Molecular Mechanisms of Thyroid Hormone Signaling in Thyroid Cancer: Oncogenesis, Progression, and Therapeutic Implications
Abstract
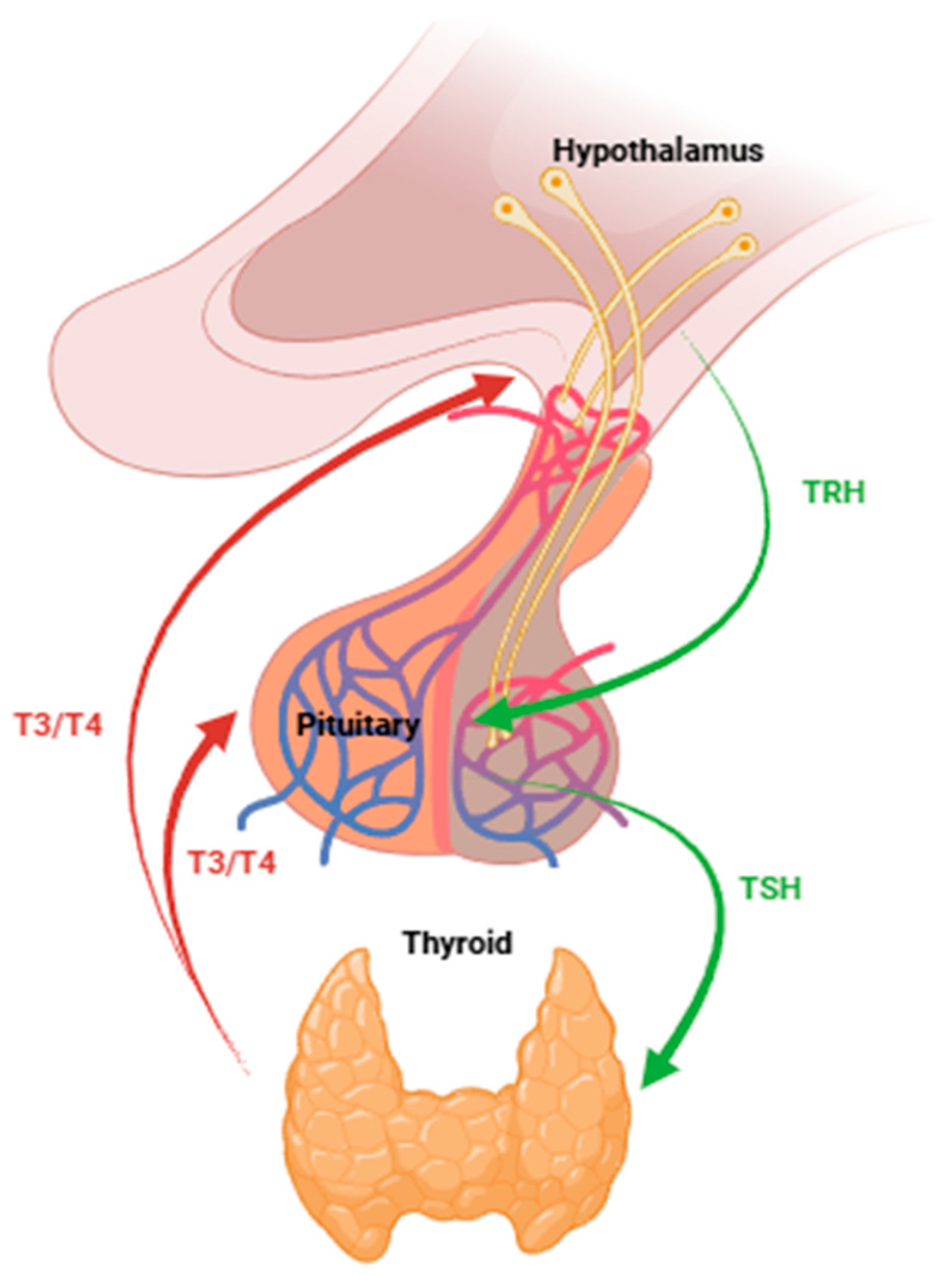
1. Introduction
2. The Molecular Biology of T3/T4 and Thyroid Cancer
2.1. Integrin αvβ3-Mediated Carcinogenic Signaling in Thyroid Cancer
2.1.1. Activation of MAPK.ERK and PI3K/Akt Pathways
2.1.2. Promotion of Proliferation and Cell Cycle Progression
2.1.3. Induction of Angiogenesis
2.1.4. Enhancement of Tumor Metastasis and Invasion
2.1.5. Inhibition of Apoptosis
2.1.6. Facilitation of Immune Evasion
2.1.7. The Effects of Tetraiodothyronine (Tetrac)
2.1.8. Novel Targeted Therapeutic Strategies Against MAPK/ERK and PI3K/Akt Pathways
2.2. The Tumor Suppressive Effect of TRβ
2.2.1. The Function of Mutant TRβ
2.2.2. PPARγ/PPRE Pathway
2.2.3. PI3K/Akt Pathway
2.2.4. Integrin-Src-FAK Pathway
2.2.5. TRβ-RUNX2
2.2.6. Prognostic Significance of TRβ Mutations
2.2.7. Preclinical Mechanism Studies of TRβ in Antitumor Therapy
3. Clinical Correlation Between Thyroid Function Status and Thyroid Cancer
3.1. Graves’ Disease
3.1.1. Graves and Nodules
3.1.2. Graves and Cancer
3.2. Potential Risks of Hypothyroidism
Elevated TSH Promotes Cancer Development
4. TSH Suppression Therapy
4.1. Risk-Stratified Targets and Clinical Outcomes
4.2. Risk of Overtreatment
4.3. Therapeutic Limitations
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Oppenheimer, J.H.; Schwartz, H.L.; Lane, J.T.; Thompson, M.P. Functional relationship of thyroid hormone-induced lipogenesis, lipolysis, and thermogenesis in the rat. J. Clin. Investig. 1991, 87, 125–132. [Google Scholar] [CrossRef]
- Fox, C.S.; Pencina, M.J.; D’Agostino, R.B.; Murabito, J.M.; Seely, E.W.; Pearce, E.N.; Vasan, R.S. Relations of thyroid function to body weight: Cross-sectional and longitudinal observations in a community-based sample. Arch. Intern. Med. 2008, 168, 587–592. [Google Scholar] [CrossRef]
- Lee, S.Y.; Pearce, E.N. Hyperthyroidism: A Review. JAMA-J. Am. Med. Assoc. 2023, 330, 1472–1483. [Google Scholar] [CrossRef]
- Persani, L. Clinical review: Central hypothyroidism: Pathogenic, diagnostic, and therapeutic challenges. J. Clin. Endocr. Metab. 2012, 97, 3068–3078. [Google Scholar] [CrossRef]
- Mariotti, S.; Beck-Peccoz, P. Physiology of the Hypothalamic-Pituitary-Thyroid Axis. 2000. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK278958/ (accessed on 20 January 2020).
