Diagnostic Accuracy of the Cobas® MTB and Cobas MTB/RIF-INH Assays on Sputum and the Cobas MTB Assay on Tongue Swabs for Mycobacterium tuberculosis Complex Detection in Symptomatic Adults in South Africa
Abstract
1. Introduction
2. Materials and Methods
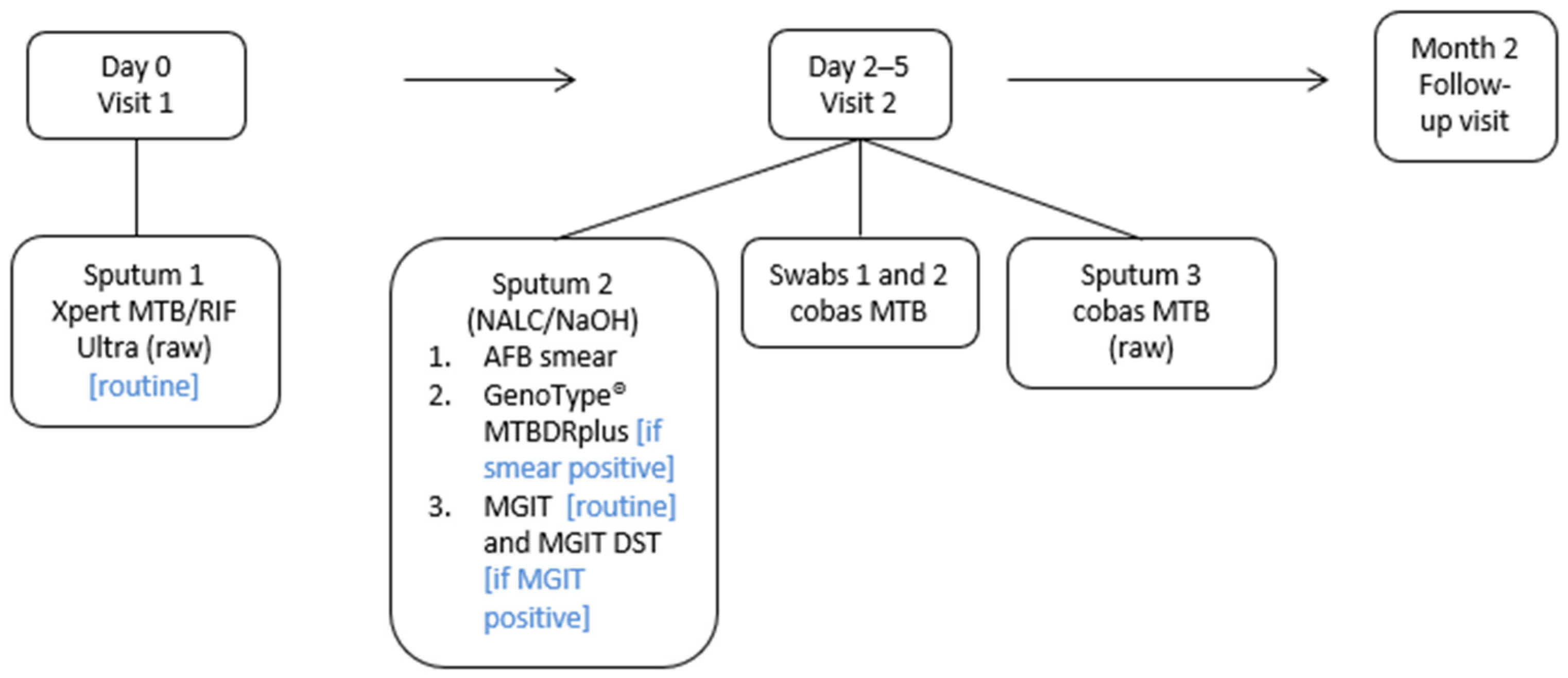
2.1. Study Design and Procedures
2.2. Laboratory Testing
2.3. Outcomes and Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Clinical Performance Evaluation of the Cobas MTB Assay on Sputum
3.3. Comparison of the Cobas MTB Assay to Xpert Ultra for Detection of MTBC
3.4. Clinical Performance Evaluation of the Cobas MTB/RIF-INH Assay on Sputum
3.5. Diagnostic Performance of Tongue Swabs on the Cobas MTB Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFB | Acid fast bacilli |
| BD | Becton Dickinson |
| BMI | Body mass index |
| CI | Confidence interval |
| DNA | Deoxyribonucleic acid |
| DST | Drug susceptibility testing |
| HCHC | Hillbrow Community Health Centre |
| INH | Isoniazid |
| LPA | Line probe assay |
| MDR-TB | Multi-drug-resistant TB |
| MGIT | Mycobacterial growth inidicator tube |
| MIS | Microbial inactivation solution |
| MTBC | Mycobacterium tuberculosis complex |
| NAAT | Nucleic acid amplification test |
| NALC-NaOH | N-acetyl-l-cysteine-sodium hydroxide |
| NPV | Negative predictive value |
| PCR | Polymerase chain reaction |
| pDST | Phenotypic drug susceptibility testing |
| PLHIV | People living with HIV |
| PPV | Positive predictive value |
| SIRE | Streptomycin [S], isoniazid [I], rifampicin [R], and ethambutol [E] |
