Plagued by the Past, Pressed by the Present: A One Health Perspective on Yersinia pestis
Abstract
1. Introduction
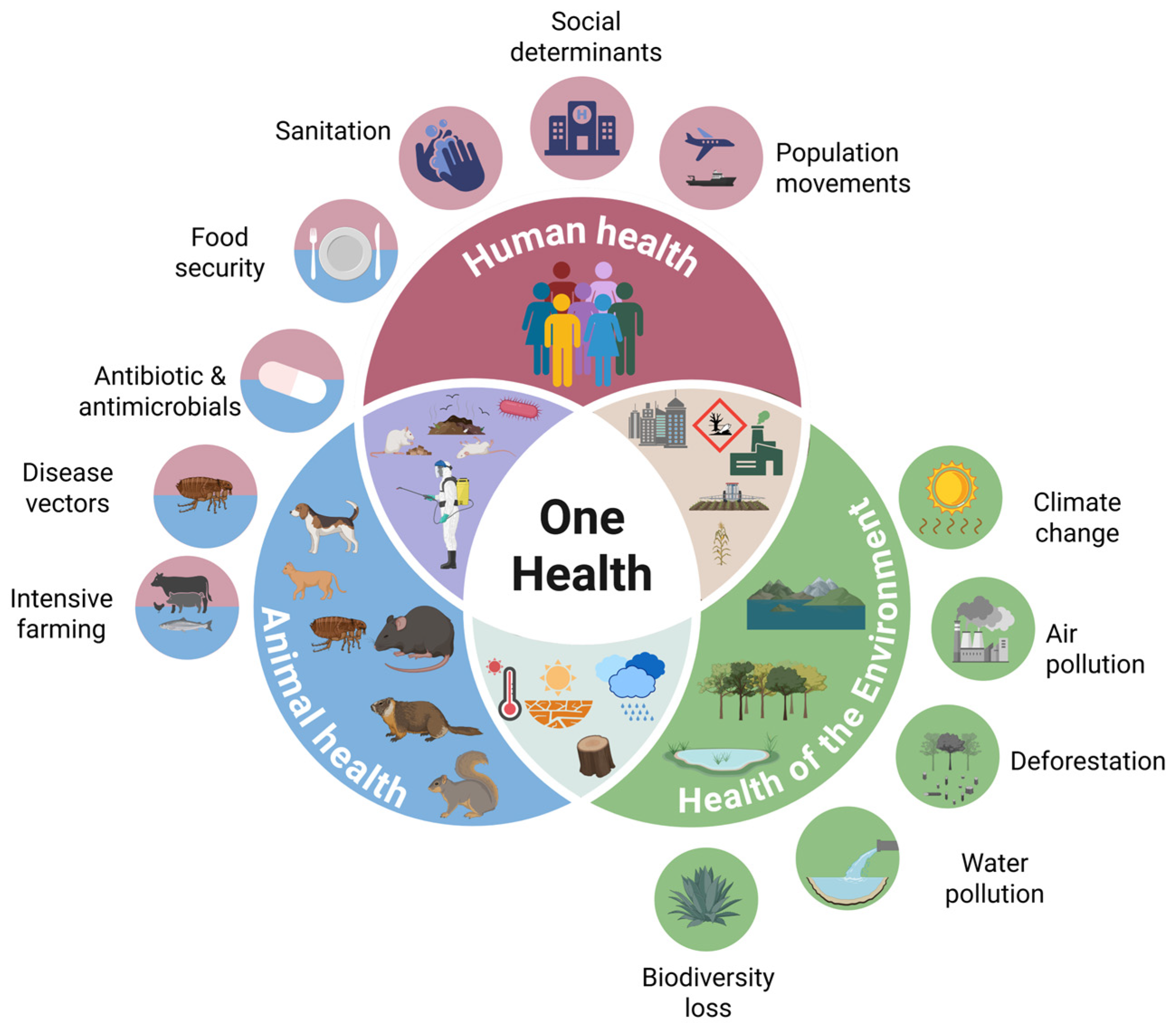
2. One Health: Humans, Animals, and the Environment
2.1. Animal Factors
2.1.1. Veterinary medicine and Yersinia pestis—A One Health approach
2.1.2. Rodents and Other Sylvatic Reservoirs
2.1.3. Vectors of Y. pestis
2.1.4. Animal: Epizootic and Enzootic Plague
2.2. Environmental Factors
2.3. Human Factors
2.4. Plague Impact Assessment and Prediction
3. Epidemiological Surveillance and Preventive Strategies
4. Epidemiology
5. Microbiological Profile of Y. pestis
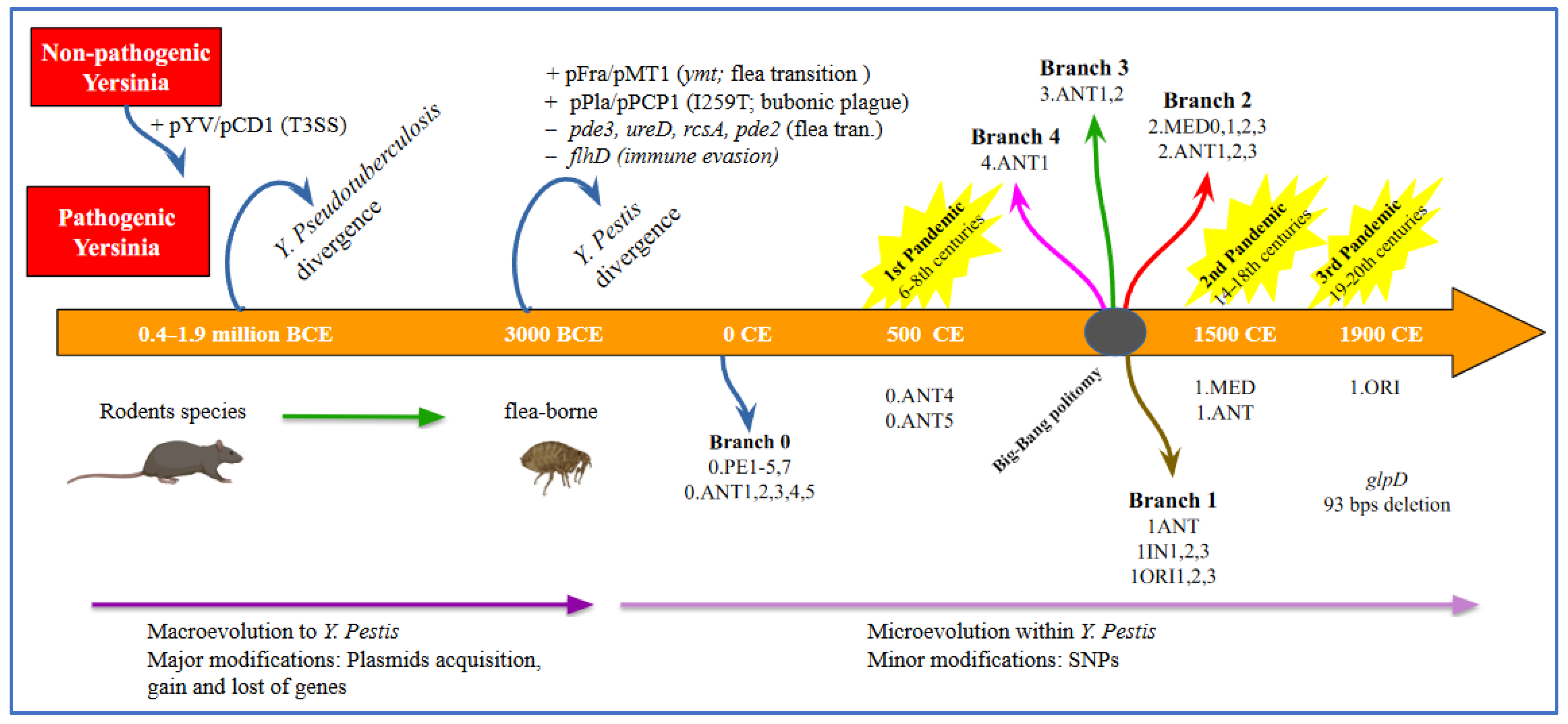
6. History and Evolution of Y. pestis and Plague
6.1. Phylogeny of Y. pestis over Time
6.2. A Historical Overview of Yersinia pestis and Its Impact on Human History Through the Plague Pandemics
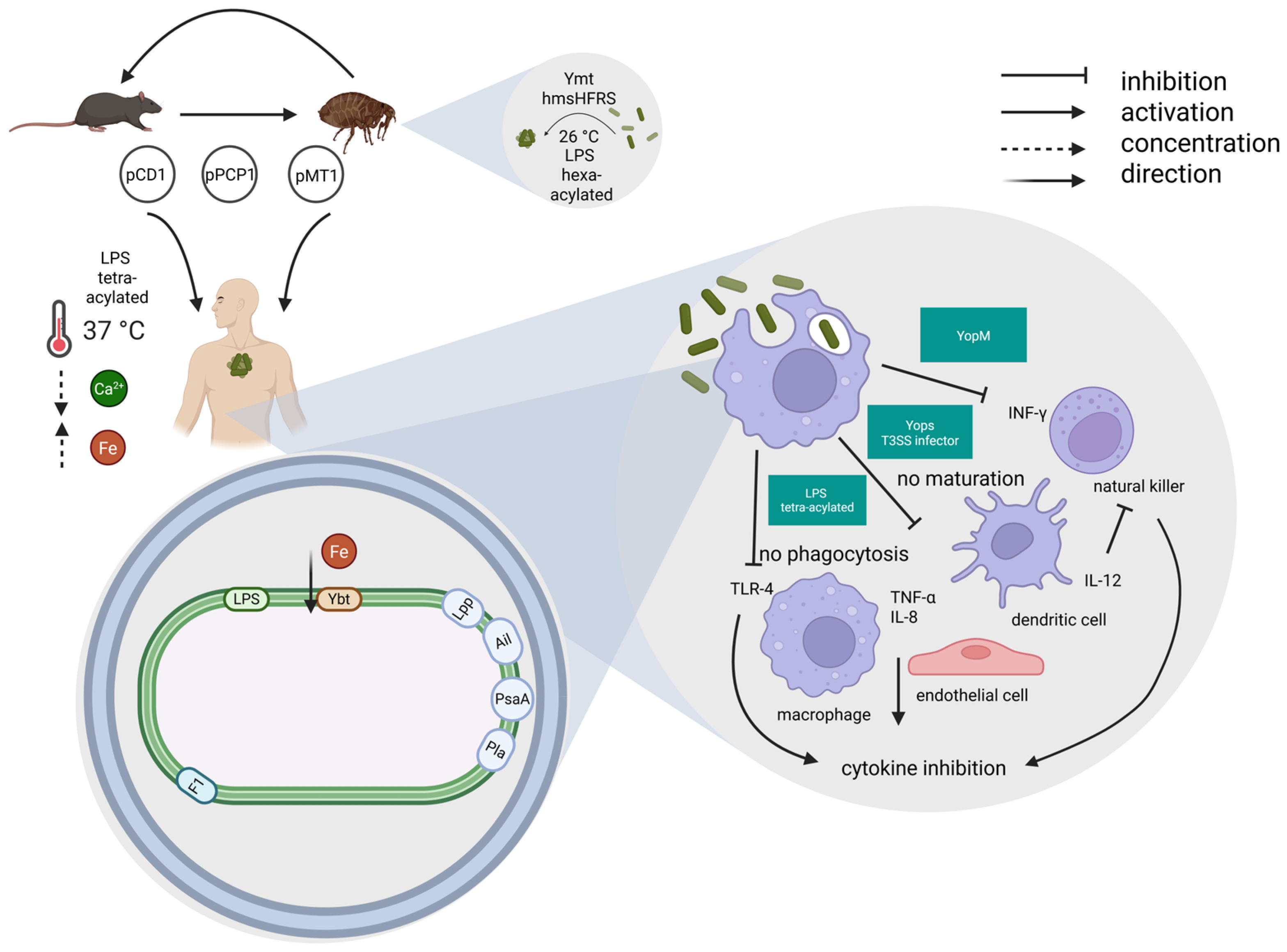
7. Pathogenesis, Y. pestis Virulence Factors, Escape Mechanisms, Host Immune Response, Clinical Forms
7.1. Pathogenesis, Y. pestis Virulence Factors, Escape Mechanisms, Host Immune Response
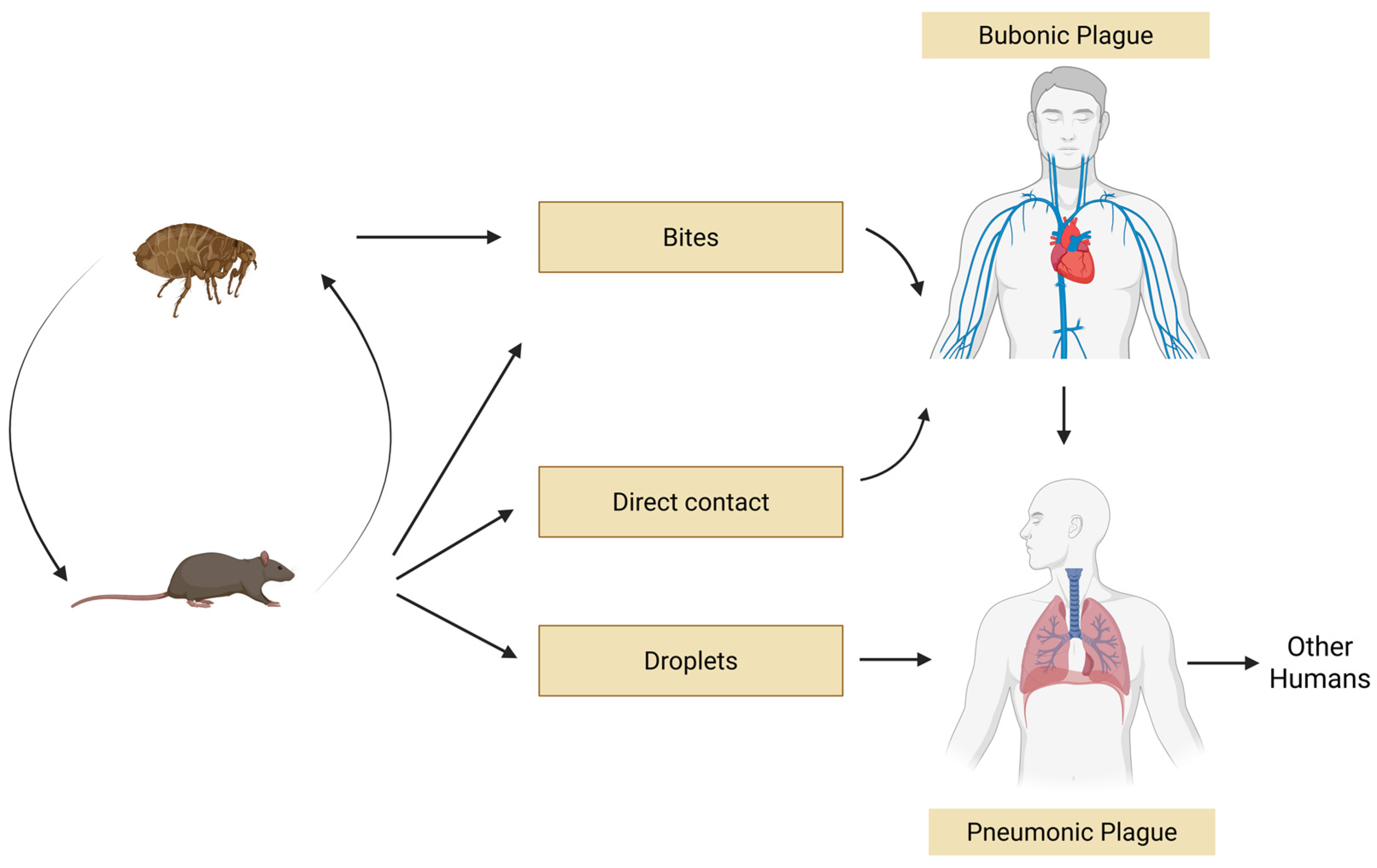
7.2. Transmission and Clinical Forms
8. Yersinia pestis as a Biological Weapon
9. Diagnosis
10. Prophylaxis and Therapy
10.1. Vaccines
10.2. Passive Immunization
10.3. Antibiotics
10.4. Bacteriophages
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Manual for Plague Surveillance, Diagnosis, Prevention and Control; World Health Organization: Geneva, Switzerland, 2024; Available online: https://apps.who.int/iris/handle/10665/68572 (accessed on 11 March 2025).
- Glatter, K.A.; Finkelman, P. History of the Plague: An Ancient Pandemic for the Age of COVID-19. Am. J. Med. 2021, 134, 176–181. [Google Scholar] [CrossRef]
- Baril, L.; Vallès, X.; Stenseth, N.C.; Rajerison, M.; Ratsitorahina, M.; Pizarro-Cerdá, J.; Demeure, C.; Belmain, S.; Scholz, H.; Girod, R.; et al. Can we make human plague history? A call to action. BMJ Glob. Health 2019, 10, e001984. [Google Scholar] [CrossRef]
- Semenza, J.C.; Zeller, H. Integrated surveillance for prevention and control of emerging vector-borne diseases in Europe. Eurosurveillance 2014, 19, 20757. [Google Scholar] [CrossRef]
- Heymann, D.L.; Dixon, M. The value of the One Health approach: Shifting from emergency response to prevention of zoonotic disease threats at their source. Microbiol. Spectr. 2013, 1, 17–31. [Google Scholar] [CrossRef]
- Zinsstag, J.; Crump, L.; Schelling, E.; Hattendorf, J.; Maidane, Y.O.; Ali, K.O.; Muhummed, A.; Umer, A.A.; Aliyi, F.; Nooh, F.; et al. Climate change and One Health. FEMS Microbiol. Lett. 2018, 365, fny085. [Google Scholar] [CrossRef]
- Sterk, A.; Schijven, J.; de Nijs, T.; de Roda Husman, A.M. Direct and indirect effects of climate change on the risk of infection by water-transmitted pathogens. Environ. Sci. Technol. 2013, 47, 12648–12660. [Google Scholar] [CrossRef]
- One Health High-Level Expert Panel (OHHLEP); Adisasmito, W.B.; Almuhairi, S.; Behravesh, C.B.; Bilivogui, P.; Bukachi, S.A.; Casas, N.; Cediel Becerra, N.; Charron, D.F.; Chaudhary, A.; et al. One Health: A new definition for a sustainable and healthy future. PLoS Pathog. 2022, 18, e1010537. [Google Scholar] [CrossRef]
- Eysenbach, G. Infodemiology and infoveillance. Am. J. Prev. Med. 2011, 40 (Suppl. 2), S154–S158. [Google Scholar] [CrossRef]
- Jean-Richard, V.; Crump, L.; Moto Daugla, D.; Hattendorf, J.; Schelling, E.; Zinsstag, J. The use of mobile phones for demographic surveillance of mobile pastoralists and their animals in Chad: Proof of principle. Glob. Health Action 2014, 7, 23209. [Google Scholar] [CrossRef]
- Kim, Y.; Huang, J.; Emery, S. Garbage in, garbage out: Data collection, quality assessment and reporting standards for social media data use in health research, infodemiology and digital disease detection. J. Med. Internet Res. 2016, 18, e41. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Kryštufek, B.; Sludsky, A.; Schmid, B.V.; De Almeida, A.M.P.; Lei, X.; Ramasindrazana, B.; Bertherat, E.; Yeszhanov, A.; Stenseth, N.C.; et al. Plague reservoir species throughout the world. Integr. Zool. 2021, 16, 820–833. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Eads, D.A.; Biggins, D.E.; Wimsatt, J.; Eisen, R.J.; Hinnebusch, B.J.; Matchett, M.R.; Goldberg, A.R.; Livieri, T.M.; Hacker, G.M.; Novak, M.G.; et al. Exploring and Mitigating Plague for One Health Purposes. Curr. Trop. Med. Rep. 2022, 9, 169–184. [Google Scholar] [CrossRef]
- Gage, K.L.; Kosoy, M.Y. Natural history of plague: Perspectives from more than a century of research. Annu. Rev. Entomol. 2005, 50, 505–528. [Google Scholar] [CrossRef]
- Biggins, D.E.; Kosoy, M.Y. Influences of introduced plague on North American mammals: Implications from ecology of plague in Asia. J. Mammal. 2001, 82, 906–916. [Google Scholar] [CrossRef]
- Vallès, X.; Stenseth, N.C.; Demeure, C.; Horby, P.; Mead, P.S.; Cabanillas, O.; Ratsitorahina, M.; Rajerison, M.; Andrianaivoarimanana, V.; Ramasindrazana, B.; et al. Human plague: An old scourge that needs new answers. PLoS Negl. Trop. Dis. 2020, 14, e0008251. [Google Scholar] [CrossRef]
- Esmaeili, S.; Esmaeili, P.; Mahmoudi, A.; Ghasemi, A.; Mohammadi, A.; Bagheri, A.; Sohrabi, A.; Rezaei, F.; Hanifi, H.; Neamati, A.H.; et al. Serological evidence of Yersinia pestis infection in rodents and carnivores in Northwestern Iran. PLoS Negl. Trop. Dis. 2023, 17, e0011021. [Google Scholar] [CrossRef]
- Gubler, D.J.; Reiter, P.; Ebi, K.L.; Yap, W.; Nasci, R.; Patz, J.A. Climate variability and change in the United States: Potential impacts on vector-borne and rodent-borne diseases. Environ. Health Perspect. 2001, 109 (Suppl. 2), 223–233. [Google Scholar]
- Zeppelini, C.G.; de Almeida, A.M.; Cordeiro-Estrela, P. Zoonoses as ecological entities: A case review of plague. PLoS Negl. Trop. Dis. 2016, 10, e0004949. [Google Scholar] [CrossRef]
- Perry, R.D.; Fetherston, J.D. Yersinia pestis-etiologic agent of plague. Clin. Microbiol. Rev. 1997, 10, 35–66. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Biodiversity series: The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. 2000, 78, 2061–2078. [Google Scholar] [CrossRef]
- Snäll, T.; O’Hara, R.B.; Ray, C.; Collinge, S.K. Climate-driven spatial dynamics of plague among prairie dog colonies. Am. Nat. 2008, 171, 238–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laudisoit, A.; Neerinckx, S.; Makundi, R.H.; Leirs, H.; Kras, B.R. Are local plague endemicity and ecological characteristics of vectors and reservoirs related? A case study in north-east Tanzania. Curr. Zool. 2009, 55, 200–211. [Google Scholar] [CrossRef]
- Dunn, R.R.; Davies, T.J.; Harris, N.C.; Gavin, M.C. Global drivers of human pathogen richness and prevalence. Proc. R. Soc. B Biol. Sci. 2010, 277, 2587–2595. [Google Scholar] [CrossRef]
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef]
- Han, B.A.; Schmidt, J.P.; Bowden, S.E.; Drake, J.M. Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA 2015, 112, 7039–7044. [Google Scholar] [CrossRef]
- Bennasar-Figueras, A. The Natural and Clinical History of Plague: From the Ancient Pandemics to Modern Insights. Microorganisms 2024, 12, 146. [Google Scholar] [CrossRef]
