CAR-T Cell Therapy for Prostate Cancer: Current Advances and Future Perspectives
Abstract
1. Introduction
2. Current Immunotherapeutic Approaches for PCa
2.1. Immune Checkpoint Inhibitors
2.2. Bispecific Antibodies
2.3. Cancer Vaccines
2.4. Oncolytic Viruses
3. Chimeric Antigen Receptor T-Cells
3.1. Production of CAR-T Cells
3.2. Prostate Tumor Antigens as Target for CAR-T Therapy
3.2.1. Prostate-Specific Membrane Antigen (PSMA)
3.2.2. Prostate Stem Cell Antigen (PSCA)
3.2.3. Six-Transmembrane Epithelial Antigen of Prostate 1 (STEAP1)
3.2.4. Six-Transmembrane Epithelial Antigen of Prostate 2 (STEAP2)
3.2.5. Epithelial Cell Adhesion Molecule (EpCAM)
3.2.6. Kallikrein-Related Peptidase 2 (KLK2)
3.2.7. Type I Transmembrane Protein B7-H3 (CD276)
3.2.8. Non-Functional P2X Purinoceptor 7 (nfP2X7)
3.2.9. Natural Killer Group 2 Member D Ligand (NKG2DL)
3.2.10. F77 Antigen
4. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABD | Antigen-Binding Domain |
| ADAM17 | Disintegrin And Metalloproteinase 17 |
| ADC | Antibody–drug Conjugate |
| ADT | Androgen Deprivation Therapy |
| AE | Adverse Effect |
| APC | Antigen Presenting Cell |
| BBIR | Biotin-Binding Immune Receptor |
| BRCA | Breast Cancer |
| bsAb | bispecific Antibody |
| CAF | Cancer-Associated Fibroblast |
| CAR | Chimeric Antigen Receptor |
| CD | Cluster of Differentiation |
| CID | Chemical Inducer of Dimerization |
| CMPC | Castration-sensitive Metastatic Prostate Cancer |
| CRISPR/Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats/Caspase 9 |
| CRPC | Castration-Resistant Prostate Cancer |
| CRS | Cytokine Release Syndrome |
| CTC | Circulating Tumor Cell |
| CTLA-4 | Cytotoxic T Lymphocyte-Associated protein 4 |
| DAMP | Danger-Associated Molecular Pattern |
| DLT | Dose Limiting Toxicity |
| dMMR | Mismatch Repair deficiency |
| dnTGF-βRII | Dominant-Negative TGF-β Receptor II |
| DOR | Duration Of Response |
| ECM | ExtraCellular Matrix |
| EMT | Epithelial–Mesenchymal Transition |
| EpCAM | Epithelial Cell Adhesion Molecule |
| ERG | EST-Related Gene |
| GD2 | disialoganglioside |
| GM-CSF | Granulocyte Macrophage Colony-Stimulating Factor |
| GPI | GlycosylPhosphatidylInositol |
| HD | Hinge Domain |
| KLK | Kallikrein |
| KO | knockout |
| FcRn | neonatal Fc Receptor |
| ICAM-1 | InterCellular Adhesion Molecule-1 |
| ICI | Immune Checkpoint Inhibitor |
| ICOS | Inducible T Cell Costimulator |
| IL | interleukin |
| iMC | inducible Costimulatory Module |
| ISD | Intracellular Signaling Domain |
| ITAM | Immunoreceptor Tyrosine-based Activation Motif |
| LFA-3 | Leukocyte Function-associated Antigen-3 |
| mAb | monoclonal Antibody |
| MAS | Macrophage Activation Syndrome |
| mCRPC | metastatic Castration-Resistant Prostate Cancer |
| MDSC | Myeloid-Derived Suppressor Cell |
| mPCa | metastatic Prostate Cancer |
| MSI-H | MicroSatellite Instability-High |
| MTD | Maximum Tolerated Dose; |
| NFAT | Nuclear Factor of Activated T cells |
| NKG2DL | Natural Killer Group 2 Member D Ligand |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PAP | Prostatic Acid Phosphatase |
| PCa | Prostate Cancer |
| PCLA | Prostate Cancer Lipid Antigen |
| PD | Pharmacodynamics |
| PD-1 | Programmed cell Death protein-1 |
