Spatial Proximity of Cancer-Associated Fibroblasts to Tumor and Osteoclasts Suggests a Coordinating Role in OSCC-Induced Bone Invasion: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Specimens
2.2. Histological Observation
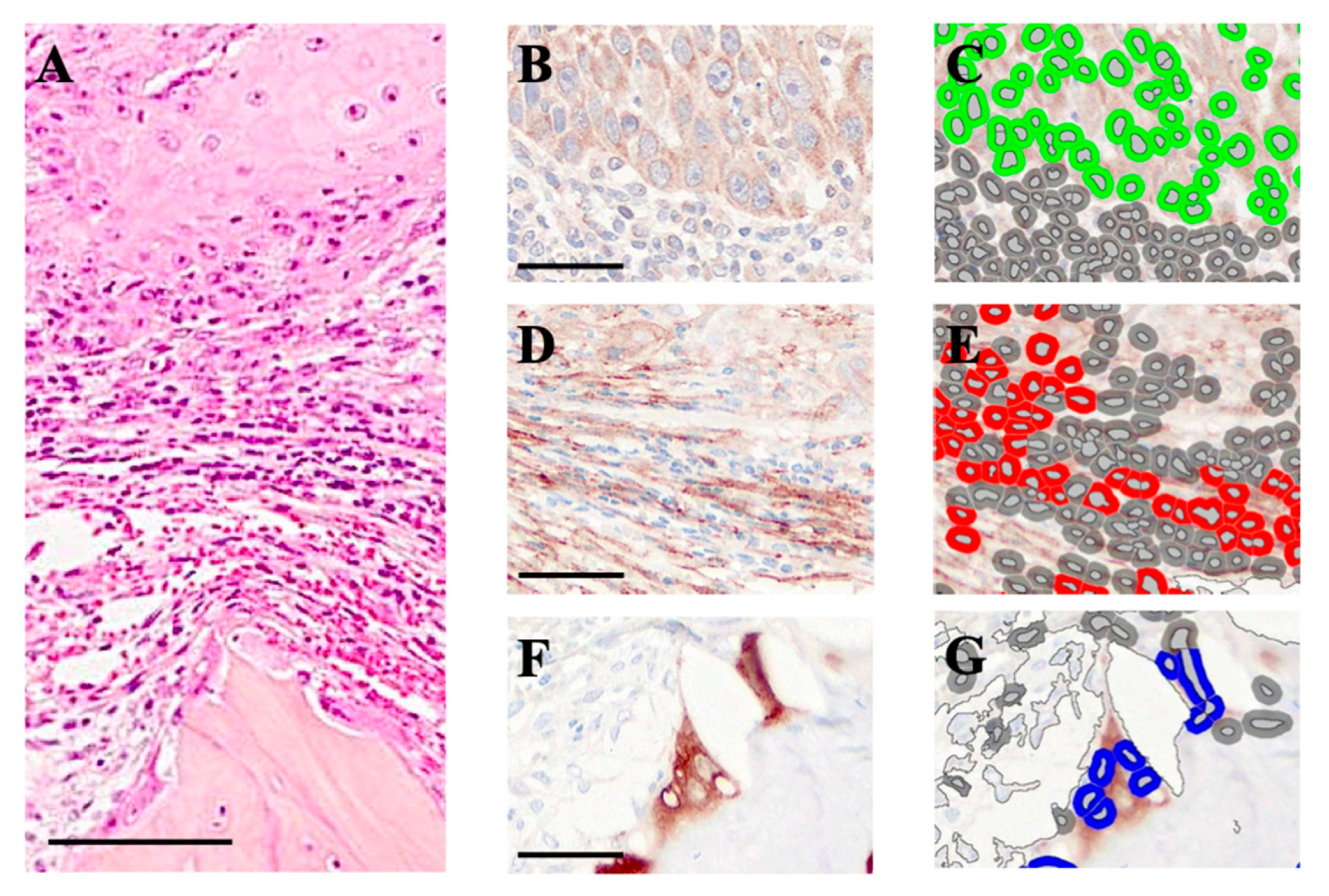
2.3. Immunohistochemical Analysis
2.4. Quantification of OSCC Cells, Cancer-Associated Fibroblasts (CAFs), and Osteoclasts at the Invasive Front of the Jawbone
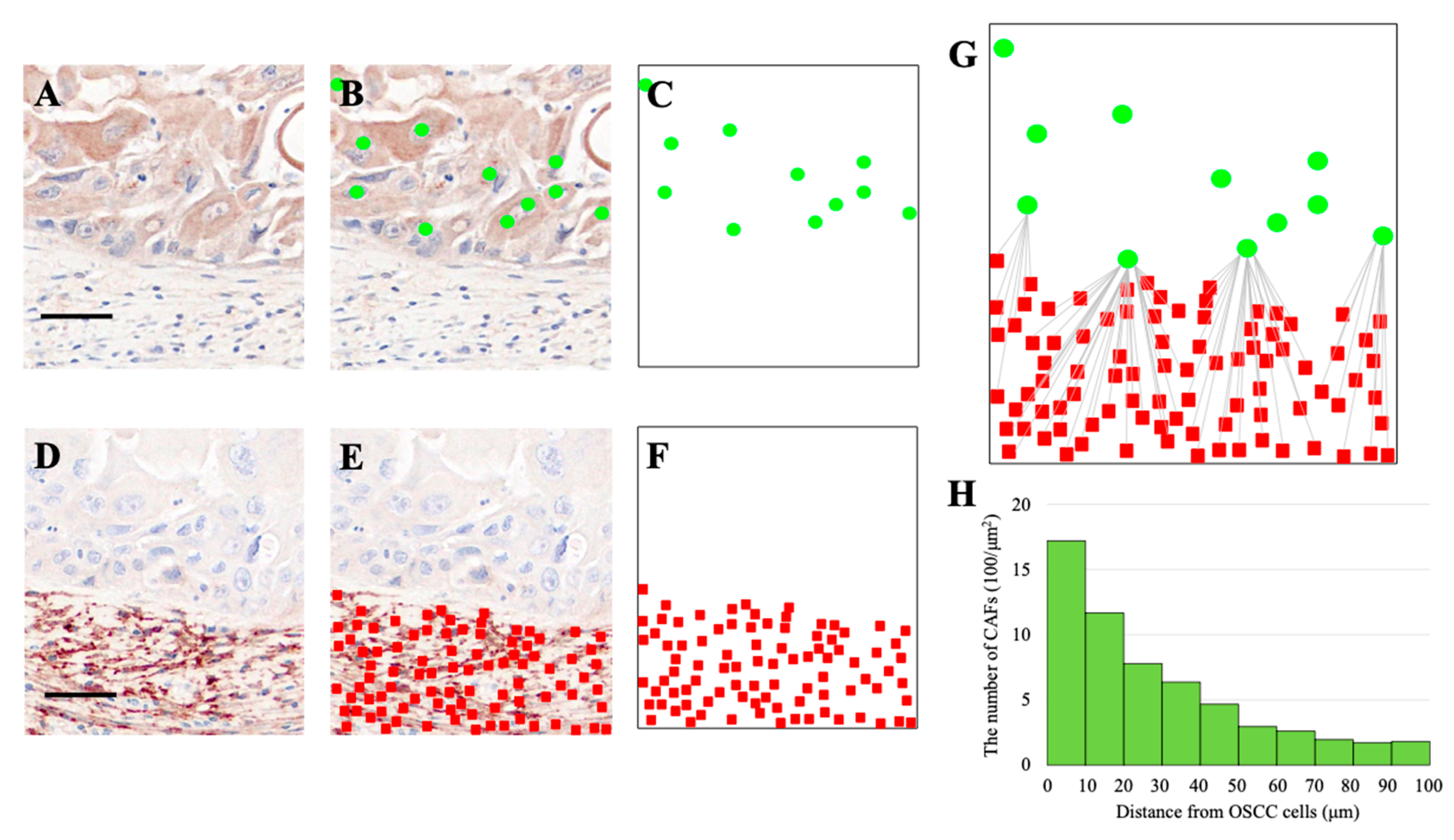
2.5. Analysis of Spatial Relationships Between OSCC Cells, CAFs, and Osteoclasts
3. Results
3.1. Induction of Cancer-Associated Fibroblasts (CAFs) Between the OSCC Growth Area and the Jawbone
3.2. Quantification of CAFs in the Vicinity of OSCC Cells and Osteoclasts
3.3. Spatial Distribution of CAFs Relative to OSCC Cells
3.4. Spatial Distribution of CAFs Relative to Osteoclasts
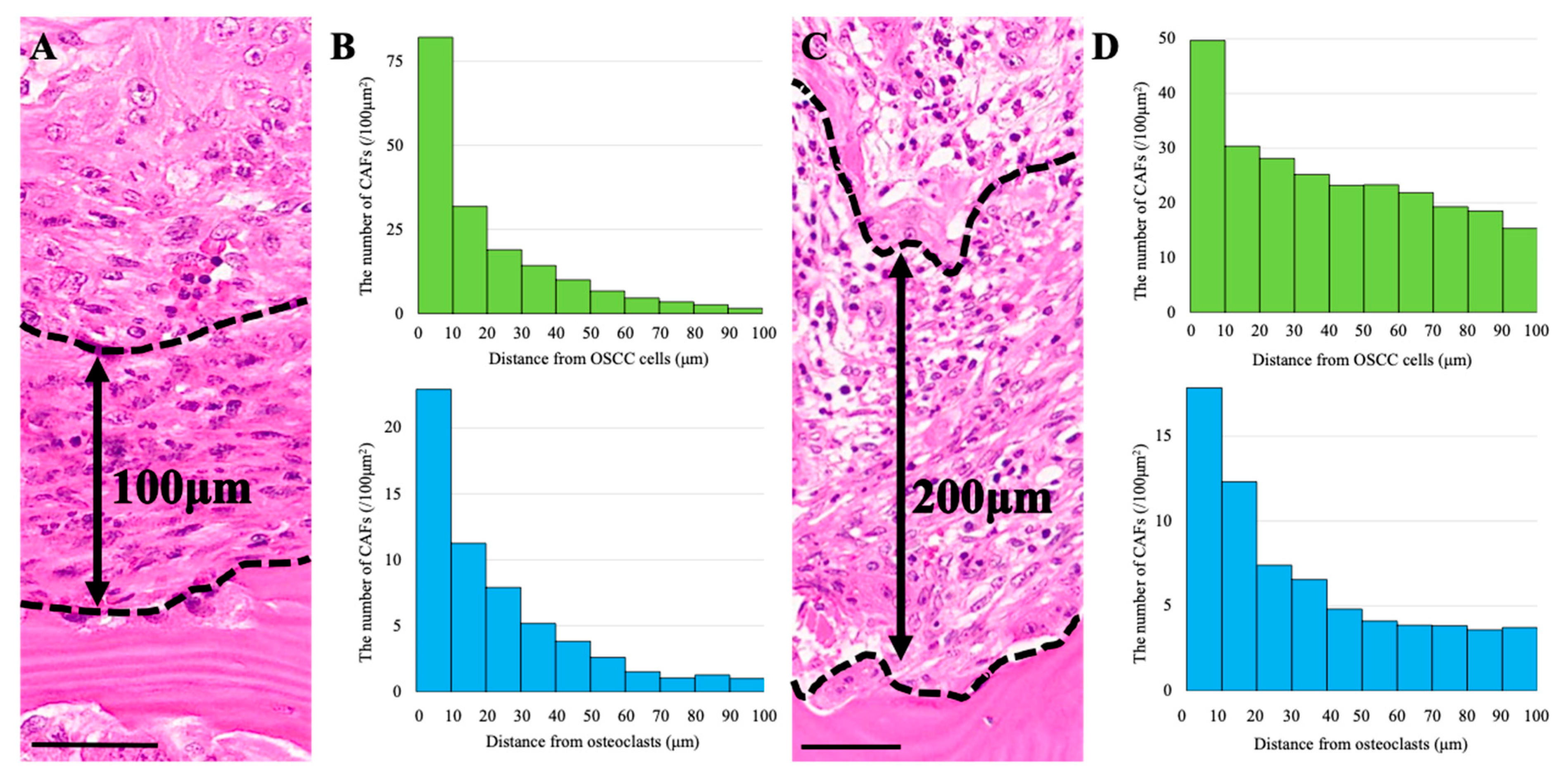
3.5. CAF Distribution and Stromal Thickness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalogirou, E.M.; Tosios, K.I.; Christopoulos, P.F. The Role of Macrophages in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 611115. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [PubMed]
- Elmusrati, A.A.; Pilborough, A.E.; Khurram, S.A.; Lambert, D.W. Cancer-associated fibroblasts promote bone invasion in oral squamous cell carcinoma. Br. J. Cancer 2017, 117, 867–875. [Google Scholar] [CrossRef]
- Jordan, R.C.; Daley, T. Oral squamous cell carcinoma: New insights. J. Can. Dent. Assoc. 1997, 63, 517–518. [Google Scholar]
- Chen, Y.L.; Kuo, S.W.; Fang, K.H.; Hao, S.P. Prognostic impact of marginal mandibulectomy in the presence of superficial bone invasion and the nononcologic outcome. Head Neck 2011, 33, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Murali, R.; Gao, K.; Elliott, M.S.; Clark, J.R. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer 2011, 117, 4460–4467. [Google Scholar] [CrossRef]
