler, J.P.; Frasch, M. Tbx20-Related Genes, Mid and H15, Are Required for Tinman Expression, Proper Patterning, and Normal Differentiation of Cardioblasts in Drosophila. Mech. Dev. 2005, 122, 1056–1069. [Google Scholar] [CrossRef]
- Qian, L.; Liu, J.; Bodmer, R. Neuromancer Tbx20-Related Genes (H15/Midline) Promote Cell Fate Specification and Morphogenesis of the Drosophila Heart. Dev. Biol. 2005, 279, 509–524. [Google Scholar] [CrossRef]
- Qian, L.; Mohapatra, B.; Akasaka, T.; Liu, J.; Ocorr, K.; Towbin, J.A.; Bodmer, R. Transcription Factor Neuromancer/TBX20 Is Required for Cardiac Function in Drosophila with Implications for Human Heart Disease. Proc. Natl. Acad. Sci. USA 2008, 105, 19833–19838. [Google Scholar] [CrossRef]
- Ocorr, K.; Vogler, G.; Bodmer, R. Methods to Assess Drosophila Heart Development, Function and Aging. Methods 2014, 68, 265–272. [Google Scholar] [CrossRef]
- Stennard, F.A.; Costa, M.W.; Elliott, D.A.; Rankin, S.; Haast, S.J.P.; Lai, D.; McDonald, L.P.A.; Niederreither, K.; Dolle, P.; Bruneau, B.G.; et al. Cardiac T-Box Factor Tbx20 Directly Interacts with Nkx2-5, GATA4, and GATA5 in Regulation of Gene Expression in the Developing Heart. Dev. Biol. 2003, 262, 206–224. [Google Scholar] [CrossRef]
- Gao, X.; Yan, B. The Mechanism and Diagnostic Value of Tbx20 in Cardiovascular Diseases. Gene Rep. 2023, 30, 101723. [Google Scholar] [CrossRef]
- Takeuchi, J.K.; Mileikovskaia, M.; Koshiba-Takeuchi, K.; Heidt, A.B.; Mori, A.D.; Arruda, E.P.; Gertsenstein, M.; Georges, R.; Davidson, L.; Mo, R.; et al. Tbx20 Dose-Dependently Regulates Transcription Factor Networks Required for Mouse Heart and Motoneuron Development. Development 2005, 132, 2463–2474. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Q.; Qin, Z.; Wen, Y.; Liu, C. The Role of TBX20 Gene Mutations in the Pathogenesis of Congenital Heart Disease: Functional Analysis and Genetic Association Study. Cardiology 2024, 150, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Hadjantonakis, A.-K.; Pisano, E.; Papaioannou, V.E. Tbx6 Regulates Left/Right Patterning in Mouse Embryos through Effects on Nodal Cilia and Perinodal Signaling. PLoS ONE 2008, 3, e2511. [Google Scholar] [CrossRef] [PubMed]
- Windner, S.E.; Doris, R.A.; Ferguson, C.M.; Nelson, A.C.; Valentin, G.; Tan, H.; Oates, A.C.; Wardle, F.C.; Devoto, S.H. Tbx6, Mesp-b and Ripply1 Regulate the Onset of Skeletal Myogenesis in Zebrafish. Development 2015, 142, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Harrelson, Z.; Kelly, R.G.; Goldin, S.N.; Gibson-Brown, J.J.; Bollag, R.J.; Silver, L.M.; Papaioannou, V.E. Tbx2 Is Essential for Patterning the Atrioventricular Canal and for Morphogenesis of the Outflow Tract during Heart Development. Development 2004, 131, 5041–5052. [Google Scholar] [CrossRef]
- Sparrow, D.B.; McInerney-Leo, A.; Gucev, Z.S.; Gardiner, B.; Marshall, M.; Leo, P.J.; Chapman, D.L.; Tasic, V.; Shishko, A.; Brown, M.A.; et al. Autosomal Dominant Spondylocostal Dysostosis Is Caused by Mutation in TBX6. Hum. Mol. Genet. 2013, 22, 1625–1631. [Google Scholar] [CrossRef]
- Xie, H.; Hong, N.; Zhang, E.; Li, F.; Sun, K.; Yu, Y. Identification of Rare Copy Number Variants Associated With Pulmonary Atresia With Ventricular Septal Defect. Front. Genet. 2019, 10, 15. [Google Scholar] [CrossRef]
- Singh, R.; Hoogaars, W.M.; Barnett, P.; Grieskamp, T.; Rana, M.S.; Buermans, H.; Farin, H.F.; Petry, M.; Heallen, T.; Martin, J.F.; et al. Tbx2 and Tbx3 Induce Atrioventricular Myocardial Development and Endocardial Cushion Formation. Cell. Mol. Life Sci. 2012, 69, 1377–1389. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, E.; Hong, N.; Fu, Q.; Li, F.; Chen, S.; Yu, Y.; Sun, K. Identification of TBX2 and TBX3 Variants in Patients with Conotruncal Heart Defects by Target Sequencing. Hum. Genom. 2018, 12, 44. [Google Scholar] [CrossRef]
- Goodman, F.R.; Majewski, F.; Collins, A.L.; Scambler, P.J. A 117-Kb Microdeletion Removing HOXD9–HOXD13 and EVX2 Causes Synpolydactyly. Am. J. Hum. Genet. 2002, 70, 547–555. [Google Scholar] [CrossRef]
- Yang, A.; Alankarage, D.; Cuny, H.; Ip, E.K.K.; Almog, M.; Lu, J.; Das, D.; Enriquez, A.; Szot, J.O.; Humphreys, D.T.; et al. CHDgene: A Curated Database for Congenital Heart Disease Genes. Circ. Genom. Precis. Med. 2022, 15, e003539. [Google Scholar] [CrossRef] [PubMed]
- Dohn, T.E.; Ravisankar, P.; Tirera, F.T.; Martin, K.E.; Gafranek, J.T.; Duong, T.B.; VanDyke, T.L.; Touvron, M.; Barske, L.A.; Crump, J.G.; et al. Nr2f-Dependent Allocation of Ventricular Cardiomyocyte and Pharyngeal Muscle Progenitors. PLoS Genet. 2019, 15, e1007962. [Google Scholar] [CrossRef] [PubMed]
- Al Turki, S.; Manickaraj, A.K.; Mercer, C.L.; Gerety, S.S.; Hitz, M.-P.; Lindsay, S.; D’Alessandro, L.C.A.; Swaminathan, G.J.; Bentham, J.; Arndt, A.-K.; et al. Rare Variants in NR2F2 Cause Congenital Heart Defects in Humans. Am. J. Hum. Genet. 2014, 94, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, W.; Heidari, M.M.; Khatami, M.; Hadadzadeh, M.; Tabrizi, F.; Darvand Araghi, M.H. Rare Pathogenic NR2F2 (COUP-TFII) Variants as Potential Etiological Causes in Pediatric Patients with Congenital Heart Diseases (CHDs). Hell. J. Cardiol. 2025; in press. [Google Scholar] [CrossRef]
- Wang, J.; Abhinav, P.; Xu, Y.-J.; Li, R.-G.; Zhang, M.; Qiu, X.-B.; Di, R.-M.; Qiao, Q.; Li, X.-M.; Huang, R.-T.; et al. NR2F2 Loss-of-function Mutation Is Responsible for Congenital Bicuspid Aortic Valve. Int. J. Mol. Med. 2019, 43, 1839–1846. [Google Scholar] [CrossRef]
- George, R.M.; Firulli, B.A.; Podicheti, R.; Rusch, D.B.; Mannion, B.J.; Pennacchio, L.A.; Osterwalder, M.; Firulli, A.B. Single Cell Evaluation of Endocardial Hand2 Gene Regulatory Networks Reveals HAND2-Dependent Pathways That Impact Cardiac Morphogenesis. Development 2023, 150, dev201341. [Google Scholar] [CrossRef]
- Clapham, K.R.; Singh, I.; Capuano, I.S.; Rajagopal, S.; Chun, H.J. MEF2 and the Right Ventricle: From Development to Disease. Front. Cardiovasc. Med. 2019, 6, 29. [Google Scholar] [CrossRef]
- Materna, S.C.; Sinha, T.; Barnes, R.M.; Lammerts van Bueren, K.; Black, B.L. Cardiovascular Development and Survival Require Mef2c Function in the Myocardial but Not the Endothelial Lineage. Dev. Biol. 2019, 445, 170–177. [Google Scholar] [CrossRef]
- Li, F.-F.; Han, Y.; Shi, S.; Li, X.; Zhu, X.-D.; Zhou, J.; Shao, Q.-L.; Li, X.-Q.; Liu, S.-L. Characterization of Transcriptional Repressor Gene MSX1 Variations for Possible Associations with Congenital Heart Diseases. PLoS ONE 2015, 10, e0142666. [Google Scholar] [CrossRef]
- Jamsheer, A.; Sowińska, A.; Simon, D.; Jamsheer-Bratkowska, M.; Trzeciak, T.; Latos-Bieleńska, A. Bilateral Radial Agenesis with Absent Thumbs, Complex Heart Defect, Short Stature, and Facial Dysmorphism in a Patient with Pure Distal Microduplication of 5q35.2-5q35.3. BMC Med. Genet. 2013, 14, 13. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Wang, J.; Qiu, X.-B.; Yuan, F.; Li, R.-G.; Xu, Y.-J.; Qu, X.-K.; Shi, H.-Y.; Hou, X.-M.; Huang, R.-T.; et al. A HAND2 Loss-of-Function Mutation Causes Familial Ventricular Septal Defect and Pulmonary Stenosis. G3 Genes Genomes Genet. 2016, 6, 987–992. [Google Scholar] [CrossRef]
- Lu, C.-X.; Wang, W.; Wang, Q.; Liu, X.-Y.; Yang, Y.-Q. A Novel MEF2C Loss-of-Function Mutation Associated with Congenital Double Outlet Right Ventricle. Pediatr. Cardiol. 2018, 39, 794–804. [Google Scholar] [CrossRef]
- Zeng, Z.-H.; Chen, H.-X.; Liu, X.-C.; Yang, Q.; He, G.-W. Functional Significance of Novel Variants of the MEF2C Gene Promoter in Congenital Ventricular Septal Defects. Am. J. Med. Genet. Part A 2022, 188, 2397–2405. [Google Scholar] [CrossRef]
- Qiao, X.-H.; Wang, F.; Zhang, X.-L.; Huang, R.-T.; Xue, S.; Wang, J.; Qiu, X.-B.; Liu, X.-Y.; Yang, Y.-Q. MEF2C Loss-of-Function Mutation Contributes to Congenital Heart Defects. Int. J. Med. Sci. 2017, 14, 1143–1153. [Google Scholar] [CrossRef]
- Kodo, K.; Nishizawa, T.; Furutani, M.; Arai, S.; Ishihara, K.; Oda, M.; Makino, S.; Fukuda, K.; Takahashi, T.; Matsuoka, R.; et al. Genetic Analysis of Essential Cardiac Transcription Factors in 256 Patients With Non-Syndromic Congenital Heart Defects. Circ. J. 2012, 76, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Han, Z.; Li, X.; Olson, E.N. The Mevalonate Pathway Controls Heart Formation in Drosophila by Isoprenylation of Gγ1. Science 2006, 313, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.L.; Vogler, G.; Missinato, M.A.; Li, X.; Nielsen, T.; Zeng, X.-X.I.; Martinez-Fernandez, A.; Walls, S.M.; Kervadec, A.; Kezos, J.N.; et al. Patient-Specific Genomics and Cross-Species Functional Analysis Implicate LRP2 in Hypoplastic Left Heart Syndrome. eLife 2020, 9, e59554. [Google Scholar] [CrossRef] [PubMed]
- Riedel, F.; Vorkel, D.; Eaton, S. Megalin-Dependent Yellow Endocytosis Restricts Melanization in the Drosophila Cuticle. Development 2011, 138, 149–158. [Google Scholar] [CrossRef]
- Basu, M.; Zhu, J.-Y.; LaHaye, S.; Majumdar, U.; Jiao, K.; Han, Z.; Garg, V. Epigenetic Mechanisms Underlying Maternal Diabetes-Associated Risk of Congenital Heart Disease. JCI Insight 2017, 2, e95085. [Google Scholar] [CrossRef]
- Schroeder, A.M.; Allahyari, M.; Vogler, G.; Missinato, M.A.; Nielsen, T.; Yu, M.S.; Theis, J.L.; Larsen, L.A.; Goyal, P.; Rosenfeld, J.A.; et al. Model System Identification of Novel Congenital Heart Disease Gene Candidates: Focus on RPL13. Hum. Mol. Genet. 2019, 28, 3954–3969. [Google Scholar] [CrossRef]
- Edison, R.J.; Muenke, M. Central Nervous System and Limb Anomalies in Case Reports of First-Trimester Statin Exposure. N. Engl. J. Med. 2004, 350, 1579–1582. [Google Scholar] [CrossRef]
- Edison, R.J.; Muenke, M. Gestational Exposure to Lovastatin Followed by Cardiac Malformation Misclassified as Holoprosencephaly. N. Engl. J. Med. 2005, 352, 2759. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Ocorr, K.; Lin, L.; Vogler, G.; Bodmer, R.; Grossfeld, P. Overexpression of Kif1A in the Developing Drosophila Heart Causes Valvar and Contractility Defects: Implications for Human Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Birker, K.; Ge, S.; Kirkland, N.J.; Theis, J.L.; Marchant, J.; Fogarty, Z.C.; Missinato, M.A.; Kalvakuri, S.; Grossfeld, P.; Engler, A.J.; et al. Mitochondrial MICOS Complex Genes, Implicated in Hypoplastic Left Heart Syndrome, Maintain Cardiac Contractility and Actomyosin Integrity. eLife 2023, 12, e83385. [Google Scholar] [CrossRef] [PubMed]
- Boukhatmi, H.; Schaub, C.; Bataillé, L.; Reim, I.; Frendo, J.-L.; Frasch, M.; Vincent, A. An Org-1–Tup Transcriptional Cascade Reveals Different Types of Alary Muscles Connecting Internal Organs in Drosophila. Development 2014, 141, 3761–3771. [Google Scholar] [CrossRef]
- Lilly, B.; Zhao, B.; Ranganayakulu, G.; Paterson, B.M.; Schulz, R.A.; Olson, E.N. Requirement of MADS Domain Transcription Factor D-MEF2 for Muscle Formation in Drosophila. Science 1995, 267, 688–693. [Google Scholar] [CrossRef]
- Ryan, K.M.; Hendren, J.D.; Helander, L.A.; Cripps, R.M. The NK Homeodomain Transcription Factor Tinman Is a Direct Activator of Seven-up in the Drosophila Dorsal Vessel. Dev. Biol. 2007, 302, 694–702. [Google Scholar] [CrossRef]
- Schaub, C.; Nagaso, H.; Jin, H.; Frasch, M. Org-1, the Drosophila Ortholog of Tbx1, Is a Direct Activator of Known Identity Genes during Muscle Specification. Development 2012, 139, 1001–1012. [Google Scholar] [CrossRef]
- Pareek, G.; Thomas, R.E.; Pallanck, L.J. Loss of the Drosophila M-AAA Mitochondrial Protease Paraplegin Results in Mitochondrial Dysfunction, Shortened Lifespan, and Neuronal and Muscular Degeneration. Cell Death Dis. 2018, 9, 304. [Google Scholar] [CrossRef]