- Fischer, S.; Ehlert, U. Hypothalamic-pituitary-thyroid (HPT) axis functioning in anxiety disorders. A systematic review. Depress. Anxiety 2018, 35, 98–110. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef]
- Wells, S.J. Progress in Endocrine Neoplasia. Clin. Cancer Res. 2016, 22, 4981–4988. [Google Scholar] [CrossRef]
- Morris, L.G.; Sikora, A.G.; Tosteson, T.D.; Davies, L. The increasing incidence of thyroid cancer: The influence of access to care. Thyroid 2013, 23, 885–891. [Google Scholar] [CrossRef]
- Rahbari, R.; Zhang, L.; Kebebew, E. Thyroid cancer gender disparity. Future Oncol. 2010, 6, 1771–1779. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.; Osamura, R.; Rosai, J. WHO Classification of Tumours Editorial Board: Endocrine and Neuroendocrine Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- Juhlin, C.C.; Mete, O.; Baloch, Z.W. The 2022 WHO classification of thyroid tumors: Novel concepts in nomenclature and grading. Endocr.-Relat. Cancer 2023, 30, e220293. [Google Scholar] [PubMed]
- Guidelines Working Committee of Chinese Society of Clinical Oncology. Guidelines of Chinese Society of Clinical Oncology (CSCO) Differentiated Thyroid Cancer. J. Cancer Control Treat. 2021, 34, 1164–1201. [Google Scholar]
- Leandro-García, L.J.; Landa, I. Mechanistic Insights of Thyroid Cancer Progression. Endocrinology 2023, 164, bqad118. [Google Scholar] [CrossRef]
- Prabhash, K.; Saldanha, E.; Patil, V.; Bal, M.; Reddy P, S.; Sanjeev, A.; Kumar, R.; Poojary, D.; Noronha, V.; Menon, N.; et al. RET Alterations Differentiate Molecular Profile of Medullary Thyroid Cancer. JCO Precis. Oncol. 2024, 8, e2300622. [Google Scholar] [CrossRef]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J.J.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef]
- Kondo, T.; Asa, S.L.; Ezzat, S. Epigenetic dysregulation in thyroid neoplasia. Endocrinol. Metab. Clin. 2008, 37, 389–400. [Google Scholar] [CrossRef]
- Poke, F.S.; Qadi, A.; Holloway, A.F. Reversing aberrant methylation patterns in cancer. Curr. Med. Chem. 2010, 17, 1246–1254. [Google Scholar] [CrossRef]
- Chi, P.; Allis, C.D.; Wang, G.G. Covalent histone modifications–Miswritten, misinterpreted and mis-erased in human cancers. Nat. Rev. Cancer 2010, 10, 457–469. [Google Scholar] [CrossRef]
- Gérard, A.; Daumerie, C.; Mestdagh, C.; Gohy, S.; De Burbure, C.; Costagliola, S.; Miot, F.; Nollevaux, M.; Denef, J.; Rahier, J.; et al. Correlation between the loss of thyroglobulin iodination and the expression of thyroid-specific proteins involved in iodine metabolism in thyroid carcinomas. J. Clin. Endocr. Metab. 2003, 88, 4977–4983. [Google Scholar] [CrossRef]
- Alvarez-Nuñez, F.; Bussaglia, E.; Mauricio, D.; Ybarra, J.; Vilar, M.; Lerma, E.; de Leiva, A.; Matias-Guiu, X. PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid 2006, 16, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Faam, B.; Ghaffari, M.A.; Khorsandi, L.; Ghadiri, A.A.; Totonchi, M.; Amouzegar, A.; Fanaei, S.A.; Azizi, F.; Shahbazian, H.B.; Hashemi Tabar, M. RAP1GAP Functions as a Tumor Suppressor Gene and Is Regulated by DNA Methylation in Differentiated Thyroid Cancer. Cytogenet. Genome Res. 2021, 161, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Kunstman, J.W.; Korah, R.; Healy, J.M.; Prasad, M.; Carling, T. Quantitative assessment of RASSF1A methylation as a putative molecular marker in papillary thyroid carcinoma. Surgery 2013, 154, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Poli, R.; Pugliese, M.; Fortunati, N.; Boccuzzi, G. Emerging molecular therapies of advanced thyroid cancer. Mol. Asp. Med. 2010, 31, 215–226. [Google Scholar] [CrossRef]
- Kato, Y.; Ying, H.; Willingham, M.C.; Cheng, S.Y. A tumor suppressor role for thyroid hormone beta receptor in a mouse model of thyroid carcinogenesis. Endocrinology 2004, 145, 4430–4438. [Google Scholar] [CrossRef]
- Kunjumohamed, F.P.; Al-Busaidi, N.B.; Al-Musalhi, H.N.; Al-Shereiqi, S.Z.; Al-Salmi, I.S. The prevalence of thyroid cancer in patients with hyperthyroidism. Saudi Med. J. 2015, 36, 874–877. [Google Scholar] [CrossRef]
- Bolf, E.L.; Gillis, N.E.; Davidson, C.D.; Cozzens, L.M.; Kogut, S.; Tomczak, J.A.; Frietze, S.; Carr, F.E. Common tumor-suppressive signaling of thyroid hormone receptor beta in breast and thyroid cancer cells. Mol. Carcinog. 2021, 60, 874–885. [Google Scholar] [CrossRef]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef]
- Lin, H.Y.; Davis, F.B.; Gordinier, J.K.; Martino, L.J.; Davis, P.J. Thyroid hormone induces activation of mitogen-activated protein kinase in cultured cells. Am. J. Physiol. 1999, 276, C1014–C1024. [Google Scholar] [CrossRef]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef]
- Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W. Ligand binding to integrins. J. Biol. Chem. 2000, 275, 21785–21788. [Google Scholar]
- Flores, K.; Yadav, S.S.; Katz, A.A.; Seger, R. The Nuclear Translocation of Mitogen-Activated Protein Kinases: Molecular Mechanisms and Use as Novel Therapeutic Target. Neuroendocrinology 2019, 108, 121–131. [Google Scholar]
- Vanhaesebroeck, B.; Ali, K.; Bilancio, A.; Geering, B.; Foukas, L.C. Signalling by PI3K isoforms: Insights from gene-targeted mice. Trends Biochem. Sci. 2005, 30, 194–204. [Google Scholar] [PubMed]
- Stokoe, D. The phosphoinositide 3-kinase pathway and cancer. Expert. Rev. Mol. Med. 2005, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nozhat, Z.; Hedayati, M. PI3K/AKT Pathway and Its Mediators in Thyroid Carcinomas. Mol. Diagn. Ther. 2016, 20, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.R.; Toker, A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell. Signal. 2009, 21, 470–476. [Google Scholar] [CrossRef]
- Illario, M.; Cavallo, A.L.; Monaco, S.; Di Vito, E.; Mueller, F.; Marzano, L.A.; Troncone, G.; Fenzi, G.; Rossi, G.; Vitale, M. Fibronectin-induced proliferation in thyroid cells is mediated by alphavbeta3 integrin through Ras/Raf-1/MEK/ERK and calcium/CaMKII signals. J. Clin. Endocr. Metab. 2005, 90, 2865–2873. [Google Scholar]
- Salehi, B.; Zucca, P.; Sharifi-Rad, M.; Pezzani, R.; Rajabi, S.; Setzer, W.N.; Varoni, E.M.; Iriti, M.; Kobarfard, F.; Sharifi-Rad, J. Phytotherapeutics in cancer invasion and metastasis. Phytother. Res. 2018, 32, 1425–1449. [Google Scholar] [CrossRef]
- Guzy, R.D.; Schumacker, P.T. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006, 91, 807–819. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Pallini, R.; Biffoni, M.; Todaro, M.; Invernici, G.; Cenci, T.; Maira, G.; Parati, E.A.; Stassi, G.; Larocca, L.M.; et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010, 468, 824–828, Erratum in Nature 2011, 469, 432. [Google Scholar] [CrossRef]
- Mousa, S.S.; Mousa, S.S.; Mousa, S.A. Effect of resveratrol on angiogenesis and platelet/fibrin-accelerated tumor growth in the chick chorioallantoic membrane model. Nutr. Cancer 2005, 52, 59–65. [Google Scholar] [PubMed]
- Yalcin, M.; Bharali, D.J.; Lansing, L.; Dyskin, E.; Mousa, S.S.; Hercbergs, A.; Davis, F.B.; Davis, P.J.; Mousa, S.A. Tetraidothyroacetic Acid (Tetrac) and Tetrac Nanoparticles Inhibit Growth of Human Renal Cell Carcinoma Xenografts. Anticancer Res. 2009, 29, 3825–3831. [Google Scholar] [PubMed]
- Li, L.; Pang, Y.; Zhang, L.; Li, M.; Zhu, C.; Fang, S.; Yin, Z. Triiodothyronine potentiates angiogenesis-related factor expression through PI3K/AKT signaling pathway in human osteoarthritic osteoblasts. Iran. J. Basic Med. Sci. 2020, 23, 819–825. [Google Scholar] [PubMed]
- Soh, E.Y.; Duh, Q.Y.; Sobhi, S.A.; Young, D.M.; Epstein, H.D.; Wong, M.G.; Garcia, Y.K.; Min, Y.D.; Grossman, R.F.; Siperstein, A.E.; et al. Vascular endothelial growth factor expression is higher in differentiated thyroid cancer than in normal or benign thyroid. J. Clin. Endocr. Metab. 1997, 82, 3741–3747. [Google Scholar] [CrossRef]
- Lin, J.D.; Chao, T.C. Vascular endothelial growth factor in thyroid cancers. Cancer Biother. Radio. 2005, 20, 648–661. [Google Scholar] [CrossRef]
- Davis, P.J.; Glinsky, G.V.; Lin, H.Y.; Leith, J.T.; Hercbergs, A.; Tang, H.Y.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Cancer Cell Gene Expression Modulated from Plasma Membrane Integrin αvβ3 by Thyroid Hormone and Nanoparticulate Tetrac. Front. Endocrinol. 2014, 5, 240. [Google Scholar]
- Chen, J.; Ortmeier, S.B.; Savinova, O.V.; Nareddy, V.B.; Beyer, A.J.; Wang, D.; Gerdes, A.M. Thyroid hormone induces sprouting angiogenesis in adult heart of hypothyroid mice through the PDGF-Akt pathway. J. Cell. Mol. Med. 2012, 16, 2726–2735. [Google Scholar]
- Bièche, I.; Franc, B.; Vidaud, D.; Vidaud, M.; Lidereau, R. Analyses of MYC, ERBB2, and CCND1 genes in benign and malignant thyroid follicular cell tumors by real-time polymerase chain reaction. Thyroid 2001, 11, 147–152. [Google Scholar]
- Moore, D.; Ohene-Fianko, D.; Garcia, B.; Chakrabarti, S. Apoptosis in thyroid neoplasms: Relationship with p53 and bcl-2 expression. Histopathology 1998, 32, 35–42. [Google Scholar]
- Xiao, G.; Unger, P.D.; Burstein, D.E. Immunohistochemical detection of X-linked inhibitor of apoptosis (XIAP) in neoplastic and other thyroid disorders. Ann. Diagn. Pathol. 2007, 11, 235–240. [Google Scholar] [CrossRef]