| PD-L1 | Programmed Death Ligand-1 |
| PFS | Progression-Free Survival |
| PK | Pharmacokinetics |
| PSA | Prostate-Specific Antigen |
| PSCA | Prostate Stem Cell Antigen |
| PSMA | Prostate-Specific Membrane Antigen |
| PTEN | Phosphatase and Tensin homolog |
| RB | RetinoBlastoma |
| RECIST | Response Evaluation Criteria In Solid Tumors |
| RhoC | Ras homolog gene family member C |
| RP2D | Recommended Phase 2 Dose |
| SAE | Serious Adverse Event |
| scFv | single-chain variable Fragment |
| STEAP | Six-Transmembrane Epithelial Antigen of Prostate |
| TAA | Tumor-Associated Antigen |
| TACE | TNF-α converting enzyme |
| TAM | Tumor-Associated Macrophage |
| TCR | T-Cell Receptor |
| TGF-β | Transforming Growth Factor-beta |
| TIL | Tumor-Infiltrating Lymphocyte |
| TM | targeting molecule |
| TMB | Tumor Mutational Burden |
| TMD | TransMembrane Domain |
| TME | Tumor MicroEnvironment |
| TMPRSS2 | 5′-Transmembrane Protein Serine Proteinase-2 |
| TNF | Tumor Necrosis Factor |
| TRAE | Treatment-Related Adverse Event |
| Treg | Regulatory T cell |
| TRUCK | T cells Redirected for Universal Cytokine Killing |
| TSCM | Stem Cell Memory T cell |
| TTR | Time To Response |
| VEGF | Vascular Endothelial Growth Factor |
References
- Mallah, H.; Diabasana, Z.; Soultani, S.; Idoux-Gillet, Y.; Massfelder, T. Prostate Cancer: A Journey Through Its History and Recent Developments. Cancers 2025, 17, 194. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, W.; Zhang, L.; Qu, Y.; Xu, Z.; Tan, Z.; Yan, P.; Tang, M.; Yang, C.; Wang, Y.; et al. Risk Factors for Prostate Cancer: An Umbrella Review of Prospective Observational Studies and Mendelian Randomization Analyses. PLoS Med. 2024, 21, e1004362. [Google Scholar] [CrossRef]
- Schafer, E.J.; Laversanne, M.; Sung, H.; Soerjomataram, I.; Briganti, A.; Dahut, W.; Bray, F.; Jemal, A. Recent Patterns and Trends in Global Prostate Cancer Incidence and Mortality: An Update. Eur. Urol. 2025, 87, 302–313. [Google Scholar] [CrossRef]
- Quigley, D.A.; Dang, H.X.; Zhao, S.G.; Lloyd, P.; Aggarwal, R.; Alumkal, J.J.; Foye, A.; Kothari, V.; Perry, M.D.; Bailey, A.M.; et al. Genomic Hallmarks and Structural Variation in Metastatic Prostate Cancer. Cell 2018, 174, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Montanari, M.; Rossetti, S.; Cavaliere, C.; D’Aniello, C.; Malzone, M.G.; Vanacore, D.; Di Franco, R.; La Mantia, E.; Iovane, G.; Piscitelli, R.; et al. Epithelial-mesenchymal Transition in Prostate Cancer: An Overview. Oncotarget 2017, 8, 35376–35389. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.T.W.; Bryant, R.J.; Parkes, E.E. The Tumor Microenvironment and Immune Responses in Prostate Cancer Patients. Endocr. Relat. Cancer 2021, 28, T95–T107. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Jalloul, M.; Azar, J.; Moubarak, M.M.; Samad, T.A.; Mukherji, D.; Al-Sayegh, M.; Abou-Kheir, W. Tumor Microenvironment in Prostate Cancer: Toward Identification of Novel Molecular Biomarkers for Diagnosis, Prognosis, and Therapy Development. Front. Genet. 2021, 12, 652747. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A Framework for Advancing our Understanding of Cancer-associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Levesque, C.; Nelson, P.S. Cellular Constituents of the Prostate Stroma: Key Contributors to Prostate Cancer Progression and Therapy Resistance. Cold Spring Harb. Perspect. Med. 2018, 8, a030510. [Google Scholar] [CrossRef]