- He, L.; Wu, B.; Shi, J.; Du, J.; Zhao, Z. Regulation of Feeding and Energy Homeostasis by Clock-Mediated Gart in Drosophila. Cell Rep. 2023, 42, 112912. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Q.; Wang, H.; Yang, X.; Mu, H. Alternative Splicing and Related RNA Binding Proteins in Human Health and Disease. Signal Transduct. Target. Ther. 2024, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Blockus, H.; Chédotal, A. Slit-Robo Signaling. Development 2016, 143, 3037–3044. [Google Scholar] [CrossRef] [PubMed]
- Medioni, C.; Astier, M.; Zmojdzian, M.; Jagla, K.; Sémériva, M. Genetic Control of Cell Morphogenesis during Drosophila melanogaster Cardiac Tube Formation. J. Cell Biol. 2008, 182, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Maartens, A.P.; Brown, N.H. The Many Faces of Cell Adhesion during Drosophila Muscle Development. Dev. Biol. 2015, 401, 62–74. [Google Scholar] [CrossRef]
- Vanderploeg, J.; Jacobs, J.R. Mapping Heart Development in Flies: Src42A Acts Non-Autonomously to Promote Heart Tube Formation in Drosophila. Vet. Sci. 2017, 4, 23. [Google Scholar] [CrossRef]
- Santiago-Martínez, E.; Soplop, N.H.; Patel, R.; Kramer, S.G. Repulsion by Slit and Roundabout Prevents Shotgun/E-Cadherin-Mediated Cell Adhesion during Drosophila Heart Tube Lumen Formation. J. Cell Biol. 2008, 182, 241–248. [Google Scholar] [CrossRef]
- Chartier, A.; Zaffran, S.; Astier, M.; Sémériva, M.; Gratecos, D. Pericardin, a Drosophila Type IV Collagen-like Protein Is Involved in the Morphogenesis and Maintenance of the Heart Epithelium during Dorsal Ectoderm Closure. Development 2002, 129, 3241–3253. [Google Scholar] [CrossRef]
- Vogler, G.; Liu, J.; Iafe, T.W.; Migh, E.; Mihály, J.; Bodmer, R. Cdc42 and Formin Activity Control Non-Muscle Myosin Dynamics during Drosophila Heart Morphogenesis. J. Cell Biol. 2014, 206, 909–922. [Google Scholar] [CrossRef]
- Hansen, S.D.; Mullins, R.D. Lamellipodin Promotes Actin Assembly by Clustering Ena/VASP Proteins and Tethering Them to Actin Filaments. eLife 2015, 4, e06585. [Google Scholar] [CrossRef]
- Raza, Q.S.; Vanderploeg, J.L.; Jacobs, J.R. Matrix Metalloproteinases Are Required for Membrane Motility and Lumenogenesis during Drosophila Heart Development. PLoS ONE 2017, 12, e0171905. [Google Scholar] [CrossRef]
- Hughes, C.J.R.; Turner, S.; Andrews, R.M.; Vitkin, A.; Jacobs, J.R. Matrix Metalloproteinases Regulate ECM Accumulation but Not Larval Heart Growth in Drosophila melanogaster. J. Mol. Cell. Cardiol. 2020, 140, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Linask, K.K.; Han, M.; Cai, D.H.; Brauer, P.R.; Maisastry, S.M. Cardiac Morphogenesis: Matrix Metalloproteinase Coordination of Cellular Mechanisms Underlying Heart Tube Formation and Directionality of Looping. Dev. Dyn. 2005, 233, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Singh, A.; Singh, J.; Mutsuddi, M.; Mukherjee, A. Regulation of Notch Signaling by Non-Muscle Myosin II Zipper in Drosophila. Cell Mol. Life Sci. 2024, 81, 195. [Google Scholar] [CrossRef]
- Molnár, I.; Migh, E.; Szikora, S.; Kalmár, T.; Végh, A.G.; Deák, F.; Barkó, S.; Bugyi, B.; Orfanos, Z.; Kovács, J.; et al. DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila. PLoS Genet. 2014, 10, e1004166. [Google Scholar] [CrossRef]
- Migh, E.; Götz, T.; Földi, I.; Szikora, S.; Gombos, R.; Darula, Z.; Medzihradszky, K.F.; Maléth, J.; Hegyi, P.; Sigrist, S.; et al. Microtubule Organization in Presynaptic Boutons Relies on the Formin DAAM. Development 2018, 145, dev158519. [Google Scholar] [CrossRef]
- Gombos, R.; Migh, E.; Antal, O.; Mukherjee, A.; Jenny, A.; Mihály, J. The Formin DAAM Functions as Molecular Effector of the Planar Cell Polarity Pathway during Axonal Development in Drosophila. J. Neurosci. 2015, 35, 10154–10167. [Google Scholar] [CrossRef]
- Qian, L.; Wythe, J.D.; Liu, J.; Cartry, J.; Vogler, G.; Mohapatra, B.; Otway, R.T.; Huang, Y.; King, I.N.; Maillet, M.; et al. Tinman/Nkx2-5 Acts via miR-1 and Upstream of Cdc42 to Regulate Heart Function across Species. J. Cell Biol. 2011, 193, 1181–1196. [Google Scholar] [CrossRef]
- Kadam, S.; McMahon, A.; Tzou, P.; Stathopoulos, A. FGF Ligands in Drosophila Have Distinct Activities Required to Support Cell Migration and Differentiation. Development 2009, 136, 739–747. [Google Scholar] [CrossRef]
- Dorey, K.; Amaya, E. FGF Signalling: Diverse Roles during Early Vertebrate Embryogenesis. Development 2010, 137, 3731–3742. [Google Scholar] [CrossRef]
- Hubert, F.; Payan, S.M.; Rochais, F. FGF10 Signaling in Heart Development, Homeostasis, Disease and Repair. Front. Genet. 2018, 9, 599. [Google Scholar] [CrossRef]
- Hutson, M.R.; Zeng, X.L.; Kim, A.J.; Antoon, E.; Harward, S.; Kirby, M.L. Arterial Pole Progenitors Interpret Opposing FGF/BMP Signals to Proliferate or Differentiate. Development 2010, 137, 3001–3011. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Golden, K.; Bodmer, R. Heart Development in Drosophila Requires the Segment Polarity Gene Wingless. Dev. Biol. 1995, 169, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Frasch, M. Regulation and Function of Tinman during Dorsal Mesoderm Induction and Heart Specification in Drosophila. Dev. Genet. 1998, 22, 187–200. [Google Scholar] [CrossRef]
- Johnson, A.N.; Burnett, L.A.; Sellin, J.; Paululat, A.; Newfeld, S.J. Defective Decapentaplegic Signaling Results in Heart Overgrowth and Reduced Cardiac Output in Drosophila. Genetics 2007, 176, 1609–1624. [Google Scholar] [CrossRef]
- Lockwood, W.K.; Bodmer, R. The Patterns of Wingless, Decapentaplegic, and Tinman Position the Drosophila Heart. Mech. Dev. 2002, 114, 13–26. [Google Scholar] [CrossRef]
- Mazzotta, S.; Neves, C.; Bonner, R.J.; Bernardo, A.S.; Docherty, K.; Hoppler, S. Distinctive Roles of Canonical and Noncanonical Wnt Signaling in Human Embryonic Cardiomyocyte Development. Stem Cell Rep. 2016, 7, 764–776. [Google Scholar] [CrossRef]
- Ueno, S.; Weidinger, G.; Osugi, T.; Kohn, A.D.; Golob, J.L.; Pabon, L.; Reinecke, H.; Moon, R.T.; Murry, C.E. Biphasic Role for Wnt/β-Catenin Signaling in Cardiac Specification in Zebrafish and Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9685–9690. [Google Scholar] [CrossRef]
- Cohen, E.D.; Tian, Y.; Morrisey, E.E. Wnt Signaling: An Essential Regulator of Cardiovascular Differentiation, Morphogenesis and Progenitor Self-Renewal. Development 2008, 135, 789–798. [Google Scholar] [CrossRef]
- Tanegashima, K.; Zhao, H.; Dawid, I.B. WGEF Activates Rho in the Wnt–PCP Pathway and Controls Convergent Extension in Xenopus Gastrulation. EMBO J. 2008, 27, 606–617. [Google Scholar] [CrossRef]
- Shi, Y.; Katsev, S.; Cai, C.; Evans, S. BMP Signaling Is Required for Heart Formation in Vertebrates. Dev. Biol. 2000, 224, 226–237. [Google Scholar] [CrossRef]
- Vasudevarao, M.D.; Posadas Pena, D.; Ihle, M.; Bongiovanni, C.; Maity, P.; Geissler, D.; Mohammadi, H.F.; Rall-Scharpf, M.; Niemann, J.; Mommersteeg, M.T.M.; et al. BMP Signaling Promotes Zebrafish Heart Regeneration via Alleviation of Replication Stress. Nat. Commun. 2025, 16, 1708. [Google Scholar] [CrossRef]
- Bhanot, P.; Fish, M.; Jemison, J.A.; Nusse, R.; Nathans, J.; Cadigan, K.M. Frizzled and DFrizzled-2 Function as Redundant Receptors for Wingless during Drosophila Embryonic Development. Development 1999, 126, 4175–4186. [Google Scholar] [CrossRef]
- Chen, C.; Struhl, G. Wingless Transduction by the Frizzled and Frizzled2 Proteins of Drosophila. Development 1999, 126, 5441–5452. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, J.; Fu, Y.; Richman, A.; Han, Z. Wnt4 Is Required for Ostia Development in the Drosophila Heart. Dev. Biol. 2016, 413, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, G.V.; Nodal, D.H.; Lovato, C.V.; Hendren, J.D.; Helander, L.A.; Lovato, T.L.; Bodmer, R.; Cripps, R.M. The Canonical Wingless Signaling Pathway Is Required but Not Sufficient for Inflow Tract Formation in the Drosophila melanogaster Heart. Dev. Biol. 2016, 413, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Schleiffarth, J.R.; Person, A.D.; Martinsen, B.J.; Sukovich, D.J.; Neumann, A.; Baker, C.V.H.; Lohr, J.L.; Cornfield, D.N.; Ekker, S.C.; Petryk, A. Wnt5a Is Required for Cardiac Outflow Tract Septation in Mice. Pediatr. Res. 2007, 61, 386–391. [Google Scholar] [CrossRef]
- van Vliet, P.P.; Lin, L.; Boogerd, C.J.; Martin, J.F.; Andelfinger, G.; Grossfeld, P.D.; Evans, S.M. Tissue Specific Requirements for WNT11 in Developing Outflow Tract and Dorsal Mesenchymal Protrusion. Dev. Biol. 2017, 429, 249–259. [Google Scholar] [CrossRef]
- Touma, M.; Kang, X.; Gao, F.; Zhao, Y.; Cass, A.A.; Biniwale, R.; Xiao, X.; Eghbali, M.; Coppola, G.; Reemtsen, B.; et al. Wnt11 Regulates Cardiac Chamber Development and Disease during Perinatal Maturation. JCI Insight 2017, 2, e94904. [Google Scholar] [CrossRef]
- Mohamed, I.A.; El-Badri, N.; Zaher, A. Wnt Signaling: The Double-Edged Sword Diminishing the Potential of Stem Cell Therapy in Congenital Heart Disease. Life Sci. 2019, 239, 116937. [Google Scholar] [CrossRef]
- Von Ohlen, T.; Hooper, J.E. Hedgehog Signaling Regulates Transcription through Gli/Ci Binding Sites in the Wingless Enhancer. Mech. Dev. 1997, 68, 149–156. [Google Scholar] [CrossRef]
- Liu, J.; Qian, L.; Wessells, R.J.; Bidet, Y.; Jagla, K.; Bodmer, R. Hedgehog and RAS Pathways Cooperate in the Anterior–Posterior Specification and Positioning of Cardiac Progenitor Cells. Dev. Biol. 2006, 290, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Rowton, M.; Perez-Cervantes, C.; Hur, S.; Jacobs-Li, J.; Lu, E.; Deng, N.; Guzzetta, A.; Hoffmann, A.D.; Stocker, M.; Steimle, J.D.; et al. Hedgehog Signaling Activates a Mammalian Heterochronic Gene Regulatory Network Controlling Differentiation Timing across Lineages. Dev. Cell 2022, 57, 2181–2203.e9. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Nosetani, M.; Nakajima, Y.; Sakaki, S.; Kato, H.; Saba, R.; Takeshita, N.; Nishikawa, K.; Ueyama, A.; Matsuo, K.; et al. Sonic Hedgehog Signaling Regulates the Optimal Differentiation Pace from Early-Stage Mesoderm to Cardiogenic Mesoderm in Mice. Dev. Growth Differ. 2025, 67, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Constable, S.; Mukhopadhyay, S. Ubiquitin Tunes Hedgehog in Matters of the Heart. Dev. Cell 2020, 55, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Soria, P.; Camenisch, T.D. ErbB Signaling in Cardiac Development and Disease. Semin. Cell Dev. Biol. 2010, 21, 929–935. [Google Scholar] [CrossRef]
- MacGrogan, D.; Münch, J.; de la Pompa, J.L. Notch and Interacting Signalling Pathways in Cardiac Development, Disease, and Regeneration. Nat. Rev. Cardiol. 2018, 15, 685–704. [Google Scholar] [CrossRef]
- Bray, S.; Furriols, M. Notch Pathway: Making Sense of Suppressor of Hairless. Curr. Biol. 2001, 11, R217–R221. [Google Scholar] [CrossRef]
- Rones, M.S.; McLaughlin, K.A.; Raffin, M.; Mercola, M. Serrate and Notch Specify Cell Fates in the Heart Field by Suppressing Cardiomyogenesis. Development 2000, 127, 3865–3876. [Google Scholar] [CrossRef]
- Chau, M.D.L.; Tuft, R.; Fogarty, K.; Bao, Z.-Z. Notch Signaling Plays a Key Role in Cardiac Cell Differentiation. Mech. Dev. 2006, 123, 626–640. [Google Scholar] [CrossRef]
- Zhao, L.; Borikova, A.L.; Ben-Yair, R.; Guner-Ataman, B.; MacRae, C.A.; Lee, R.T.; Burns, C.G.; Burns, C.E. Notch Signaling Regulates Cardiomyocyte Proliferation during Zebrafish Heart Regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 1403–1408. [Google Scholar] [CrossRef]