- Klein, M.; Picard, E.; Vignaud, J.M.; Marie, B.; Bresler, L.; Toussaint, B.; Weryha, G.; Duprez, A.; Leclère, J. Vascular endothelial growth factor gene and protein: Strong expression in thyroiditis and thyroid carcinoma. J. Endocrinol. 1999, 161, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Shingu, K.; Sugenoya, A.; Itoh, N.; Kato, R. Expression of basic fibroblast growth factor in thyroid disorders. World J. Surg. 1994, 18, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lin, J.; Liou, M.; Weng, H.; Chang, C.A.; Chan, E. An aberrant autocrine activation of the platelet-derived growth factor alpha-receptor in follicular and papillary thyroid carcinoma cell lines. Cancer Lett. 2006, 231, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.; Resch, J.; Cowen, R.L.; von Wasielewski, R.; Hoang-Vu, C.; West, C.M.; Williams, K.J.; Brabant, G. Expression of hypoxia-inducible factor 1 alpha in thyroid carcinomas. Endocr.-Relat. Cancer 2010, 17, 61–72. [Google Scholar] [CrossRef]
- Nose, F.; Ichikawa, T.; Fujiwara, M.; Okayasu, I. Up-regulation of cyclooxygenase-2 expression in lymphocytic thyroiditis and thyroid tumors: Significant correlation with inducible nitric oxide synthase. Am. J. Clin. Pathol. 2002, 117, 546–551. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Zeng, Y.; Ruan, X.; Tao, M.; Lin, W.; Liu, C.; Chen, H.; Liu, H.; Wu, Y. Prognostic value of EMT-related genes and immune cell infiltration in thyroid carcinoma. Front. Immunol. 2024, 15, 1463258. [Google Scholar] [CrossRef]
- Kraiem, Z.; Korem, S. Matrix metalloproteinases and the thyroid. Thyroid 2000, 10, 1061–1069. [Google Scholar] [CrossRef]
- Hong, M.; Cheng, H.; Song, L.; Wang, W.; Wang, Q.; Xu, D.; Xing, W. Wogonin Suppresses the Activity of Matrix Metalloproteinase-9 and Inhibits Migration and Invasion in Human Hepatocellular Carcinoma. Molecules 2018, 23, 384. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, H.; Li, Y. PKCζ, MMP-2 and MMP-9 expression in lung adenocarcinoma and association with a metastatic phenotype. Mol. Med. Rep. 2017, 16, 8301–8306. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef]
- Hall, A. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 2005, 33 Pt 5, 891–895. [Google Scholar] [CrossRef]
- Keely, P.J.; Westwick, J.K.; Whitehead, I.P.; Der, C.J.; Parise, L.V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature 1997, 390, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Glinskii, A.B.; Glinsky, G.V.; Lin, H.; Tang, H.; Sun, M.; Davis, F.B.; Luidens, M.K.; Mousa, S.A.; Hercbergs, A.H.; Davis, P.J. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle 2009, 8, 3562–3570. [Google Scholar] [CrossRef] [PubMed]
- Vial, E.; Sahai, E.; Marshall, C.J. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell 2003, 4, 67–79. [Google Scholar] [CrossRef]
- Hetmanski, J.H.; Zindy, E.; Schwartz, J.M.; Caswell, P.T. A MAPK-Driven Feedback Loop Suppresses Rac Activity to Promote RhoA-Driven Cancer Cell Invasion. PLoS Comput. Biol. 2016, 12, e1004909. [Google Scholar]
- Shih, A.; Davis, F.B.; Lin, H.; Davis, P.J. Resveratrol Induces Apoptosis in Thyroid Cancer Cell Lines via a MAPK- and p53-Dependent Mechanism. J. Clin. Endocr. Metab. 2002, 87, 1223–1232. [Google Scholar] [CrossRef]
- Davis, P.J.; Shih, A.; Lin, H.; Martino, L.J.; Davis, F.B. Thyroxine Promotes Association of Mitogen-activated Protein Kinase and Nuclear Thyroid Hormone Receptor (TR) and Causes Serine Phosphorylation of, T.R. J. Biol. Chem. 2000, 275, 38032–38039. [Google Scholar] [CrossRef]
- Gore, J.; Imasuen-Williams, I.E.; Conteh, A.M.; Craven, K.E.; Cheng, M.; Korc, M. Combined targeting of TGF-β, EGFR and HER2 suppresses lymphangiogenesis and metastasis in a pancreatic cancer model. Cancer Lett. 2016, 379, 143–153. [Google Scholar] [CrossRef]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Bai, Y.; Niu, D.; Huang, X.; Kang, Q.; Dou, F.; Ji, X.; Xue, W.; Liu, Y.; Li, Z.; Feng, Q.; et al. PD-L1 and PD-1 expression are correlated with distinctive clinicopathological features in papillary thyroid carcinoma. Diagn. Pathol. 2017, 12, 72. [Google Scholar] [CrossRef]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Mousa, S.A.; Hercbergs, A.; Lin, H.Y.; Keating, K.A.; Davis, P.J. Actions of Thyroid Hormones on Thyroid Cancers. Front. Endocrinol. 2021, 12, 691736. [Google Scholar] [CrossRef]