- Shah, J.P.; Gil, Z. Current concepts in management of oral cancer–surgery. Oral Oncol. 2009, 45, 394–401. [Google Scholar] [CrossRef]
- Epstein, J.B.; Thariat, J.; Bensadoun, R.J.; Barasch, A.; Murphy, B.A.; Kolnick, L.; Popplewell, L.; Maghami, E. Oral complications of cancer and cancer therapy: From cancer treatment to survivorship. CA Cancer J. Clin. 2012, 62, 400–422. [Google Scholar] [CrossRef]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Jimi, E.; Furuta, H.; Matsuo, K.; Tominaga, K.; Takahashi, T.; Nakanishi, O. The cellular and molecular mechanisms of bone invasion by oral squamous cell carcinoma. Oral Dis. 2011, 17, 462–468. [Google Scholar] [CrossRef]
- Ding, L.; Ren, J.; Zhang, D.; Li, Y.; Huang, X.; Hu, Q.; Wang, H.; Song, Y.; Ni, Y.; Hou, Y. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis 2018, 39, 397–406. [Google Scholar] [CrossRef]
- Shan, Q.; Takabatake, K.; Omori, H.; Kawai, H.; Oo, M.W.; Sukegawa, S.; Fujii, M.; Inada, Y.; Sano, S.; Nakano, K.; et al. Investigation of bone invasion and underlying mechanisms of oral cancer using a cell line-derived xenograft model. Oncol. Lett. 2022, 24, 382. [Google Scholar] [CrossRef]
- Wong, R.J.; Keel, S.B.; Glynn, R.J.; Varvares, M.A. Histological pattern of mandibular invasion by oral squamous cell carcinoma. Laryngoscope 2000, 110, 65–72. [Google Scholar] [CrossRef]
- da Silva, M.; Manhaes, V.P.R.; Gasparotto Junior, L.; Tsukumo, D.M.L.; Lalli, C.A. Pancytopenia as an initial manifestation of prostate cancer: A case report. J. Med. Case Rep. 2021, 15, 247. [Google Scholar] [CrossRef]