- Kwon, C.; Qian, L.; Cheng, P.; Nigam, V.; Arnold, J.; Srivastava, D. A Regulatory Pathway Involving Notch1/β-Catenin/Isl1 Determines Cardiac Progenitor Cell Fate. Nat. Cell Biol. 2009, 11, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Watanabe, Y.; Smyth, G.; Miyagawa-Tomita, S.; Meyers, E.; Klingensmith, J.; Camenisch, T.; Buckingham, M.; Moon, A.M. An FGF Autocrine Loop Initiated in Second Heart Field Mesoderm Regulates Morphogenesis at the Arterial Pole of the Heart. Development 2008, 135, 3599–3610. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, K.; Choi, C.Y.; Kim, Y.; Schulz, R.A. Genetically Distinct Cardial Cells within the Drosophila Heart. Genesis 2000, 28, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Salomone, J.; Farrow, E.; Gebelein, B. Homeodomain Complex Formation and Biomolecular Condensates in Hox Gene Regulation. Semin. Cell Dev. Biol. 2024, 152–153, 93–100. [Google Scholar] [CrossRef]
- Wellik, D.M. Hox Genes and Patterning the Vertebrate Body. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2024; Volume 159, pp. 1–27. ISBN 978-0-12-823461-7. [Google Scholar]
- Holland, P.W.; Booth, H.A.F.; Bruford, E.A. Classification and Nomenclature of All Human Homeobox Genes. BMC Biol. 2007, 5, 47. [Google Scholar] [CrossRef]
- Hubert, K.A.; Wellik, D.M. Hox Genes in Development and Beyond. Development 2023, 150, dev192476. [Google Scholar] [CrossRef]
- Deschamps, J.; Duboule, D. Embryonic Timing, Axial Stem Cells, Chromatin Dynamics, and the Hox Clock. Genes Dev. 2017, 31, 1406–1416. [Google Scholar] [CrossRef]
- Epstein, M.; Pillemer, G.; Yelin, R.; Yisraeli, J.K.; Fainsod, A. Patterning of the Embryo along the Anterior-Posterior Axis: The Role of the Caudal Genes. Development 1997, 124, 3805–3814. [Google Scholar] [CrossRef]
- Maeda, R.K.; Karch, F. Chapter 1 The Bithorax Complex of Drosophila. In Current Topics in Developmental Biology; Genes; Academic Press: Cambridge, MA, USA, 2009; Volume 88, pp. 1–33. [Google Scholar]
- Rosales-Vega, M.; Hernández-Becerril, A.; Murillo-Maldonado, J.M.; Zurita, M.; Vázquez, M. The Role of the Trithorax Group TnaA Isoforms in Hox Gene Expression, and in Drosophila Late Development. PLoS ONE 2018, 13, e0206587. [Google Scholar] [CrossRef]
- Mark, M.; Rijli, F.M.; Chambon, P. Homeobox Genes in Embryogenesis and Pathogenesis. Pediatr. Res. 1997, 42, 421–429. [Google Scholar] [CrossRef]
- Mulhair, P.O.; Holland, P.W.H. Evolution of the Insect Hox Gene Cluster: Comparative Analysis across 243 Species. Semin. Cell Dev. Biol. 2024, 152–153, 4–15. [Google Scholar] [CrossRef]
- Rosales-Vega, M.; Reséndez-Pérez, D.; Vázquez, M. Antennapedia: The Complexity of a Master Developmental Transcription Factor. Genesis 2024, 62, e23561. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Monier, B.; Ponzielli, R.; Astier, M.; Semeriva, M. Drosophila Cardiac Tube Organogenesis Requires Multiple Phases of Hox Activity. Dev. Biol. 2004, 272, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Ponzielli, R.; Astier, M.; Chartier, A.; Gallet, A.; Thérond, P.; Sémériva, M. Heart Tube Patterning in Drosophila Requires Integration of Axial and Segmental Information Provided by the Bithorax Complex Genes and Hedgehog Signaling. Development 2002, 129, 4509–4521. [Google Scholar] [CrossRef] [PubMed]
- Maeda, R.K.; Karch, F. The ABC of the BX-C: The Bithorax Complex Explained. Development 2006, 133, 1413–1422. [Google Scholar] [CrossRef]
- LaBeau, E.M.; Trujillo, D.L.; Cripps, R.M. Bithorax Complex Genes Control Alary Muscle Patterning along the Cardiac Tube of Drosophila. Mech. Dev. 2009, 126, 478–486. [Google Scholar] [CrossRef]
- Schroeder, A.M.; Nielsen, T.; Lynott, M.; Vogler, G.; Colas, A.R.; Bodmer, R. Nascent Polypeptide-Associated Complex and Signal Recognition Particle Have Cardiac-Specific Roles in Heart Development and Remodeling. PLoS Genet. 2022, 18, e1010448. [Google Scholar] [CrossRef]
- Ryan, K.M.; Hoshizaki, D.K.; Cripps, R.M. Homeotic Selector Genes Control the Patterning of Seven-up Expressing Cells in the Drosophila Dorsal Vessel. Mech. Dev. 2005, 122, 1023–1033. [Google Scholar] [CrossRef]
- Duboule, D. The Rise and Fall of Hox Gene Clusters. Development 2007, 134, 2549–2560. [Google Scholar] [CrossRef]
- Lescroart, F.; Wang, X.; Lin, X.; Swedlund, B.; Gargouri, S.; Sànchez-Dànes, A.; Moignard, V.; Dubois, C.; Paulissen, C.; Kinston, S.; et al. Defining the Earliest Step of Cardiovascular Lineage Segregation by Single-Cell RNA-Seq. Science 2018, 359, 1177–1181. [Google Scholar] [CrossRef]
- Makki, N.; Capecchi, M.R. Cardiovascular Defects in a Mouse Model of HOXA1 Syndrome. Hum. Mol. Genet. 2012, 21, 26–31. [Google Scholar] [CrossRef]
- Tischfield, M.A.; Bosley, T.M.; Salih, M.A.M.; Alorainy, I.A.; Sener, E.C.; Nester, M.J.; Oystreck, D.T.; Chan, W.-M.; Andrews, C.; Erickson, R.P.; et al. Homozygous HOXA1 Mutations Disrupt Human Brainstem, Inner Ear, Cardiovascular and Cognitive Development. Nat. Genet. 2005, 37, 1035–1037. [Google Scholar] [CrossRef]
- Roux, M.; Laforest, B.; Capecchi, M.; Bertrand, N.; Zaffran, S. Hoxb1 Regulates Proliferation and Differentiation of Second Heart Field Progenitors in Pharyngeal Mesoderm and Genetically Interacts with Hoxa1 during Cardiac Outflow Tract Development. Dev. Biol. 2015, 406, 247–258. [Google Scholar] [CrossRef]
- Roux, M.; Laforest, B.; Eudes, N.; Bertrand, N.; Stefanovic, S.; Zaffran, S. Hoxa1 and Hoxb1 Are Required for Pharyngeal Arch Artery Development. Mech. Dev. 2017, 143, 1–8. [Google Scholar] [CrossRef]
- Lufkin, T.; Dierich, A.; LeMeur, M.; Mark, M.; Chambon, P. Disruption of the Hox-1.6 Homeobox Gene Results in Defects in a Region Corresponding to Its Rostral Domain of Expression. Cell 1991, 66, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Godwin, A.R.; Stadler, H.S.; Nakamura, K.; Capecchi, M.R. Detection of Targeted GFP-Hox Gene Fusions during Mouse Embryogenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13042–13047. [Google Scholar] [CrossRef] [PubMed]
- Makki, N.; Capecchi, M.R. Hoxa1 Lineage Tracing Indicates a Direct Role for Hoxa1 in the Development of the Inner Ear, the Heart, and the Third Rhombomere. Dev. Biol. 2010, 341, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Chisaka, O.; Capecchi, M.R. Regionally Restricted Developmental Defects Resulting from Targeted Disruption of the Mouse Homeobox Gene Hox-1.5. Nature 1991, 350, 473–479. [Google Scholar] [CrossRef]
- Kameda, Y.; Watari-Goshima, N.; Nishimaki, T.; Chisaka, O. Disruption of the Hoxa3 Homeobox Gene Results in Anomalies of the Carotid Artery System and the Arterial Baroreceptors. Cell Tissue Res. 2003, 311, 343–352. [Google Scholar] [CrossRef]
- Chisaka, O.; Kameda, Y. Hoxa3 Regulates the Proliferation and Differentiation of the Third Pharyngeal Arch Mesenchyme in Mice. Cell Tissue Res. 2005, 320, 77–89. [Google Scholar] [CrossRef]
- Sharifi-Zarchi, A.; Gerovska, D.; Adachi, K.; Totonchi, M.; Pezeshk, H.; Taft, R.J.; Schöler, H.R.; Chitsaz, H.; Sadeghi, M.; Baharvand, H.; et al. DNA Methylation Regulates Discrimination of Enhancers from Promoters through a H3K4me1-H3K4me3 Seesaw Mechanism. BMC Genom. 2017, 18, 964. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, C.; Huang, X.; van de Leemput, J.; Lee, H.; Han, Z. H3K36 Di-Methylation Marks, Mediated by Ash1 in Complex with Caf1-55 and MRG15, Are Required during Drosophila Heart Development. J. Cardiovasc. Dev. Dis. 2023, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y.; van de Leemput, J.; Han, Z. Distinct Roles of COMPASS Subunits to Drosophila Heart Development. Biol. Open 2024, 13, bio061736. [Google Scholar] [CrossRef]
- Krogan, N.J.; Dover, J.; Khorrami, S.; Greenblatt, J.F.; Schneider, J.; Johnston, M.; Shilatifard, A. COMPASS, a Histone H3 (Lysine 4) Methyltransferase Required for Telomeric Silencing of Gene Expression. J. Biol. Chem. 2002, 277, 10753–10755. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, J.; Fu, Y.; van de Leemput, J.; Han, Z. Lpt, Trr, and Hcf Regulate Histone Mono- and Dimethylation That Are Essential for Drosophila Heart Development. Dev. Biol. 2022, 490, 53–65. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Lee, H.; Huang, X.; van de Leemput, J.; Han, Z. Distinct Roles for COMPASS Core Subunits Set1, Trx, and Trr in the Epigenetic Regulation of Drosophila Heart Development. Int. J. Mol. Sci. 2023, 24, 17314. [Google Scholar] [CrossRef]
- Borland, S.; Tenin, G.; Williams, S.; Monaghan, R.; Baxter, M.; Ray, D.; Abraham, S.; Keavney, B. BS9 KMT2C—A Tetralogy of Fallot Candidate Gene. Heart 2019, 105, A145–A146. [Google Scholar] [CrossRef]
- Ang, S.-Y.; Uebersohn, A.; Spencer, C.I.; Huang, Y.; Lee, J.-E.; Ge, K.; Bruneau, B.G. KMT2D Regulates Specific Programs in Heart Development via Histone H3 Lysine 4 Di-Methylation. Development 2016, 143, 810–821. [Google Scholar] [CrossRef]
- Yuan, S.-M. Congenital Heart Defects in Kabuki Syndrome. Cardiol. J. 2013, 20, 121–124. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Wong, C.N. Epigenetics in Heart Failure: Role of DNA Methylation in Potential Pathways Leading to Heart Failure with Preserved Ejection Fraction. Biomedicines 2023, 11, 2815. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, J.; Du, D.; Zhang, D.; Jin, Z.; Qiu, W.; Zhou, X.; Dong, S.; Zhou, M.; Zhao, H.; et al. Altered DNA Methylation Pattern Reveals Epigenetic Regulation of Hox Genes in Thoracic Aortic Dissection and Serves as a Biomarker in Disease Diagnosis. Clin. Epigenetics 2021, 13, 124. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.; Guo, Y. Deciphering the Emerging Landscape of HOX Genes in Cardiovascular Biology, Atherosclerosis and beyond (Review). Int. J. Mol. Med. 2024, 53, 17. [Google Scholar] [CrossRef] [PubMed]
- Behrens, A.N.; Iacovino, M.; Lohr, J.L.; Ren, Y.; Zierold, C.; Harvey, R.P.; Kyba, M.; Garry, D.J.; Martin, C.M. Nkx2-5 Mediates Differential Cardiac Differentiation through Interaction with Hoxa10. Stem Cells Dev. 2013, 22, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Diman, N.Y.S.-G.; Remacle, S.; Bertrand, N.; Picard, J.J.; Zaffran, S.; Rezsohazy, R. A Retinoic Acid Responsive Hoxa3 Transgene Expressed in Embryonic Pharyngeal Endoderm, Cardiac Neural Crest and a Subdomain of the Second Heart Field. PLoS ONE 2011, 6, e27624. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.R.; Seo, E.-J.; Lee, J.-O.; Yoo, H.-W.; Park, I.-S.; Yoon, H.-K. Molecular Cytogenetic and Clinical Characterization of a Patient with a 5.6-Mb Deletion in 7p15 Including HOXA Cluster. Am. J. Med. Genet. Part A 2011, 155, 642–647. [Google Scholar] [CrossRef]
- Munabi, N.C.O.; Mikhail, S.; Toubat, O.; Webb, M.; Auslander, A.; Sanchez-Lara, P.A.; Manojlovic, Z.; Schmidt, R.J.; Craig, D.; Magee, W.P.; et al. High Prevalence of Deleterious Mutations in Concomitant Nonsyndromic Cleft and Outflow Tract Heart Defects. Am. J. Med. Genet. Part A 2022, 188, 2082–2095. [Google Scholar] [CrossRef]
- Smedts, H.P.M.; van Uitert, E.M.; Valkenburg, O.; Laven, J.S.E.; Eijkemans, M.J.C.; Lindemans, J.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. A Derangement of the Maternal Lipid Profile Is Associated with an Elevated Risk of Congenital Heart Disease in the Offspring. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 477–485. [Google Scholar] [CrossRef]
- Su, M.-T.; Venkatesh, T.V.; Wu, X.; Golden, K.; Bodmer, R. The Pioneer Gene, Apontic, Is Required for Morphogenesis and Function of the Drosophila Heart. Mech. Dev. 1999, 80, 125–132. [Google Scholar] [CrossRef]