- Davis, P.J.; Lin, H.Y.; Hercbergs, A.; Mousa, S.A. Actions of L-thyroxine (T4) and Tetraiodothyroacetic Acid (Tetrac) on Gene Expression in Thyroid Cancer Cells. Genes 2020, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.; Dyskin, E.; Lansing, L.; Bharali, D.J.; Mousa, S.S.; Bridoux, A.; Hercbergs, A.H.; Lin, H.Y.; Davis, F.B.; Glinsky, G.V.; et al. Tetraiodothyroacetic acid (tetrac) and nanoparticulate tetrac arrest growth of medullary carcinoma of the thyroid. J. Clin. Endocr. Metab. 2010, 95, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Sudha, T.; Rehman, M.U.; Darwish, N.; Coskun, M.D.; Satti, J.A.; Davis, P.J.; Mousa, S.A. Nano-Targeting of Thyrointegrin αvβ3 Receptor in Solid Tumors and Impact on Radiosensitization. Radiat. Res. 2021, 196, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Nilubol, N.; Boufraqech, M. New Therapies for Advanced Thyroid Cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Brose, M.S.; Cabanillas, M.E.; Cohen, E.E.W.; Wirth, L.J.; Riehl, T.; Yue, H.; Sherman, S.I.; Sherman, E.J. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: A non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1272–1282. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients with Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef]
- Brauner, E.; Gunda, V.; Vanden Borre, P.; Zurakowski, D.; Kim, Y.S.; Dennett, K.V.; Amin, S.; Freeman, G.J.; Parangi, S. Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti tumor immunity in an immunocompetent murine model of anaplastic thyroid cancer. Oncotarget 2016, 7, 17194–17211. [Google Scholar] [CrossRef]
- Gunda, V.; Gigliotti, B.; Ashry, T.; Ndishabandi, D.; McCarthy, M.; Zhou, Z.; Amin, S.; Lee, K.E.; Stork, T.; Wirth, L.; et al. Anti-PD-1/PD-L1 therapy augments lenvatinib’s efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int. J. Cancer 2019, 144, 2266–2278. [Google Scholar] [CrossRef]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef]
- Yen, P.M.; Ando, S.; Feng, X.; Liu, Y.; Maruvada, P.; Xia, X. Thyroid hormone action at the cellular, genomic and target gene levels. Mol. Cell. Endocrinol. 2006, 246, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, C.; Thompson, C.C.; Ong, E.S.; Lebo, R.; Gruol, D.J.; Evans, R.M. The c-erb-A gene encodes a thyroid hormone receptor. Nature 1986, 324, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Sap, J.; Muñoz, A.; Damm, K.; Goldberg, Y.; Ghysdael, J.; Leutz, A.; Beug, H.; Vennström, B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 1986, 324, 635–640. [Google Scholar] [PubMed]
- Yen, P.M.; Ikeda, M.; Brubaker, J.H.; Forgione, M.; Sugawara, A.; Chin, W.W. Roles of v-erbA homodimers and heterodimers in mediating dominant negative activity by v-erbA. J. Biol. Chem. 1994, 269, 903–909. [Google Scholar] [CrossRef]
- Hörlein, A.J.; Näär, A.M.; Heinzel, T.; Torchia, J.; Gloss, B.; Kurokawa, R.; Ryan, A.; Kamei, Y.; Söderström, M.; Glass, C.K.; et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 1995, 377, 397–404. [Google Scholar] [CrossRef]
- Gandrillon, O.; Jurdic, P.; Pain, B.; Desbois, C.; Madjar, J.J.; Moscovici, M.G.; Moscovici, C.; Samarut, J. Expression of the v-erbA product, an altered nuclear hormone receptor, is sufficient to transform erythrocytic cells in vitro. Cell 1989, 58, 115–121. [Google Scholar] [CrossRef]
- Kim, W.G.; Cheng, S.Y. Thyroid hormone receptors and cancer. Biochim. Biophys. Acta 2013, 1830, 3928–3936. [Google Scholar] [CrossRef]
- Lin, K.; Shieh, H.; Chen, S.; Hsu, H. Expression of mutant thyroid hormone nuclear receptors in human hepatocellular carcinoma cells. Mol. Carcinog. 1999, 26, 53–61. [Google Scholar] [CrossRef]
- Wallin, G.; Brönnegård, M.; Grimelius, L.; McGUIRE, J.; Tørring, O. Expression of the Thyroid Hormone Receptor, the Oncogenes c-myc and H-ras, and the 90 kD Heat Shock Protein in Normal. Hyperplastic, and Neoplastic Human Thyroid Tissue. Thyroid 1992, 2, 307–313. [Google Scholar]
- Suzuki, H.; Willingham, M.C.; Cheng, S. Mice with a Mutation in the Thyroid Hormone Receptor β Gene Spontaneously Develop Thyroid Carcinoma: A Mouse Model of Thyroid Carcinogenesis. Thyroid 2002, 12, 963–969. [Google Scholar] [CrossRef]
- Araki, O.; Ying, H.; Furuya, F.; Zhu, X.; Cheng, S.Y. Thyroid hormone receptor beta mutants: Dominant negative regulators of peroxisome proliferator-activated receptor gamma action. Proc. Natl. Acad. Sci. USA 2005, 102, 16251–16256. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iglesias, O.A.; Alonso-Merino, E.; Gómez-Rey, S.; Velasco-Martín, J.P.; Martín, O.R.; Luengo, E.; García, M.R.; Ibáñez, D.C.I.; Fernández, A.F.; Fraga, M.F.; et al. Autoregulatory loop of nuclear corepressor 1 expression controls invasion, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, E328–E337. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iglesias, O.; Alonso-Merino, E.; Aranda, A. Tumor suppressive actions of the nuclear receptor corepressor 1. Pharmacol. Res. 2016, 108, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.A.; Dickstein, B.M.; Ashizawa, K.; McClaskey, J.H.; Muchmore, P.; Ransom, S.C.; Menke, J.B.; Hao, E.H.; Usala, S.J.; Bercu, B.B.; et al. Variable transcriptional activity and ligand binding of mutant beta 1 3,5,3′-triiodothyronine receptors from four families with generalized resistance to thyroid hormone. Mol. Endocrinol. 1992, 6, 248–258. [Google Scholar]