- Barbieri, R.; Signoli, M.; Chevé, D.; Costedoat, C.; Tzortzis, S.; Aboudharam, G.; Raoult, D.; Drancourt, M. Yersinia pestis: The Natural History of Plague. Clin. Microbiol. Rev. 2020, 34, e00044-19. [Google Scholar] [CrossRef]
- Petrov, V.S. USSR Natural Foci of Plague (Typing, Structure, Genesis). Ph.D. Thesis, University of Almaty, Almaty, Kazakhstan, 1968; p. 40. [Google Scholar]
- Burdelov, L.A. Hostal and Functional Structure of the Central Asian Desert Plague Focus (on the Example of the Pre-Aral Area). Ph.D. Thesis, All-Union Anti-plague Research Institute “Microbe”, Saratov, Russia, 1991; p. 42. [Google Scholar]
- Miarinjara, A.; Raveloson, A.O.; Mugel, S.G.; An, N.; Andriamiadanarivo, A.; Rajerison, M.E.; Randremanana, R.V.; Girod, R.; Gillespie, T.R. Socio-ecological risk factors associated with human flea infestations of rural household in plague-endemic areas of Madagascar. PLoS Negl. Trop. Dis. 2024, 18, e0012036. [Google Scholar] [CrossRef]
- Hufthammer, A.K.; Walløe, L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in northern Europe. J. Archaeol. Sci. 2013, 40, 1752–1759. [Google Scholar] [CrossRef]
- Yue, R.P.H.; Lee, H.F. Pre-industrial plague transmission is mediated by the synergistic effect of temperature and aridity index. BMC Infect. Dis. 2018, 18, 134. [Google Scholar] [CrossRef]
- Hinnebusch, B.J.; Jarrett, C.O.; Bland, D.M. “Fleaing” the plague: Adaptations of Yersinia pestis to its insect vector that lead to transmission. Annu. Rev. Microbiol. 2017, 71, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Bacot, A.W.; Martin, C.J. LXVII. Observations on the mechanism of the transmission of plague by fleas. J. Hyg. 1914, 13, 423–439. [Google Scholar] [PubMed]
- Jarrett, C.O.; Deak, E.; Isherwood, K.E.; Oyston, P.C.; Fischer, E.R.; Whitney, A.R.; Kobayashi, S.D.; DeLeo, F.R.; Hinnebusch, B.J. Transmission of Yersinia pestis from an infectious biofilm in the flea vector. J. Infect. Dis. 2004, 190, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Bacot, A.W. LXXXI. Further notes on the mechanism of the transmission of plague by fleas. J. Hyg. 1915, 14, 774–776.3. [Google Scholar]
- Biggins, D.E.; Godbey, J.L.; Gage, K.L.; Carter, L.G.; Montenieri, J.A. Vector control improves survival of three species of prairie dogs (Cynomys) in areas considered enzootic for plague. Vector Borne Zoonotic Dis. 2010, 10, 17–26. [Google Scholar] [CrossRef]
- Wimsatt, J.; Biggins, D.E. A review of plague persistence with special emphasis on fleas. J. Vector Borne Dis. 2009, 46, 85–99. [Google Scholar]
- Smith, P.; Singh, P.K.; Ballal, V.P.; Cherubini, F.; Díaz-José, J.; Duchková, H.; Gupta, H.; Hori, M.; Ito, A.; Khan, S.; et al. Impacts of Climate Change Interventions on Biodiversity, Water, the Food System and Human Health and Well-Being. Glob. Change Biol. 2025, 31, e70444. [Google Scholar] [CrossRef]
- Watts, N.; Adger, W.N.; Ayeb-Karlsson, S.; Bai, Y.; Byass, P.; Campbell-Lendrum, D.; Colbourn, T.; Cox, P.; Davies, M.; Depledge, M.; et al. The Lancet Countdown: Tracking progress on health and climate change. Lancet 2017, 389, 1151–1164. [Google Scholar] [CrossRef]
- Gage, K.L. Factors Affecting the Spread and Maintenance of Plague. Adv. Exp. Med. Biol. 2012, 954, 79–94. [Google Scholar] [CrossRef]
- Gage, K.L.; Burkot, T.R.; Eisen, R.J.; Hayes, E.B. Climate and vectorborne diseases. Am. J. Prev. Med. 2008, 35, 436–450. [Google Scholar] [CrossRef]
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.; Kutz, S.; Harvell, C.D. Climate change and infectious diseases: From evidence to a predictive framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef]
- Davis, S.; Begon, M.; De Bruyn, L.; Ageyev, V.S.; Klassovskiy, N.L.; Pole, S.B.; Viljugrein, H.; Stenseth, N.C.; Leirs, H. Predictive thresholds for plague in Kazakhstan. Science 2004, 304, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Ben Ari, T.; Neerinckx, S.; Gage, K.L.; Kreppel, K.; Laudisoit, A.; Leirs, H.; Stenseth, N.C. Plague and climate: Scales matter. PLoS Pathog. 2011, 7, e1002160, Correction in PLoS Pathog. 2011, 8, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Stige, L.C.; Kausrud, K.L.; Ben Ari, T.; Wang, S.; Fang, X.; Schmid, B.V.; Liu, Q.; Stenseth, N.C.; Zhang, Z. Wet climate and transportation routes accelerate spread of human plague. Proc. R. Soc. B Biol. Sci. 2014, 8, 85–94. [Google Scholar] [CrossRef]
- Rogers, L. The yearly variations in plague in India in relation to climate: Forecasting epidemics. Proc. R. Soc. B Biol. Sci. 1928, 83, 624–632. [Google Scholar] [CrossRef]
- Cavanaugh, D.C.; Marshall, J.D. The influence of climate on the seasonal prevalence of plague in the Republic of Vietnam. J. Wildl. Dis. 1972, 8, 85–94. [Google Scholar] [CrossRef]
- Xu, L.; Stige, L.C.; Leirs, H.; Neerinckx, S.; Gage, K.L.; Yang, R.; Liu, Q.; Bramanti, B.; Dean, K.R.; Tang, H.; et al. Historical and genomic data reveal the influencing factors on global transmission velocity of plague during the Third Pandemic. Proc. Natl. Acad. Sci. USA 2019, 116, 11833–11838. [Google Scholar] [CrossRef]
- Davis, D.H. Plague in Africa from 1935 to 1949, a survey of wild rodents in African territories. Bull. World Health Organ. 1953, 9, 665–700. [Google Scholar] [PubMed]
- Schotthoefer, A.M.; Bearden, S.W.; Vetter, S.M.; Holmes, J.; Montenieri, J.A.; Graham, C.B.; Woods, M.E.; Eisen, R.J.; Gage, K.L. Effects of temperature on early-phase transmission of Yersinia pestis by the flea, Xenopsylla cheopis. J. Med. Entomol. 2011, 48, 411–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williams, S.K.; Schotthoefer, A.M.; Montenieri, J.A.; Holmes, J.L.; Vetter, S.M.; Gage, K.L.; Bearden, S.W. Effects of low-temperature flea maintenance on the transmission of Yersinia pestis by Oropsylla montana. Vector Borne Zoonotic Dis. 2013, 13, 468–478. [Google Scholar] [CrossRef]
- Kreppel, K.S.; Telfer, S.; Rajerison, M.; Morse, A.; Baylis, M. Effect of temperature and relative humidity on the development times and survival of Synopsyllus fonquerniei and Xenopsylla cheopis, the flea vectors of plague in Madagascar. Parasit. Vectors 2016, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Tennant, W.S.D.; Tildesley, M.J.; Spencer, S.E.F.; Keeling, M.J. Climate drivers of plague epidemiology in British India, 1898-1949. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200538. [Google Scholar] [CrossRef]
- Carlson, C.J.; Bevins, S.N.; Schmid, B.V. Plague risk in the western United States over seven decades of environmental change. Glob. Change Biol. 2022, 28, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Carniel, E. Plague today. Med. Hist. Suppl. 2008, 27, 115–122. [Google Scholar] [CrossRef]
- Arotolu, T.E.; Wang, H.; Lv, J.; Kun, S.; Huang, L.; Wang, X. Environmental suitability of Yersinia pestis and the spatial dynamics of plague in the Qinghai Lake region, China. Vet. Med. 2022, 67, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; Belden, L.K.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.D.; Hudson, P.; Jolles, A.; Jones, K.E.; Mitchell, C.E.; et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Samia, N.I.; Viljugrein, H.; Kausrud, K.L.; Begon, M.; Davis, S.; Leirs, H.; Dubyanskiy, V.M.; Esper, J.; Ageyev, V.S.; et al. Plague dynamics are driven by climate variation. Proc. Natl. Acad. Sci. USA 2006, 103, 13110–13115. [Google Scholar] [CrossRef]