- Liu, Q.-X.; Wang, X.-F.; Ikeo, K.; Hirose, S.; Gehring, W.J.; Gojobori, T. Evolutionarily Conserved Transcription Factor Apontic Controls the G1/S Progression by Inducing Cyclin E during Eye Development. Proc. Natl. Acad. Sci. USA 2014, 111, 9497–9502. [Google Scholar] [CrossRef]
- Gregory, G.D.; Vakoc, C.R.; Rozovskaia, T.; Zheng, X.; Patel, S.; Nakamura, T.; Canaani, E.; Blobel, G.A. Mammalian ASH1L Is a Histone Methyltransferase That Occupies the Transcribed Region of Active Genes. Mol. Cell Biol. 2007, 27, 8466–8479. [Google Scholar] [CrossRef]
- Ji, W.; Ferdman, D.; Copel, J.; Scheinost, D.; Shabanova, V.; Brueckner, M.; Khokha, M.K.; Ment, L.R. De Novo Damaging Variants Associated with Congenital Heart Diseases Contribute to the Connectome. Sci. Rep. 2020, 10, 7046. [Google Scholar] [CrossRef]
- Jin, S.C.; Homsy, J.; Zaidi, S.; Lu, Q.; Morton, S.; DePalma, S.R.; Zeng, X.; Qi, H.; Chang, W.; Sierant, M.C.; et al. Contribution of Rare Inherited and de Novo Variants in 2,871 Congenital Heart Disease Probands. Nat. Genet. 2017, 49, 1593–1601. [Google Scholar] [CrossRef]
- Homsy, J.; Zaidi, S.; Shen, Y.; Ware, J.S.; Samocha, K.E.; Karczewski, K.J.; DePalma, S.R.; McKean, D.; Wakimoto, H.; Gorham, J.; et al. De Novo Mutations in Congenital Heart Disease with Neurodevelopmental and Other Birth Defects. Science 2015, 350, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- Stoller, J.Z.; Huang, L.; Tan, C.C.; Huang, F.; Zhou, D.D.; Yang, J.; Gelb, B.D.; Epstein, J.A. Ash2l Interacts with Tbx1 and Is Required during Early Embryogenesis. Exp. Biol. Med. 2010, 235, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Barish, S.; Berg, K.; Drozd, J.; Berglund-Brown, I.; Khizir, L.; Wasson, L.K.; Seidman, C.E.; Seidman, J.G.; Chen, S.; Brueckner, M. The H2Bub1-Deposition Complex Is Required for Human and Mouse Cardiogenesis. Development 2023, 150, dev201899. [Google Scholar] [CrossRef] [PubMed]
- VanDusen, N.J.; Lee, J.Y.; Gu, W.; Butler, C.E.; Sethi, I.; Zheng, Y.; King, J.S.; Zhou, P.; Suo, S.; Guo, Y.; et al. Massively Parallel in Vivo CRISPR Screening Identifies RNF20/40 as Epigenetic Regulators of Cardiomyocyte Maturation. Nat. Commun. 2021, 12, 4442. [Google Scholar] [CrossRef]
- Robson, A.; Makova, S.Z.; Barish, S.; Zaidi, S.; Mehta, S.; Drozd, J.; Jin, S.C.; Gelb, B.D.; Seidman, C.E.; Chung, W.K.; et al. Histone H2B Monoubiquitination Regulates Heart Development via Epigenetic Control of Cilia Motility. Proc. Natl. Acad. Sci. USA 2019, 116, 14049–14054. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Li, J.; Wang, R.; Tharakan, B.; Zhang, S.L.; Tong, C.W.; Peng, X. Deletion of Cdc42 in Embryonic Cardiomyocytes Results in Right Ventricle Hypoplasia. Clin. Transl. Med. 2017, 6, 40. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Jin, Y.; Wang, R.; Wang, J.; Lu, S.; VanBuren, V.; Dostal, D.E.; Zhang, S.L.; Peng, X. Essential Role of Cdc42 in Cardiomyocyte Proliferation and Cell-Cell Adhesion during Heart Development. Dev. Biol. 2017, 421, 271–283. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, Y.; Li, J.; Seto, E.; Kuo, E.; Yu, W.; Schwartz, R.J.; Blazo, M.; Zhang, S.L.; Peng, X. Inactivation of Cdc42 in Neural Crest Cells Causes Craniofacial and Cardiovascular Morphogenesis Defects. Dev. Biol. 2013, 383, 239–252. [Google Scholar] [CrossRef]
- Martinelli, S.; Krumbach, O.H.F.; Pantaleoni, F.; Coppola, S.; Amin, E.; Pannone, L.; Nouri, K.; Farina, L.; Dvorsky, R.; Lepri, F.; et al. Functional Dysregulation of CDC42 Causes Diverse Developmental Phenotypes. Am. J. Hum. Genet. 2018, 102, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.M.; Garone, C.; Emmanuele, V.; Tadesse, S.; Krishna, S.; Dorado, B.; Hirano, M. Tissue-Specific Oxidative Stress and Loss of Mitochondria in CoQ-Deficient Pdss2 Mutant Mice. FASEB J. 2013, 27, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Iványi, B.; Rácz, G.Z.; Gál, P.; Brinyiczki, K.; Bódi, I.; Kalmár, T.; Maróti, Z.; Bereczki, C. Diffuse Mesangial Sclerosis in a PDSS2 Mutation-Induced Coenzyme Q10 Deficiency. Pediatr. Nephrol. 2018, 33, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.P.; Lubbers, E.R.; Mohler, P.J. Advancing Our Understanding of AnkRD1 in Cardiac Development and Disease. Cardiovasc. Res. 2020, 116, 1402–1404. [Google Scholar] [CrossRef]
- Almontashiri, N.A.M.; Chen, H.-H.; Mailloux, R.J.; Tatsuta, T.; Teng, A.C.T.; Mahmoud, A.B.; Ho, T.; Stewart, N.A.S.; Rippstein, P.; Harper, M.E.; et al. SPG7 Variant Escapes Phosphorylation-Regulated Processing by AFG3L2, Elevates Mitochondrial ROS, and Is Associated with Multiple Clinical Phenotypes. Cell Rep. 2014, 7, 834–847. [Google Scholar] [CrossRef]
- Lin, Q.; Schwarz, J.; Bucana, C.; N Olson, E. Control of Mouse Cardiac Morphogenesis and Myogenesis by Transcription Factor MEF2C. Science 1997, 276, 1404–1407. [Google Scholar] [CrossRef]
- Pavone, P.; Falsaperla, R.; Ruggieri, M.; Marino, S.D.; Parano, E.; Pappalardo, X.G. A Young Boy with 21q21.1 Microdeletion Showing Speech Delay, Spastic Diplegia, and MRI Abnormalities: Original Case Report. Glob. Med. Genet. 2023, 10, 234–239. [Google Scholar] [CrossRef]
- Weisfeld-Adams, J.D.; Tkachuk, A.K.; Maclean, K.N.; Meeks, N.L.; Scott, S.A. A de Novo 2.78-Mb Duplication on Chromosome 21q22.11 Implicates Candidate Genes in the Partial Trisomy 21 Phenotype. npj Genom. Med. 2016, 1, 16003. [Google Scholar] [CrossRef]
- Chapman, D.L.; Cooper-Morgan, A.; Harrelson, Z.; Papaioannou, V.E. Critical Role for Tbx6 in Mesoderm Specification in the Mouse Embryo. Mech. Dev. 2003, 120, 837–847. [Google Scholar] [CrossRef]
- Peralta, T.M.; Zelarayán, L.C. Dot1L-H3K79me2-Tbx6 Axis: A Novel Therapeutic Target for Preventing Cardiac Failure. Circ. Res. 2025, 137, 513–515. [Google Scholar] [CrossRef]
- Ma, L.; Lu, M.-F.; Schwartz, R.J.; Martin, J.F. Bmp2 Is Essential for Cardiac Cushion Epithelial-Mesenchymal Transition and Myocardial Patterning. Development 2005, 132, 5601–5611. [Google Scholar] [CrossRef]
- Bobos, D.; Soufla, G.; Angouras, D.C.; Lekakis, I.; Georgopoulos, S.; Melissari, E. Investigation of the Role of BMP2 and -4 in ASD, VSD and Complex Congenital Heart Disease. Diagnostics 2023, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Dunlevy, L.; Bennett, M.; Slender, A.; Lana-Elola, E.; Tybulewicz, V.L.; Fisher, E.M.C.; Mohun, T. Down’s Syndrome-like Cardiac Developmental Defects in Embryos of the Transchromosomic Tc1 Mouse. Cardiovasc. Res. 2010, 88, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.R.; Gamliel, A.; Wessells, R.J.; Taghli-Lamallem, O.; Jepsen, K.; Ocorr, K.; Korenberg, J.R.; Peterson, K.L.; Rosenfeld, M.G.; Bodmer, R.; et al. Over-Expression of DSCAM and COL6A2 Cooperatively Generates Congenital Heart Defects. PLoS Genet. 2011, 7, e1002344. [Google Scholar] [CrossRef] [PubMed]
- Raza, Q.; Jacobs, J.R. Guidance Signalling Regulates Leading Edge Behaviour during Collective Cell Migration of Cardiac Cells in Drosophila. Dev. Biol. 2016, 419, 285–297. [Google Scholar] [CrossRef]
- Mollo, N.; Scognamiglio, R.; Conti, A.; Paladino, S.; Nitsch, L.; Izzo, A. Genetics and Molecular Basis of Congenital Heart Defects in Down Syndrome: Role of Extracellular Matrix Regulation. Int. J. Mol. Sci. 2023, 24, 2918. [Google Scholar] [CrossRef]
- Brown, G.S.; Jang, J.; Li, D. Growth Factors and Their Roles in Cardiac Development and Regeneration: A Narrative Review. Pediatr. Med. 2023, 6, 35. [Google Scholar] [CrossRef]
- Iwamoto, R.; Mine, N.; Mizushima, H.; Mekada, E. ErbB1 and ErbB4 Generate Opposing Signals Regulating Mesenchymal Cell Proliferation during Valvulogenesis. J. Cell Sci. 2017, 130, 1321–1332. [Google Scholar] [CrossRef]
- McBride, K.L.; Zender, G.A.; Fitzgerald–Butt, S.M.; Seagraves, N.J.; Fernbach, S.D.; Zapata, G.; Lewin, M.; Towbin, J.A.; Belmont, J.W. Association of Common Variants in ERBB4 with Congenital Left Ventricular Outflow Tract Obstruction Defects. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 162–168. [Google Scholar] [CrossRef]
- Giannakou, A.; Sicko, R.J.; Kay, D.M.; Zhang, W.; Romitti, P.A.; Caggana, M.; Shaw, G.M.; Jelliffe-Pawlowski, L.L.; Mills, J.L. Copy Number Variants in Hypoplastic Right Heart Syndrome. Am. J. Med. Genet. Part A 2018, 176, 2760–2767. [Google Scholar] [CrossRef]
- Foth, R.; Shomroni, O.; Sigler, M.; Hörer, J.; Cleuziou, J.; Paul, T.; Eildermann, K. Screening for Potential Targets to Reduce Stenosis in Bioprosthetic Heart Valves. Sci. Rep. 2021, 11, 2464. [Google Scholar] [CrossRef] [PubMed]
- Alrefaei, A.F. Frizzled Receptors (FZD) Play Multiple Cellular Roles in Development, in Diseases, and as Potential Therapeutic Targets. J. King Saud. Univ. Sci. 2021, 33, 101613. [Google Scholar] [CrossRef]
- Yu, H.; Smallwood, P.M.; Wang, Y.; Vidaltamayo, R.; Reed, R.; Nathans, J. Frizzled 1 and Frizzled 2 Genes Function in Palate, Ventricular Septum and Neural Tube Closure: General Implications for Tissue Fusion Processes. Development 2010, 137, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-M.; Guo, M.; Salas, M.; Schupf, N.; Silverman, W.; Zigman, W.B.; Husain, S.; Warburton, D.; Thaker, H.; Tycko, B. Cell Type-Specific over-Expression of Chromosome 21 Genes in Fibroblasts and Fetal Hearts with Trisomy 21. BMC Med. Genet. 2006, 7, 24. [Google Scholar] [CrossRef]
- Eisa-Beygi, S.; Hatch, G.; Noble, S.; Ekker, M.; Moon, T.W. The 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGCR) Pathway Regulates Developmental Cerebral-Vascular Stability via Prenylation-Dependent Signalling Pathway. Dev. Biol. 2013, 373, 258–266. [Google Scholar] [CrossRef]
- Holm, A.; Graus, M.S.; Wylie-Sears, J.; Tan, J.W.H.; Alvarez-Harmon, M.; Borgelt, L.; Nasim, S.; Chung, L.; Jain, A.; Sun, M.; et al. An Endothelial SOX18–Mevalonate Pathway Axis Enables Repurposing of Statins for Infantile Hemangioma. J. Clin. Invest. 2025, 135, e179782. [Google Scholar] [CrossRef]
- Nishimura, S.; Mishra-Gorur, K.; Park, J.; Surovtseva, Y.V.; Sebti, S.M.; Levchenko, A.; Louvi, A.; Gunel, M. Combined HMG-COA Reductase and Prenylation Inhibition in Treatment of CCM. Proc. Natl. Acad. Sci. USA 2017, 114, 5503–5508. [Google Scholar] [CrossRef]
- Torregrosa-Carrión, R.; Piñeiro-Sabarís, R.; Siguero-Álvarez, M.; Grego-Bessa, J.; Luna-Zurita, L.; Fernandes, V.S.; MacGrogan, D.; Stainier, D.Y.R.; de la Pompa, J.L. Adhesion G Protein-Coupled Receptor Gpr126/Adgrg6 Is Essential for Placental Development. Sci. Adv. 2021, 7, eabj5445. [Google Scholar] [CrossRef]
- Patel, M.V.; Zhu, J.; Jiang, Z.; Richman, A.; VanBerkum, M.F.A.; Han, Z. Gia/Mthl5 Is an Aorta Specific GPCR Required for Drosophila Heart Tube Morphology and Normal Pericardial Cell Positioning. Dev. Biol. 2016, 414, 100–107. [Google Scholar] [CrossRef]
- Lu, S.; Liu, S.; Wietelmann, A.; Kojonazarov, B.; Atzberger, A.; Tang, C.; Schermuly, R.T.; Gröne, H.-J.; Offermanns, S. Developmental Vascular Remodeling Defects and Postnatal Kidney Failure in Mice Lacking Gpr116 (Adgrf5) and Eltd1 (Adgrl4). PLoS ONE 2017, 12, e0183166. [Google Scholar] [CrossRef]
- Tanaka, K.; Chen, M.; Prendergast, A.; Zhuang, Z.; Nasiri, A.; Joshi, D.; Hintzen, J.; Chung, M.; Kumar, A.; Mani, A.; et al. Latrophilin-2 Mediates Fluid Shear Stress Mechanotransduction at Endothelial Junctions. EMBO J. 2024, 43, 3175–3191. [Google Scholar] [CrossRef]
- Chiba, Y.; Yoshizaki, K.; Saito, K.; Ikeuchi, T.; Iwamoto, T.; Rhodes, C.; Nakamura, T.; de Vega, S.; Morell, R.J.; Boger, E.T.; et al. G Protein-Coupled Receptor Gpr115 (Adgrf4) Is Required for Enamel Mineralization Mediated by Ameloblasts. J. Biol. Chem. 2020, 295, 15328–15341. [Google Scholar] [CrossRef] [PubMed]