- Brown, H.K.; Ottewell, P.D.; Evans, C.A.; Holen, I. Location matters: Osteoblast and osteoclast distribution is modified by the presence and proximity to breast cancer cells in vivo. Clin. Exp. Metastasis 2012, 29, 927–938. [Google Scholar] [CrossRef]
- Shiirevnyamba, A.; Takahashi, T.; Shan, H.; Ogawa, H.; Yano, S.; Kanayama, H.; Izumi, K.; Uehara, H. Enhancement of osteoclastogenic activity in osteolytic prostate cancer cells by physical contact with osteoblasts. Br. J. Cancer 2011, 104, 505–513. [Google Scholar] [CrossRef]
- Sun, L.; Ke, M.; Yin, M.; Zeng, Y.; Ji, Y.; Hu, Y.; Fu, S.; Zhang, C. Extracellular vesicle-encapsulated microRNA-296-3p from cancer-associated fibroblasts promotes ovarian cancer development through regulation of the PTEN/AKT and SOCS6/STAT3 pathways. Cancer Sci. 2024, 115, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Coppey, M.; Berezhkovskii, A.M.; Sealfon, S.C.; Shvartsman, S.Y. Time and length scales of autocrine signals in three dimensions. Biophys. J. 2007, 93, 1917–1922. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, D.; Mulcrone, P.L.; Owens, P.; Sterling, J.A. The Bone Microenvironment: A Fertile Soil for Tumor Growth. Curr. Osteoporos. Rep. 2016, 14, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fan, X.; Zhang, Q.; Shi, X.; Xu, G.; Zou, C. Cancer-associated fibroblasts secrete FGF-1 to promote ovarian proliferation, migration, and invasion through the activation of FGF-1/FGFR4 signaling. Tumour Biol. 2017, 39, 1010428317712592. