Association of Intracranial Plaque Features with the Severity of White Matter Hyperintensities in Middle-Aged and Older Community-Dwelling Adults
Abstract
1. Introduction
2. Materials and Methods
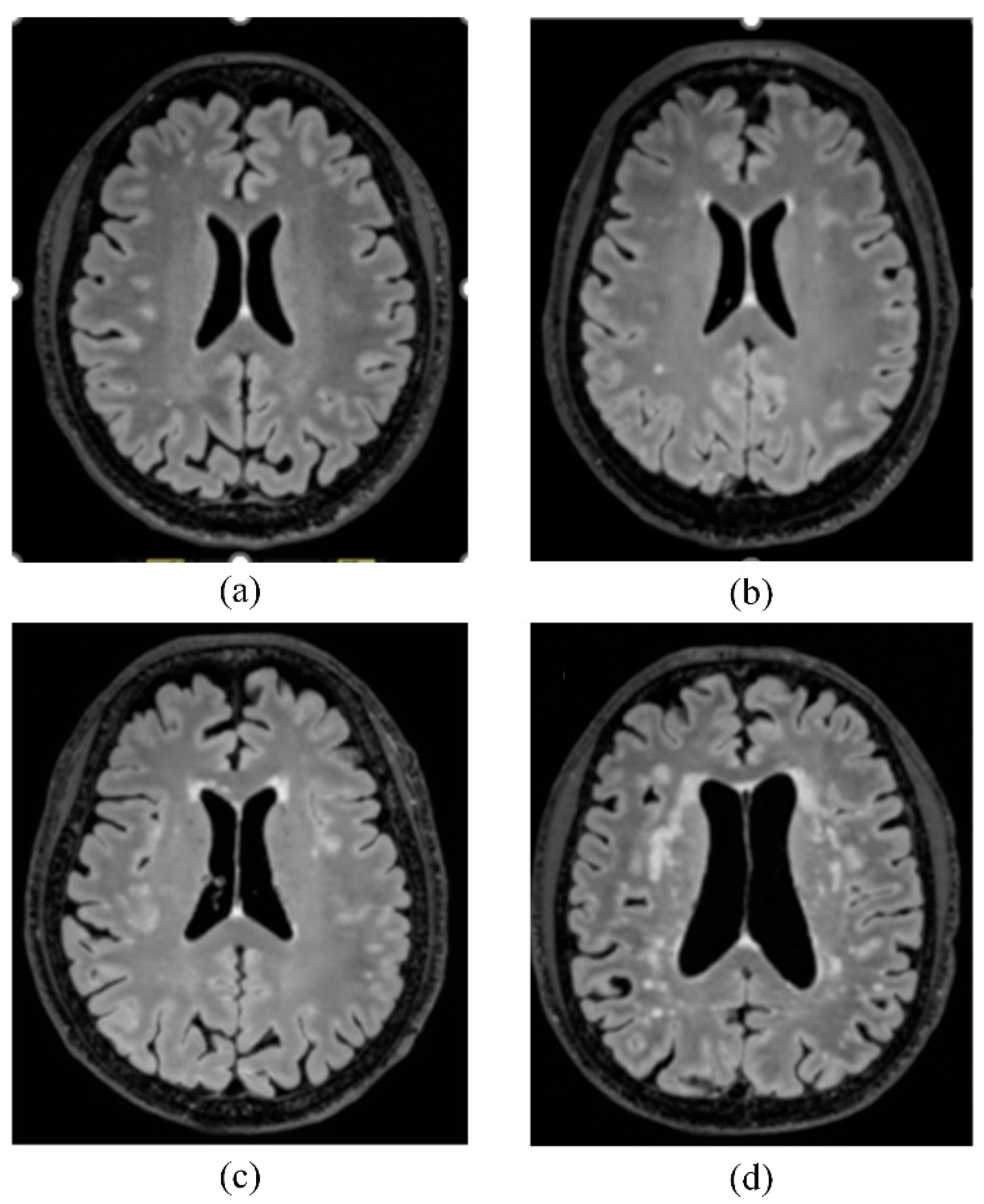
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, H.M.; Lynn, M.J.; Turan, T.N.; Derdeyn, C.P.; Fiorella, D.; Lane, B.F.; Montgomery, J.; Janis, L.S.; Rumboldt, Z.; Chimowitz, M.I.; et al. Frequency, Risk Factors, and Outcome of Coexistent Small Vessel Disease and Intracranial Arterial Stenosis: Results From the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial. JAMA Neurol. 2016, 73, 36–42. [Google Scholar] [CrossRef]
- Chojdak-Łukasiewicz, J.; Dziadkowiak, E.; Zimny, A.; Paradowski, B. Cerebral small vessel disease: A review. Adv. Clin. Exp. Med. 2021, 30, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-S.; Kwon, H.-M. Risk of “silent stroke” in patients older than 60 years: Risk assessment and clinical perspectives. Clin. Interv. Aging 2010, 5, 239–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benjamin, P.; Lawrence, A.J.; Lambert, C.; Patel, B.; Chung, A.W.; MacKinnon, A.D.; Morris, R.G.; Barrick, T.R.; Markus, H.S. Strategic lacunes and their relationship to cognitive impairment in cerebral small vessel disease. Neuroimage Clin. 2014, 4, 828–837. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, Y.; Wang, Y.; Yang, L.; Wang, Y.; Li, Y.; Liang, X.; Zhao, Q.; Wu, J.; Chu, S.; et al. White matter hyperintensities associated with progression of cerebral small vessel disease: A 7-year Chinese urban community study. Aging 2020, 12, 8506–8522. [Google Scholar] [CrossRef]
- Du, H.; Zheng, J.; Li, X.; Dong, Y.; Cheng, Y.; Liu, C.; Hu, J.; Chen, X. The correlation between medial pattern of intracranial arterial calcification and white matter hyperintensities. Atherosclerosis 2023, 381, 117247. [Google Scholar] [CrossRef]
- Zhuang, F.-J.; Chen, Y.; He, W.-B.; Cai, Z.-Y. Prevalence of white matter hyperintensities increases with age. Neural Regen. Res. 2018, 13, 2141–2146. [Google Scholar] [CrossRef]
- Li, S.; Fang, F.; Cui, M.; Jiang, Y.; Wang, Y.; Kong, X.; Tian, W.; Fan, M.; Yuan, Z.; Chen, J.; et al. Incidental findings on brain MRI among Chinese at the age of 55-65 years: The Taizhou Imaging Study. Sci. Rep. 2019, 9, 464. [Google Scholar] [CrossRef]
- Schmidt, R.; Schmidt, H.; Haybaeck, J.; Loitfelder, M.; Weis, S.; Cavalieri, M.; Seiler, S.; Enzinger, C.; Ropele, S.; Erkinjuntti, T.; et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011, 122, 171–185. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, R.; Liu, G.; Cao, C.; Chen, F.; Jin, K.; Ji, X.; Cao, G. Intracranial atherosclerotic disease. Neurobiol. Dis. 2019, 124, 118–132. [Google Scholar] [CrossRef]
- Hamilton, O.K.L.; Cox, S.R.; Okely, J.A.; Conte, F.; Ballerini, L.; Bastin, M.E.; Corley, J.; Taylor, A.M.; Page, D.; Gow, A.J.; et al. Cerebral small vessel disease burden and longitudinal cognitive decline from age 73 to 82: The Lothian Birth Cohort 1936. Transl. Psychiatry 2021, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Pinter, D.; Ritchie, S.J.; Doubal, F.; Gattringer, T.; Morris, Z.; Bastin, M.E.; Hernández, M.d.C.V.; Royle, N.A.; Corley, J.; Maniega, S.M.; et al. Impact of small vessel disease in the brain on gait and balance. Sci. Rep. 2017, 7, 41637. [Google Scholar] [CrossRef] [PubMed]