- Janani, C.; Ranjitha, K.B. PPAR gamma gene—A review. Diabetes Metab. Synd. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Kroll, T.G.; Sarraf, P.; Pecciarini, L.; Chen, C.; Mueller, E.; Spiegelman, B.M.; Fletcher, J.A. PAX8-PPARγ1 Fusion in Oncogene Human Thyroid Carcinoma. Science 2000, 289, 1357–1360. [Google Scholar] [CrossRef]
- Hao, Y.; Suzuki, H.; Li, Z.; Willingham, M.C.; Meltzer, P.; Cheng, S. Mutant thyroid hormone receptor β represses the expression and transcriptional activity of peroxisome proliferator-activated receptor γ during thyroid carcinogenesis. Cancer Res. 2003, 63, 5274–5280. [Google Scholar]
- Furuya, F.; Hanover, J.A.; Cheng, S. Activation of Phosphatidylinositol 3-kinase Signaling by a Mutant Thyroid Hormone β Receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 1780–1785. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, L.; Ying, H.; Willingham, M.C.; Cheng, S. Growth Activation Alone Is Not Sufficient to Cause Metastatic Thyroid Cancer in a Mouse Model of Follicular Thyroid Carcinoma. Endocrinology 2010, 151, 1929–1939. [Google Scholar] [CrossRef]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.C.; Yamaguchi-Iwai, Y.; Ogawa, E.; Maruyama, M.; Inuzuka, M.; Kagoshima, H.; Shigesada, K.; Satake, M.; Ito, Y. Isolation of PEBP2 alpha B cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene 1993, 8, 809–814. [Google Scholar] [PubMed]
- Kagoshima, H.; Shigesada, K.; Satake, M.; Ito, Y.; Miyoshi, H.; Ohki, M.; Pepling, M.; Gergen, P. The Runt domain identifies a new family of heteromeric transcriptional regulators. Trends Genet. 1993, 9, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, H.; Browne, G.; O’Donovan, K.M.; Byrne, N.M.; Worthington, J.; McKeown, S.R.; McKenna, D.J. Nitric Oxide Up-Regulates RUNX2 in LNCaP Prostate Tumours: Implications for Tumour Growth In Vitro and In Vivo. J. Cell. Physiol. 2016, 231, 473–482. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, P.; Zhao, Y.; Liu, R.; Zhang, Y. Overexpressed circRANBP17 acts as an oncogene to facilitate nasopharyngeal carcinoma via the miR-635/RUNX2 axis. J. Cancer 2021, 12, 4322–4331. [Google Scholar] [CrossRef]
- Foley, J.M.; Scholten, D.N.; Monks, N.R.; Cherba, D.; Monsma, D.J.; Davidson, P.; Dylewski, D.; Dykema, K.; Winn, M.E.; Steensma, M.R. Anoikis-resistant subpopulations of human osteosarcoma display significant chemoresistance and are sensitive to targeted epigenetic therapies predicted by expression profiling. J. Transl. Med. 2015, 13, 110. [Google Scholar] [CrossRef]
- Carr, F.E.; Tai, P.W.; Barnum, M.S.; Gillis, N.E.; Evans, K.G.; Taber, T.H.; White, J.H.; Tomczak, J.A.; Jaworski, D.M.; Zaidi, S.K.; et al. Thyroid Hormone Receptor-β (TRβ) Mediates Runt-Related Transcription Factor 2 (Runx2) Expression in Thyroid Cancer Cells: A Novel Signaling Pathway in Thyroid Cancer. Endocrinology 2016, 157, 3278–3292. [Google Scholar] [CrossRef]
- Gillis, N.E.; Taber, T.H.; Bolf, E.L.; Beaudet, C.M.; Tomczak, J.A.; White, J.H.; Stein, J.L.; Stein, G.S.; Lian, J.B.; Frietze, S.; et al. Thyroid Hormone Receptor β Suppression of RUNX2 Is Mediated by Brahma-Related Gene 1-Dependent Chromatin Remodeling. Endocrinology 2018, 159, 2484–2494. [Google Scholar] [CrossRef]
- Furuya, F.; Guigon, C.J.; Zhao, L.; Lu, C.; Hanover, J.A.; Cheng, S. Nuclear receptor corepressor is a novel regulator of phosphatidylinositol 3-kinase signaling. Mol. Cell. Biol. 2007, 27, 6116–6126. [Google Scholar] [CrossRef][Green Version]
- Ying, H.; Furuya, F.; Zhao, L.; Araki, O.; West, B.L.; Hanover, J.A.; Willingham, M.C.; Cheng, S. Aberrant accumulation of PTTG1 induced by a mutated thyroid hormone beta receptor inhibits mitotic progression. J. Clin. Investig. 2006, 116, 2972–2984. [Google Scholar] [CrossRef]
- Guigon, C.J.; Zhao, L.; Lu, C.; Willingham, M.C.; Cheng, S. Regulation of beta-catenin by a novel nongenomic action of thyroid hormone beta receptor. Mol. Cell. Biol. 2008, 28, 4598–4608. [Google Scholar] [CrossRef] [PubMed]
- Guigon, C.J.; Cheng, S. Novel non-genomic signaling of thyroid hormone receptors in thyroid carcinogenesis. Mol. Cell. Endocrinol. 2009, 308, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, M.; Yamasaki, S.; Tsuyuguchi, M. A case of resistance to thyroid hormone diagnosed after total thyroidectomy for thyroid cancer. J. Med. Investig. 2015, 62, 268–271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gillis, N.E.; Cozzens, L.M.; Wilson, E.R.; Smith, N.M.; Tomczak, J.A.; Bolf, E.L.; Carr, F.E. TRβ Agonism Induces Tumor Suppression and Enhances Drug Efficacy in Anaplastic Thyroid Cancer in Female Mice. Endocrinology 2023, 164, bqad135. [Google Scholar] [CrossRef]