| SR | Sample reagent |
| RIF | Rifampicin |
| TB | Tuberculosis |
| TS | Tongue swab |
| W4SS | WHO-recommended four-symptom screen |
| WitsDIH | Wits Diagnostic Innovation Hub |
References
- Hans, L.; Cassim, N.; Sarang, S.; Hardie, D.; Ndlovu, S.; Venter, W.D.F.; da Silva, P.; Stevens, W. HIV Viral Load Testing in the South African Public Health Setting in the Context of Evolving ART Guidelines and Advances in Technology, 2013–2022. Diagnostics 2023, 13, 2731. [Google Scholar] [CrossRef] [PubMed]
- Hans, L.; Marins, E.G.; Simon, C.O.; Magubane, D.; Seiverth, B.; Carmona, S. Classification of HIV-1 virological treatment failure using the Roche cobas plasma separation card on cobas 8800 compared to dried blood spots on Abbott RealTime HIV-1. J. Clin. Virol. 2021, 140, 104839. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024 (accessed on 4 November 2024).
- Treatment Action Group. Pipeline Report 2022; Treatment Action Group: New York, NY, USA, 2022; Available online: https://www.treatmentactiongroup.org/resources/pipeline-report/2022-pipeline-report/ (accessed on 26 November 2022).
- World Health Organization. Module 3: Diagnostics, Rapid Diagnostic for Tuberculosis Detection, WHO Consolidated Guidelines on Tuberculosis; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240029415 (accessed on 26 November 2022).
- NICD. Yes, We Can End TB; NICD: Johannesburg, South Africa, 2023; Available online: https://www.nicd.ac.za/wp-content/uploads/2023/03/World-TB-Day-2023.pdf (accessed on 26 November 2022).