- Clancy, U.; Gilmartin, D.; Jochems, A.C.C.; Knox, L.; Doubal, F.N.; Wardlaw, J.M. Neuropsychiatric symptoms associated with cerebral small vessel disease: A systematic review and meta-analysis. Lancet Psychiatry 2021, 8, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Rensma, S.P.; van Sloten, T.T.; Launer, L.J.; Stehouwer, C.D.A. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 164–173. [Google Scholar] [CrossRef]
- Debette, S.; Schilling, S.; Duperron, M.G.; Larsson, S.C.; Markus, H.S. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 81–94. [Google Scholar] [CrossRef]
- Debette, S.; Markus, H. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef]
- Hiremath, N.; Kate, M.; Mohimen, A.; Kesavadas, C.; Sylaja, P.N. Risk factors of white matter hyperintensities in South Asian patients with transient ischemic attack and minor stroke. Neuroradiology 2020, 62, 1279–1284. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, X.; Li, H.; Jin, A.; Jiang, L.; Chen, W.; Jing, J.; Mei, L.; Li, S.; Meng, X.; et al. Association of intracranial atherosclerosis with cerebral small vessel disease in a community-based population. Eur. J. Neurol. 2023, 30, 2700–2712. [Google Scholar] [CrossRef]
- Fazekas, F.; Schmidt, R.; Kleinert, R.; Kapeller, P.; Roob, G.; Flooh, E. The spectrum of age-associated brain abnormalities: Their measurement and histopathological correlates. J. Neural Transm. Suppl. 1998, 53, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Wright, C.B.; Nickolas, T.L.; Yoshita, M.; Paik, M.C.; Kranwinkel, G.; Sacco, R.L.; DeCarli, C. Chronic kidney disease is associated with white matter hyperintensity volume: The Northern Manhattan Study (NOMAS). Stroke 2007, 38, 3121–3126. [Google Scholar] [CrossRef] [PubMed]
- Grueter, B.E.; Schulz, U.G. Age-related cerebral white matter disease (leukoaraiosis): A review. Postgrad. Med. J. 2012, 88, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Porcu, M.; Cocco, L.; Cocozza, S.; Pontillo, G.; Operamolla, A.; Defazio, G.; Suri, J.S.; Brunetti, A.; Saba, L. The association between white matter hyperintensities, cognition and regional neural activity in healthy subjects. Eur. J. Neurosci. 2021, 54, 5427–5443. [Google Scholar] [CrossRef]
- Wu, L.Y.; Chai, Y.L.; Cheah, I.K.; Chia, R.S.L.; Hilal, S.; Arumugam, T.V.; Chen, C.P.; Lai, M.K.P. Blood-based biomarkers of cerebral small vessel disease. Ageing Res. Rev. 2024, 95, 102247. [Google Scholar] [CrossRef]
- Yang, W.J.; Fisher, M.; Zheng, L.; Niu, C.B.; Paganini-Hill, A.; Zhao, H.L.; Xu, Y.; Wong, K.S.; Ng, H.K.; Chen, X.Y. Histological Characteristics of Intracranial Atherosclerosis in a Chinese Population: A Postmortem Study. Front. Neurol. 2017, 8, 488. [Google Scholar] [CrossRef]