- Vitobello, A.; Mazel, B.; Lelianova, V.G.; Zangrandi, A.; Petitto, E.; Suckling, J.; Salpietro, V.; Meyer, R.; Elbracht, M.; Kurth, I.; et al. ADGRL1 Haploinsufficiency Causes a Variable Spectrum of Neurodevelopmental Disorders in Humans and Alters Synaptic Activity and Behavior in a Mouse Model. Am. J. Hum. Genet. 2022, 109, 1436–1457. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.G.; Rosa-E-Silva, J.C.; Gomes, A.G.; Grzesiuk, J.D.; Vidotto, T.; Squire, J.A.; Panepucci, R.A.; Meola, J.; Martelli, L. Identification of a Rare Copy Number Polymorphic Gain at 3q12.2 with Candidate Genes for Familial Endometriosis. Rev. Bras. Ginecol. Obs. 2024, 46, e-rbgo12. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-Q.; Tang, Y. Gene mutations in congenital bilateral absence of the vas deferens: An update. Zhonghua Nan Ke Xue 2021, 27, 450–455. [Google Scholar]
- Vidal, O.M.; Vélez, J.I.; Arcos-Burgos, M. ADGRL3 Genomic Variation Implicated in Neurogenesis and ADHD Links Functional Effects to the Incretin Polypeptide GIP. Sci. Rep. 2022, 12, 15922. [Google Scholar] [CrossRef]
- Quintana, A.M.; Geiger, E.A.; Achilly, N.; Rosenblatt, D.S.; Maclean, K.N.; Stabler, S.P.; Artinger, K.B.; Appel, B.; Shaikh, T.H. Hcfc1b, a Zebrafish Ortholog of HCFC1, Regulates Craniofacial Development by Modulating Mmachc Expression. Dev. Biol. 2014, 396, 94–106. [Google Scholar] [CrossRef]
- Yu, H.-C.; Sloan, J.L.; Scharer, G.; Brebner, A.; Quintana, A.M.; Achilly, N.P.; Manoli, I.; Coughlin, C.R.; Geiger, E.A.; Schneck, U.; et al. An X-Linked Cobalamin Disorder Caused by Mutations in Transcriptional Coregulator HCFC1. Am. J. Hum. Genet. 2013, 93, 506–514. [Google Scholar] [CrossRef]
- Reynolds, J.J.; Bicknell, L.S.; Carroll, P.; Higgs, M.R.; Shaheen, R.; Murray, J.E.; Papadopoulos, D.K.; Leitch, A.; Murina, O.; Tarnauskaitė, Ž.; et al. Mutations in DONSON Disrupt Replication Fork Stability and Cause Microcephalic Dwarfism. Nat. Genet. 2017, 49, 537–549. [Google Scholar] [CrossRef]
- Washington Smoak, I.; Byrd, N.A.; Abu-Issa, R.; Goddeeris, M.M.; Anderson, R.; Morris, J.; Yamamura, K.; Klingensmith, J.; Meyers, E.N. Sonic Hedgehog Is Required for Cardiac Outflow Tract and Neural Crest Cell Development. Dev. Biol. 2005, 283, 357–372. [Google Scholar] [CrossRef]
- Dell’Era, P.; Ronca, R.; Coco, L.; Nicoli, S.; Metra, M.; Presta, M. Fibroblast Growth Factor Receptor-1 Is Essential for In Vitro Cardiomyocyte Development. Circ. Res. 2003, 93, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; Alembik, Y.; Dott, B.; Roth, M.-P. Associated Anomalies in Cases with Achondroplasia. Eur. J. Med. Genet. 2022, 65, 104612. [Google Scholar] [CrossRef] [PubMed]
- Marguerie, A.; Bajolle, F.; Zaffran, S.; Brown, N.A.; Dickson, C.; Buckingham, M.E.; Kelly, R.G. Congenital Heart Defects in Fgfr2-IIIb and Fgf10 Mutant Mice. Cardiovasc. Res. 2006, 71, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Vega-Hernández, M.; Kovacs, A.; De Langhe, S.; Ornitz, D.M. FGF10/FGFR2b Signaling Is Essential for Cardiac Fibroblast Development and Growth of the Myocardium. Development 2011, 138, 3331–3340. [Google Scholar] [CrossRef]
- Mysliwiec, M.R.; Bresnick, E.; Lee, Y. Abstract 21584: Jarid2/Jumonji Dependent Epigenetic Control of Notch1 Expression Is Required for Normal Cardiac Development. Circulation 2010, 122, A21584. [Google Scholar] [CrossRef]
- van der Laan, L.; Rooney, K.; Haghshenas, S.; Silva, A.; McConkey, H.; Relator, R.; Levy, M.A.; Valenzuela, I.; Trujillano, L.; Lasa-Aranzasti, A.; et al. Functional Insight into and Refinement of the Genomic Boundaries of the JARID2-Neurodevelopmental Disorder Episignature. Int. J. Mol. Sci. 2023, 24, 14240. [Google Scholar] [CrossRef]
- Bajpai, R.; Chen, D.A.; Rada-Iglesias, A.; Zhang, J.; Xiong, Y.; Helms, J.; Chang, C.-P.; Zhao, Y.; Swigut, T.; Wysocka, J. CHD7 Cooperates with PBAF to Control Multipotent Neural Crest Formation. Nature 2010, 463, 958–962. [Google Scholar] [CrossRef]
- Han, P.; Hang, C.T.; Yang, J.; Chang, C.-P. Chromatin Remodeling in Cardiovascular Development and Physiology. Circ. Res. 2011, 108, 378–396. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, L.; Chi, C.; Wu, X.; Yang, X.; Han, M.; Xu, T.; Zhuang, Y.; Deng, K. Adam10 Is Essential for Early Embryonic Cardiovascular Development. Dev. Dyn. 2010, 239, 2594–2602. [Google Scholar] [CrossRef]
- Farber, G.; Parks, M.M.; Lustgarten Guahmich, N.; Zhang, Y.; Monette, S.; Blanchard, S.C.; Di Lorenzo, A.; Blobel, C.P. ADAM10 Controls the Differentiation of the Coronary Arterial Endothelium. Angiogenesis 2019, 22, 237–250. [Google Scholar] [CrossRef]
- Albrecht, S.; Wang, S.; Holz, A.; Bergter, A.; Paululat, A. The ADAM Metalloprotease Kuzbanian Is Crucial for Proper Heart Formation in Drosophila melanogaster. Mech. Dev. 2006, 123, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ma, A.; Wang, B.; Peng, R.; Jing, Y.; Wang, D.; Finnell, R.H.; Qiao, B.; Wang, Y.; Wang, H.; et al. Rare Mutations of ADAM17 from TOFs Induce Hypertrophy in Human Embryonic Stem Cell-Derived Cardiomyocytes via HB-EGF Signaling. Clin Sci 2019, 133, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Kaimori, J.-Y.; Kikkawa, Y.; Motooka, D.; Namba-Hamano, T.; Takuwa, A.; Okazaki, A.; Kobayashi, K.; Tanigawa, A.; Kotani, Y.; Uno, Y.; et al. A Heterozygous LAMA5 Variant May Contribute to Slowly Progressive, Vinculin-Enhanced Familial FSGS and Pulmonary Defects. JCI Insight 2022, 7, e158378. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.A.; Yee, G.H.; Roote, C.E.; Williams, E.L.; Zusman, S.; Hynes, R.O. A Novel α Integrin Subunit Associates with β PS and Functions in Tissue Morphogenesis and Movement during Drosophila Development. Development 1997, 124, 4583–4594. [Google Scholar] [CrossRef]
- Nishiyama, M.; Takase, M.; Tanaka, Y.; Gamo, S. Ether-Resistant Mutant of Laminin Alpha Subunit (LanA) in Drosophila melanogaster. Int. Congr. Ser. 2005, 1283, 260–262. [Google Scholar] [CrossRef]
- Deogharia, M.; Venegas-Zamora, L.; Agrawal, A.; Shi, M.; Jain, A.K.; McHugh, K.J.; Altamirano, F.; Marian, A.J.; Gurha, P. Histone Demethylase KDM5 Regulates Cardiomyocyte Maturation by Promoting Fatty Acid Oxidation, Oxidative Phosphorylation, and Myofibrillar Organization. Cardiovasc. Res. 2024, 120, 630–643. [Google Scholar] [CrossRef]
- Szot, J.O.; Cuny, H.; Blue, G.M.; Humphreys, D.T.; Ip, E.; Harrison, K.; Sholler, G.F.; Giannoulatou, E.; Leo, P.; Duncan, E.L.; et al. A Screening Approach to Identify Clinically Actionable Variants Causing Congenital Heart Disease in Exome Data. Circ. Genom. Precis. Med. 2018, 11, e001978. [Google Scholar] [CrossRef]
- Zhu, J.; van de Leemput, J.; Han, Z. The Roles of Histone Lysine Methyltransferases in Heart Development and Disease. J. Cardiovasc. Dev. Dis. 2023, 10, 305. [Google Scholar] [CrossRef]
- Sun, H.; Yi, T.; Hao, X.; Yan, H.; Wang, J.; Li, Q.; Gu, X.; Zhou, X.; Wang, S.; Wang, X.; et al. Contribution of Single-Gene Defects to Congenital Cardiac Left-Sided Lesions in the Prenatal Setting. Ultrasound Obstet. Gynecol. 2020, 56, 225–232. [Google Scholar] [CrossRef]
- Whitford, W.; Taylor, J.; Hayes, I.; Smith, W.; Snell, R.G.; Lehnert, K.; Jacobsen, J.C. A Novel 11 Base Pair Deletion in KMT2C Resulting in Kleefstra Syndrome 2. Mol. Genet. Genom. Med. 2024, 12, e2350. [Google Scholar] [CrossRef]
- Rots, D.; Choufani, S.; Faundes, V.; Dingemans, A.J.M.; Joss, S.; Foulds, N.; Jones, E.A.; Stewart, S.; Vasudevan, P.; Dabir, T.; et al. Pathogenic Variants in KMT2C Result in a Neurodevelopmental Disorder Distinct from Kleefstra and Kabuki Syndromes. Am. J. Hum. Genet. 2024, 111, 1626–1642. [Google Scholar] [CrossRef]
- Cantemir, V.; Cai, D.H.; Reedy, M.V.; Brauer, P.R. Tissue Inhibitor of Metalloproteinase-2 (TIMP-2) Expression during Cardiac Neural Crest Cell Migration and Its Role in proMMP-2 Activation. Dev. Dyn. 2004, 231, 709–719. [Google Scholar] [CrossRef]
- Muñoz-Sáez, E.; Moracho, N.; Learte, A.I.R.; Arroyo, A.G.; Sánchez-Camacho, C. Dynamic Expression of Membrane Type 1-Matrix Metalloproteinase (Mt1-Mmp/Mmp14) in the Mouse Embryo. Cells 2021, 10, 2448. [Google Scholar] [CrossRef]
- Fealey, M.E.; Edwards, W.D.; Miller, D.V.; Maleszewski, J.J. Unicommissural Aortic Valves: Gross, Histological, and Immunohistochemical Analysis of 52 Cases (1978-2008). Cardiovasc. Pathol. 2012, 21, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Levay, A.K.; Gridley, T.; Lincoln, J. Mmp15 Is a Direct Target of Snai1 during Endothelial to Mesenchymal Transformation and Endocardial Cushion Development. Dev. Biol. 2011, 359, 209–221. [Google Scholar] [CrossRef]
- Abdelrahman, H.A.; Akawi, N.; Al-Shamsi, A.M.; Ali, A.; Al-Jasmi, F.; John, A.; Hertecant, J.; Al-Gazali, L.; Ali, B.R. Bi-Allelic Null Variant in Matrix Metalloproteinase-15, Causes Congenital Cardiac Defect, Cholestasis Jaundice, and Failure to Thrive. Clin. Genet. 2022, 101, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.J.; Siomos, A.K.; Garcia, A.M.; Nguyen, H.; SooHoo, M.; Galambos, C.; Nunley, K.; Stauffer, B.L.; Sucharov, C.C.; Miyamoto, S.D. Fibrosis-Related Gene Expression in Single Ventricle Heart Disease. J. Pediatr. 2017, 191, 82–90.e2. [Google Scholar] [CrossRef] [PubMed]
- Gorący, I.; Grudniewicz, S.; Safranow, K.; Ciechanowicz, A.; Jakubiszyn, P.; Gorący, A.; Brykczyński, M. Genetic Polymorphisms of MMP1, MMP9, COL1A1, and COL1A2 in Polish Patients with Thoracic Aortopathy. Dis. Markers 2020, 2020, 9567239. [Google Scholar] [CrossRef]
- Song, C.; Wei, S.; Fan, Y.; Jiang, S. Bioinformatic-Based Identification of Genes Associated with Aortic Valve Stenosis. Heart Surg. Forum 2022, 25, E069–E078. [Google Scholar] [CrossRef]
- Bertolino, P.; Radovanovic, I.; Casse, H.; Aguzzi, A.; Wang, Z.-Q.; Zhang, C.-X. Genetic Ablation of the Tumor Suppressor Menin Causes Lethality at Mid-Gestation with Defects in Multiple Organs. Mech. Dev. 2003, 120, 549–560. [Google Scholar] [CrossRef]
- Ishii, M.; Han, J.; Yen, H.-Y.; Sucov, H.M.; Chai, Y.; Maxson, R.E. Combined Deficiencies of Msx1 and Msx2 Cause Impaired Patterning and Survival of the Cranial Neural Crest. Development 2005, 132, 4937–4950. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, L.; Castori, M.; Capalbo, A.; Mokini, V.; Mingarelli, R.; Simi, P.; Bertuccelli, A.; Novelli, A.; Dallapiccola, B. Syndromic Craniosynostosis Due to Complex Chromosome 5 Rearrangement and MSX2 Gene Triplication. Am. J. Med. Genet. Part A 2007, 143A, 2937–2943. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Randall, W.R.; Du, S.-J. skNAC (Skeletal Naca), a Muscle-Specific Isoform of Naca (Nascent Polypeptide-Associated Complex Alpha), Is Required for Myofibril Organization. FASEB J. 2009, 23, 1988–2000. [Google Scholar] [CrossRef]
- Murayama, E.; Sarris, M.; Redd, M.; Le Guyader, D.; Vivier, C.; Horsley, W.; Trede, N.; Herbomel, P. NACA Deficiency Reveals the Crucial Role of Somite-Derived Stromal Cells in Haematopoietic Niche Formation. Nat. Commun. 2015, 6, 8375. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.-D.; Cui, C.-Y.; Qin, Y.-Y.; Fan, T.-B.; Peng, B.-T.; Zhang, L.-Z.; Wang, C.-Z. Whole Exome Sequencing Identifies Novel Mutation in Eight Chinese Children with Isolated Tetralogy of Fallot. Oncotarget 2017, 8, 106976–106988. [Google Scholar] [CrossRef]
- Opitz, R.; Hitz, M.-P.; Vandernoot, I.; Trubiroha, A.; Abu-Khudir, R.; Samuels, M.; Désilets, V.; Costagliola, S.; Andelfinger, G.; Deladoëy, J. Functional Zebrafish Studies Based on Human Genotyping Point to Netrin-1 as a Link Between Aberrant Cardiovascular Development and Thyroid Dysgenesis. Endocrinology 2015, 156, 377–388. [Google Scholar] [CrossRef]