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Yang, X.; Li, X.; Kong, J.; Qi, D.; Zhang, F.; Sun, B.; Liu, Y.; Liu, T. Carcinoma-associated fibroblast-derived lysyl oxidase-rich extracellular vesicles mediate collagen crosslinking and promote epithelial-mesenchymal transition via p-FAK/p-paxillin/YAP signaling. Int. J. Oral Sci. 2023, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Ohlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Yao, Y.; Lv, R.; Dong, J.; Chen, Q. CAFs-derived TIAM1 Promotes OSCC Cell Growth and Metastasis by Regulating ZEB2. Cell Biochem. Biophys. 2025, 83, 729–740. [Google Scholar] [CrossRef]
- Gao, A.; Kim, D.W.; Pei, M.; Kim, K.Y.; Park, Y.J.; Nam, W.; Kim, H.J.; Kim, H.S.; Cha, I.H.; Zhang, X. Implications of TP-positive CAFs in the Bone Invasion of Oral Squamous Cell Carcinoma. Anticancer Res. 2024, 44, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFbeta to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019, 9, 282–301. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef]
- Schurch, C.M.; Bhate, S.S.; Barlow, G.L.; Phillips, D.J.; Noti, L.; Zlobec, I.; Chu, P.; Black, S.; Demeter, J.; McIlwain, D.R.; et al. Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front. Cell 2020, 182, 1341–1359.e1319. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.M.; Asselin-Labat, M.L.; Nguyen, Q.; Berthelet, J.; Tan, X.; Wimmer, V.C.; Merino, D.; Rogers, K.L.; Naik, S.H. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods 2021, 18, 997–1012. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef]
- Lynch, C.C.; Hikosaka, A.; Acuff, H.B.; Martin, M.D.; Kawai, N.; Singh, R.K.; Vargo-Gogola, T.C.; Begtrup, J.L.; Peterson, T.E.; Fingleton, B.; et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 2005, 7, 485–496. [Google Scholar] [CrossRef]