- Fang, B.; Lu, Y.; Li, X.; Wei, Y.; Ye, D.; Wei, G.; Zhu, Y. Targeting the Tumor Microenvironment, a New Therapeutic Approach for Prostate Cancer. Prostate Cancer Prostatic Dis. 2024, 28, 260–269. [Google Scholar] [CrossRef]
- Stark, T.; Livas, L.; Kyprianou, N. Inflammation in Prostate Cancer Progression and Therapeutic Targeting. Transl. Androl. Urol. 2015, 4, 455–463. [Google Scholar] [CrossRef]
- Ene, C.; Nicolae, I.; Ene, C.D. Angiogenic Systemic Response to the Hypoxic Microenvironment in Prostate Tumorigenesis: A Pilot Study. Exp. Ther. Med. 2023, 26, 483. [Google Scholar] [CrossRef]
- Ge, R.; Wang, Z.; Cheng, L. Tumor Microenvironment Heterogeneity an Important Mediator of Prostate Cancer Progression and Therapeutic Resistance. NPJ Precis. Oncol. 2022, 6, 31. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, S.; Takata, R.; Obara, W. Molecular Mechanisms of Prostate Cancer Development in the Precision Medicine Era: A Comprehensive Review. Cancers 2024, 16, 523. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Hess, J.; Yamada, Y.; Ku, S.Y.; Beltran, H. Epigenetics in Prostate Cancer: Clinical Implications. Transl. Androl. Urol. 2021, 10, 3104–3116. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, G.L.; Gupta, V.K.; Prasad, K.; Kim, E.; Tej, M.B.; Mohanty, P.; Verma, H.K.; Raju, G.S.R.; Bhaskar, L.; Huh, Y.S. Recent Advances and Future Perspectives in the Therapeutics of Prostate Cancer. Exp. Hematol. Oncol. 2023, 12, 80. [Google Scholar] [CrossRef]
- Achard, V.; Panje, C.M.; Engeler, D.; Zilli, T.; Putora, P.M. Localized and Locally Advanced Prostate Cancer: Treatment Options. Oncology 2021, 99, 413–421. [Google Scholar] [CrossRef]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Yu, E.M.; Aragon-Ching, J.B. Advances with Androgen Deprivation Therapy for Prostate Cancer. Expert Opin. Pharmacother. 2022, 23, 1015–1033. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Li, D.; Wu, R.; Huang, J.; Ye, L.; Tuo, Z.; Yu, Q.; Shao, F.; Wusiman, D.; et al. Novel Hormone Therapies for Advanced Prostate Cancer: Understanding and Countering Drug Resistance. J. Pharm. Anal. 2025, 15, 101232. [Google Scholar] [CrossRef]
- Liu, D.; Wang, L.; Guo, Y. Advances in and Prospects of Immunotherapy for Prostate Cancer. Cancer Lett. 2024, 601, 217155. [Google Scholar] [CrossRef]
- Tang, L.; Huang, Z.; Mei, H.; Hu, Y. Immunotherapy in hematologic malignancies: Achievements, challenges and future prospects. Signal Transduct. Target. Ther. 2023, 8, 306. [Google Scholar] [CrossRef]
- Pham, T.; Roth, S.; Kong, J.; Guerra, G.; Narasimhan, V.; Pereira, L.; Desai, J.; Heriot, A.; Ramsay, R. An Update on Immunotherapy for Solid Tumors: A Review. Ann. Surg. Oncol. 2018, 25, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Taefehshokr, S.; Parhizkar, A.; Hayati, S.; Mousapour, M.; Mahmoudpour, A.; Eleid, L.; Rahmanpour, D.; Fattahi, S.; Shabani, H.; Taefehshokr, N. Cancer Immunotherapy: Challenges and Limitations. Pathol. Res. Pract. 2022, 229, 153723. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.; Denlinger, N.; Yang, Y. Recent Advances and Challenges in Cancer Immunotherapy. Cancers 2022, 14, 3972. [Google Scholar] [CrossRef]