- Matos-Nieves, A.; Greskovich, S.C.; Choudhury, T.Z.; Manivannan, S.; Ueyama, Y.; Rao, A.S.; Cameron, E.M.; Garg, V. Expression of Netrin-1 in the Developing Mouse Heart. Gene Expr. Patterns 2025, 56, 119398. [Google Scholar] [CrossRef]
- Theodoris, C.V.; Li, M.; White, M.P.; Liu, L.; He, D.; Pollard, K.S.; Bruneau, B.G.; Srivastava, D. Human Disease Modeling Reveals Integrated Transcriptional and Epigenetic Mechanisms of NOTCH1 Haploinsufficiency. Cell 2015, 160, 1072–1086. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, N.; Kennard, S.; Lilly, B. Notch2 and Notch3 Function Together to Regulate Vascular Smooth Muscle Development. PLoS ONE 2012, 7, e37365. [Google Scholar] [CrossRef]
- Stanley, K.J.; Kalbfleisch, K.J.; Moran, O.M.; Chaturvedi, R.R.; Roifman, M.; Chen, X.; Manshaei, R.; Martin, N.; McDermott, S.; McNiven, V.; et al. Expanding the Phenotypic Spectrum of NOTCH1 Variants: Clinical Manifestations in Families with Congenital Heart Disease. Eur. J. Hum. Genet. 2024, 32, 795–803. [Google Scholar] [CrossRef]
- Meester, J.A.N.; Verstraeten, A.; Alaerts, M.; Schepers, D.; Van Laer, L.; Loeys, B.L. Overlapping but Distinct Roles for NOTCH Receptors in Human Cardiovascular Disease. Clin. Genet. 2019, 95, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guo, H.; Li, J.; Myint, T.; Pittman, W.; Yang, L.; Zhong, W.; Schwartz, R.J.; Schwarz, J.J.; Singer, H.A.; et al. Numb Family Proteins Are Essential for Cardiac Morphogenesis and Progenitor Differentiation. Development 2014, 141, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cao, R.; Xu, Y.; Li, T.; Li, F.; Chen, S.; Xu, R.; Sun, K. Rare Copy Number Variants Analysis Identifies Novel Candidate Genes in Heterotaxy Syndrome Patients with Congenital Heart Defects. Genome Med. 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Nomaru, H.; Liu, Y.; De Bono, C.; Righelli, D.; Cirino, A.; Wang, W.; Song, H.; Racedo, S.E.; Dantas, A.G.; Zhang, L.; et al. Single Cell Multi-Omic Analysis Identifies a Tbx1-Dependent Multilineage Primed Population in Murine Cardiopharyngeal Mesoderm. Nat. Commun. 2021, 12, 6645. [Google Scholar] [CrossRef]
- Afouda, B.A. Towards Understanding the Gene-Specific Roles of GATA Factors in Heart Development: Does GATA4 Lead the Way? Int. J. Mol. Sci. 2022, 23, 5255. [Google Scholar] [CrossRef]
- Theis, J.L.; Niaz, T.; Sundsbak, R.S.; Fogarty, Z.C.; Bamlet, W.R.; Hagler, D.J.; Olson, T.M. CELSR1 Risk Alleles in Familial Bicuspid Aortic Valve and Hypoplastic Left Heart Syndrome. Circ. Genom. Precis. Med. 2022, 15, e003523. [Google Scholar] [CrossRef]
- Cantù, C.; Felker, A.; Zimmerli, D.; Prummel, K.D.; Cabello, E.M.; Chiavacci, E.; Méndez-Acevedo, K.M.; Kirchgeorg, L.; Burger, S.; Ripoll, J.; et al. Mutations in Bcl9 and Pygo Genes Cause Congenital Heart Defects by Tissue-Specific Perturbation of Wnt/β-Catenin Signaling. Genes Dev. 2018, 32, 1443–1458. [Google Scholar] [CrossRef]
- Itoh, N.; Ohta, H.; Nakayama, Y.; Konishi, M. Roles of FGF Signals in Heart Development, Health, and Disease. Front. Cell Dev. Biol. 2016, 4, 110. [Google Scholar] [CrossRef]
- Moon, A. The Role of Fgf8 in Cardiovascular Development and Human Congenital Heart Disease. FASEB J. 2007, 21, A34. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, R.; Li, G.; Huang, P.; Zhang, Y.; Zhu, J.; Kuang, J.; Hutchins, A.P.; Qin, D.; Zhu, P.; et al. C-JUN Is a Barrier in hESC to Cardiomyocyte Transition. Life Sci. Alliance 2023, 6, e202302121. [Google Scholar] [CrossRef]
- Feldman, E.R.; Li, Y.; Cutler, D.J.; Rosser, T.C.; Wechsler, S.B.; Sanclemente, L.; Rachubinski, A.L.; Elliott, N.; Vyas, P.; Roberts, I.; et al. Genome-Wide Association Studies of Down Syndrome Associated Congenital Heart Defects Suggests a Genetically Heterogeneous Risk for CHD in DS. Genet. Epidemiol. 2025, 49, e70010. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.M.; Yeh, M.L.; Parnavelas, J.G.; Andrews, W.D. Disrupted Slit-Robo Signalling Results in Membranous Ventricular Septum Defects and Bicuspid Aortic Valves. Cardiovasc. Res. 2015, 106, 55–66. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.M.; Andrews, W.D.; Ypsilanti, A.R.; Zelina, P.; Yeh, M.L.; Norden, J.; Kispert, A.; Chédotal, A.; Christoffels, V.M.; Parnavelas, J.G. Slit–Roundabout Signaling Regulates the Development of the Cardiac Systemic Venous Return and Pericardium. Circ. Res. 2013, 112, 465–475. [Google Scholar] [CrossRef]
- MacMullin, A.; Jacobs, J.R. Slit Coordinates Cardiac Morphogenesis in Drosophila. Dev. Biol. 2006, 293, 154–164. [Google Scholar] [CrossRef]
- Zhao, J.; Mommersteeg, M.T.M. Slit–Robo Signalling in Heart Development. Cardiovasc. Res. 2018, 114, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Kruszka, P.; Tanpaiboon, P.; Neas, K.; Crosby, K.; Berger, S.I.; Martinez, A.F.; Addissie, Y.A.; Pongprot, Y.; Sittiwangkul, R.; Silvilairat, S.; et al. Loss of Function in ROBO1 Is Associated with Tetralogy of Fallot and Septal Defects. J. Med. Genet. 2017, 54, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Jaouadi, H.; Gérard, H.; Théron, A.; Collod-Béroud, G.; Collart, F.; Avierinos, J.-F.; Zaffran, S. Identification of Non-Synonymous Variations in ROBO1 and GATA5 Genes in a Family with Bicuspid Aortic Valve Disease. J. Hum. Genet. 2022, 67, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Jaouadi, H.; Jopling, C.; Bajolle, F.; Théron, A.; Faucherre, A.; Gerard, H.; Al Dybiat, S.; Ovaert, C.; Bonnet, D.; Avierinos, J.-F.; et al. Expanding the Phenome and Variome of the ROBO-SLIT Pathway in Congenital Heart Defects: Toward Improving the Genetic Testing Yield of CHD. J. Transl. Med. 2023, 21, 160. [Google Scholar] [CrossRef]
- Ţuţulan-Cuniţă, A.C.; Papuc, S.M.; Arghir, A.; Rötzer, K.M.; Deshpande, C.; Lungeanu, A.; Budişteanu, M. 3p Interstitial Deletion: Novel Case Report and Review. J. Child. Neurol. 2012, 27, 1062–1066. [Google Scholar] [CrossRef]
- Digilio, M.C.; Pugnaloni, F.; De Luca, A.; Calcagni, G.; Baban, A.; Dentici, M.L.; Versacci, P.; Dallapiccola, B.; Tartaglia, M.; Marino, B. Atrioventricular Canal Defect and Genetic Syndromes: The Unifying Role of Sonic Hedgehog. Clin. Genet. 2019, 95, 268–276. [Google Scholar] [CrossRef]
- Iyer, K.R.; Clarke, S.L.; Guarischi-Sousa, R.; Gjoni, K.; Heath, A.S.; Young, E.P.; Stitziel, N.O.; Laurie, C.; Broome, J.G.; Khan, A.T.; et al. Unveiling the Genetic Landscape of Coronary Artery Disease Through Common and Rare Structural Variants. J. Am. Heart Assoc. 2025, 14, e036499. [Google Scholar] [CrossRef]
- Jin, L.; Mo, W.; Yan, Y.; Wang, Y. Novel Mutation in the SETD1A Gene in a Newborn Patient Associating with Congenital Airway and Heart Defeats: A Case Report. Medicine 2023, 102, e33449. [Google Scholar] [CrossRef]
- Chen, F.; Chen, J.; Wang, H.; Tang, H.; Huang, L.; Wang, S.; Wang, X.; Fang, X.; Liu, J.; Li, L.; et al. Histone Lysine Methyltransferase SETD2 Regulates Coronary Vascular Development in Embryonic Mouse Hearts. Front. Cell Dev. Biol. 2021, 9, 651655. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Liu, Y.; Li, P.; Chen, Z.; Li, H.; Yang, X.; Finnell, R.H.; Yang, Z.; Zhang, T.; Qiao, B.; et al. Genetic Analysis of Rare Coding Mutations of CELSR1–3 in Congenital Heart and Neural Tube Defects in Chinese People. Clin Sci 2016, 130, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Conceição, R.; Evans, R.S.; Pearson, C.S.; Hänzi, B.; Osborne, A.; Deshpande, S.S.; Martin, K.R.; Barber, A.C. Expression of Developmentally Important Axon Guidance Cues in the Adult Optic Chiasm. Invest. Ophthalmol. Vis. Sci. 2019, 60, 4727–4739. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Wang, D.; Shen, H.; Jiang, M.; Mei, P.; Hayden, P.S.; Sedor, J.R.; Hu, H. Congenital Diaphragmatic Hernia, Kidney Agenesis and Cardiac Defects Associated with Slit3-Deficiency in Mice. Mech. Dev. 2003, 120, 1059–1070. [Google Scholar] [CrossRef]
- Sanna-Cherchi, S.; Khan, K.; Westland, R.; Krithivasan, P.; Fievet, L.; Rasouly, H.M.; Ionita-Laza, I.; Capone, V.P.; Fasel, D.A.; Kiryluk, K.; et al. Exome-Wide Association Study Identifies GREB1L Mutations in Congenital Kidney Malformations. Am. J. Hum. Genet. 2017, 101, 789–802. [Google Scholar] [CrossRef]
- Qiao, X.-H.; Wang, Q.; Wang, J.; Liu, X.-Y.; Xu, Y.-J.; Huang, R.-T.; Xue, S.; Li, Y.-J.; Zhang, M.; Qu, X.-K.; et al. A Novel NR2F2 Loss-of-Function Mutation Predisposes to Congenital Heart Defect. Eur. J. Med. Genet. 2018, 61, 197–203. [Google Scholar] [CrossRef]
- Tong, W.; Xue, Q.; Li, Y.; Zhang, L. Maternal Hypoxia Alters Matrix Metalloproteinase Expression Patterns and Causes Cardiac Remodeling in Fetal and Neonatal Rats. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2113–H2121. [Google Scholar] [CrossRef]
- Corbitt, H.; Morris, S.A.; Gravholt, C.H.; Mortensen, K.H.; Tippner-Hedges, R.; Silberbach, M.; Maslen, C.L. GenTAC Registry Investigators TIMP3 and TIMP1 Are Risk Genes for Bicuspid Aortic Valve and Aortopathy in Turner Syndrome. PLoS Genet. 2018, 14, e1007692. [Google Scholar] [CrossRef]
- Corbitt, H.; Gutierrez, J.; Silberbach, M.; Maslen, C.L. The Genetic Basis of Turner Syndrome Aortopathy. Am. J. Med. Genet. Part C Semin. Med. Genet. 2019, 181, 117–125. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Shi, L.; McDonald-McGinn, D.M.; Crowley, T.B.; McGinn, D.E.; Tran, O.T.; Miller, D.; Lin, J.-R.; Zackai, E.; et al. Chromatin Regulators in the TBX1 Network Confer Risk for Conotruncal Heart Defects in 22q11.2DS. npj Genom. Med. 2023, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Ogino, J.; Dou, Y. Histone Methyltransferase KMT2A: Developmental Regulation to Oncogenic Transformation. J. Biol. Chem. 2024, 300, 107791. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.E.; Campbell, I.M.; Harr, M.H.; Gold, N.; Li, D.; Bjornsson, H.T.; Cohen, J.S.; Fahrner, J.A.; Fatemi, A.; Harris, J.R.; et al. Expanding the Genotypic and Phenotypic Spectrum in a Diverse Cohort of 104 Individuals with Wiedemann-Steiner Syndrome. Am. J. Med. Genet. Part A 2021, 185, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Lindo, C.; Peoples, J.; Cox, A.; Lytle, E.; Nguyen, V.; Mehta, M.; Alvarez, J.D.; Yooseph, S.; Pacher, P.; et al. Maternal Binge Alcohol Consumption Leads to Distinctive Acute Perturbations in Embryonic Cardiac Gene Expression Profiles. Alcohol Clin. Exp. Res. 2022, 46, 1433–1448. [Google Scholar] [CrossRef]
- Argào, E.A.; Kern, M.J.; Branford, W.W.; Scott, W.J.; Potter, S.S. Malformations of the Heart, Kidney, Palate, and Skeleton in α-MHC-Hoxb-7 Transgenic Mice. Mech. Dev. 1995, 52, 291–303. [Google Scholar] [CrossRef]
- Bergwerff, M.; DeRuiter, M.C.; Gittenberger-de Groot, A.C. Comparative Anatomy and Ontogeny of the Ductus Arteriosus, a Vascular Outsider. Anat Embryol 1999, 200, 559–571. [Google Scholar] [CrossRef]
- Wu, J.; Dou, B.; Meng, G.; Wang, H.; Hou, Y.; Xia, J.; Bai, Y.; Kong, X. Phenotypic and genetic characteristics of a child with 7p15 deletion syndrome. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2020, 37, 855–858. [Google Scholar] [CrossRef]
- Gong, L.; Qiu, G.; Jiang, H.; Xu, X.; Zhu, H.; Sun, K. Analysis of Single Nucleotide Polymorphisms and Haplotypes in HOXC Gene Cluster within Susceptible Region 12q13 of Simple Congenital Heart Disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2005, 22, 497–501. [Google Scholar]
- Schatteman, G.C.; Loushin, C.; Li, T.; Hart, C.E. PDGF-A Is Required for Normal Murine Cardiovascular Development. Dev. Biol. 1996, 176, 133–142. [Google Scholar] [CrossRef]