- Kawamura, I.; Ohe, R.; Suzuki, K.; Kabasawa, T.; Kitaoka, T.; Takahara, D.; Kono, M.; Uchiyama, N.; Musha, H.; Futakuchi, M.; et al. Neighboring macrophage-induced alteration in the phenotype of colorectal cancer cells in the tumor budding area. Cancer Cell Int. 2024, 24, 107. [Google Scholar] [CrossRef]
- Kitaoka, T.; Ohe, R.; Kabasawa, T.; Kaneko, M.; Sasahara, N.; Kono, M.; Suzuki, K.; Uchiyama, N.; Ogawa, R.; Futakuchi, M. Activation of fibroblasts by plasma cells via PDGF/PDGFR signaling in IgG4-related sialadenitis. J. Clin. Exp. Hematop. 2024, 64, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ohe, R.; Kabasawa, T.; Kitaoka, T.; Kawai, M.; Motoi, F.; Futakuchi, M. Histological spatial analysis on the induction of PD-L1(+) macrophages by CD8(+) T cells at the marginal microenvironment of triple-negative breast cancer. Breast Cancer 2023, 30, 1094–1104. [Google Scholar] [CrossRef]
- Jimi, E.; Shin, M.; Furuta, H.; Tada, Y.; Kusukawa, J. The RANKL/RANK system as a therapeutic target for bone invasion by oral squamous cell carcinoma (Review). Int. J. Oncol. 2013, 42, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Tsuchiya, H.; Miyazaki, Y.; Kikuchi, K.; Ide, F.; Sakashita, H.; Kusama, K. Podoplanin expressing cancer-associated fibroblasts in oral cancer. Tumour Biol. 2014, 35, 11345–11352. [Google Scholar] [CrossRef]
- Jin, W.; Yu, Q.; Yu, L.; Zhou, T.; Wang, X.; Lu, W.; Zhang, X.; Ding, L.; Hu, Q.; Ni, Y. HAS1(high) cancer associated fibroblasts located at the tumor invasion front zone promote oral squamous cell carcinoma invasion via ECM remodeling. J. Exp. Clin. Cancer Res. 2025, 44, 238. [Google Scholar] [CrossRef]