- Runcie, K.D.; Dallos, M.C. Prostate Cancer Immunotherapy-Finally in From the Cold? Curr. Oncol. Rep. 2021, 23, 88. [Google Scholar] [CrossRef]
- Khosravi, G.R.; Mostafavi, S.; Bastan, S.; Ebrahimi, N.; Gharibvand, R.S.; Eskandari, N. Immunologic Tumor Microenvironment Modulators for Turning Cold Tumors Hot. Cancer Commun. 2024, 44, 521–553. [Google Scholar] [CrossRef]
- Bandara, S.; Raveendran, S. Current Landscape and Future Directions in Cancer Immunotherapy: Therapies, Trials, and Challenges. Cancers 2025, 17, 821. [Google Scholar] [CrossRef]
- Desai, I.; Thakur, S.; Pagariya, P. Current Advances in Immunotherapy for Cancer. Oral Oncol. Rep. 2024, 12, 100652. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Y.; Mortazavi, A.; Zhang, J. Emerging Immunotherapy Approaches for Treating Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 14347. [Google Scholar] [CrossRef] [PubMed]
- Castle, J.C.; Uduman, M.; Pabla, S.; Stein, R.B.; Buell, J.S. Mutation-Derived Neoantigens for Cancer Immunotherapy. Front. Immunol. 2019, 10, 1856. [Google Scholar] [CrossRef] [PubMed]
- Vitkin, N.; Nersesian, S.; Siemens, D.R.; Koti, M. The Tumor Immune Contexture of Prostate Cancer. Front. Immunol. 2019, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Palazon, A.; Noguera-Ortega, E.; Powell, D.J., Jr.; Guedan, S. CAR-T Cells Hit the Tumor Microenvironment: Strategies to Overcome Tumor Escape. Front. Immunol. 2020, 11, 1109. [Google Scholar] [CrossRef]
- Fay, A.P.; Antonarakis, E.S. Blocking the PD-1/PD-L1 Axis in Advanced Prostate Cancer: Are we Moving in the Right Direction? Ann. Transl. Med. 2019, 7, S7. [Google Scholar] [CrossRef] [PubMed]
- Claps, M.; Mennitto, A.; Guadalupi, V.; Sepe, P.; Stellato, M.; Zattarin, E.; Gillessen, S.S.; Sternberg, C.N.; Berruti, A.; De Braud, F.G.M.; et al. Immune-checkpoint Inhibitors and Metastatic Prostate Cancer Therapy: Learning by Making Mistakes. Cancer Treat. Rev. 2020, 88, 102057. [Google Scholar] [CrossRef]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Subudhi, S.K.; Vence, L.; Zhao, H.; Blando, J.; Yadav, S.S.; Xiong, Q.; Reuben, A.; Aparicio, A.; Corn, P.G.; Chapin, B.F.; et al. Neoantigen Responses, Immune Correlates, and Favorable Outcomes After Ipilimumab Treatment of Patients with Prostate Cancer. Sci. Transl. Med. 2020, 12, eaaz3577. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab Versus Placebo After Radiotherapy in Patients with Metastatic Castration-Resistant Prostate Cancer that Had Progressed After Docetaxel Chemotherapy (CA184-043): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.P.; Aggarwal, R.; et al. Pembrolizumab for Advanced Prostate Adenocarcinoma: Findings of the KEYNOTE-028 Study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.C.; Vaishampayan, U.N.; De Wit, R.; Alanko, T.V.; Fukasawa, S.; Tabata, K.; Feyerabend, S. 611P Pembrolizumab (Pembro) Monotherapy for Docetaxel-Petreated Metastatic Castration-Resistant Prostate Cancer (mCRPC): Updated Analyses with 4 Years of Follow-up from Cohorts 1-3 of the KEYNOTE-199 Study. Ann. Oncol. 2021, 32, S651–S652. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qin, Y.; Li, B. CD8(+) T Cell Exhaustion and Cancer Immunotherapy. Cancer Lett. 2023, 559, 216043. [Google Scholar] [CrossRef]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Flechon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Yoon, D.H.; Osborn, M.J.; Tolar, J.; Kim, C.J. Incorporation of Immune Checkpoint Blockade into Chimeric Antigen Receptor T Cells (CAR-Ts): Combination or Built-In CAR-T. Int. J. Mol. Sci. 2018, 19, 340. [Google Scholar] [CrossRef]
- Heitmann, J.S.; Pfluegler, M.; Jung, G.; Salih, H.R. Bispecific Antibodies in Prostate Cancer Therapy: Current Status and Perspectives. Cancers 2021, 13, 549. [Google Scholar] [CrossRef]
- Brinkmann, U.; Kontermann, R.E. The Making of Bispecific Antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef]
- Lee, S.C.; Ma, J.S.Y.; Kim, M.S.; Laborda, E.; Choi, S.H.; Hampton, E.N.; Yun, H.; Nunez, V.; Muldong, M.T.; Wu, C.N.; et al. A PSMA-targeted Bispecific Antibody for Prostate Cancer Driven by a Small-molecule Targeting Ligand. Sci. Adv. 2021, 7, eabi8193. [Google Scholar] [CrossRef]
- Miyahira, A.K.; Soule, H.R. The 27th Annual Prostate Cancer Foundation Scientific Retreat Report. Prostate 2021, 81, 1107–1124. [Google Scholar] [CrossRef]
- Hummel, H.D.; Kufer, P.; Grullich, C.; Seggewiss-Bernhardt, R.; Deschler-Baier, B.; Chatterjee, M.; Goebeler, M.E.; Miller, K.; de Santis, M.; Loidl, W.; et al. Pasotuxizumab, a BiTE((R)) Immune Therapy for Castration-resistant Prostate Cancer: Phase I, Dose-escalation Study Findings. Immunotherapy 2021, 13, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.S.; Walz, J.S.; Pflugler, M.; Kauer, J.; Schlenk, R.F.; Jung, G.; Salih, H.R. Protocol of a Prospective, Multicentre Phase I Study to Evaluate the Safety, Tolerability and Preliminary Efficacy of the Bispecific PSMAxCD3 Antibody CC-1 in Patients with Castration-Resistant Prostate Carcinoma. BMJ Open 2020, 10, e039639. [Google Scholar] [CrossRef] [PubMed]
- Dorff, T.; Horvath, L.G.; Autio, K.; Bernard-Tessier, A.; Rettig, M.B.; Machiels, J.P.; Bilen, M.A.; Lolkema, M.P.; Adra, N.; Rottey, S.; et al. A Phase I Study of Acapatamab, a Half-life Extended, PSMA-Targeting Bispecific T-cell Engager for Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2024, 30, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04631601 (accessed on 5 May 2025).
- Lund, M.E.; Howard, C.B.; Thurecht, K.J.; Campbell, D.H.; Mahler, S.M.; Walsh, B.J. A Bispecific T Cell Engager Targeting Glypican-1 Redirects T Cell Cytolytic Activity to Kill Prostate Cancer Cells. BMC Cancer 2020, 20, 1214. [Google Scholar] [CrossRef]
- Yamamoto, K.; Trad, A.; Baumgart, A.; Huske, L.; Lorenzen, I.; Chalaris, A.; Grotzinger, J.; Dechow, T.; Scheller, J.; Rose-John, S. A Novel Bispecific Single-chain Antibody for ADAM17 and CD3 Induces T-cell-mediated Lysis of Prostate Cancer Cells. Biochem. J. 2012, 445, 135–144. [Google Scholar] [CrossRef]
- Feldmann, A.; Arndt, C.; Topfer, K.; Stamova, S.; Krone, F.; Cartellieri, M.; Koristka, S.; Michalk, I.; Lindemann, D.; Schmitz, M.; et al. Novel Humanized and Highly Efficient Bispecific Antibodies Mediate Killing of Prostate Stem Cell Antigen-expressing Tumor Cells by CD8+ and CD4+ T Cells. J. Immunol. 2012, 189, 3249–3259. [Google Scholar] [CrossRef]