- Pourvali, K.; Shimi, G.; Ghorbani, A.; Shakery, A.; Shirazi, F.H.; Zand, H. Selective thyroid hormone receptor beta agonist, GC-1, is capable to reduce growth of colorectal tumor in syngeneic mouse models. J. Recept. Signal Transduct. 2022, 42, 495–502. [Google Scholar] [CrossRef]
- Bolf, E.L.; Gillis, N.E.; Davidson, C.D.; Rodriguez, P.D.; Cozzens, L.; Tomczak, J.A.; Frietze, S.; Carr, F.E. Thyroid Hormone Receptor Beta Induces a Tumor-Suppressive Program in Anaplastic Thyroid Cancer. Mol. Cancer Res. 2020, 18, 1443–1452. [Google Scholar] [CrossRef]
- Rustad, J.L.; Gillis, N.E.; Lignos, J.; Bright, K.A.; Frietze, S.; Carr, F.E. Epigenomic Modulators and Thyroid Hormone Receptor β Agonists: A New Paradigm for Tumor Suppression in Thyroid Cancer. Endocrinology 2025, 166, bqaf116. [Google Scholar] [CrossRef]
- Nyström, H.F.; Jansson, S.; Berg, G. Incidence rate and clinical features of hyperthyroidism in a long-term iodine sufficient area of Sweden (Gothenburg) 2003–2005. Clin. Endocrinol. 2013, 78, 768–776. [Google Scholar] [CrossRef]
- Bartalena, L.; Fatourechi, V. Extrathyroidal manifestations of Graves’ disease: A 2014 update. J. Endocrinol. Investig. 2014, 37, 691–700. [Google Scholar] [CrossRef]
- Kraimps, J.L.; Bouin-Pineau, M.H.; Mathonnet, M.; De Calan, L.; Ronceray, J.; Visset, J.; Marechaud, R.; Barbier, J. Multicentre study of thyroid nodules in patients with Graves’ disease. Br. J. Surg. 2000, 87, 1111–1113. [Google Scholar] [CrossRef]
- Kirnap, N.; Anil, C.; Bozkus, Y. Graves’ disease does not pose an increased risk of thyroid cancer. Ann. Med. Res. 2019, 26, 2553. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Papanastasiou, A.; Sapalidis, K.; Goulis, D.G.; Michalopoulos, N.; Mareti, E.; Mantalovas, S.; Kesisoglou, I. Thyroid nodules as a risk factor for thyroid cancer in patients with Graves’ disease: A systematic review and meta-analysis of observational studies in surgically treated patients. Clin. Endocrinol. 2019, 91, 571–577. [Google Scholar] [CrossRef]
- Belfiore, A.; Garofalo, M.R.; Giuffrida, D.; Runello, F.; Filetti, S.; Fiumara, A.; Ippolito, O.; Vigneri, R. Increased aggressiveness of thyroid cancer in patients with Graves’ disease. J. Clin. Endocr. Metab. 1990, 70, 830–835. [Google Scholar] [CrossRef]
- Rees, S.B.; McLachlan, S.M.; Furmaniak, J. Autoantibodies to the thyrotropin receptor. Endocr. Rev. 1988, 9, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferri, E.L. Thyroid cancer and Graves’ disease. J. Clin. Endocr. Metab. 1990, 70, 826–829. [Google Scholar] [PubMed]
- Filetti, S.; Belfiore, A.; Amir, S.M.; Daniels, G.H.; Ippolito, O.; Vigneri, R.; Ingbar, S.H. The role of thyroid-stimulating antibodies of Graves’ disease in differentiated thyroid cancer. N. Engl. J. Med. 1988, 318, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Dias Lopes, N.M.; Mendonça Lens, H.H.; Armani, A.; Marinello, P.C.; Cecchini, A.L. Thyroid cancer and thyroid autoimmune disease: A review of molecular aspects and clinical outcomes. Pathol. Res. Pract. 2020, 216, 153098. [Google Scholar] [CrossRef]
- Jacobson, D.L.; Gange, S.J.; Rose, N.R.; Graham, N.M.H. Epidemiology and Estimated Population Burden of Selected Autoimmune Diseases in the United States. Clin. Immunol. Immunopathol. 1997, 84, 223–243. [Google Scholar] [CrossRef]
- Vanderpump, M.P.J. The epidemiology of thyroid disease. Brit Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Resende De Paiva, C.; Grønhøj, C.; Feldt-Rasmussen, U.; von Buchwald, C. Association between Hashimoto’s Thyroiditis and Thyroid Cancer in 64,628 Patients. Front. Oncol. 2017, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Targovnik, H.M.; Scheps, K.G.; Rivolta, C.M. Defects in protein folding in congenital hypothyroidism. Mol. Cell. Endocrinol. 2020, 501, 110638. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Rubio, I.; Brust, E.S.; Cazarin, J.; Hecht, F.; Alkmim, N.R.; Rajão, K.; Ramos, H.E. Congenital hypothyroidism and thyroid cancer. Endocr.-Relat. Cancer 2021, 28, R217–R230. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Dionigi, G.; Sun, H. The relationship between subclinical hypothyroidism and invasive papillary thyroid cancer. Front. Endocrinol. 2023, 14, 1294441. [Google Scholar] [CrossRef]