- de Vos, M.; Scott, L.; David, A.; Trollip, A.; Hoffmann, H.; Georghiou, S.B.; Carmona, S.; Ruhwald, M.; Stevens, W.; Denkinger, C.M.; et al. Comparative analytical evaluation of four centralized platforms for the detection of M. tuberculosis complex and resistance to rifampicin and isoniazid. J. Clin. Microbiol. 2021, 59, e02168-20. [Google Scholar] [CrossRef] [PubMed]
- de Vos, M.; David, A.; Duraisamy, K.; Nadarajan, D.; Noroc, E.; Penn-Nicholson, A.; Crudu, V.; Giri, S.; Maurer, F.P.; Pati, S.; et al. Accuracy of cobas MTB and MTB-RIF/INH for Detection of Mycobacterium tuberculosis and Drug Resistance. J. Mol. Diagn. 2024, 26, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.; David, A.; Govender, L.; Furrer, J.; Rakgokong, M.; Waja, Z.; Martinson, N.; Eisenberg, G.; Marlowe, E.; Stevens, W. Performance of the Roche Cobas MTB Assay for the Molecular Diagnosis of Pulmonary Tuberculosis in a High HIV Burden Setting. J. Mol. Diagn. 2020, 22, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Nadarajan, D.; Hillemann, D.; Kamara, R.; Foray, L.; Conteh, O.S.; Merker, M.; Niemann, S.; Lau, J.; Njoya, M.; Kranzer, K.; et al. Evaluation of the Roche cobas MTB and MTB-RIF/INH Assays in Samples from Germany and Sierra Leone. J. Clin. Microbiol. 2021, 20, e02983-20. [Google Scholar] [CrossRef]
- Aono, A.; Murase, Y.; Minegishi, M.; Ohtawa, S.; Yano, M.; Chikamatsu, K.; Shimomura, Y.; Hosoya, M.; Igarashi, Y.; Morishige, Y.; et al. Clinical evaluation of the cobas® MTB-RIF/INH reagent and the cobas® 6800 for the detection of isoniazid and rifampicin resistance. Tuberculosis 2022, 134, 102199. [Google Scholar] [CrossRef] [PubMed]
- South African National Department of Health. National Tuberculosis Management Guidelines; South African National Department of Health: Pretoria, South Africa, 2014. Available online: https://knowledgehub.health.gov.za/elibrary/national-tuberculosis-management-guidelines (accessed on 22 May 2023).
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- David, A.; de Vos, M.; Scott, L.; da Silva, P.; Trollip, A.; Ruhwald, M.; Schumacher, S.; Stevens, W. Feasibility, Ease-of-Use, and Operational Characteristics of World Health Organization-Recommended Moderate-Complexity Automated Nucleic Acid Amplification Tests for the Detection of Tuberculosis and Resistance to Rifampicin and Isoniazid. J. Mol. Diagn. 2023, 25, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Mvusi, L.; Nanoo, A.; Dreyer, A.; Omar, S.V.; Babatunde, S.; Molebatsi, T.; van de Walt, M.; Adelekan, A.; Deyde, V.; et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: A national and sub-national cross-sectional survey. Lancet Infect. Dis. 2018, 18, 779–787. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 4 May 2022).
- Cohen, K.A.; Manson, A.L.; Desjardins, C.A.; Abeel, T.; Earl, A.M. Deciphering drug resistance in Mycobacterium tuberculosis using whole-genome sequencing: Progress, promise, and challenges. Genome Med. 2019, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Church, E.C.; Steingart, K.R.; Cangelosi, G.A.; Ruhwald, M.; Kohli, M.; Shapiro, A.E. Oral swabs with a rapid molecular diagnostic test for pulmonary tuberculosis in adults and children: A systematic review. Lancet Glob. Health 2024, 12, e45–e54. [Google Scholar] [CrossRef] [PubMed]