- Stein, M.N.; Graham, L.S.; Baldini, C.; Vinceneux, A.; Kessler, E.; Runcie, K.; Wei, A.Z.; Papadopoulos, K.P.; Bernard-Tessier, A.; Laurent, M.; et al. A Phase I, First-in-human, Open-label, Multicenter, Trial-in-progress of the Safety, Tolerability, and Preliminary Efficacy of JNJ-87189401 (PSMA-CD28 Bispecific Antibody) Combined with JNJ-78278343 (KLK2-CD3 Bispecific Antibody) for Advanced Prostate Cancer. Ann. Oncol. 2024, 35, S1000. [Google Scholar] [CrossRef]
- Kelly, W.K.; Danila, D.C.; Lin, C.C.; Lee, J.L.; Matsubara, N.; Ward, P.J.; Armstrong, A.J.; Pook, D.; Kim, M.; Dorff, T.B.; et al. Xaluritamig, a STEAP1 x CD3 XmAb 2+1 Immune Therapy for Metastatic Castration-Resistant Prostate Cancer: Results from Dose Exploration in a First-in-Human Study. Cancer Discov. 2024, 14, 76–89. [Google Scholar] [CrossRef]
- Sam, J.; Colombetti, S.; Fauti, T.; Roller, A.; Biehl, M.; Fahrni, L.; Nicolini, V.; Perro, M.; Nayak, T.; Bommer, E.; et al. Combination of T-Cell Bispecific Antibodies with PD-L1 Checkpoint Inhibition Elicits Superior Anti-Tumor Activity. Front. Oncol. 2020, 10, 575737. [Google Scholar] [CrossRef]
- Kobold, S.; Pantelyushin, S.; Rataj, F.; Vom Berg, J. Rationale for Combining Bispecific T Cell Activating Antibodies With Checkpoint Blockade for Cancer Therapy. Front. Oncol. 2018, 8, 285. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer Vaccines as Promising Immuno-therapeutics: Platforms and Current Progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Handy, C.E.; Antonarakis, E.S. Sipuleucel-T for the Treatment of Prostate Cancer: Novel Insights and Future Directions. Future Oncol. 2018, 14, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Muniyan, S.; Chaturvedi, N.K.; Dwyer, J.G.; Lagrange, C.A.; Chaney, W.G.; Lin, M.F. Human Prostatic Acid Phosphatase: Structure, Function and Regulation. Int. J. Mol. Sci. 2013, 14, 10438–10464. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Fratesi, P.; Reese, D.M.; Strang, G.; Laus, R.; Peshwa, M.V.; Valone, F.H. Immunotherapy of Hormone-refractory Prostate Cancer with Antigen-loaded Dendritic Cells. J. Clin. Oncol. 2000, 18, 3894–3903. [Google Scholar] [CrossRef]
- Peshwa, M.V.; Shi, J.D.; Ruegg, C.; Laus, R.; van Schooten, W.C.A. Induction of Prostate Tumor-specific CD8+ Cytotoxic T-lymphocytes in Vitro Using Antigen-presenting Cells Pulsed with Prostatic Acid Phosphatase Peptide. Prostate 1998, 36, 129–138. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S.; Small, E.J.; Schellhammer, P.; Yasothan, U.; Gubernick, S.; Kirkpatrick, P.; Kantoff, P.W. Sipuleucel-T. Nat. Rev. Drug Discov. 2010, 9, 513–514. [Google Scholar] [CrossRef]
- Hall, S.J.; Klotz, L.; Pantuck, A.J.; George, D.J.; Whitmore, J.B.; Frohlich, M.W.; Sims, R.B. Integrated Safety Data From 4 Randomized, Double-Blind, Controlled Trials of Autologous Cellular Immunotherapy with Sipuleucel-T in Patients with Prostate Cancer. J. Urol. 2011, 186, 877–881. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/provenge-sipuleucel-t (accessed on 26 May 2025).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/overview/provenge-epar-summary-public_en.pdf (accessed on 20 May 2025).