- Steurer, M.A.; Norton, M.E.; Baer, R.J.; Shaw, G.M.; Keating, S.; Moon-Grady, A.J.; Chambers, C.D.; Jelliffe-Pawlowski, L.L. The Association of Maternal Lymphatic Markers and Critical Congenital Heart Defects in the Fetus-A Population Based Case-Control Study. Am. J. Med. Genet. Part A 2017, 173, 1231–1236. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Khokha, M.K. WDR5 Regulates Left-Right Patterning via Chromatin-Dependent and -Independent Functions. Development 2018, 145, dev159889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, M.; Li, Z.; Li, H.; Yuan, D.; Zhang, X.; Guo, M.; Qian, W.; Cheng, D. Wds-Mediated H3K4me3 Modification Regulates Lipid Synthesis and Transport in Drosophila. Int. J. Mol. Sci. 2023, 24, 6125. [Google Scholar] [CrossRef] [PubMed]
- Crucean, A.; Alqahtani, A.; Barron, D.J.; Brawn, W.J.; Richardson, R.V.; O’Sullivan, J.; Anderson, R.H.; Henderson, D.J.; Chaudhry, B. Re-Evaluation of Hypoplastic Left Heart Syndrome from a Developmental and Morphological Perspective. Orphanet J. Rare Dis. 2017, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H.; Zheng, Y.; Qiao, B.; Duan, W.; Huang, L.; Liu, W.; Wang, H. Variants in the Regulatory Region of WNT5A Reduced Risk of Cardiac Conotruncal Malformations in the Chinese Population. Sci. Rep. 2015, 5, 13120. [Google Scholar] [CrossRef]
- Lee, A.; Wei, S.; Schwertani, A. A Notch More: Molecular Players in Bicuspid Aortic Valve Disease. J. Mol. Cell Cardiol. 2019, 134, 62–68. [Google Scholar] [CrossRef]
- Nelson, J.S.; Kwok, C.; Braganca, N.E.; Lopez, D.L.; Espina Rey, A.P.; Robinson, M.; Ebert, S.N. Comparison of DNA Methylation Patterns across Tissue Types in Infants with Tetralogy of Fallot. Birth Defects Res. 2022, 114, 1101–1111. [Google Scholar] [CrossRef]
- Goddard, L.M.; Duchemin, A.-L.; Ramalingan, H.; Wu, B.; Chen, M.; Bamezai, S.; Yang, J.; Li, L.; Morley, M.; Wang, T.; et al. Hemodynamic Forces Sculpt Developing Heart Valves through a KLF2-WNT9B Paracrine Signaling Axis. Dev. Cell 2017, 43, 274–289.e5. [Google Scholar] [CrossRef]
- Spinner, N.B.; Loomes, K.M.; Krantz, I.D.; Gilbert, M.A. Alagille Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Broberg, M.; Ampuja, M.; Jones, S.; Ojala, T.; Rahkonen, O.; Kivelä, R.; Priest, J.; Palotie, A.; Ollila, H.M.; Helle, E.; et al. Genome-Wide Association Studies Highlight Novel Risk Loci for Septal Defects and Left-Sided Congenital Heart Defects. BMC Genom. 2024, 25, 256. [Google Scholar] [CrossRef]
- Escalante-Alcalde, D.; Morales, S.L.; Stewart, C.L. Generation of a Reporter-Null Allele of Ppap2b/Lpp3and Its Expression during Embryogenesis. Int. J. Dev. Biol. 2009, 53, 139–147. [Google Scholar] [CrossRef]
- Grazioli, A.; Alves, C.S.; Konstantopoulos, K.; Yang, J.T. Defective Blood Vessel Development and Pericyte/pvSMC Distribution in A4 Integrin-Deficient Mouse Embryos. Dev. Biol. 2006, 293, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Palmquist-Gomes, P.; Ruiz-Villalba, A.; Guadix, J.A.; Romero, J.P.; Bessiéres, B.; MacGrogan, D.; Conejo, L.; Ortiz, A.; Picazo, B.; Houyel, L.; et al. Origin of Congenital Coronary Arterio-Ventricular Fistulae from Anomalous Epicardial and Myocardial Development. Exp. Mol. Med. 2023, 55, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.G.A.; Jacinto, A.; Prag, S. Drosophila Integrin Adhesion Complexes Are Essential for Hemocyte Migration In Vivo. Biol. Open 2013, 2, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.A.; Wright, Z.A.; Owen, M.L.; Bredemeier, N.O.; Sumanas, S. Integrin A5 and Integrin A4 Cooperate to Promote Endocardial Differentiation and Heart Morphogenesis. Dev. Biol. 2020, 465, 46–57. [Google Scholar] [CrossRef]
- Vickers, A.; Tewary, M.; Laddach, A.; Poletti, M.; Salameti, V.; Fraternali, F.; Danovi, D.; Watt, F.M. Plating Human iPSC Lines on Micropatterned Substrates Reveals Role for ITGB1 nsSNV in Endoderm Formation. Stem Cell Rep. 2021, 16, 2628–2641. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, X.; Ithychanda, S.S.; Wu, T.; Gu, Y.; Chen, C.; Wang, L.; Bogomolovas, J.; Qin, J.; Chen, J. Interaction of Filamin C With Actin Is Essential for Cardiac Development and Function. Circ. Res. 2023, 133, 400–411. [Google Scholar] [CrossRef]
- Rastogi, S.; Liberles, D.A. Subfunctionalization of Duplicated Genes as a Transition State to Neofunctionalization. BMC Evol. Biol. 2005, 5, 28. [Google Scholar] [CrossRef]
- Clarence, T.; Robert, N.S.M.; Sarigol, F.; Fu, X.; Bates, P.A.; Simakov, O. Robust 3D Modeling Reveals Spatiosyntenic Properties of Animal Genomes. iScience 2023, 26, 106136. [Google Scholar] [CrossRef]
- Irie, N.; Sehara-Fujisawa, A. The Vertebrate Phylotypic Stage and an Early Bilaterian-Related Stage in Mouse Embryogenesis Defined by Genomic Information. BMC Biol. 2007, 5, 1. [Google Scholar] [CrossRef]
- Papaioannou, V.E.; Behringer, R.R. Early Embryonic Lethality in Genetically Engineered Mice: Diagnosis and Phenotypic Analysis. Vet. Pathol. 2012, 49, 64–70. [Google Scholar] [CrossRef]
- Asadzadeh, J.; Neligan, N.; Kramer, S.G.; Labrador, J.-P. Tinman Regulates NetrinB in the Cardioblasts of the Drosophila Dorsal Vessel. PLoS ONE 2016, 11, e0148526. [Google Scholar] [CrossRef]
- Li, H.; Janssens, J.; De Waegeneer, M.; Kolluru, S.S.; Davie, K.; Gardeux, V.; Saelens, W.; David, F.P.A.; Brbić, M.; Spanier, K.; et al. Fly Cell Atlas: A Single-Nucleus Transcriptomic Atlas of the Adult Fruit Fly. Science 2022, 375, eabk2432. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bouhours, M.; Gracheva, E.O.; Liao, E.H.; Xu, K.; Sengar, A.S.; Xin, X.; Roder, J.; Boone, C.; Richmond, J.E.; et al. ITSN-1 Controls Vesicle Recycling at the Neuromuscular Junction and Functions in Parallel with DAB-1. Traffic 2008, 9, 742. [Google Scholar] [CrossRef]
- Bandura, J.L.; Beall, E.L.; Bell, M.; Silver, H.R.; Botchan, M.R.; Calvi, B.R. Humpty Dumpty Is Required for Developmental DNA Amplification and Cell Proliferation in Drosophila. Curr. Biol. 2005, 15, 755–759. [Google Scholar] [CrossRef]
- Grant, J.; Saldanha, J.W.; Gould, A.P. A Drosophila Model for Primary Coenzyme Q Deficiency and Dietary Rescue in the Developing Nervous System. Dis. Models Mech. 2010, 3, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vaquero, D.; Parra-Peralbo, E.; Mejía-Morales, J.E.; Culi, J. Drosophila Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake. PLoS Genet. 2015, 11, e1005356. [Google Scholar] [CrossRef]
- Ding, M.; Zheng, L.; Li, Q.F.; Wang, W.L.; Peng, W.D.; Zhou, M. Exercise-Training Regulates Apolipoprotein B in Drosophila to Improve HFD-Mediated Cardiac Function Damage and Low Exercise Capacity. Front. Physiol. 2021, 12, 650959. [Google Scholar] [CrossRef]
- Lai, K.; Amsterdam, A.; Farrington, S.; Bronson, R.T.; Hopkins, N.; Lees, J.A. Many Ribosomal Protein Mutations Are Associated with Growth Impairment and Tumor Predisposition in Zebrafish. Dev. Dyn. 2009, 238, 76–85. [Google Scholar] [CrossRef]
- Johnson, A.N.; Mokalled, M.H.; Haden, T.N.; Olson, E.N. JAK/Stat Signaling Regulates Heart Precursor Diversification in Drosophila. Development 2011, 138, 4627–4638. [Google Scholar] [CrossRef]
- Daubresse, G.; Deuring, R.; Moore, L.; Papoulas, O.; Zakrajsek, I.; Waldrip, W.R.; Scott, M.P.; Kennison, J.A.; Tamkun, J.W. The Drosophila Kismet Gene Is Related to Chromatin-Remodeling Factors and Is Required for Both Segmentation and Segment Identity. Development 1999, 126, 1175–1187. [Google Scholar] [CrossRef]
- Koemans, T.S.; Kleefstra, T.; Chubak, M.C.; Stone, M.H.; Reijnders, M.R.F.; de Munnik, S.; Willemsen, M.H.; Fenckova, M.; Stumpel, C.T.R.M.; Bok, L.A.; et al. Functional Convergence of Histone Methyltransferases EHMT1 and KMT2C Involved in Intellectual Disability and Autism Spectrum Disorder. PLoS Genet. 2017, 13, e1006864. [Google Scholar] [CrossRef]
- Karpe, F.; Pinnick, K.E. Biology of Upper-Body and Lower-Body Adipose Tissue--Link to Whole-Body Phenotypes. Nat. Rev. Endocrinol. 2015, 11, 90–100. [Google Scholar] [CrossRef]
- Joll, J.E.; Riley, L.A.; Bersi, M.R.; Nyman, J.S.; Merryman, W.D. Sclerostin Ablation Prevents Aortic Valve Stenosis in Mice. Am. J. Physiol.-Heart Circ. Physiol. 2022, 323, H1037–H1047. [Google Scholar] [CrossRef]
- Vann, K.R.; Sharma, R.; Hsu, C.-C.; Devoucoux, M.; Tencer, A.H.; Zeng, L.; Lin, K.; Zhu, L.; Li, Q.; Lachance, C.; et al. Structure-Function Relationship of ASH1L and Histone H3K36 and H3K4 Methylation. Nat. Commun. 2025, 16, 2235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Canagarajah, B.; Zhao, Y.; Baibakov, B.; Tokuhiro, K.; Maric, D.; Dean, J. BTBD18 Regulates a Subset of piRNA-Generating Loci through Transcription Elongation in Mice. Dev. Cell 2017, 40, 453–466.e5. [Google Scholar] [CrossRef] [PubMed]
- Fritz, K.R.; Zhang, Y.; Ruest, L.B. Cdc42 Activation by Endothelin Regulates Neural Crest Cell Migration in the Cardiac Outflow Tract. Dev. Dyn. 2019, 248, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Bakovic, P.; Mirosevic, V.; Svagusa, T.; Sepac, A.; Kulic, A.; Milicic, D.; Gasparovic, H.; Rudez, I.; Urlic, M.; Sikiric, S.; et al. Reduced Expression of UPRmt Proteins HSP10, HSP60, HTRA2, OMA1, SPG7, and YME1L Is Associated with Accelerated Heart Failure in Humans. Biomedicines 2025, 13, 1142. [Google Scholar] [CrossRef]
- Jahncke, J.N.; Wright, K.M. The Many Roles of Dystroglycan in Nervous System Development and Function: Dystroglycan and Neural Circuit Development: Dystroglycan and Neural Circuit Development. Dev. Dyn. 2023, 252, 61–80. [Google Scholar] [CrossRef]
- Bertero, A.; Madrigal, P.; Galli, A.; Hubner, N.C.; Moreno, I.; Burks, D.; Brown, S.; Pedersen, R.A.; Gaffney, D.; Mendjan, S.; et al. Activin/Nodal Signaling and NANOG Orchestrate Human Embryonic Stem Cell Fate Decisions by Controlling the H3K4me3 Chromatin Mark. Genes Dev. 2015, 29, 702–717. [Google Scholar] [CrossRef]
- Yang, Z.; Shah, K.; Khodadadi-Jamayran, A.; Jiang, H. Dpy30 Is Critical for Maintaining the Identity and Function of Adult Hematopoietic Stem Cells. J. Exp. Med. 2016, 213, 2349–2364. [Google Scholar] [CrossRef]
- Sadeghi, M.B.; Nakhaee, A.; Saravani, R.; Sargazi, S. Significant Association of LXRβ (NR1H2) Polymorphisms (Rs28514894, Rs2303044) with Type 2 Diabetes Mellitus and Laboratory Characteristics. J. Diabetes Metab. Disord. 2021, 20, 261–270. [Google Scholar] [CrossRef]
- Zheng, Z.-G.; Zhu, S.-T.; Cheng, H.-M.; Zhang, X.; Cheng, G.; Thu, P.M.; Wang, S.P.; Li, H.-J.; Ding, M.; Qiang, L.; et al. Discovery of a Potent SCAP Degrader That Ameliorates HFD-Induced Obesity, Hyperlipidemia and Insulin Resistance via an Autophagy-Independent Lysosomal Pathway. Autophagy 2021, 17, 1592–1613. [Google Scholar] [CrossRef]
- Guo, X.; Zhong, J.; Zhao, Y.; Fu, Y.; Sun, L.-Y.; Yuan, A.; Liu, J.; Chen, A.F.; Pu, J. LXRα Promotes Abdominal Aortic Aneurysm Formation Through UHRF1 Epigenetic Modification of miR-26b-3p. Circulation 2024, 150, 30–46. [Google Scholar] [CrossRef]
- Lammers, S.; Barrera, V.; Brennecke, P.; Miller, C.; Yoon, J.; Balolong, J.; Anderson, M.S.; Ho Sui, S.; Steinmetz, L.M.; von Andrian, U.H.; et al. Ehf and Fezf2 Regulate Late Medullary Thymic Epithelial Cell and Thymic Tuft Cell Development. Front. Immunol. 2023, 14, 1277365. [Google Scholar] [CrossRef]