- Roche, B.; Rohani, P.; Dobson, A.P.; Guegan, J.F. The impact of community organization on vector-borne pathogens. Am. Nat. 2013, 181, 1–11. [Google Scholar] [CrossRef]
- Rivière-Cinnamond, A.; Santandreu, A.; Luján, A.; Mertens, F.; Espinoza, J.O.; Carpio, Y.; Bravo, J.; Gabastou, J.M. Identifying the social and environmental determinants of plague endemicity in Peru: Insights from a case study in Ascope, La Libertad. BMC Public Health 2018, 18, 220. [Google Scholar] [CrossRef]
- Alderson, J.; Quastel, M.; Wilson, E.; Bellamy, D. Factors influencing the re-emergence of plague in Madagascar. Emerg. Top. Life Sci. 2020, 4, 423–433. [Google Scholar] [CrossRef]
- Nyirenda, S.S.; Hang’ombe, B.M.; Machang’u, R.; Mwanza, J.; Kilonzo, B.S. Identification of Risk Factors Associated with Transmission of Plague Disease in Eastern Zambia. Am. J. Trop. Med. Hyg. 2017, 97, 826–830. [Google Scholar] [CrossRef]
- Semenza, J.C.; Paz, S. Climate change and infectious disease in Europe: Impact, projection and adaptation. Lancet Reg. Health Eur. 2021, 9, 100230. [Google Scholar] [CrossRef]
- Ben-Ari, T.; Gershunov, A.; Gage, K.L.; Snäll, T.; Ettestad, P.; Kausrud, K.L.; Stenseth, N.C. Human plague in the USA: The importance of regional and local climate. Biol. Lett. 2008, 4, 737–740. [Google Scholar] [CrossRef]
- Ben-Ari, T.; Gershunov, A.; Tristan, R.; Cazelles, B.; Gage, K.; Stenseth, N.C. Interannual variability of human plague occurrence in the Western United States explained by tropical and North Pacific Ocean climate variability. Am. J. Trop. Med. Hyg. 2010, 83, 624–632. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Q.; Stige, L.C.; Ari, T.B.; Fang, X.; Chan, K.S.; Wang, S.; Stenseth, N.C.; Zhang, Z. Nonlinear effect of climate on plague during the third pandemic in China. Proc. Natl. Acad. Sci. USA 2011, 108, 10214–10219. [Google Scholar] [CrossRef] [PubMed]
- Stapp, P.; Antolin, M.F.; Ball, M. Patterns of Extinction in Prairie Dog Metapopulations: Plague Outbreaks Follow El Niño Events. Front. Ecol. Environ. 2004, 2, 235–240. [Google Scholar] [CrossRef]
- Bevins, S.N.; Baroch, J.A.; Nolte, D.L.; Zhang, M.; He, H. Yersinia pestis: Examining wildlife plague surveillance in China and the USA. Integr. Zool. 2012, 7, 99–109. [Google Scholar] [CrossRef]
- Xu, L.; Schmid, B.V.; Liu, J.; Si, X.; Stenseth, N.C.; Zhang, Z. The trophic responses of two different rodent-vector-plague systems to climate change. Proc. R. Soc. B Biol. Sci. 2015, 282, 20141846. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Salathé, M.; Stapp, P.; Jones, J.H. Plague outbreaks in prairie dog populations explained by percolation thresholds of alternate host abundance. Proc. Natl. Acad. Sci. USA 2010, 107, 14247–14250. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- World Health Organization. Pathogens Prioritization: A Scientific Framework for Epidemic and Pandemic Research Preparedness; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Ukoaka, B.M.; Okesanya, O.J.; Daniel, F.M. Updated WHO list of emerging pathogens for a potential future pandemic: Implications for public health and global preparedness. Infez. Med. 2024, 32, 463–477. [Google Scholar] [CrossRef]
- World Health Organization. One Health; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Andrianaivoarimanana, V.; Piola, P.; Wagner, D.M.; Rakotomanana, F.; Maheriniaina, V.; Andrianalimanana, S.; Chanteau, S.; Rahalison, L.; Ratsitorahina, M.; Rajerison, M. Trends of human plague, Madagascar, 1998–2016. Emerg. Infect. Dis. 2019, 25, 220–228. [Google Scholar] [CrossRef]
- Moore, S.M.; Monaghan, A.; Borchert, J.N.; Mpanga, J.T.; Atiku, L.A.; Boegler, K.A.; Montenieri, J.; MacMillan, K.; Gage, K.L.; Eisen, R.J. Seasonal fluctuations of small mammal and flea communities in a Ugandan plague focus: Evidence to implicate Arvicanthis niloticus and Crocidura spp. as key hosts in Yersinia pestis transmission. Parasites Vectors 2015, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, Y.; Guo, Z.; Liang, Y.; Zhu, Z.; Zhou, Q.; Yan, Y.; Song, Z.; Yang, R. Serologic survey of the sentinel animals for plague surveillance and screening for complementary diagnostic markers to F1 antigen by protein microarray. Am. J. Trop. Med. Hyg. 2008, 79, 799–802. [Google Scholar] [CrossRef]
- Brown, H.E.; Levy, C.E.; Enscore, R.E.; Schriefer, M.E.; DeLiberto, T.J.; Gage, K.L.; Eisen, R.J. Annual seroprevalence of Yersinia pestis in coyotes as predictors of interannual variation in reports of human plague cases in Arizona, United States. Vector Borne Zoonotic Dis. 2011, 11, 1439–1446. [Google Scholar] [CrossRef]
- Rahelinirina, S.; Harimalala, M.; Rakotoniaina, J.; Randriamanantsoa, M.G.; Dentinger, C.; Zohdy, S.; Girod, R.; Rajerison, M. Tracking of Mammals and Their Fleas for Plague Surveillance in Madagascar, 2018-2019. Am. J. Trop. Med. Hyg. 2022, 106, 1601–1609. [Google Scholar] [CrossRef]
- Rajerison, M.; Andrianaivoarimanana, V.; Ratsitorahina, M.; Rahelinirina, S.; Chanteau, S.; Telfer, S.; Rahalison, L. Field assessment of dog as sentinel animal for plague in endemic foci of Madagascar. Integr. Zool. 2021, 16, 886–892. [Google Scholar] [CrossRef]
- Ramalingaswami, V. Plague in India. Nat. Med. 1995, 1, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Bertherat, E.; Bekhoucha, S.; Chougrani, S.; Razik, F.; Duchemin, J.B.; Houti, L.; Deharib, L.; Fayolle, C.; Makrerougrass, B.; Dali-Yahia, R.; et al. Plague reappearance in Algeria after 50 years, 2003. Emerg Infect. Dis. 2007, 13, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Cabanel, N.; Leclercq, A.; Chenal-Francisque, V.; Annajar, B.; Rajerison, M.; Bekkhoucha, S.; Bertherat, E.; Carniel, E. Plague outbreak in Libya, 2009, unrelated to plague in Algeria. Emerg. Infect. Dis. 2013, 19, 230–236. [Google Scholar] [CrossRef]
- Nyirenda, S.S.; Hang’ombe, B.M.; Mulenga, E.; Kilonzo, B.S. Serological and PCR investigation of Yersinia pestis in potential reservoir hosts from a plague outbreak focus in Zambia. BMC Res. Notes 2017, 10, 345. [Google Scholar] [CrossRef]
- Diaz, J.H. Regional Rodent-Borne Infectious Diseases in North America: What Wilderness Medicine Providers Need to Know. Wilderness Environ Med. 2021, 32, 365–376. [Google Scholar] [CrossRef]
- Baeten, L.A.; Pappert, R.; Young, J.; Schriefer, M.E.; Gidlewski, T.; Kohler, D.; Bowen, R.A. Immunological and clinical response of coyotes (Canis latrans) to experimental inoculation with Yersinia pestis. J. Wildl. Dis. 2013, 49, 932–939. [Google Scholar] [CrossRef]
- Miarinjara, A.; Boyer, S. Current Perspectives on Plague Vector Control in Madagascar: Susceptibility Status of Xenopsylla cheopis to 12 Insecticides. PLoS Negl. Trop. Dis. 2016, 10, e0004414. [Google Scholar] [CrossRef]