- McLeod, D.S.A.; Watters, K.F.; Carpenter, A.D.; Ladenson, P.W.; Cooper, D.S.; Ding, E.L. Thyrotropin and Thyroid Cancer Diagnosis: A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Endocr. Metab. 2012, 97, 2682–2692. [Google Scholar] [CrossRef]
- Hiasa, Y.; Kitahori, Y.; Kato, Y.; Ohshima, M.; Konishi, N.; Shimoyama, T.; Sakaguchi, Y.; Hshimoto, H.; Minami, S.; Murata, Y. Potassium Perchlorate, Potassium Iodide, and Propylthiouracil: Promoting Effect on the Development of Thyroid Tumors in Rats Treated with N-Bis(2-hydroxypropyl)nitrosamine. Jpn. J. Cancer Res. Gann 1987, 78, 1335–1340. [Google Scholar]
- Ringel, M.D.; Anderson, J.; Souza, S.L.; Tambascia, M.; Shriver, C.D.; Tuttle, R.M. Expression of the Sodium Iodide Symporter and Thyroglobulin Genes Are Reduced in Papillary Thyroid Cancer. Mod. Pathol. 2001, 14, 289–296. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Amdur, R.J. Essentials of Thyroid Cancer Management; Springer: New York, NY, USA, 2005. [Google Scholar]
- Biondi, B.; Cooper, D.S. Thyroid Hormone Suppression Therapy. Endocrin Metab. Clin. 2019, 48, 227–237. [Google Scholar] [CrossRef]
- Biondi, B.; Fazio, S.; Cuocolo, A.; Sabatini, D.; Nicolai, E.; Lombardi, G.; Salvatore, M.; Saccà, L. Impaired cardiac reserve and exercise capacity in patients receiving long-term thyrotropin suppressive therapy with levothyroxine. J. Clin. Endocr. Metab. 1996, 81, 4224–4228. [Google Scholar]
- Lamartina, L.; Durante, C.; Lucisano, G.; Grani, G.; Bellantone, R.; Lombardi, C.P.; Pontecorvi, A.; Arvat, E.; Felicetti, F.; Zatelli, M.C.; et al. Are Evidence-Based Guidelines Reflected in Clinical Practice? An Analysis of Prospectively Collected Data of the Italian Thyroid Cancer Observatory. Thyroid 2017, 27, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Do Cao, C.; Wémeau, J.L. Risk-benefit ratio for TSH- suppressive Levothyroxine therapy in differentiated thyroid cancer. Ann. Endocrinol.-Paris 2015, 76 (Suppl. 1), 1S–47S. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, N.; Gao, X.; Liang, J.; Fan, X.; Zhao, Y. Meta-analysis of TSH suppression therapy and the risk of cardiovascular events after thyroid cancer surgery. Front. Endocrinol. 2022, 13, 991876. [Google Scholar] [CrossRef] [PubMed]
- Ku, E.J.; Yoo, W.S.; Lee, E.K.; Ahn, H.Y.; Woo, S.H.; Hong, J.H.; Chung, H.K.; Park, J. Effect of TSH Suppression Therapy on Bone Mineral Density in Differentiated Thyroid Cancer: A Systematic Review and Meta-analysis. J. Clin. Endocr. Metab. 2021, 106, 3655–3667. [Google Scholar] [CrossRef]
- Park, H.; Park, J.; Yoo, H.; Kim, S.; Koh, J.H.; Jee, J.H.; Min, Y.; Chung, J.H.; Kim, T.H.; Kang, M.; et al. Bone-density testing interval and transition to osteoporosis in differentiated thyroid carcinoma patients on TSH suppression therapy. Clin. Endocrinol. 2022, 97, 130–136. [Google Scholar] [CrossRef]
- Wenter, V.; Jellinek, A.; Unterrainer, M.; Ahmaddy, F.; Lehner, S.; Albert, N.L.; Bartenstein, P.; Knösel, T.; Spitzweg, C.; Ilhan, H.; et al. Long-term outcome of rare oncocytic papillary (Hürthle cell) thyroid carcinoma following (adjuvant) initial radioiodine therapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2526–2535. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer-Am. Cancer Soc. 2017, 123, 372–381. [Google Scholar] [CrossRef]

| Gene | Effect | References |
|---|---|---|
| Promoting proliferation genes | ||
| CCND1 | ↑ | [50] |
| Apotosis | ||
| Bcl-2 | ↑ | [51] |
| XIAP | ↑ | [52] |
| Angiogenesis genes | ||
| VEGF | ↑ | [53] |
| bFGF | ↑ | [54] |
| PDGF | ↑ | [55] |
| HIF-1α | ↑ | [56] |
| NOS2 | ↑ | [57] |
| Tumor metastasis genes | ||
| SNAL1 | ↑ | [58] |
| MMPs | ↑ | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Liu, W.; Zheng, J.; Wu, Q.; Ai, Z. Molecular Mechanisms of Thyroid Hormone Signaling in Thyroid Cancer: Oncogenesis, Progression, and Therapeutic Implications. Biomedicines 2025, 13, 2552. https://doi.org/10.3390/biomedicines13102552
Zhou C, Liu W, Zheng J, Wu Q, Ai Z. Molecular Mechanisms of Thyroid Hormone Signaling in Thyroid Cancer: Oncogenesis, Progression, and Therapeutic Implications. Biomedicines. 2025; 13(10):2552. https://doi.org/10.3390/biomedicines13102552
Chicago/Turabian StyleZhou, Changhao, Wei Liu, Jiaojiao Zheng, Qiao Wu, and Zhilong Ai. 2025. "Molecular Mechanisms of Thyroid Hormone Signaling in Thyroid Cancer: Oncogenesis, Progression, and Therapeutic Implications" Biomedicines 13, no. 10: 2552. https://doi.org/10.3390/biomedicines13102552
APA StyleZhou, C., Liu, W., Zheng, J., Wu, Q., & Ai, Z. (2025). Molecular Mechanisms of Thyroid Hormone Signaling in Thyroid Cancer: Oncogenesis, Progression, and Therapeutic Implications. Biomedicines, 13(10), 2552. https://doi.org/10.3390/biomedicines13102552