- Ahls, C.L.; Emsweller, D.; Helfers, S.J.; Niu, X.; Wilson, D.; Padgett, L.R.; Drain, P.K. No extraction? No problem. Direct to PCR processing of tongue swabs for diagnosis of tuberculosis disease as an alternative to sputum collection. Microbiol. Spectr. 2024, 12, e03107-23. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | All (n = 354) |
|---|---|
| Demographics | |
| Age, mean (range), years | 39 (18–70) |
| Male sex, n (%) | 213 (63.6) |
| BMI kg/m2, n (%) | |
| <18.5 (Underweight) | 94 (26.6) |
| 18.5–24.9 (Healthy weight) | 186 (52.5) |
| 25–29.9 (Overweight) | 44 (12.4) |
| >30 (Obese) | 30 (8.5) |
| HIV-related information | |
| HIV-positive, n (%) | 199 (56.6) |
| HIV-negative, n (%) | 152 (42.9) |
| Status unknown, n (%) | 3 (0.9) |
| TB History | |
| Previously diagnosed with TB, n (%) | 42 (11.9) |
| Clinical signs and symptoms of TB at presentation | |
| Cough (any duration), n (%) | 353 (99.7) |
| Unexplained weight loss, n (%) | 265 (74.9) |
| Nights sweats, n (%) | 263 (74.2) |
| Fever, n (%) | 236 (66.9) |
| * Other, n (%) | 257 (72.6) |
| Bacteriological confirmation (sputum 2), n (%) | |
| Smear and culture positive | 44 (12.4) |
| Smear negative and culture positive | 20 (5.6) |
| Smear and culture negative | 284 (80.2) |
| Smear positive and culture negative | 6 (1.7) |
| Variable | Smear Microscopy | Xpert MTB/RIF Ultra (Raw Sputum) | Cobas MTB (Raw Sputum) |
|---|---|---|---|
| All (including trace) (n = 354) | |||
| Sensitivity, % (95% CI) | 68.8 (55.9–79.8) | 93.8 (84.8–98.3) | 93.8 (84.8–98.3) |
| Specificity, % (95% CI) | 97.9 (95.6–99.2) | 97.9 (95.6–99.2) | 100 (98.7–100) |
| PPV, % (95% CI) | 88.2 (76.1–95.6) | 90.9 (81.3–96.6) | 100 (94.0–100) |
| NPV, % (95% CI) | 93.4 (90.0–95.9) | 98.6 (96.5–99.6) | 98.6 (96.6–99.6) |
| All (excluding trace) (n = 347) | |||
| Sensitivity, % (95% CI) | 70.5 (57.4–81.5) | 93.4 (84.1–98.2) | 96.7 (88.7–99.6) |
| Specificity, % (95% CI) | 97.9 (95.5–99.2) | 99.3 (97.5–99.9) | 100 (98.7–100) |
| PPV, % (95% CI) | 87.8 (75.2–95.4) | 96.6 (88.3–99.6) | 100 (93.9–100) |
| NPV, % (95% CI) | 94.0 (90.6–96.4) | 98.6 (96.5–99.6) | 99.3 (97.5–99.9) |
| Specimens from HIV-positive individuals (n = 199) | |||
| Sensitivity, % (95% CI) | 58.8 (40.7–75.4) | 91.2 (76.3–98.1) | 88.2 (72.5–96.7) |
| Specificity, % (95% CI) | 98.2 (94.8–99.6) | 97.6 (93.9–99.3) | 100 (97.8–100) |
| PPV, % (95% CI) | 87.0 (66.4–97.2) | 88.6 (73.3–96.8) | 100 (88.4–100) |
| NPV, % (95% CI) | 92.0 (87.0–95.6) | 98.2 (94.7–99.6) | 97.6 (94.1–99.4) |
| Specimens from HIV-negative individuals (n = 152) | |||
| Sensitivity, % (95% CI) | 82.1 (63.1–93.9) | 96.4 (81.7–99.9) | 100 (87.7–100) |
| Specificity, % (95% CI) | 97.6 (93.1–99.5) | 98.4 (94.0–99.8) | 100 (97.1–100) |
| PPV, % (95% CI) | 88.5 (69.8–97.6) | 93.1 (77.2–99.2) | 100 (87.7–100) |
| NPV, % (95% CI) | 96.0 (91.0–98.7) | 99.1 (95.3–100) | 100 (97.1–100) |
| Smear microscopy negative specimens (n = 304) | |||
| Sensitivity, % (95% CI) | n/a | 85.0 (62.1–96.8) | 85.0 (62.1–96.8) |
| Specificity, % (95% CI) | 97.9 (95.5–99.2) | 100 (98.7–100) | |
| PPV, % (95% CI) | 73.9 (51.6–89.8) | 100 (80.5–100) | |