- Rosario-Acevedo, R.; Biryukov, S.S.; Bozue, J.A.; Cote, C.K. Plague prevention and therapy: Perspectives on Current and Future Strategies. Biomedicines 2021, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Madagascar Plague Outbreak: External Situation Report #14; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Enscore, R.E.; Biggerstaff, B.J.; Brown, T.L.; Fulgham, R.E.; Reynolds, P.J.; Engelthaler, D.M.; Levy, C.E.; Parmenter, R.R.; Montenieri, J.A.; Cheek, J.E.; et al. Modeling relationships between climate and the frequency of human plague cases in the southwestern United States, 1960-1997. Am. J. Trop. Med. Hyg. 2002, 66, 186–196. [Google Scholar] [CrossRef][Green Version]
- Jullien, S.; de Silva, N.L.; Garner, P. Plague transmission from corpses and carcasses. Emerg. Infect. Dis. 2021, 27, 2033–2041. [Google Scholar] [CrossRef]
- Yang, R. Plague: Recognition, treatment, and prevention. J. Clin. Microbiol. 2017, 56, e01519-17. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Lehr, T. Big epidemiology: The birth, life, death, and resurgence of diseases on a global timescale. Epidemiologia 2024, 5, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Blaschko, M.B.; Saarakkala, S.; Tiulpin, A. Clinically-inspired multi-agent transformers for disease trajectory forecasting from multimodal data. IEEE Trans. Med. Imaging 2024, 43, 529–541. [Google Scholar] [CrossRef]
- Dubyanskiy, V.M.; Yeszhanov, A.B. Ecology of Yersinia pestis and the Epidemiology of Plague. Adv. Exp. Med. Biol. 2016, 918, 101–170. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for South-East Asia. Operational Guidelines on Plague Surveillance, Diagnosis, Prevention and Control; WHO Regional Office for South-East Asia: New Delhi, India, 2010; Available online: https://iris.who.int/handle/10665/205593 (accessed on 18 March 2025).
- Randremanana, R.; Andrianaivoarimanana, V.; Nikolay, B.; Ramasindrazana, B.; Paireau, J.; Ten Bosch, Q.A.; Rakotondramanga, J.M.; Rahajandraibe, S.; Rahelinirina, S.; Rakotomanana, F.; et al. Epidemiological characteristics of an urban plague epidemic in Madagascar, August–November, 2017: An outbreak report. Lancet Infect. Dis. 2019, 19, 537–545. [Google Scholar] [CrossRef]
- Riehm, J.M.; Vergnaud, G.; Kiefer, D.; Damdindorj, T.; Dashdavaa, O.; Khurelsukh, T.; Zöller, L.; Wölfel, R.; Le Flèche, P.; Scholz, H.C. Yersinia pestis lineages in Mongolia. PLoS ONE 2012, 7, e30624. [Google Scholar] [CrossRef]
- Galdan, B.; Baatar, U.; Molotov, B.; Dashdavaa, O. Plague in Mongolia. Vector Borne Zoonotic Dis. 2010, 10, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Eroshenko, G.A.; Nosov, N.Y.; Kras, Y.M.; Oglodin, Y.G.; Kukleva, L.M.; Guseva, N.P.; Kuznetsov, A.A.; Abdikarimov, S.T.; Dzhaparova, A.K.; Kutyrev, V.V. Yersinia pestis strains of ancient phylogenetic branch 0.ANT are widely spread in the high-mountain plague foci of Kyrgyzstan. PLoS ONE 2017, 12, e0187230. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, A.P. Faktory Yersinia pestis, obespechivaiushchie tsirkuliatsiiu i sokhranenie vozbutelia chumy v ékosistemakh prirodnykh ochagov. Soobshchenie 1. Mol. Gen. Mikrobiol. Virusol. 2002, 3, 3–23. [Google Scholar]
- Stenseth, N.C.; Atshabar, B.B.; Begon, M.; Belmain, S.R.; Bertherat, E.; Carniel, E.; Gage, K.L.; Leirs, H.; Rahalison, L. Plague: Past, present, and future. PLoS Med. 2008, 5, e3. [Google Scholar] [CrossRef]
- Seabaugh, J.A.; Anderson, D.M. Pathogenicity and Virulence of Yersinia. Virulence 2024, 15, 2316439. [Google Scholar] [CrossRef]
- Avril, A.; Guillier, S.; Rasetti-Escargueil, C. Development of Effective Medical Countermeasures Against the Main Biowarfare Agents: The Importance of Antibodies. Microorganisms 2024, 12, 2622. [Google Scholar] [CrossRef]
- Achtman, M.; Morelli, G.; Zhu, P.; Wirth, T.; Diehl, I.; Kusecek, B.; Vogler, A.J.; Wagner, D.M.; Allender, C.J.; Easterday, W.R.; et al. Microevolution and history of the plague bacillus, Yersinia pestis. Proc. Natl. Acad. Sci. USA 2004, 101, 17837–17842. [Google Scholar] [CrossRef]
- Zhou, D.; Han, Y.; Song, Y.; Huang, P.; Yang, R. Comparative and evolutionary genomics of Yersinia pestis. Microbes Infect. 2004, 6, 1226–1234. [Google Scholar] [CrossRef]
- Drancourt, M.; Aboudharam, G.; Signoli, M.; Dutour, O.; Raoult, D. Detection of 400-year-old Yersinia pestis DNA in human dental pulp. Proc. Natl. Acad. Sci. USA 1998, 95, 12637–12640. [Google Scholar] [CrossRef]
- Demeure, C.E.; Dussurget, O.; Mas Fiol, G.; Le Guern, A.S.; Savin, C.; Pizarro-Cerdá, J. Yersinia pestis and plague: An updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes Immun. 2019, 20, 357–370. [Google Scholar] [CrossRef] [PubMed]
- American Society for Microbiology. Sentinel Level Clinical Laboratory Guidelines for Suspected Agents of Bioterrorism and Emerging Infectious Diseases: Yersinia pestis; ASM (American Society for Microbiology): Washington, DC, USA, 2016. [Google Scholar]
- Vadyvaloo, V.; Jarrett, C.; Sturdevant, D.E.; Sebbane, F.; Hinnebusch, B.J. Transit through the flea vector induces a pre transmission innate immunity resistance phenotype in Yersinia pestis. PLoS Pathog. 2010, 6, e1000783. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; The Williams & Wilkins Co.: Baltimore, MD, USA, 1994; pp. 175–289. [Google Scholar]
- Hare, J.M.; McDonough, K.A. High-frequency RecA-dependent and -independent mechanisms of Congo red binding mutations in Yersinia pestis. J. Bacteriol. 1999, 181, 4896–4904. [Google Scholar] [CrossRef]
- Cornelis, G.R. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 2000, 97, 8778–8783. [Google Scholar] [CrossRef]
- Wren, B.W. The Yersiniae—A model genus to study the rapid evolution of bacterial pathogens. Nat. Rev. Microbiol. 2003, 1, 55–64. [Google Scholar] [CrossRef]
- Brubaker, R.R. Factors promoting acute and chronic diseases caused by Yersiniae. Clin. Microbiol. Rev. 1991, 4, 309–324. [Google Scholar] [CrossRef]
- Devignat, R. Variétés de le spece Pasteurella pestis. Bull. World Health Organ. 1951, 4, 241–263. [Google Scholar]
- Plato, M.E.; Evseeva, V.V.; Dentovskaya, S.V.; Anisimov, A.P. Molecular Typing of Yersinia pestis. Mol. Gen. Mikrobiol. Virusol. 2013, 28, 3–12. [Google Scholar]
- Anisimov, A.P.; Lindler, L.E.; Pier, G.B. Intraspecific diversity of Yersinia pestis. Clin. Microbiol. Rev. 2004, 17, 434–464. [Google Scholar] [CrossRef]
- Vogler, A.J.; Keim, P.; Wagner, D.M. A review of methods for subtyping Yersinia pestis: From phenotypes to whole genome sequencing. Infect. Genet. Evol. 2016, 37, 21–36. [Google Scholar] [CrossRef]
- Achtman, M.; Zurth, K.; Morelli, G.; Torrea, G.; Guiyoule, A.; Carniel, E. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 1999, 96, 14043–14048. [Google Scholar] [CrossRef]
- Morelli, G.; Song, Y.; Mazzoni, C.J.; Eppinger, M.; Roumagnac, P.; Wagner, D.M.; Feldkamp, M.; Kusecek, B.; Vogler, A.J.; Li, Y.; et al. Yersinia pestis genome sequencing identifies patterns of global phylogenetic diversity. Nat. Genet. 2010, 42, 1140–1143. [Google Scholar] [CrossRef]