- Lange, M.C.; Ribas, G.; Scavasine, V.; Ducci, R.D.; Mendes, D.C.; Zétola, V.H.F.; Cabral, N.; Rundek, T. Stroke recurrence in the different subtypes of ischemic stroke. The importance of the intracranial disease. Arq. Neuro-Psiquiatr. 2018, 76, 649–653. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Liu, L.; Soo, Y.O.; Pu, Y.; Pan, Y.; Wang, Y.; Zou, X.; Leung, T.W.; Cai, Y.; et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: The Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014, 45, 663–669. [Google Scholar] [CrossRef]
- Moroni, F.; Ammirati, E.; Magnoni, M.; D’Ascenzo, F.; Anselmino, M.; Anzalone, N.; Rocca, M.A.; Falini, A.; Filippi, M.; Camici, P.G. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 223, 681–687. [Google Scholar] [CrossRef]
- Ni, L.; Zhou, F.; Qing, Z.; Zhang, X.; Li, M.; Zhu, B.; Zhang, B.; Xu, Y. The Asymmetry of White Matter Hyperintensity Burden Between Hemispheres Is Associated With Intracranial Atherosclerotic Plaque Enhancement Grade. Front. Aging Neurosci. 2020, 12, 163. [Google Scholar] [CrossRef]
- Park, J.H.; Kwon, H.M.; Lee, J.; Kim, D.S.; Ovbiagele, B. Association of intracranial atherosclerotic stenosis with severity of white matter hyperintensities. Eur. J. Neurol. 2015, 22, 44-e3. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Mera, R.M.; Del Brutto, V.J.; Hill, J.P.; Torpey, A.P.; Peralta, L.D.; Generale, L.M.; Matcha, G.; Costa, A.F.; Recalde, B.Y.; et al. Cerebral small vessel disease score and atherosclerosis burden—A population study in community-dwelling older adults. Clin. Neurol. Neurosurg. 2020, 194, 105795. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Spagnolo-Allende, A.; Yang, D.; Qiao, Y.; Gutierrez, J. Epidemiology, Pathophysiology, and Imaging of Atherosclerotic Intracranial Disease. Stroke 2024, 55, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, J.; Yang, W.; Bos, D.; Zheng, L.; Wong, L.K.S.; Leung, T.W.; Chen, X. Intracranial Arterial Calcification and Intracranial Atherosclerosis: Close but Different. Front. Neurol. 2022, 13, 799429. [Google Scholar] [CrossRef]
- Zheng, L.; Li, J.; Yang, W.; Lam, H.C.; Wong, K.S.L.; Chu, W.; Leung, T.W.H.; Chen, X. Patterns and Implications of Intracranial Atherosclerosis in Anterior and Posterior Circulation Identified by High-Resolution Vessel Wall Imaging. Cerebrovasc. Dis. 2024, 53, 403–410. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.S.; Chung, S.W.; Kim, B.S.; Ahn, K.J.; Lee, K.S. White matter hyperintensities (WMH) are associated with intracranial atherosclerosis rather than extracranial atherosclerosis. Arch. Gerontol. Geriatr. 2011, 53, e129–e132. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.S.; Lee, K.S.; An, J.Y.; Kim, W.; Kim, Y.I.; Kim, B.S.; Jung, S.L. The leukoaraiosis is more prevalent in the large artery atherosclerosis stroke subtype among Korean patients with ischemic stroke. BMC Neurol. 2008, 8, 31. [Google Scholar] [CrossRef]
- Jung, K.W.; Shon, Y.M.; Yang, D.W.; Kim, B.S.; Cho, A.H. Coexisting carotid atherosclerosis in patients with intracranial small- or large-vessel disease. J. Clin. Neurol. 2012, 8, 104–108. [Google Scholar] [CrossRef]