- Linz, C.; Brands, R.C.; Kertels, O.; Dierks, A.; Brumberg, J.; Gerhard-Hartmann, E.; Hartmann, S.; Schirbel, A.; Serfling, S.; Zhi, Y.; et al. Targeting fibroblast activation protein in newly diagnosed squamous cell carcinoma of the oral cavity—Initial experience and comparison to [(18)F]FDG PET/CT and MRI. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3951–3960. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasahara, N.; Kaneko, M.; Kitaoka, T.; Kohno, M.; Kabasawa, T.; Aung, N.Y.; Ohe, R.; Iino, M.; Futakuchi, M. Spatial Proximity of Cancer-Associated Fibroblasts to Tumor and Osteoclasts Suggests a Coordinating Role in OSCC-Induced Bone Invasion: A Preliminary Study. Biomedicines 2025, 13, 2554. https://doi.org/10.3390/biomedicines13102554
Sasahara N, Kaneko M, Kitaoka T, Kohno M, Kabasawa T, Aung NY, Ohe R, Iino M, Futakuchi M. Spatial Proximity of Cancer-Associated Fibroblasts to Tumor and Osteoclasts Suggests a Coordinating Role in OSCC-Induced Bone Invasion: A Preliminary Study. Biomedicines. 2025; 13(10):2554. https://doi.org/10.3390/biomedicines13102554
Chicago/Turabian StyleSasahara, Nobuyuki, Masayuki Kaneko, Takumi Kitaoka, Michihisa Kohno, Takanobu Kabasawa, Naing Ye Aung, Rintaro Ohe, Mitsuyoshi Iino, and Mitsuru Futakuchi. 2025. "Spatial Proximity of Cancer-Associated Fibroblasts to Tumor and Osteoclasts Suggests a Coordinating Role in OSCC-Induced Bone Invasion: A Preliminary Study" Biomedicines 13, no. 10: 2554. https://doi.org/10.3390/biomedicines13102554
APA StyleSasahara, N., Kaneko, M., Kitaoka, T., Kohno, M., Kabasawa, T., Aung, N. Y., Ohe, R., Iino, M., & Futakuchi, M. (2025). Spatial Proximity of Cancer-Associated Fibroblasts to Tumor and Osteoclasts Suggests a Coordinating Role in OSCC-Induced Bone Invasion: A Preliminary Study. Biomedicines, 13(10), 2554. https://doi.org/10.3390/biomedicines13102554