- Cui, Y.; Yu, C.; Yan, Y.; Li, D.; Li, Y.; Jombart, T.; Weinert, L.A.; Wang, Z.; Guo, Z.; Xu, L.; et al. Historical variations in mutation rate in an epidemic pathogen, Yersinia pestis. Proc. Natl. Acad. Sci. USA 2013, 110, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Morozova, I.; Kasia, A.; Bruskin, S.; Neukamm, J.; Molak, M.; Batieva, E.; Pudło, A.; Rühli, F.J.; Schuenemann, V.J. New Ancient Eastern European Yersinia pestis Genomes Illuminate the Dispersal of Plague in Europe. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190569. [Google Scholar] [CrossRef] [PubMed]
- Spyrou, M.A.; Keller, M.; Tukhbatova, R.I.; Scheib, C.L.; Nelson, E.A.; Andrades Valtueña, A.; Neumann, G.U.; Walker, D.; Alterauge, A.; Carty, N.; et al. Phylogeography of the Second Plague Pandemic Revealed through Analysis of Historical Yersinia pestis Genomes. Nat. Commun. 2019, 10, 4470. [Google Scholar] [CrossRef]
- Rasmussen, S.; Allentoft, M.E.; Nielsen, K.; Orlando, L.; Sikora, M.; Sjögren, K.G.; Pedersen, A.G.; Schubert, M.; Van Dam, A.; Kapel, C.M.; et al. Early Divergent Strains of Yersinia pestis in Eurasia 5000 Years Ago. Cell 2015, 163, 571–582. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Hauck, Y.; Platonov, M.E.; Dai, E.; Song, Y.; Guo, Z.; Pourcel, C.; Dentovskaya, S.V.; Anisimov, A.P.; et al. Genotyping and phylogenetic analysis of Y. pestis by MLVA: Insights into the worldwide expansion of Central Asia plague foci. PLoS ONE 2009, 4, e6000. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.; Thomson, N.R.; Reuter, S.; Wren, B.W. ‘Add, stir and reduce’: Yersinia spp. as model bacteria for pathogen evolution. Nat. Rev. Microbiol. 2016, 14, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Bland, D.M.; Miarinjara, A.; Bosio, C.F.; Calarco, J.; Hinnebusch, B.J. Acquisition of yersinia murine toxin enabled Yersinia pestis to expand the range of mammalian hosts that sustain flea-borne plague. PLoS Pathog. 2021, 17, e1009995. [Google Scholar] [CrossRef]
- Williamson, E.D.; Kilgore, P.B.; Hendrix, E.K.; Neil, B.H.; Sha, J.; Chopra, A.K. Progress on the research and development of plague vaccines with a call to action. NPJ Vaccines 2024, 9, 162. [Google Scholar] [CrossRef]
- Wagner, D.M.; Klunk, J.; Harbeck, M.; Devault, A.; Waglechner, N.; Sahl, J.W.; Enk, J.; Birdsell, D.N.; Kuch, M.; Lumibao, C.; et al. Yersinia pestis and the Plague of Justinian 541–543 AD: A Genomic Analysis. Lancet Infect. Dis. 2014, 14, 319–326. [Google Scholar] [CrossRef]
- Torrea, G.; Chenal-Francisque, V.; Leclercq, A.; Carniel, E. Efficient tracing of global isolates of Yersinia pestis by restriction fragment length polymorphism analysis using three insertion sequences as probes. J. Clin. Microbiol. 2006, 44, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, C.; Mu, K.; Guo, Y.; Song, Y.; Yang, R.; Cui, Y. Insights into Yersinia pestis evolution through rearrangement analysis of 242 complete genomes. Nat. Genet. 2025, 57, 1994–2003. [Google Scholar] [CrossRef]
- Bos, K.I.; Schuenemann, V.J.; Golding, G.B.; Burbano, H.A.; Waglechner, N.; Coombes, B.K.; McPhee, J.B.; DeWitte, S.N.; Meyer, M.; Schmedes, S.; et al. A Draft Genome of Yersinia pestis from Victims of the Black Death. Nature 2011, 478, 506–510. [Google Scholar] [CrossRef]
- Keeling, M.J.; Gilligan, C.A. Metapopulation dynamics of bubonic plague. Nature 2000, 407, 903–906. [Google Scholar] [CrossRef]
- Joosten, M.H.; Cozijnsen, T.J.; De Wit, P.J. Host resistance to a fungal tomato pathogen lost by a single base-pair change in an avirulence gene. Nature 1994, 367, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Nodari, R.; Perini, M.; Luconi, E.; Fois, L.; Vaglienti, F.; Bandi, C.; Biganzoli, E.; Comandatore, F. A Spatiotemporal Reconstruction of the 1630 Plague Epidemic in Milan. iScience 2023, 26, 106704. [Google Scholar] [CrossRef] [PubMed]
- Kohn, G.C. Encyclopedia of Plague and Pestilence: From Ancient Times to the Present, 3rd ed.; Facts On File Inc.: New York, NY, USA, 2007; p. 177. [Google Scholar]
- Collingwood, F. The Great Plague of London, 1665. Nurs. Times 1965, 61, 591. [Google Scholar]
- Signoli, M. History of the Plague of 1720–1722, in Marseille. Presse Med. 2022, 51, 104138. [Google Scholar] [CrossRef] [PubMed]
- Musa, S.S.; Zhao, S.; Mkandawire, W.; Colubri, A.; He, D. An Epidemiological Modeling Investigation of the Long-Term Changing Dynamics of the Plague Epidemics in Hong Kong. Math. Biosci. Eng. 2024, 21, 7435–7453. [Google Scholar] [CrossRef]
- Spyrou, M.A.; Tukhbatova, R.I.; Feldman, M.; Drath, J.; Kacki, S.; Beltrán de Heredia, J.; Arnold, S.; Sitdikov, A.G.; Castex, D.; Wahl, J.; et al. Historical Yersinia pestis Genomes Reveal the European Black Death as the Source of Ancient and Modern Plague Pandemics. Cell Host Microbe 2016, 19, 874–881. [Google Scholar] [CrossRef]
- Tsiamis, C.; Poulakou-Rebelakou, E.; Petridou, E. The Red Sea and the Port of Clysma. A Possible Gate of Justinian’s Plague. Gesnerus 2009, 66, 209–217. [Google Scholar] [CrossRef]
- Mordechai, L.; Eisenberg, M.; Newfield, T.P.; Izdebski, A.; Kay, J.E.; Poinar, H. The Justinianic Plague: An Inconsequential Pandemic? Proc. Natl. Acad. Sci. USA 2019, 116, 25546–25554. [Google Scholar] [CrossRef]
- Wheelis, M. Biological Warfare at the 1346 Siege of Caffa. Emerg. Infect. Dis. 2002, 8, 971–975. [Google Scholar] [CrossRef]
- Spyrou, M.A.; Musralina, L.; Gnecchi Ruscone, G.A.; Kocher, A.; Borbone, P.G.; Khartaich, V.I.; Buzhilova, A.; Djansugurova, L.; Bos, K.I.; Kühnert, D.; et al. The Source of the Black Death in Fourteenth-Century Central Eurasia. Nature 2022, 606, 718–724. [Google Scholar] [CrossRef]
- Zietz, B.P.; Dunkelberg, H. The History of the Plague and the Research on the Causative Agent Yersinia pestis. Int. J. Hyg. Environ. Health 2004, 207, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M. Ueber die Pestepidemie in Formosa. Zbl. Bakt. 1897, 21, 769–777. [Google Scholar]
- Sebbane, F.; Gardner, D.; Long, D.; Gowen, B.B.; Hinnebusch, B.J. Kinetics of disease progression and host response in a rat model of bubonic plague. Am. J. Pathol. 2005, 166, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Butler, T. Plague into the 21st century. Clin. Infect. Dis. 2009, 49, 736–742. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, Z.; Yang, R. Yersinia pestis: Mechanisms of entry into and resistance to the host cell. Front. Cell. Infect. Microbiol. 2013, 3, 106. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, S.; Zhou, Y.; Wang, T.; Jiao, Y.; Tan, Y.; Wu, Y.; Ren, Y.; Song, Y.; Zhang, J.R.; et al. Molecular turn in Yersinia pestis pathogenesis: Implications of the gppA frameshift for bacterial survival in human macrophage. Emerg Microbes Infect. 2025, 14, 2467778. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S. Plague: From natural disease to bioterrorism. Bayl. Univ. Med. Cent. Proc. 2005, 18, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.J.; Lane, M.C.; Wagner, N.J.; Weening, E.H.; Miller, V.L. Dissemination of a highly virulent pathogen: Tracking the early events that define infection. PLoS Pathog. 2015, 11, e1004587. [Google Scholar] [CrossRef]
- Hinnebusch, B.J.; Rudolph, A.E.; Cherepanov, P.; Dixon, J.E.; Schwan, T.G.; Forsberg, A. Role of Yersinia murine toxin in survival of Yersinia pestis in the midgut of the flea vector. Science 2002, 296, 733–735. [Google Scholar] [CrossRef]