- Zhu, K.L.; Shang, Z.Y.; Liu, B.J.; Wang, Y.; Li, J.; Yang, B.Q.; Ntaios, G.; Chen, H.S. The association of intracranial atherosclerosis with cerebral small vessel disease imaging markers: A high-resolution magnetic resonance imaging study. Sci. Rep. 2023, 13, 17017. [Google Scholar] [CrossRef]
- de Boer, L.; Poos, J.M.; Van Den Berg, E.; De Houwer, J.F.H.; Swartenbroekx, T.; Dopper, E.G.P.; Boesjes, P.; Tahboun, N.; Bouzigues, A.; Foster, P.H.; et al. Montreal Cognitive Assessment vs the Mini-Mental State Examination as a Screening Tool for Patients with Genetic Frontotemporal Dementia. Neurology 2025, 104, e213401. [Google Scholar] [CrossRef]
- Freitas, S.; Simões, M.R.; Alves, L.; Duro, D.; Santana, I. Montreal Cognitive Assessment (MoCA): Validation study for frontotemporal dementia. J. Geriatr. Psychiatry Neurol. 2012, 25, 146–154. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, H.; Cheng, Y.; Li, X.; Gao, Q.; Chen, X. Serum phosphorus concentration and its association with the degree and pattern of intracranial arterial calcification. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.; Timpson, N.J.; Dimou, N. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Kowal, P.; Chatterji, S.; Naidoo, N.; Biritwum, R.; Fan, W.; Lopez Ridaura, R.; Maximova, T.; Arokiasamy, P.; Phaswana-Mafuya, N.; Williams, S.; et al. Data resource profile: The World Health Organization Study on global AGEing and adult health (SAGE). Int. J. Epidemiol. 2012, 41, 1639–1649. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. 1), S13–S27. [Google Scholar] [CrossRef]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 2017, 23 (Suppl. 9), S139–S148. [Google Scholar] [PubMed]
- Li, J.; Yang, W.J.; Zheng, L.; Du, H.; Chu, W.C.; Leung, T.W.; Chen, X.Y. Vertebrobasilar Junction Angle Over 90°: A Potential Imaging Marker Associated With Vertebrobasilar Atherosclerosis. Front. Neurosci. 2021, 15, 789852. [Google Scholar] [CrossRef]
- Wang, M.; Wu, F.; Yang, Y.; Miao, H.; Fan, Z.; Ji, X.; Li, D.; Guo, X.; Yang, Q. Quantitative assessment of symptomatic intracranial atherosclerosis and lenticulostriate arteries in recent stroke patients using whole-brain high-resolution cardiovascular magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2018, 20, 35. [Google Scholar] [CrossRef]
- Yang, W.J.; Abrigo, J.; Soo, Y.O.; Wong, S.; Wong, K.S.; Leung, T.W.; Chu, W.C.; Chen, X.Y. Regression of Plaque Enhancement Within Symptomatic Middle Cerebral Artery Atherosclerosis: A High-Resolution MRI Study. Front. Neurol. 2020, 11, 755. [Google Scholar] [CrossRef]
- Qiao, Y.; Anwar, Z.; Intrapiromkul, J.; Liu, L.; Zeiler, S.R.; Leigh, R.; Zhang, Y.; Guallar, E.; Wasserman, B.A. Patterns and Implications of Intracranial Arterial Remodeling in Stroke Patients. Stroke 2016, 47, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Ackah, J.A.; Du, H.; Yang, W.; Zeng, H.; Chan, J.T.L.; Lo, M.L.C.; Chen, X. The burden of intracranial atherosclerosis on cerebral small vessel disease: A community cohort study. Ann. Clin. Transl. Neurol. 