- Zhou, J.; Chehab, R.; Tkalcevic, J.; Naylor, M.J.; Harris, J.; Wilson, T.J.; Tsao, S.; Tellis, I.; Zavarsek, S.; Xu, D.; et al. Elf5 Is Essential for Early Embryogenesis and Mammary Gland Development during Pregnancy and Lactation. EMBO J. 2005, 24, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, H. The Transcription Factor ELF5 Is Essential for Early Preimplantation Development. Mol. Biol. Rep. 2023, 50, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Faiella, A.; D’Esposito, M.; Rambaldi, M.; Acampora, D.; Balsfiore, S.; Stornaiuolo, A.; Mallamaci, A.; Migliaccio, E.; Gulisano, M.; Simeone, A.; et al. Isolation and Mapping of EVx1, a Human Homeobox Gene Homologus to Even-Skipped, Localized at the 5′ End of Hox1 Locus on Chromosome 7. Nucleic Acids Res. 1991, 19, 6541–6545. [Google Scholar] [CrossRef]
- Szabo, L.; Morey, R.; Palpant, N.J.; Wang, P.L.; Afari, N.; Jiang, C.; Parast, M.M.; Murry, C.E.; Laurent, L.C.; Salzman, J. Statistically Based Splicing Detection Reveals Neural Enrichment and Tissue-Specific Induction of Circular RNA during Human Fetal Development. Genome Biol. 2015, 16, 126. [Google Scholar] [CrossRef]
- Sato, A.; Scholl, A.M.; Kuhn, E.B.; Stadt, H.A.; Decker, J.R.; Pegram, K.; Hutson, M.R.; Kirby, M.L. FGF8 Signaling Is Chemotactic for Cardiac Neural Crest Cells. Dev. Biol. 2011, 354, 18–30. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, S.; Chen, L.; Yuan, R.; Nie, Y.; Ding, S.; Fang, Y.; Zhu, Q.; Chen, K.; Wei, H.; et al. Integrated miRNA-mRNA Transcriptomic Analysis Reveals Epigenetic-Mediated Embryonic Muscle Growth Differences between Wuzhishan and Landrace Pigs1. J. Anim. Sci. 2019, 97, 1967–1978. [Google Scholar] [CrossRef]
- Voges, H.K.; Foster, S.R.; Reynolds, L.; Parker, B.L.; Devilée, L.; Quaife-Ryan, G.A.; Fortuna, P.R.J.; Mathieson, E.; Fitzsimmons, R.; Lor, M.; et al. Vascular Cells Improve Functionality of Human Cardiac Organoids. Cell Rep. 2023, 42, 112322. [Google Scholar] [CrossRef]
- Jiang, D.-S.; Yi, X.; Li, R.; Su, Y.-S.; Wang, J.; Chen, M.-L.; Liu, L.-G.; Hu, M.; Cheng, C.; Zheng, P.; et al. The Histone Methyltransferase Mixed Lineage Leukemia (MLL) 3 May Play a Potential Role in Clinical Dilated Cardiomyopathy. Mol. Med. 2017, 23, 196–203. [Google Scholar] [CrossRef]
- Novotny, E.; Compton, S.; Liu, P.P.; Collins, F.S.; Chandrasekharappa, S.C. In Vitro Hematopoietic Differentiation of Mouse Embryonic Stem Cells Requires the Tumor Suppressor Menin and Is Mediated by Hoxa9. Mech. Dev. 2009, 126, 517–522. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Luo, T.-H.; Feng, L.; Zhao, Y.; Li, W.-Y.; Xu, J.; Zhang, Q.; Xu, L.-H.; Zheng, S.; Li, G.; et al. Microarray Analysis of Gene Expression in Men1 Knockout Embryoid Body Reveals Genetic Events Involved in Early Mouse Embryonic Development. Biochem. Biophys. Res. Commun. 2007, 352, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Goupille, O.; Cloment, C.S.; Lallemand, Y.; Cumano, A.; Robert, B. Msx Genes Define a Population of Mural Cell Precursors Required for Head Blood Vessel Maturation. Development 2011, 138, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Berkholz, J.; Breustedt, T.; Ploen, D.; Munz, B. Skeletal Muscle-Specific Variant of Nascent Polypeptide Associated Complex Alpha (skNAC): Implications for a Specific Role in Mammalian Myoblast Differentiation. Eur. J. Cell Biol. 2012, 91, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Azhdari, M.; zur Hausen, A. Wnt/β-Catenin and Notch Signaling Pathways in Cardiovascular Disease: Mechanisms and Therapeutics Approaches. Pharmacol. Res. 2025, 211, 107565. [Google Scholar] [CrossRef]
- Paolini, A.; Fontana, F.; Pham, V.-C.; Rödel, C.J.; Abdelilah-Seyfried, S. Mechanosensitive Notch-Dll4 and Klf2-Wnt9 Signaling Pathways Intersect in Guiding Valvulogenesis in Zebrafish. Cell Rep. 2021, 37, 109782. [Google Scholar] [CrossRef]
- Yilbas, A.; Hamilton, A.; Wang, Y.; Mach, H.; Lacroix, N.; Davis, D.R.; Chen, J.; Li, Q. Activation of GATA4 Gene Expression at the Early Stage of Cardiac Specification. Front. Chem. 2014, 2, 12. [Google Scholar] [CrossRef]
- Rivera-Feliciano, J.; Lee, K.-H.; Kong, S.W.; Rajagopal, S.; Ma, Q.; Springer, Z.; Izumo, S.; Tabin, C.J.; Pu, W.T. Development of Heart Valves Requires Gata4 Expression in Endothelial-Derived Cells. Development 2006, 133, 3607–3618. [Google Scholar] [CrossRef]
- Jászai, J.; Brand, M. Cloning and Expression of Ventrhoid, a Novel Vertebrate Homologue of the Drosophila EGF Pathway Gene Rhomboid. Mech. Dev. 2002, 113, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ma, Q. Ubiquitin-Specific Protease: An Emerging Key Player in Cardiomyopathy. Cell Commun. Signal. 2025, 23, 143. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Song, C.; Cui, J.; Li, Y.; Lei, X.; Tang, H. The Role of Deubiquitinases in Cardiovascular Diseases: Mechanisms and Therapeutic Implications. Front. Cardiovasc. Med. 2025, 12, 1582049. [Google Scholar] [CrossRef] [PubMed]
- Fraile, J.M.; Campos-Iglesias, D.; Rodríguez, F.; Astudillo, A.; Vilarrasa-Blasi, R.; Verdaguer-Dot, N.; Prado, M.A.; Paulo, J.A.; Gygi, S.P.; Martín-Subero, J.I.; et al. Loss of the Deubiquitinase USP36 Destabilizes the RNA Helicase DHX33 and Causes Preimplantation Lethality in Mice. J. Biol. Chem. 2018, 293, 2183–2194. [Google Scholar] [CrossRef]
- Kranz, A.; Anastassiadis, K. The Role of SETD1A and SETD1B in Development and Disease. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194578. [Google Scholar] [CrossRef]
- Wansleeben, C.; Meijlink, F. The Planar Cell Polarity Pathway in Vertebrate Development. Dev. Dyn. 2011, 240, 616–626. [Google Scholar] [CrossRef]
- Humeres, C.; Venugopal, H.; Frangogiannis, N.G. Smad-Dependent Pathways in the Infarcted and Failing Heart. Curr. Opin. Pharmacol. 2022, 64, 102207. [Google Scholar] [CrossRef]
- Wang, W.; Song, B.; Anbarchian, T.; Shirazyan, A.; Sadik, J.E.; Lyons, K.M. Smad2 and Smad3 Regulate Chondrocyte Proliferation and Differentiation in the Growth Plate. PLoS Genet. 2016, 12, e1006352. [Google Scholar] [CrossRef]
- Jiang, H.; Bai, L.; Song, S.; Yin, Q.; Shi, A.; Zhou, B.; Lian, H.; Chen, H.; Xu, C.-R.; Wang, Y.; et al. EZH2 Controls Epicardial Cell Migration during Heart Development. Life Sci. Alliance 2023, 6, e202201765. [Google Scholar] [CrossRef]
- França, M.M.; Mendonca, B.B. Genetics of Ovarian Insufficiency and Defects of Folliculogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101594. [Google Scholar] [CrossRef]
- Searcy, R.D.; Yutzey, K.E. Analysis of Hox gene expression during early avian heart development. Dev. Dyn. 1998, 213, 82–91. [Google Scholar] [CrossRef]
- Garcia-Padilla, C.; Dueñas, A.; Franco, D.; Garcia-Lopez, V.; Aranega, A.; Garcia-Martinez, V.; Lopez-Sanchez, C. Dynamic MicroRNA Expression Profiles During Embryonic Development Provide Novel Insights Into Cardiac Sinus Venosus/Inflow Tract Differentiation. Front. Cell Dev. Biol. 2022, 9, 767954. [Google Scholar] [CrossRef] [PubMed]
- Hrycaj, S.M.; Marty-Santos, L.; Cebrian, C.; Rasky, A.J.; Ptaschinski, C.; Lukacs, N.W.; Wellik, D.M. Hox5 Genes Direct Elastin Network Formation during Alveologenesis by Regulating Myofibroblast Adhesion. Proc. Natl. Acad. Sci. USA 2018, 115, E10605–E10614. [Google Scholar] [CrossRef] [PubMed]
- Morioka, N.; Ganier, C.; Watt, F.M. Fetal Fibroblast Heterogeneity Defines Dermal Architecture during Human Embryonic Skin Development. J. Investig. Dermatol. 2025, 145, 1081–1091.e7. [Google Scholar] [CrossRef]
- Kang, J.; Gu, Y.; Li, P.; Johnson, B.L.; Sucov, H.M.; Thomas, P.S. PDGF-A as an Epicardial Mitogen during Heart Development. Dev. Dyn. 2008, 237, 692–701. [Google Scholar] [CrossRef]
- Moore, K.; Fulmer, D.; Guo, L.; Koren, N.; Glover, J.; Moore, R.; Gensemer, C.; Beck, T.; Morningstar, J.; Stairley, R.; et al. PDGFRα: Expression and Function during Mitral Valve Morphogenesis. J. Cardiovasc. Dev. Dis. 2021, 8, 28. [Google Scholar] [CrossRef]
- Bi, Y.; Lv, Z.; Wang, Y.; Hai, T.; Huo, R.; Zhou, Z.; Zhou, Q.; Sha, J. WDR82, a Key Epigenetics-Related Factor, Plays a Crucial Role in Normal Early Embryonic Development in Mice. Biol. Reprod. 2011, 84, 756–764. [Google Scholar] [CrossRef]
- Paolini, A.; Sharipova, D.; Lange, T.; Abdelilah-Seyfried, S. Wnt9 Directs Zebrafish Heart Tube Assembly via a Combination of Canonical and Non-Canonical Pathway Signaling. Development 2023, 150, dev201707. [Google Scholar] [CrossRef]
- Smyth, S.S.; Kraemer, M.; Yang, L.; Van Hoose, P.; Morris, A.J. Roles for Lysophosphatidic Acid Signaling in Vascular Development and Disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158734. [Google Scholar] [CrossRef]
- Lu, C.; Wu, X.; Meng, X.; Liu, Y.; Yang, T.; Zeng, Y.; Chen, Y.; Huang, Y.; Fang, Z.; Yang, X.; et al. Silver Nanoparticles Exposure Impairs Cardiac Development by Suppressing the Focal Adhesion Pathway in Zebrafish. Int. J. Nanomed. 2024, 19, 9291–9304. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, J.; Pan, L.; Li, M.; Zhang, J.; Cai, X.; Chu, M. Microarray Analysis of Differential Gene Expression Profile Between Human Fetal and Adult Heart. Pediatr. Cardiol. 2017, 38, 700–706. [Google Scholar] [CrossRef]
- Barth, J.L.; Clark, C.D.; Fresco, V.M.; Knoll, E.P.; Lee, B.; Argraves, W.S.; Lee, K.-H. Jarid2 Is among a Set of Genes Differentially Regulated by Nkx2.5 during Outflow Tract Morphogenesis. Dev. Dyn. 2010, 239, 2024–2033. [Google Scholar] [CrossRef]
- Brauer, P.R.; Cai, D.H. Expression of Tissue Inhibitor of Metalloproteinases (TIMPs) during Early Cardiac Development. Mech. Dev. 2002, 113, 175–179. [Google Scholar] [CrossRef]
- Chandran, L.; Backer, W.; Schleutker, R.; Kong, D.; Beati, S.A.H.; Luschnig, S.; Müller, H.-A.J. Src42A Is Required for E-Cadherin Dynamics at Cell Junctions during Drosophila Axis Elongation. Development 2023, 150, dev201119. [Google Scholar] [CrossRef] [PubMed]
- Niikura, Y.; Tabata, Y.; Tajima, A.; Inoue, I.; Arai, K.; Watanabe, S. Zebrafish Numb Homologue: Phylogenetic Evolution and Involvement in Regulation of Left–Right Asymmetry. Mech. Dev. 2006, 123, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Abid, R.; Dababneh, S.; Bakr, M.; Aslani, T.; Cook, D.P.; Vanderhyden, B.C.; Park, J.G.; Munshi, N.V.; Hui, C.-C.; et al. Transcriptional Regulation of the Postnatal Cardiac Conduction System Heterogeneity. Nat. Commun. 2024, 15, 6550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Wang, C.; Gao, D.; Zuo, Z. Phenanthrene Exposure Produces Cardiac Defects during Embryo Development of Zebrafish (Danio rerio) through Activation of MMP-9. Chemosphere 2013, 93, 1168–1175. [Google Scholar] [CrossRef]
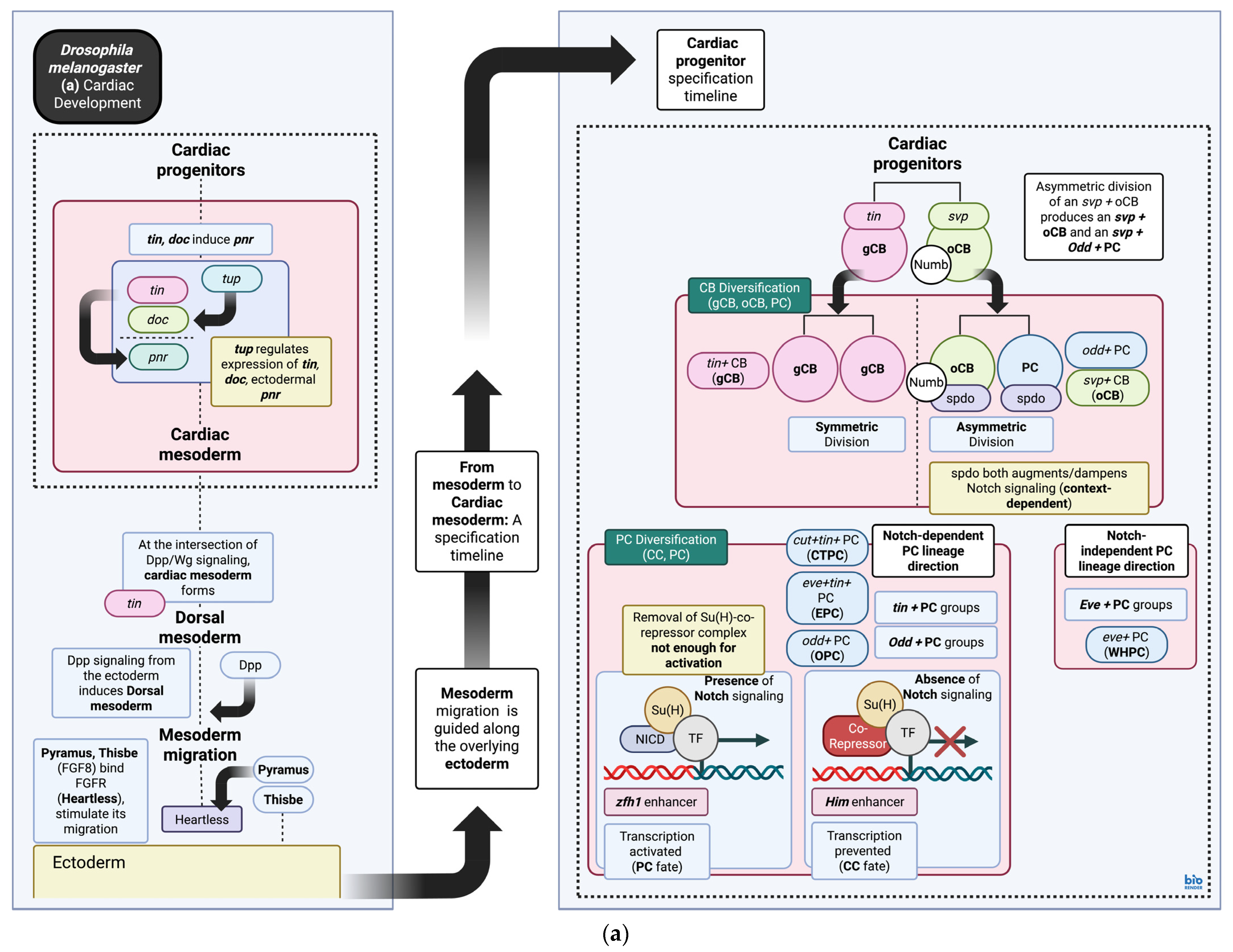
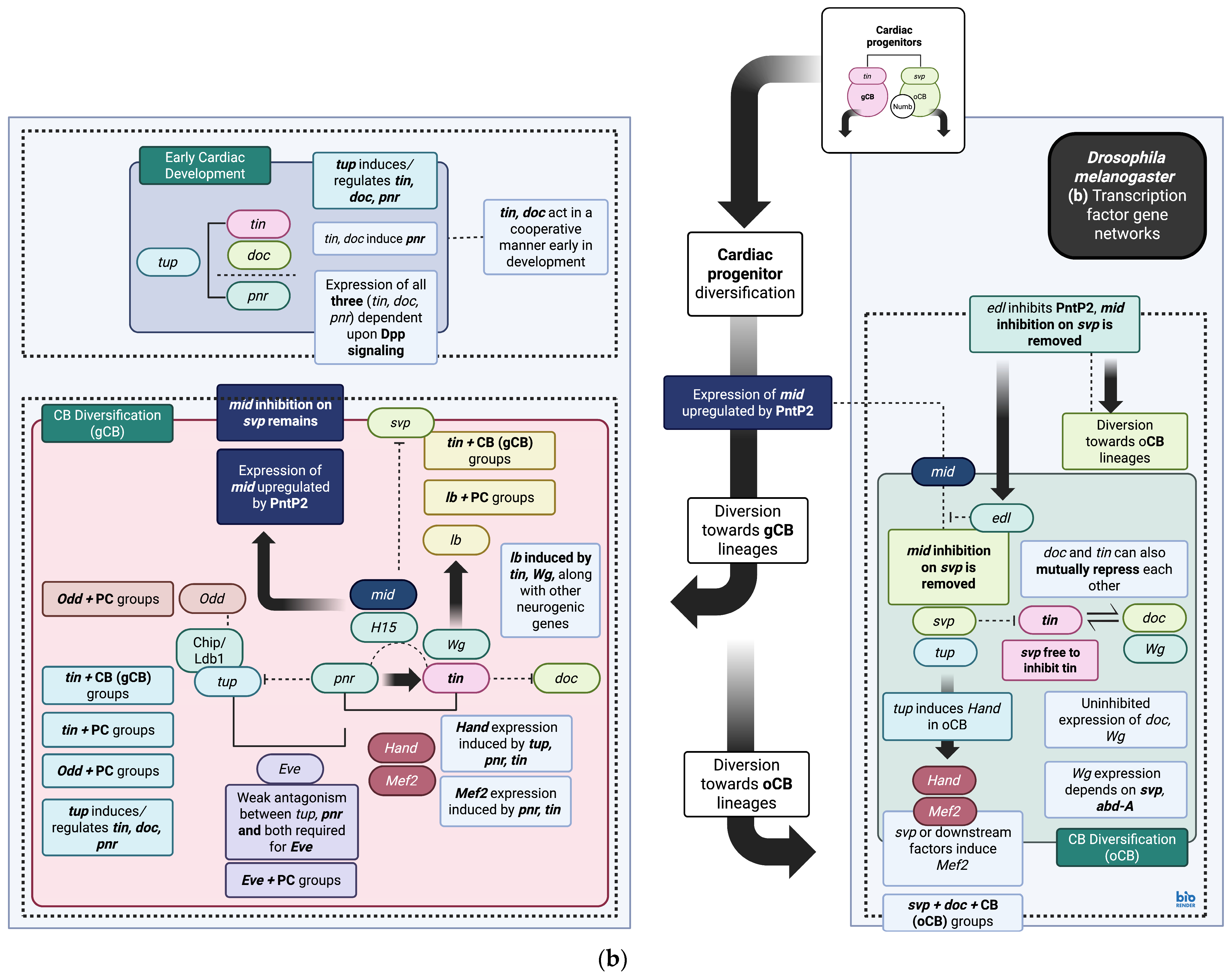
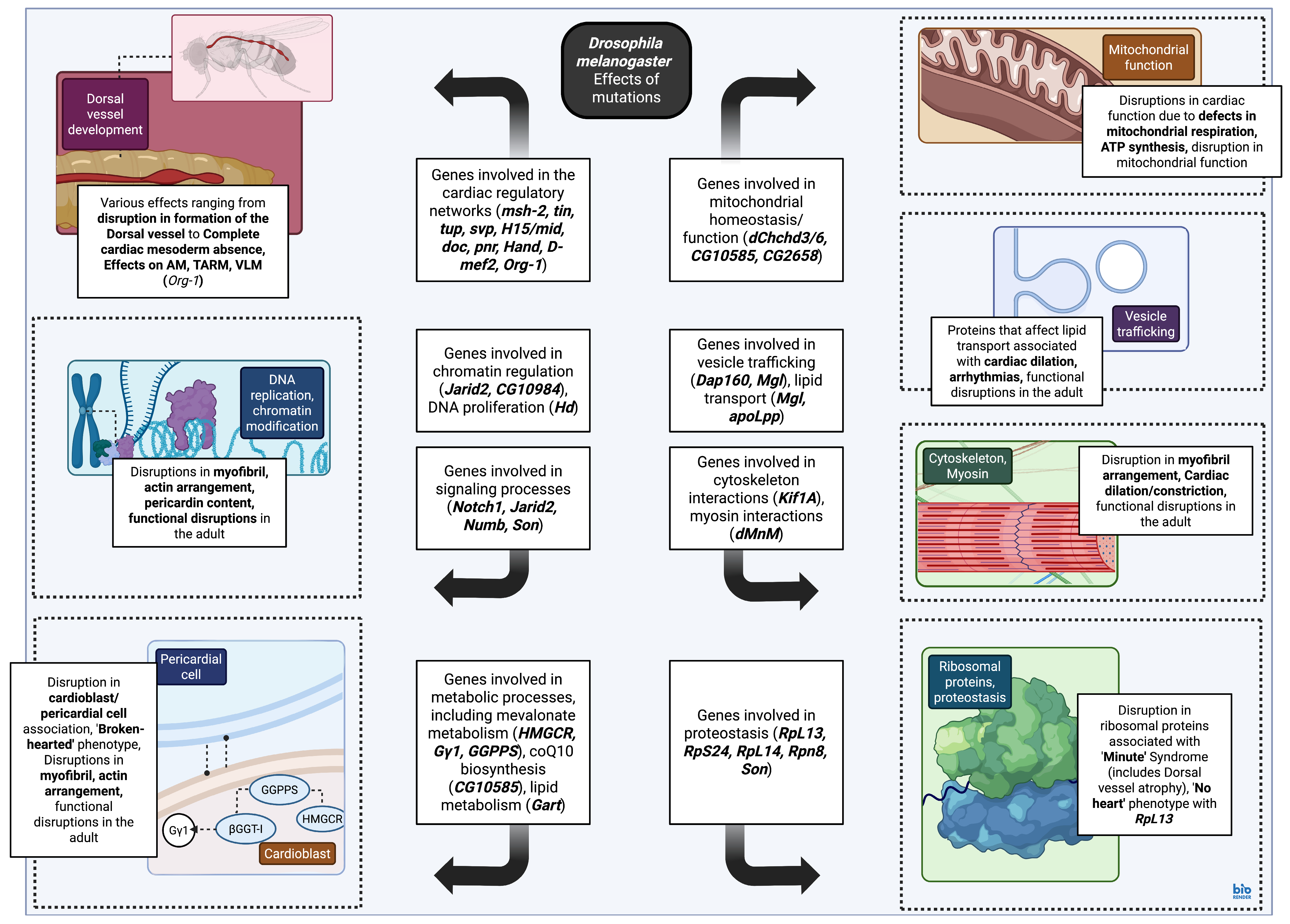
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stougiannou, T.M.; Koutini, M.; Mitropoulos, F.; Karangelis, D. In Vivo Models of Cardiovascular Disease: Drosophila melanogaster as a Genetic Model of Congenital Heart Disease. Biomedicines 2025, 13, 2569. https://doi.org/10.3390/biomedicines13102569
Stougiannou TM, Koutini M, Mitropoulos F, Karangelis D. In Vivo Models of Cardiovascular Disease: Drosophila melanogaster as a Genetic Model of Congenital Heart Disease. Biomedicines. 2025; 13(10):2569. https://doi.org/10.3390/biomedicines13102569
Chicago/Turabian StyleStougiannou, Theodora M, Maria Koutini, Fotios Mitropoulos, and Dimos Karangelis. 2025. "In Vivo Models of Cardiovascular Disease: Drosophila melanogaster as a Genetic Model of Congenital Heart Disease" Biomedicines 13, no. 10: 2569. https://doi.org/10.3390/biomedicines13102569
APA StyleStougiannou, T. M., Koutini, M., Mitropoulos, F., & Karangelis, D. (2025). In Vivo Models of Cardiovascular Disease: Drosophila melanogaster as a Genetic Model of Congenital Heart Disease. Biomedicines, 13(10), 2569. https://doi.org/10.3390/biomedicines13102569