- Smiley, S.T. Current challenges in the development of vaccines for pneumonic plague. Expert Rev. Vaccines 2008, 7, 209–221. [Google Scholar] [CrossRef]
- Prentice, M.B.; Rahalison, L. Plague. Lancet 2007, 369, 1196–1207. [Google Scholar] [CrossRef]
- Cui, Y.; Schmid, B.V.; Cao, H.; Dai, X.; Du, Z.; Easterday, W.R.; Fang, H.; Guo, C.; Huang, S.; Liu, W.; et al. Evolutionary selection of biofilm-mediated extended phenotypes in Yersinia pestis in response to a fluctuating environment. Nat. Commun. 2020, 11, 281. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Fatal laboratory-acquired infection with an attenuated Yersinia pestis Strain-Chicago, Illinois, 2009. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 201–205. [Google Scholar]
- Losada, L.; Varga, J.J.; Hostetler, J.; Radune, D.; Kim, M.; Durkin, S.; Schneewind, O.; Nierman, W.C. Genome sequencing and analysis of Yersinia pestis KIM D27, an avirulent strain exempt from select agent regulation. PLoS ONE 2011, 6, e19054. [Google Scholar] [CrossRef][Green Version]
- Marketon, M.M.; DePaolo, R.W.; DeBord, K.L.; Jabri, B.; Schneewind, O. Plague bacteria target immune cells during infection. Science 2005, 309, 1739–1741. [Google Scholar] [CrossRef]
- Huang, X.Z.; Lindler, L.E. The pH 6 antigen is an antiphagocytic factor produced by Yersinia pestis independent of Yersinia outer proteins and capsule antigen. Infect. Immun. 2004, 72, 7212–7219. [Google Scholar] [CrossRef] [PubMed]
- Montminy, S.W.; Khan, N.; McGrath, S.; Walkowicz, M.J.; Sharp, F.; Conlon, J.E.; Fukase, K.; Kusumoto, S.; Sweet, C.; Miyake, K.; et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 2006, 7, 1066–1073. [Google Scholar] [CrossRef]
- Li, B.; Yang, R. Interaction between Yersinia pestis and the host immune system. Infect. Immun. 2008, 76, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Knirel, Y.A.; Anisimov, A.P.; Kislichkina, A.A.; Kondakova, A.N.; Bystrova, O.V.; Vagaiskaya, A.S.; Shatalin, K.Y.; Shashkov, A.S.; Dentovskaya, S.V. Lipopolysaccharide of the Yersinia pseudotuberculosis Complex. Biomolecules 2021, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.K.; Bliska, J.B. Yersinia versus host immunity: How a pathogen evades or triggers a protective response. Curr. Opin. Microbiol. 2016, 29, 56–62. [Google Scholar] [CrossRef]
- Cavanaugh, D.C.; Randall, R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J. Immunol. 1959, 83, 348–363. [Google Scholar] [CrossRef]
- Shannon, J.G.; Hasenkrug, A.M.; Dorward, D.W.; Nair, V.; Carmody, A.B.; Hinnebusch, B.J. Yersinia pestis subverts the dermal neutrophil response in a mouse model of bubonic plague. mBio 2013, 4, e00170-13. [Google Scholar] [CrossRef]
- Spinner, J.L.; Winfree, S.; Starr, T.; Shannon, J.G.; Nair, V.; Steele-Mortimer, O.; Hinnebusch, B.J. Yersinia pestis survival and replication within human neutrophil phagosomes and uptake of infected neutrophils by macrophages. J. Leukoc. Biol. 2014, 95, 389–398. [Google Scholar] [CrossRef]
- Bartra, S.S.; Styer, K.L.; O’Bryant, D.M.; Nilles, M.L.; Hinnebusch, B.J.; Aballay, A.; Plano, G.V. Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein. Infect. Immun. 2008, 76, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.K.; Skurnik, M.; Blom, A.M.; Meri, S. Yersinia pestis Ail recruitment of C4b-binding protein leads to factor I-mediated inactivation of covalently and noncovalently bound C4b. Eur. J. Immunol. 2014, 44, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Kerschen, E.J.; Cohen, D.A.; Kaplan, A.M.; Straley, S.C. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 2004, 72, 4589–4602. [Google Scholar] [CrossRef]
- Chung, L.K.; Park, Y.H.; Zheng, Y.; Brodsky, I.E.; Hearing, P.; Kastner, D.L.; Chae, J.J.; Bliska, J.B. The Yersinia Virulence Factor YopM Hijacks Host Kinases to Inhibit Type III Effector-Triggered Activation of the Pyrin Inflammasome. Cell Host Microbe 2016, 20, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Patin, E. Plague as a cause for familial Mediterranean fever. Nat. Immunol. 2020, 21, 833–834. [Google Scholar] [CrossRef]
- Heilig, R.; Broz, P. Function and mechanism of the pyrin inflammasome. Eur. J. Immunol. 2018, 48, 230–238. [Google Scholar] [CrossRef]
- Park, Y.H.; Remmers, E.F.; Lee, W.; Ombrello, A.K.; Chung, L.K.; Shilei, Z.; Stone, D.L.; Ivanov, M.I.; Loeven, N.A.; Barron, K.S.; et al. Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nat. Immunol. 2020, 21, 857–867. [Google Scholar] [CrossRef]
- Sebbane, F.; Jarrett, C.O.; Gardner, D.; Long, D.; Hinnebusch, B.J. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc. Natl. Acad. Sci. USA 2006, 103, 5526–5530. [Google Scholar] [CrossRef]
- Lathem, W.W.; Price, P.A.; Miller, V.L.; Goldman, W.E. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science 2007, 315, 509–513. [Google Scholar] [CrossRef]
- Vagima, Y.; Zauberman, A.; Levy, Y.; Gur, D.; Tidhar, A.; Aftalion, M.; Shafferman, A.; Mamroud, E. Circumventing, Y. pestis Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague. PLoS Pathog. 2015, 11, e1004893. [Google Scholar] [CrossRef]
- Bi, Y.; Zhou, J.; Yang, H.; Wang, X.; Zhang, X.; Wang, Q.; Wu, X.; Han, Y.; Song, Y.; Tan, Y.; et al. IL-17A produced by neutrophils protects against pneumonic plague through orchestrating IFN-γ-activated macrophage programming. J. Immunol. 2014, 192, 704–713. [Google Scholar] [CrossRef]
- Yao, T.; Mecsas, J.; Healy, J.I.; Falkow, S.; Chien, Y. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 1999, 190, 1343–1350. [Google Scholar] [CrossRef]
- Sebbane, F.; Lemaître, N. Antibiotic Therapy of Plague: A Review. Biomolecules 2021, 11, 724. [Google Scholar] [CrossRef]
- Bourner, J.; Andriamarohasina, L.; Salam, A.; Kayem, N.D.; Randremanana, R.; Olliaro, P. A systematic review of the clinical profile of patients with bubonic plague and the outcome measures used in research settings. PLoS Negl. Trop. Dis. 2023, 17, e0011509. [Google Scholar] [CrossRef]
- Inglesby, T.V.; Dennis, D.T.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Friedlander, A.M.; Hauer, J.; Koerner, J.F.; et al. Plague as a biological weapon: Medical and public health management. Working Group on Civilian Biodefense. JAMA 2000, 283, 2281–2290. [Google Scholar] [CrossRef]
- World Health Organization. Public Health Response to Biological and Chemical Weapons—WHO Guidance; 2nd ed of Health Aspects of Chemical and Biological Weapons: Report of a WHO Group of Consultants; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Kilgore, P.B.; Sha, J.; Hendrix, E.K.; Neil, B.H.; Lawrence, W.S.; Peel, J.E.; Hittle, L.; Woolston, J.; Sulakvelidze, A.; Schwartz, J.A.; et al. A bacteriophage cocktail targeting Yersinia pestis provides strong post-exposure protection in a rat pneumonic plague model. Microbiol. Spectr. 2024, 12, e0094224. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Aspects of Chemical and Biological Weapons; World Health Organization: Geneva, Switzerland, 1970; p. 98. [Google Scholar]
- Bertherat, E.G.; Jullien, S. Revision of the international definition of plague cases. Wkly. Epidemiol. Rec. 2021, 96, 238–240. Available online: https://iris.who.int/handle/10665/341853 (accessed on 3 April 2025).