- Zhou, W.; Lu, X.; Tian, F.; Luo, Q.; Zhou, W.; Yang, S.; Li, W.; Yang, Y.; Shi, M.; Zhou, T. Vaccine Therapies for Prostate Cancer: Current Status and Future Outlook. Vaccines 2024, 12, 1384. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Busch, H.; Boerries, M.; Brummer, T.; Timme, S.; Lassmann, S.; Aktories, K.; Schmidt, G. Specific Role of RhoC in Tumor Invasion and Metastasis. Oncotarget 2017, 8, 87364–87378. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Heidu, S.; Balchen, T.; Richardson, J.R.; Schmeltz, C.; Sonne, J.; Schweiker, J.; Rammensee, H.G.; Thor Straten, P.; Roder, M.A.; et al. Vaccination Against RhoC Induces Long-lasting Immune Responses in Patients with Prostate Cancer: Results from a Phase I/II Clinical Trial. J. Immunother. Cancer 2020, 8, e001157. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pal, S.K.; Alex, A.; Agarwal, N. Development of PROSTVAC Immunotherapy in Prostate Cancer. Future Oncol. 2015, 11, 2137–2148. [Google Scholar] [CrossRef]
- Sharp, D.W.; Lattime, E.C. Recombinant Poxvirus and the Tumor Microenvironment: Oncolysis, Immune Regulation and Immunization. Biomedicines 2016, 4, 19. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Gulley, J.L.; Pico-Navarro, C. Revised Overall Survival Analysis of a Phase II, Randomized, Double-Blind, Controlled Study of PROSTVAC in Men with Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Borre, M.; Vogelzang, N.J.; Ng, S.; Agarwal, N.; Parker, C.C.; Pook, D.W.; Rathenborg, P.; Flaig, T.W.; Carles, J.; et al. Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- McNeel, D.G.; Eickhoff, J.C.; Johnson, L.E.; Roth, A.R.; Perk, T.G.; Fong, L.; Antonarakis, E.S.; Wargowski, E.; Jeraj, R.; Liu, G. Phase II Trial of a DNA Vaccine Encoding Prostatic Acid Phosphatase (pTVG-HP [MVI-816]) in Patients With Progressive, Nonmetastatic, Castration-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef]
- Melcher, A.; Parato, K.; Rooney, C.M.; Bell, J.C. Thunder and Lightning: Immunotherapy and Oncolytic Viruses Collide. Mol. Ther. 2011, 19, 1008–1016. [Google Scholar] [CrossRef]
- Sweeney, K.; Hallden, G. Oncolytic Adenovirus-mediated Therapy for Prostate Cancer. Oncolytic Virother. 2016, 5, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, S.; Luo, Z. Oncolytic Adenovirus, a New Treatment Strategy for Prostate Cancer. Biomedicines 2022, 10, 3262. [Google Scholar] [CrossRef]
- Freytag, S.O.; Stricker, H.; Peabody, J.; Pegg, J.; Paielli, D.; Movsas, B.; Barton, K.N.; Brown, S.L.; Lu, M.; Kim, J.H. Five-year Follow-up of Trial of Replication-competent Adenovirus-mediated Suicide Gene Therapy for Treatment of Prostate Cancer. Mol. Ther. 2007, 15, 636–642. [Google Scholar] [CrossRef]
- Freytag, S.O.; Barton, K.N.; Zhang, Y. Efficacy of Oncolytic Adenovirus Expressing Suicide Genes and Interleukin-12 in Preclinical Model of Prostate Cancer. Gene Ther. 2013, 20, 1131–1139. [Google Scholar] [CrossRef]
- Freytag, S.O.; Stricker, H.; Lu, M.; Elshaikh, M.; Aref, I.; Pradhan, D.; Levin, K.; Kim, J.H.; Peabody, J.; Siddiqui, F.; et al. Prospective Randomized Phase 2 Trial of Intensity Modulated Radiation Therapy With or Without Oncolytic Adenovirus-mediated Cytotoxic Gene Therapy in Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 268–276. [Google Scholar] [CrossRef]
- Ponterio, E.; Haas, T.L.; De Maria, R. Oncolytic Virus and CAR-T Cell Therapy in Solid Tumors. Front. Immunol. 2024, 15, 1455163. [Google Scholar] [CrossRef] [PubMed]
- Nyati, S.; Stricker, H.; Barton, K.N.; Li, P.; Elshaikh, M.; Ali, H.; Brown, S.L.; Hwang, C.; Peabody, J.; Freytag, S.O.; et al. A Phase I Clinical Trial of Oncolytic Adenovirus Mediated Suicide and Interleukin-12 Gene Therapy in Patients with Recurrent Localized Prostate Adenocarcinoma. PLoS ONE 2023, 18, e0291315. [Google Scholar] [CrossRef]