2025, 12, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Fan, P.; Li, Z.; Mossa-Basha, M.; Ju, Y.; Zhao, X.; Kong, Q.; Pei, X.; Zhang, X.; Sui, B.; et al. Evaluating Middle Cerebral Artery Plaque Characteristics and Lenticulostriate Artery Morphology Associated With Subcortical Infarctions at 7T MRI. J. Magn. Reson. Imaging 2023, 53, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Li, X.Q.; Hou, X.W.; Yang, B.Q.; Xia, C.; Ntaios, G.; Chen, H.S. Intracranial Atherosclerotic Plaque as a Potential Cause of Embolic Stroke of Undetermined Source. J. Am. Coll. Cardiol. 2021, 77, 680–691. [Google Scholar] [CrossRef]
- Dieleman, N.; Yang, W.; Abrigo, J.M.; Chu, W.C.; van der Kolk, A.G.; Siero, J.C.; Wong, K.S.; Hendrikse, J.; Chen, X.Y. Magnetic Resonance Imaging of Plaque Morphology, Burden, and Distribution in Patients With Symptomatic Middle Cerebral Artery Stenosis. Stroke 2016, 47, 1797–1802. [Google Scholar] [CrossRef]
- Li, F.; McDermott, M.M.; Li, D.; Carroll, T.J.; Hippe, D.S.; Kramer, C.M.; Fan, Z.; Zhao, X.; Hatsukami, T.S.; Chu, B.; et al. The association of lesion eccentricity with plaque morphology and components in the superficial femoral artery: A high-spatial-resolution, multi-contrast weighted CMR study. J. Cardiovasc. Magn. Reson. 2010, 12, 37. [Google Scholar] [CrossRef]
- Yang, W.J.; Chen, X.Y.; Zhao, H.L.; Niu, C.B.; Xu, Y.; Wong, K.S.; Ng, H.K. In Vitro Assessment of Histology Verified Intracranial Atherosclerotic Disease by 1.5T Magnetic Resonance Imaging: Concentric or Eccentric? Stroke 2016, 47, 527–530. [Google Scholar] [CrossRef]
- Alkan, O.; Kizilkilic, O.; Yildirim, T.; Atalay, H. Intracranial cerebral artery stenosis with associated coronary artery and extracranial carotid artery stenosis in Turkish patients. Eur. J. Radiol. 2009, 71, 450–455. [Google Scholar] [CrossRef]
- Samuels, O.B.; Joseph, G.J.; Lynn, M.J.; Smith, H.A.; Chimowitz, M.I. A standardized method for measuring intracranial arterial stenosis. Am. J. Neuroradiol. 2000, 21, 643–646. [Google Scholar] [PubMed]
- Yasaka, M.; Yamaguchi, T.; Shichiri, M. Distribution of atherosclerosis and risk factors in atherothrombotic occlusion. Stroke 1993, 24, 206–211. [Google Scholar] [CrossRef]
- Fazekas, F.; Kleinert, R.; Offenbacher, H.; Schmidt, R.; Kleinert, G.; Payer, F.; Radner, H.; Lechner, H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993, 43, 1683–1689. [Google Scholar] [CrossRef]
- Joo, L.; Shim, W.H.; Suh, C.H.; Lim, S.J.; Heo, H.; Kim, W.S.; Hong, E.; Lee, D.; Sung, J.; Lim, J.S.; et al. Diagnostic performance of deep learning-based automatic white matter hyperintensity segmentation for classification of the Fazekas scale and differentiation of subcortical vascular dementia. PLoS ONE 2022, 17, e0274562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Bu, W.; Meng, L.H.; Li, W.J.; Dong, Y.J.; Cao, X.Y.; Gao, Q.; Zhang, X.Y.; Ren, H.L. The correlation between intracranial atherosclerosis and white matter hyperintensities in cerebral small vessel disease: A high-resolution magnetic resonance imaging study. Front. Neurol. 2024, 15, 1485921. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wei, Q.; Zhao, X.; Hou, D.; Zhao, H.; Wang, L.; Zhang, X.; Zheng, Z.; Wu, J. Association between Short-Term Blood Pressure Variability and Intracranial Atherosclerotic Plaque Vulnerability: A High-Resolution Magnetic Resonance Imaging Study. J. Atheroscler. Thromb. 2022, 29, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Leonards, C.O.; Ipsen, N.; Malzahn, U.; Fiebach, J.B.; Endres, M.; Ebinger, M. White matter lesion severity in mild acute ischemic stroke patients and functional outcome after 1 year. Stroke 2012, 43, 3046–3051. [Google Scholar] [CrossRef]
- Ottavi, T.P.; Pepper, E.; Bateman, G.; Fiorentino, M.; Brodtmann, A. Consensus statement for the management of incidentally found brain white matter hyperintensities in general medical practice. Med. J. Aust. 2023, 219, 278–284. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, J.; Kim, Y.J.; Kwon, S.U.; Lee, D.; Jung, S.C.; Kang, D.W.; Kim, J.S. Isolated MCA disease in patients without significant atherosclerotic risk factors: A high-resolution magnetic resonance imaging study. Stroke 2015, 46, 697–703. [Google Scholar] [CrossRef]
- Ohara, T.; Toyoda, K.; Otsubo, R.; Nagatsuka, K.; Kubota, Y.; Yasaka, M.; Naritomi, H.; Minematsu, K. Eccentric stenosis of the carotid artery associated with ipsilateral cerebrovascular events. Am. J. Neuroradiol. 2008, 29, 1200–1203. [Google Scholar] [CrossRef]
- Swartz, R.H.; Bhuta, S.S.; Farb, R.I.; Agid, R.; Willinsky, R.A.; Terbrugge, K.G.; Butany, J.; Wasserman, B.A.; Johnstone, D.M.; Silver, F.L.; et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 2009, 72, 627–634. [Google Scholar] [CrossRef]
- Shi, Y.; Thrippleton, M.J.; Blair, G.W.; Dickie, D.A.; Marshall, I.; Hamilton, I.; Doubal, F.N.; Chappell, F.; Wardlaw, J.M. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J. Cereb. Blood Flow Metab. 2020, 40, 85–99. [Google Scholar] [CrossRef]
- Vasan, R.S.; Short, M.I.; Niiranen, T.J.; Xanthakis, V.; DeCarli, C.; Cheng, S.; Seshadri, S.; Mitchell, G.F. Interrelations Between Arterial Stiffness, Target Organ Damage, and Cardiovascular Disease Outcomes. J. Am. Heart Assoc. 2019, 8, e012141. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Ryu, C.W.; Yun, S.J. Vessel-Wall Magnetic Resonance Imaging of Intracranial Atherosclerotic Plaque and Ischemic Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2018, 9, 1032. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Jiang, H.; Li, T.; Qian, C.; Gong, S.; Wang, T.; Zhu, L. Predictive value of the combination between the intracranial arterial culprit plaque characteristics and the Essen Stroke Risk Score for short-term stroke recurrence. J. Stroke Cerebrovasc. Dis. 2022, 31, 106624. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Carrizzo, A.; Alfano, A.; Virtuoso, N.; Capunzo, M.; Calabrese, M.; De Simone, E.; Sciarretta, S.; Frati, G.; Oliveti, M.; et al. The Impact of Aging on Cardio and Cerebrovascular Diseases. Int. J. Mol. Sci. 2018, 19, 481. [Google Scholar] [CrossRef]