- Moses, S.; Vagima, Y.; Tidhar, A.; Aftalion, M.; Mamroud, E.; Rotem, S.; Steinberger-Levy, I. Characterization of Yersinia pestis Phage Lytic Activity in Human Whole Blood for the Selection of Efficient Therapeutic Phages. Viruses 2021, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.A.; Molineux, I.J.; Westwater, C. Diagnostic bioluminescent phage for detection of Yersinia pestis. J. Clin. Microbiol. 2009, 47, 3887–3894. [Google Scholar] [CrossRef]
- Sergueev, K.V.; He, Y.; Borschel, R.H.; Nikolich, M.P.; Filippov, A.A. Rapid and sensitive detection of Yersinia pestis using amplification of plague diagnostic bacteriophages monitored by real-time PCR. PLoS ONE 2010, 5, e11337. [Google Scholar] [CrossRef] [PubMed]
- Konyshev, I.; Dudina, L.; Belozerov, V.; Iva, S.; Dentovskaya, S.; Anisimov, A.; Byvalov, A. Biophysical and microbiological aspects of the interaction between Yersinia pestis PsaA and bacteriophage L-413C. Eur. Biophys. J. 2025, 54, 267–276. [Google Scholar] [CrossRef]
- Jullien, S.; Dissanayake, H.A.; Chaplin, M. Rapid diagnostic tests for plague. Cochrane Database Syst. Rev. 2020, 6, CD013459. [Google Scholar] [CrossRef]
- Bezerra, M.F.; Dos Santos, W.J.T.; Rocha, I.V.; Nadaes, N.R.; Dantas-Torres, F.; Sales, K.G.D.S.; De Melo Neto, O.P.; Sobreira, M.; Silva, E.D.; De Almeida, A.M.P.; et al. Performance assessment of a new indirect rapid diagnostic test for plague detection in humans and other mammalian hosts. Acta Trop. 2022, 231, 106427. [Google Scholar] [CrossRef]
- Haffkine, W.M. Remarks on the Plague Prophylactic Fluid. Br. Med. J. 1897, 1, 1461–1462. [Google Scholar] [CrossRef][Green Version]
- Kolle, W.; Otto, R. Weitere Untersuchungen uber die Pestimmunitat. Z. Hyg. 1904, 48, 399–428. [Google Scholar][Green Version]
- Strong, R.P. Vaccination against plague. Philipp. J. Sci. 1906, 1, 181–190. [Google Scholar][Green Version]
- Strong, R.P. Protective inoculation against plague. J. Med. Res. 1908, 18, 325–346. [Google Scholar][Green Version]
- Girard, G. Immunity in plague. Results of 30 years of work on the ‘Pasteurella pestis Ev’ (Girard and Robic) strain. Biol. Med. 1963, 52, 631–731. [Google Scholar][Green Version]
- Meyer, K.F. Effectiveness of live or killed plague vaccines in man. Bull. World Health Organ. 1970, 42, 653–666. [Google Scholar][Green Version]
- Sagiyev, Z.; Berdibekov, A.; Bolger, T.; Merekea, A.; Ashirova, S.; Nurgozhin, Z.; Dalibayev, Z. Human response to live plague vaccine EV, Almaty region, Kazakhstan, 2014–2015. PLoS ONE 2019, 14, e0218366. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.G.; Anderson, G.W.; Mauro, J.M.; Welkos, S.L.; Andrews, G.P.; Adamovicz, J.; Friedlander, A.M. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 1998, 16, 1131–1137. [Google Scholar] [CrossRef]
- Quenee, L.E.; Ciletti, N.A.; Elli, D.; Hermanas, T.M.; Schneewind, O. Prevention of pneumonic plague in mice, rats, guinea pigs and non-human primates with clinical grade rV10, rV10-2 or F1-V vaccines. Vaccine 2011, 29, 6572–6583. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.D.; Flick-Smith, H.C.; Lebutt, C.; Rowland, C.A.; Jones, S.M.; Waters, E.L.; Gwyther, R.J.; Miller, J.; Packer, P.J.; Irving, M. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 2005, 73, 3598–3608. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Hu, J.; Meng, F.; Li, J.; Luo, L.; Xu, J.; Yuan, Z.; Li, Z.; Chen, W.; Jiao, L.; et al. Immunogenicity and safety of subunit plague vaccine: A randomized phase 2a clinical trial. Hum. Vaccines Immunother. 2016, 12, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jiao, L.; Hu, Y.; Chu, K.; Li, J.; Zhu, F.; Li, T.; Wu, Z.; Wei, D.; Meng, F.; et al. One year immunogenicity and safety of subunit plague vaccine in Chinese healthy adults: An extended open-label study. Hum. Vaccines Immunother. 2018, 14, 2701–2705. [Google Scholar] [CrossRef]
- Hart, M.K.; Saviolakis, G.A.; Welkos, S.L.; House, R.V. Advanced Development of the rF1V and rBV A/B Vaccines: Progress and Challenges. Adv. Prev. Med. 2012, 2012, 731604. [Google Scholar] [CrossRef]
- Feodorova, V.A.; Corbel, M.J. Prospects for new plague vaccines. Expert Rev. Vaccines 2009, 8, 1721–1738. [Google Scholar] [CrossRef]
- Rosenzweig, J.A.; Hendrix, E.K.; Chopra, A.K. Plague vaccines: New developments in an ongoing search. Appl. Microbiol. Biotechnol. 2021, 105, 4931–4941. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Miquel-Clopés, A.; Wegmann, U.; Jones, E.; Stentz, R.; Telatin, A.; Walker, N.J.; Butcher, W.A.; Brown, P.J.; Holmes, S.; et al. Use of bioengineered human commensal gut bacteria-derived microvesicles for mucosal plague vaccine delivery and immunization. Clin. Exp. Immunol. 2019, 196, 287–304. [Google Scholar] [CrossRef]
- Wagner, D.A.; Kelly, S.M.; Petersen, A.C.; Peroutka-Bigus, N.; Darling, R.J.; Bellaire, B.H.; Wannemuehler, M.J.; Narasimhan, B. Single-dose combination nanovaccine induces both rapid and long-lived protection against pneumonic plague. Acta Biomater. 2019, 100, 326–337. [Google Scholar] [CrossRef]
- Dankmeyer, J.L.; Fast, R.L.; Cote, C.K.; Worsham, P.L.; Fritz, D.; Fisher, D.; Kern, S.J.; Merkel, T.; Kirschning, C.J.; Amemiya, K. Multiple roles of Myd88 in the immune response to the plague F1-V vaccine and in protection against an aerosol challenge of Yersinia pestis CO92 in mice. J. Immunol. Res. 2014, 2014, 341820. [Google Scholar] [CrossRef]
- Frey, S.E.; Lottenbach, K.; Graham, I.; Anderson, E.; Bajwa, K.; May, R.C.; Mizel, S.B.; Graff, A.; Belshe, R.B. A phase I safety and immunogenicity dose escalation trial of plague vaccine, Flagellin/F1/V, in healthy adult volunteers (DMID 08-0066). Vaccine 2017, 35 Pt B, 6759–6765. [Google Scholar] [CrossRef]
- Gallagher, T.B.; Mellado-Sanchez, G.; Jorgensen, A.L.; Moore, S.; Nataro, J.P.; Pasetti, M.F.; Baillie, L.W. Development of a multiple-antigen protein fusion vaccine candidate that confers protection against Bacillus anthracis and Yersinia pestis. PLoS Negl. Trop. Dis. 2019, 13, e0007644. [Google Scholar] [CrossRef]
- Tao, P.; Mahalingam, M.; Zhu, J.; Moayeri, M.; Sha, J.; Lawrence, W.S.; Leppla, S.H.; Chopra, A.K.; Rao, V.B. A Bacteriophage T4 Nanoparticle-Based Dual Vaccine against Anthrax and Plague. mBio. 2018, 9, e01926-18. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Kirtley, M.L.; Klages, C.; Erova, T.E.; Telepnev, M.; Ponnusamy, D.; Fitts, E.C.; Baze, W.B.; Sivasubramani, S.K.; Lawrence, W.S.; et al. A Replication-Defective Human Type 5 Adenovirus-Based Trivalent Vaccine Confers Complete Protection against Plague in Mice and Nonhuman Primates. Clin. Vaccine Immunol. 2016, 23, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, P.B.; Sha, J.; Andersson, J.A.; Motin, V.L.; Chopra, A.K. A new generation needle- and adjuvant-free trivalent plague vaccine utilizing adenovirus-5 nanoparticle platform. npj Vaccines 2021, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Elia, U.; Levy, Y.; Cohen, H.; Zauberman, A.; Gur, D.; Hazan-Halevy, I.; Aftalion, M.; Benarroch, S.; Bar-Haim, E.; Redy-Keisar, O.; et al. Novel Bivalent mRNA-LNP Vaccine for Highly Effective Protection against Pneumonic Plague. Adv. Sci. 2025, 12, e2501286. [Google Scholar] [CrossRef]
- Kon, E.; Levy, Y.; Elia, U.; Cohen, H.; Hazan-Halevy, I.; Aftalion, M.; Ezra, A.; Bar-Haim, E.; Naidu, G.S.; Diesendruck, Y.; et al. A single-dose F1-based mRNA-LNP vaccine provides protection against the lethal plague bacterium. Sci. Adv. 2023, 9, eadg1036. [Google Scholar] [CrossRef]
- WHO. Target Product Profiles. Available online: https://cdn.who.int/media/docs/default-source/documents/r-d-blueprint-meetings/global-consultation-on-plague-vaccines/3_ximena-riveros_plague-tpp.pdf?sfvrsn=edbe8135_3 (accessed on 27 April 2025).
- Von Behring, E.; Kitasato, S. Ueber das Zustandekommen der Diphtherie-Immunitat and der Tetanus-Immunitat bei Thieren. Dtsch. Med. Wochenschr. 1890, 16, 1113–1114. [Google Scholar]
- Von Behring, E. Untersuchungen uber das Zustandekommen der Diphtherie-Immunitat and der Tetanus-Immunitat bei Thieren. Dtsch. Med. Wochenschr. 1890, 16, 1145–1148. [Google Scholar]
- Yersin, A.; Calmette, A.; Borrel, A. La peste bubonique. Ann. Inst. Pasteur. 1895, 9, 589–592. [Google Scholar]
- Butler, T. Plague history: Yersin’s discovery of the causative bacterium in 1894 enabled, in the subsequent century, scientific progress in understanding the disease and the development of treatments and vaccines. Clin. Microbiol. Infect. 2014, 20, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.F.; Quan, S.F.; McCrumb, F.R.; Larson, A. Effective treatment of plague. Ann. N. Y. Acad. Sci. 1952, 55, 1228–1274. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Leary, S.E.; Griffin, K.F.; Williamson, E.D.; Titball, R.W. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 1997, 65, 4476–4482. [Google Scholar] [CrossRef]
- Carman, J.A. Prontosil in the treatment of oriental plague. East Afr. Med. J. 1938, 14, 362–366. [Google Scholar]
- Vine, R.S. Plague in the Nilgiris. J. R. Army Med. Corps 1938, 71, 382–395. [Google Scholar]
- Girard, G. Le traitement de l’infection pesteuse par les corps sulfamides. Peste expérimentale et peste humaine. Bull. Soc. Path Exot. 1941, 34, 43–54. [Google Scholar]
- Favarel, R.; Carriere, M.; Chartres, A. Guérison de trois cas de peste pulmonaire primitive par le traitement sulfamide. Bull. Soc. Path Exot. 1948, 41, 515–523. [Google Scholar]
- Karamchandi, P.V.; Rao, K.S. Streptomycin in human plague. Lancet 1948, 25, P22. [Google Scholar] [CrossRef]
- Sokhey, S.S.; Wagle, P.M.; Habbu, M.K. Treatment of bubonic plague with sulfonamides and antibiotics. Bull. World Health Organ. 1953, 9, 637–643. [Google Scholar]
- McCrumb, F.R., Jr.; Mercier, S.; Robic, J.; Bouillat, M.; Smadel, J.E.; Woodward, T.E.; Goodner, K. Chloramphenicol and terramycin in the treatment of pneumonic plague. Am. J. Med. 1953, 14, 284–293. [Google Scholar] [CrossRef]
- Nguyen-Van-Ai; Nguyen-Duc-Hanh; Pham-Van-Dien; Nguyen-Van-Le. Letter: Co-trimoxazole in bubonic plague. Br. Med. J. 1973, 4, 108–109. [Google Scholar] [CrossRef][Green Version]
- Galimand, M.; Guiyoule, A.; Gerbaud, G.; Rasoamanana, B.; Chanteau, S.; Carniel, E.; Courvalin, P. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 1997, 337, 677–680. [Google Scholar] [CrossRef]