| NPV, % (95% CI) | 98.9 (96.9–99.8) | 99.0 (97.0–99.8) | |
| pDST Result | Cobas MTB-RIF/INH Result | ||
|---|---|---|---|
| RIF-R not detected | RIF-R detected | Invalid | |
| RIF-sensitive (n = 54) | 46 | 0 | 8 |
| RIF-resistant (n = 1) | 0 | 1 | 0 |
| Invalid (n = 5) | 5 | 0 | 0 |
| pDST Result | Cobas MTB-RIF/INH Result | ||
|---|---|---|---|
| INH-R not detected | INH-R detected | Invalid | |
| INH sensitive (n = 50) | 43 | 0 | 7 |
| INH resistant (n = 5) | 3 | 1 | 1 |
| Invalid (n = 5) | 5 | 0 | 0 |
| Cobas MTB TS Result, n/N (%) | ||||
|---|---|---|---|---|
| Comparator Test Result (Sputum) | Neat MIS (TS 1) | Neat MIS (TS 2) | 66% MIS (TS 1) | 66% MIS (TS 2) |
| Xpert Ultra-positive | 25/53 (47.2) | 28/53 (52.8) | 9/13 (69.2) | 9/13 (69.2) |
| High | 16/18 (88.9) | 15/18 (83.3) | 4/6 (66.7) | 4/6 (66.7) |
| Medium | 2/6 (33.3) | 3/6 (50.0) | 1/2 (50.0) | 1/2 (50.0) |
| Low | 6/15 (40.0) | 6/15 (40.0) | 4/4 (100) | 4/4 (100) |
| Very low | 1/7 (14.2) | 4/7 (57.1) | 0/1 (0) | 0/1 (0) |
| Trace | 0/7 (0) | 0/6 (0) | - | - |
| Xpert Ultra-negative | 215/217 (99.1) | 216/217 (99.5) | 70/71 (98.6) | 71/71 (100) |
| Liquid culture-positive | 26/52 (50) | 29/52 (55.8) | 9/12 (75.0) | 9/12 (75.0) |
| Liquid culture-negative | 217/218 (99.5) | 218/218 (100) | 71/72 (98.6) | 72/72 (100) |
| Cobas MTB sputum-positive | 26/48 (54.2) | 29/48 (60.4) | 9/12 (75.0) | 9/12 (75.0) |
| Cobas sputum-negative | 221/222 (99.5) | 222/222 (100) | 71/72 (98.6) | 72/72 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, A.; Singh, L.; da Silva, M.P.; Peloakgosi-Shikwambani, K.; Nsingwane, Z.; Molepo, V.; Stevens, W.; Scott, L.E. Diagnostic Accuracy of the Cobas® MTB and Cobas MTB/RIF-INH Assays on Sputum and the Cobas MTB Assay on Tongue Swabs for Mycobacterium tuberculosis Complex Detection in Symptomatic Adults in South Africa. Biomedicines 2025, 13, 2556. https://doi.org/10.3390/biomedicines13102556
David A, Singh L, da Silva MP, Peloakgosi-Shikwambani K, Nsingwane Z, Molepo V, Stevens W, Scott LE. Diagnostic Accuracy of the Cobas® MTB and Cobas MTB/RIF-INH Assays on Sputum and the Cobas MTB Assay on Tongue Swabs for Mycobacterium tuberculosis Complex Detection in Symptomatic Adults in South Africa. Biomedicines. 2025; 13(10):2556. https://doi.org/10.3390/biomedicines13102556
Chicago/Turabian StyleDavid, Anura, Lyndel Singh, Manuel Pedro da Silva, Keneilwe Peloakgosi-Shikwambani, Zanele Nsingwane, Violet Molepo, Wendy Stevens, and Lesley Erica Scott. 2025. "Diagnostic Accuracy of the Cobas® MTB and Cobas MTB/RIF-INH Assays on Sputum and the Cobas MTB Assay on Tongue Swabs for Mycobacterium tuberculosis Complex Detection in Symptomatic Adults in South Africa" Biomedicines 13, no. 10: 2556. https://doi.org/10.3390/biomedicines13102556
APA StyleDavid, A., Singh, L., da Silva, M. P., Peloakgosi-Shikwambani, K., Nsingwane, Z., Molepo, V., Stevens, W., & Scott, L. E. (2025). Diagnostic Accuracy of the Cobas® MTB and Cobas MTB/RIF-INH Assays on Sputum and the Cobas MTB Assay on Tongue Swabs for Mycobacterium tuberculosis Complex Detection in Symptomatic Adults in South Africa. Biomedicines, 13(10), 2556. https://doi.org/10.3390/biomedicines13102556