- Pedelty, L.; Gorelick, P.B. Management of hypertension and cerebrovascular disease in the elderly. Am. J. Med. 2008, 121, S23–S31. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Y.; Xiao, S.; Qiu, L.; Yu, Y.; Zhang, Z. Association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases: Systematic review and meta-analysis. BMJ 2022, 378, e070244. [Google Scholar] [CrossRef]
- Kuipers, S.; Overmars, L.M.; van Es, B.; de Bresser, J.; Bron, E.E.; Hoefer, I.E.; Kappelle, L.J.; Teunissen, C.E.; Biessels, G.J.; Haitjema, S. A cluster of blood-based protein biomarkers reflecting coagulation relates to the burden of cerebral small vessel disease. J. Cereb. Blood Flow. Metab. 2022, 42, 1282–1293. [Google Scholar] [CrossRef]
- Bots, M.; Breteler, M.; Hofman, A.; Grobbee, D.; van Swieten, J.; van Gijn, J.; de Jong, P. Cerebral white matter lesions and atherosclerosis in the Rotterdam Study. Lancet 1993, 341, 1232–1237. [Google Scholar] [CrossRef]
- Zhong, T.; Qi, Y.; Li, R.; Zhou, H.; Ran, B.; Wang, J.; Cai, Z. Contribution of intracranial artery stenosis to white matter hyperintensities progression in elderly Chinese patients: A 3-year retrospective longitudinal study. Front. Neurol. 2022, 13, 922320. [Google Scholar] [CrossRef]
- Brisset, M.; Boutouyrie, P.; Pico, F.; Zhu, Y.; Zureik, M.; Schilling, S.; Dufouil, C.; Mazoyer, B.; Laurent, S.; Tzourio, C.; et al. Large-vessel correlates of cerebral small-vessel disease. Neurology 2013, 80, 662–669. [Google Scholar] [CrossRef]
- Foddis, M.; Winek, K.; Bentele, K.; Mueller, S.; Blumenau, S.; Reichhart, N.N.; Crespo-Garcia, S.; Harnett, D.; Ivanov, A.; Meisel, A.; et al. An exploratory investigation of brain collateral circulation plasticity after cerebral ischemia in two experimental C57BL/6 mouse models. J. Cereb. Blood Flow Metab. 2020, 40, 276–287. [Google Scholar] [CrossRef]
- Liebeskind, D.S. Collateral circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Leukoaraiosis in stroke patients: The Copenhagen Stroke Study. Stroke 1995, 26, 588–592. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Erkinjuntti, T. Small vessel disease and subcortical vascular dementia. J. Clin. Neurol. 2006, 2, 1–11. [Google Scholar] [CrossRef]

| Characteristics | Total (n = 272) | Adults Without Moderate-to-Severe WMH (n = 205) | Adults with Moderate-to-Severe WMH (n = 67) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age, year, mean ± SD | 63.4 ± 6.8 | 62.3 ± 6.2 | 66.7 ± 7.5 | <0.001 |
| Sex, male, n (%) | 118 (43.4) | 84 (41.0) | 34 (50.7) | 0.161 |
| SBP, mmHg, mean ± SD | 129.9 ± 17.1 | 128.6 ± 17.7 | 134.0 ± 14.4 | 0.024 |
| DBP, mmHg, mean ± SD | 79.9 ± 10.5 | 79.5 ± 10.7 | 81.1 ± 9.8 | 0.275 |
| Body mass index ≥ 25 kg/m2, n (%) | 87 (32.0) | 63 (30.7) | 24 (35.8) | 0.438 |
| Medical comorbidities, n (%) | ||||
| Hypertension | 80 (29.4) | 48 (23.4) | 32 (47.8) | <0.001 |
| Diabetes mellitus | 33 (12.1) | 20 (9.8) | 13 (19.4) | 0.036 |
| Coronary artery disease | 17 (6.3) | 9 (4.4) | 8 (11.9) | 0.039 |
| Hyperlipidemia | 104 (38.2) | 69 (33.7) | 35 (52.2) | 0.007 |
| Medications, n (%) | ||||
| Statins | 84 (30.9) | 58 (28.3) | 26 (38.8) | 0.090 |