- Butler, T.; Levin, J.; Linh, N.N.; Chau, D.M.; Adickman, M.; Arnold, K. Yersinia pestis infection in Vietnam. II. Quantitative blood cultures and detection of endotoxin in the cerebrospinal fluid of patients with meningitis. J. Infect. Dis. 1976, 133, 493–499. [Google Scholar] [CrossRef]
- Boulanger, L.L.; Ettestad, P.; Fogarty, J.D.; Dennis, D.T.; Romig, D.; Mertz, G. Gentamicin and tetracyclines for the treatment of human plague: Review of 75 cases in New Mexico, 1985-1999. Clin. Infect. Dis. 2004, 38, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Mwengee, W.; Butler, T.; Mgema, S.; Mhina, G.; Almasi, Y.; Bradley, C.; Formanik, J.B.; Rochester, C.G. Treatment of plague with gentamicin or doxycycline in a randomized clinical trial in Tanzania. Clin. Infect. Dis. 2006, 42, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Bonacorsi, S.P.; Scavizzi, M.R.; Guiyoule, A.; Amouroux, J.H.; Carniel, E. Assessment of a fluoroquinolone, three beta-lactams, two aminoglycosides, and a cycline in treatment of murine Yersinia pestis infection. Antimicrob. Agents Chemother. 1994, 38, 481–486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guiyoule, A.; Gerbaud, G.; Buchrieser, C.; Galimand, M.; Rahalison, L.; Chanteau, S.; Courvalin, P.; Carniel, E. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg. Infect. Dis. 2001, 7, 43–48. [Google Scholar] [CrossRef]
- Cabanel, N.; Bouchier, C.; Rajerison, M.; Carniel, E. Plasmid-mediated doxycycline resistance in a Yersinia pestis strain isolated from a rat. Int. J. Antimicrob. Agents 2018, 51, 249–254. [Google Scholar] [CrossRef]
- Cote, C.K.; Blanco, I.I.; Hunter, M.; Shoe, J.L.; Klimko, C.P.; Panchal, R.G.; Welkos, S.L. Combinations of early generation antibiotics and antimicrobial peptides are effective against a broad spectrum of bacterial biothreat agents. Microb. Pathog. 2020, 142, 104050. [Google Scholar] [CrossRef]
- Zauberman, A.; Gur, D.; Levy, Y.; Aftalion, M.; Vagima, Y.; Tidhar, A.; Chitlaru, T.; Mamroud, E. Postexposure Administration of a Yersinia pestis Live Vaccine for Potentiation of Second-Line Antibiotic Treatment Against Pneumonic Plague. J. Infect. Dis. 2019, 220, 1147–1151. [Google Scholar] [CrossRef]
- Vagima, Y.; Gur, D.; Aftalion, M.; Moses, S.; Levy, Y.; Makovitzki, A.; Holtzman, T.; Oren, Z.; Segula, Y.; Fatelevich, E.; et al. Phage Therapy Potentiates Second-Line Antibiotic Treatment against Pneumonic Plague. Viruses 2022, 14, 688. [Google Scholar] [CrossRef]
- D’Herelle, F. Essai de traîtement de la peste bubonique par le bactériophage. Presse Méd. 1925, 33, 1393–1394. [Google Scholar]
- Lehti, T.A.; Pajunen, M.I.; Skog, M.S.; Finne, J. Internalization of a polysialic acid-binding Escherichia coli bacteriophage into eukaryotic neuroblastoma cells. Nat. Commun. 2017, 8, 1915. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Revazishvili, T.; Dean, T.; Butani, A.; Verratti, K.; Bishop-Lilly, K.A.; Sozhamannan, S.; Sulakvelidze, A.; Rajanna, C. A Yersinia pestis-specific, lytic phage preparation significantly reduces viable Y. pestis on various hard surfaces experimentally contaminated with the bacterium. Bacteriophage 2012, 2, 168–177. [Google Scholar] [CrossRef]
- Suladze, T.; Jaiani, E.; Darsavelidze, M.; Elizbarashvili, M.; Gorge, O.; Kusradze, I.; Kokashvili, T.; Lashkhi, N.; Tsertsvadze, G.; Janelidze, N.; et al. New Bacteriophages with Podoviridal Morphotypes Active against Yersinia pestis: Characterization and Application Potential. Viruses 2023, 15, 1484. [Google Scholar] [CrossRef]
- Filippov, A.A.; Sergueev, K.V.; He, Y.; Huang, X.Z.; Gnade, B.T.; Mueller, A.J.; Fernandez-Prada, C.M.; Nikolich, M.P. Bacteriophage therapy of experimental bubonic plague in mice. Adv. Exp. Med. Biol. 2012, 954, 337–348. [Google Scholar] [CrossRef]
- Filippov, A.A.; Sergueev, K.V.; He, Y.; Huang, X.Z.; Gnade, B.T.; Mueller, A.J.; Fernandez-Prada, C.M.; Nikolich, M.P. Bacteriophage-resistant mutants in Yersinia pestis: Identification of phage receptors and attenuation for mice. PLoS ONE 2011, 6, e25486. [Google Scholar] [CrossRef]
- Drozdov, I.G.; Anisimov, A.P.; Samoilova, S.V.; Yezhov, I.N.; Yeremin, S.A.; Karlyshev, A.V.; Krasilnikova, V.M.; Kravchenko, V.I. Virulent non-capsulate Yersinia pestis variants constructed by insertion mutagenesis. J. Med. Microbiol. 1995, 42, 264–268. [Google Scholar] [CrossRef]

| Animal Group | Role in Plague Ecology |
|---|---|
| Rodents | Primary reservoirs; amplifying hosts during epizootics |
| Lagomorphs | Susceptible spillover hosts |
| Insectivores | Susceptible spillover hosts |
| Carnivores | Susceptible hosts; sentinels for plague activity |
| Other Mammals | Documented infections; potential transmission routes |
| Pandemic | Period | Affected Area | Estimated Deaths | Reference |
|---|---|---|---|---|
| First Plague Pandemic—Plague of Justinian | 6th-8th century | Eastern Roman Empire, Eastern and Western Mediterranean and Western Europe | 25–50 million | [144] |
| Second Plague Pandemic—Black Death | mid 14th–mid 18th century | Europe, Asia, North Africa | ~30 million | [144] |
| Plague of Milan 1629–1631 | 1629–1631 | Milan, Italy | ~60,000 | [139] |
| Great Plague of London | 1665–1666 | London, England | 100,000–200,000 | [141] |
| Plague of Marseille | 1720–1722 | Marseille, France | ~100,000 | [142] |
| Third Plague Pandemic | late 19th–mid 20th century | Global (originated in China, spread to India, Asia, Africa, and the Americas) | Over 26 million | [144] |
| Plague of Hong Kong | 1894–1895 | Hong Kong, China | ~60,000 | [144] |
| Diagnostic Technique | Biosafety Level | Turnaround Time | Sensitivity/Specificity | Notes |
|---|---|---|---|---|
| Bacterial culture | BSL-3 | 2–3 days | High (gold standard) | Requires CIN selective medium; allows antibiotic susceptibility testing |
| Gram, Giemsa or Wayson staining | BSL-2 | Rapid | Moderate | Detects pleomorphic Gram-negative/bipolar rods |
| F1 antigen detection (ELISA, immunofluorescence, hemagglutination) | BSL-2 | Rapid | Good | Used on blood, buboes, sputum |
| PCR (caf1, pla, 3a) | BSL-2 | Rapid | High, but some targets are no longer reliable | Rapid alternative to culture |
| Phage lysis test (φA1122, L-413C) | BSL-2/3 | hours | φA1122: high; L-413C: more specific but less sensitive. | Also used with qPCR or modified with luxAB |
| Phage-qPCR (φA1122 + qPCR) | BSL-2 | 4 h | Very high | Detects 1 cell/μL without DNA extraction |
| Rapid immunochromatographic test (anti-F1 dipstick) | BSL-2 | 15 min | Sensitivity 100%, Specificity 70% | Suitable for endemic, resource-limited countries |
| Seroconversion or 4-fold change in anti-F1 | BSL-2 | ≥2 weeks | High | Requires two serum samples at least 2 weeks apart |
| luxAB-modified phage (bioluminescence) | BSL-2 | <4 h | High (under 105 CFU/mL) | Can be miniaturized for point-of-care |
| Phage PST (active in blood) | BSL-2 | hours | High in complex matrices | Does not require pre-culture; active in biological fluids |
| Phage cocktail YPP 401 | BSL-2 | hours | High (broad genomic coverage) | Indirect, diagnostic potential due to broad genomic coverage |
| Antibiotics | Recipients | Dose Amount | Doses No. | Route of Administration |
|---|---|---|---|---|
| Ciprofloxacin | Adults | 400 mg | 3 | Intravenous/oral |
| 750 mg | 2 | |||
| Children | 10 mg/kg (max. 400 mg/dose) | 2–3 | Intravenous/oral | |
| 15 mg/kg (max. 750 mg/dose) | 2 | |||
| Levofloxacin | Adults | 750 mg | 1 | intravenous/oral |
| Children ≥ 6 months | <50 kg: 8 mg/kg (max. 250 mg/dose) | 2 | intravenous/oral | |
| ≥50 kg: 500–750 mg | 1 | intravenous/oral | ||
| Moxifloxacin | Adults | 400 mg | 1 | intravenous/oral |
| Gentamicin | Adults | 5 mg/kg | 1 | intravenous/intramuscular |
| Children | 4.5–7.5 mg/kg | 1 | intravenous/intramuscular | |
| Streptomycin | Adults | 1 g | 2 | intravenous/intramuscular |
| Children | 15 mg/kg (max. 1 g/dose) | 2 | intravenous/intramuscular |
| Antibiotics | Recipients | Dose Amount | Doses No. | Route of Administration |
|---|---|---|---|---|
| Ciprofloxacin | Adults | 400 mg | 3 | Intravenous/oral |
| 750 mg | 2 | |||
| Children | 10 mg/kg (max. 400 mg/dose) | 2–3 | Intravenous/oral | |
| 15 mg/kg (max. 750 mg/dose) | 2 | |||
| Levofloxacin | Adults | 750 mg | 1 | intravenous/oral |
| Children ≥ 6 months | <50 kg: 8 mg/kg (max. 250 mg/dose) | 2 | intravenous/oral | |
| ≥50 kg: 500–750 mg | 1 | intravenous/oral | ||
| Moxifloxacin | Adults | 400 mg | 1 | intravenous/oral |
| Doxycycline | Adults and Children ≥ 45 kg | 200 mg loading dose followed by 100 mg | 2 | intravenous/oral |
| Children < 45 kg | 4.4 mg/kg (max. 200 mg) loading dose followed by 2.2 mg/kg (max. 100 mg) | 2 | intravenous/oral | |
| Gentamicin | Adults | 5 mg/kg | 1 | intravenous/intramuscular |
| Children | 4.5–7.5 mg/kg | 1 | intravenous/intramuscular | |
| Streptomycin | Adults | 1 g | 2 | intravenous/intramuscular |
| Children | 15 mg/kg (max. 1 g/dose) | 2 | intravenous/intramuscular |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciammaruconi, A.; Di Spirito, M.; Pascolini, C.; Molinari, F.; Rozov, O.; Cavalli, M.; Campoli, G.; Totaro, N.; Recchia, E.; Chimienti, S.; et al. Plagued by the Past, Pressed by the Present: A One Health Perspective on Yersinia pestis. Biomedicines 2025, 13, 2555. https://doi.org/10.3390/biomedicines13102555
Ciammaruconi A, Di Spirito M, Pascolini C, Molinari F, Rozov O, Cavalli M, Campoli G, Totaro N, Recchia E, Chimienti S, et al. Plagued by the Past, Pressed by the Present: A One Health Perspective on Yersinia pestis. Biomedicines. 2025; 13(10):2555. https://doi.org/10.3390/biomedicines13102555
Chicago/Turabian StyleCiammaruconi, Andrea, Maria Di Spirito, Chiara Pascolini, Filippo Molinari, Orr Rozov, Marzia Cavalli, Giulia Campoli, Nathalie Totaro, Elisa Recchia, Silvia Chimienti, and et al. 2025. "Plagued by the Past, Pressed by the Present: A One Health Perspective on Yersinia pestis" Biomedicines 13, no. 10: 2555. https://doi.org/10.3390/biomedicines13102555
APA StyleCiammaruconi, A., Di Spirito, M., Pascolini, C., Molinari, F., Rozov, O., Cavalli, M., Campoli, G., Totaro, N., Recchia, E., Chimienti, S., Monte, A., Spagnolo, F., Lista, F., D’Amelio, R., & Fillo, S. (2025). Plagued by the Past, Pressed by the Present: A One Health Perspective on Yersinia pestis. Biomedicines, 13(10), 2555. https://doi.org/10.3390/biomedicines13102555