| Antiplatelet | 13 (4.8) | 8 (3.9) | 5 (7.5) | 0.316 |
| Anticoagulant | 31 (11.4) | 19 (9.3) | 12 (17.9) | 0.048 |
| Habits, n (%) | ||||
| Current Smoker | 14 (5.1) | 10 (4.9) | 4 (6.0) | 0.752 |
| Alcohol Drinking | 20 (7.4) | 15 (7.3) | 5 (7.5) | 0.968 |
| Total (n = 272) | Adults Without Moderate-to-Severe WMH (n = 205) | Adults with Moderate-to-Severe WMH (n = 67) | p | |
|---|---|---|---|---|
| The presence of ICAS, n (%) | 152 (55.9) | 107 (52.2) | 45 (67.2) | 0.032 |
| ICAS in MCA, n (%) | 105 (38.6) | 70 (34.1) | 35 (52.2) | 0.008 |
| ICAS in VA, n (%) | 68 (25.0) | 46 (22.4) | 22 (32.8) | 0.088 |
| ICAS in BA, n (%) | 36 (13.2) | 21 (10.2) | 15 (22.4) | 0.011 |
| The degree of stenosis, n (%) | 0.072 | |||
| <25% | 154 (56.6) | 121 (59.0) | 33(49.3) | |
| 25–49% | 95 (34.9) | 71 (34.6) | 24 (35.8) | |
| ≥50% | 23 (8.5) | 13 (6.3) | 10 (14.9) | |
| Morphological features | ||||
| Irregular surface (n, %) | 65 (23.9) | 46 (22.4) | 19 (28.4) | 0.110 |
| Diffuse lesion (n, %) | 110 (40.4) | 79 (38.5) | 31 (46.3) | 0.032 |
| Eccentric lesion (n, %) | 119 (43.8) | 82 (40.0) | 37 (55.2) | 0.029 |
| Positive remodeling index (n, %) | 94 (34.6) | 64 (31.2) | 30 (44.8) | 0.087 |
| The Severity of WMH Odds Ratio (95%CI) | p | |
|---|---|---|
| Characteristics | ||
| Stenosis degree (%) | 1.52 (1.01–2.30) | 0.046 |
| Plaque burden (%) | 1.01 (1.00–1.02) | 0.055 |
| Irregular surface | 0.74 (0.54–1.01) | 0.060 |
| Diffuse thickening pattern | 1.41 (1.01–1.96) | 0.041 |
| Eccentric lesion | 1.59 (1.13–2.22) | 0.007 |
| Model-2 Adjusted for confounding factors (age, hypertension, diabetes, hyperlipidemia, sex, smoking, and antithrombotic drugs) | ||
| Stenosis degree (%) | 1.28 (0.82–2.02) | 0.281 |
| Plaque burden (%) | 1.01 (0.99–1.01) | 0.259 |
| Irregular surface | 0.87 (0.62–1.22) | 0.418 |
| Diffuse thickening pattern | 1.19 (0.83–1.71) | 0.334 |
| Eccentric lesion | 1.47 (1.04–2.10) | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Lai, L.; Luo, J.; Ying, M.T.C. Association of Intracranial Plaque Features with the Severity of White Matter Hyperintensities in Middle-Aged and Older Community-Dwelling Adults. Biomedicines 2025, 13, 2553. https://doi.org/10.3390/biomedicines13102553
Cheng Y, Lai L, Luo J, Ying MTC. Association of Intracranial Plaque Features with the Severity of White Matter Hyperintensities in Middle-Aged and Older Community-Dwelling Adults. Biomedicines. 2025; 13(10):2553. https://doi.org/10.3390/biomedicines13102553
Chicago/Turabian StyleCheng, Yangyang, Lihua Lai, Jieqi Luo, and Michael Tin Cheung Ying. 2025. "Association of Intracranial Plaque Features with the Severity of White Matter Hyperintensities in Middle-Aged and Older Community-Dwelling Adults" Biomedicines 13, no. 10: 2553. https://doi.org/10.3390/biomedicines13102553
APA StyleCheng, Y., Lai, L., Luo, J., & Ying, M. T. C. (2025). Association of Intracranial Plaque Features with the Severity of White Matter Hyperintensities in Middle-Aged and Older Community-Dwelling Adults. Biomedicines, 13(10), 2553. https://doi.org/10.3390/biomedicines13102553