- Brachtlova, T.; Abramovitch, A.; Giddens, J.; Incze, P.; Jansz, K.; Casey, R.; Beusechem, V.v.; Dong, W. Clinical Results from a Phase I Dose Escalation Study in Treatment-naïve Early Stage Prostate Cancer Patients with ORCA-010, a Potency Enhanced Oncolytic Replication Competent Adenovirus. J. Immunotherap. Cancer 2021, 9, A1004. [Google Scholar] [CrossRef]
- Sun, D.; Shi, X.; Li, S.; Wang, X.; Yang, X.; Wan, M. CAR-T Cell Therapy: A Breakthrough in Traditional Cancer Treatment Strategies (Review). Mol. Med. Rep. 2024, 29, 47. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Riviere, I.; Park, J.H.; Davila, M.L.; Wang, X.; Stefanski, J.; Taylor, C.; Yeh, R.; Bartido, S.; Borquez-Ojeda, O.; et al. Safety and Persistence of Adoptively Transferred Autologous CD19-Targeted T Cells in Patients with Relapsed or Chemotherapy Refractory B-Cell Leukemias. Blood 2011, 118, 4817–4828. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric Antigen Receptor-modified T Cells for Acute Lymphoid Leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef]
- Nagarsheth, N.B.; Norberg, S.M.; Sinkoe, A.L.; Adhikary, S.; Meyer, T.J.; Lack, J.B.; Warner, A.C.; Schweitzer, C.; Doran, S.L.; Korrapati, S.; et al. TCR-engineered T Cells Targeting E7 for Patients with Metastatic HPV-associated Epithelial Cancers. Nat. Med. 2021, 27, 419–425. [Google Scholar] [CrossRef]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Giorgioni, L.; Ambrosone, A.; Cometa, M.F.; Salvati, A.L.; Magrelli, A. CAR-T State of the Art and Future Challenges, a Regulatory Perspective. Int. J. Mol. Sci. 2023, 24, 11803. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Maus, M.V.; Hinrichs, C.S. CAR T Cells and T-Cell Therapies for Cancer: A Translational Science Review. JAMA 2024, 332, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Calderon, H.; Mamonkin, M.; Guedan, S. Analysis of CAR-Mediated Tonic Signaling. Methods Mol. Biol. 2020, 2086, 223–236. [Google Scholar] [CrossRef]
- Stoiber, S.; Cadilha, B.L.; Benmebarek, M.R.; Lesch, S.; Endres, S.; Kobold, S. Limitations in the Design of Chimeric Antigen Receptors for Cancer Therapy. Cells 2019, 8, 472. [Google Scholar] [CrossRef]
- Xie, Y.J.; Dougan, M.; Jailkhani, N.; Ingram, J.; Fang, T.; Kummer, L.; Momin, N.; Pishesha, N.; Rickelt, S.; Hynes, R.O.; et al. Nanobody-based CAR T Cells that Target the Tumor Microenvironment Inhibit the Growth of Solid Tumors in Immunocompetent Mice. Proc. Natl. Acad. Sci. USA 2019, 116, 7624–7631. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Gao, Q.; Li, L.L.; Han, L.; Zhang, B.; Ding, Y.; Song, Z.; Zhang, R.; Zhang, J.; Wu, X.H. The Application of Nanobody in CAR-T Therapy. Biomolecules 2021, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Guest, R.D.; Hawkins, R.E.; Kirillova, N.; Cheadle, E.J.; Arnold, J.; O’Neill, A.; Irlam, J.; Chester, K.A.; Kemshead, J.T.; Shaw, D.M.; et al. The Role of Extracellular Spacer Regions in the Optimal Design of Chimeric Immune Receptors: Evaluation of Four Different scFvs and Antigens. J. Immunother. 2005, 28, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hudecek, M.; Sommermeyer, D.; Kosasih, P.L.; Silva-Benedict, A.; Liu, L.; Rader, C.; Jensen, M.C.; Riddell, S.R. The Nonsignaling Extracellular Spacer Domain of Chimeric Antigen Receptors is Decisive for in Vivo Antitumor Activity. Cancer Immunol. Res. 2015, 3, 125–135. [Google Scholar] [CrossRef]
- Hombach, A.; Hombach, A.A.; Abken, H. Adoptive Immunotherapy with Genetically Engineered T Cells: Modification of the Igg1 Fc ‘Spacer’ Domain in the Extracellular Moiety of Chimeric Antigen Receptors Avoids ‘Off-Target’ Activation and Unintended Initiation of an Innate Immune Response. Gene Ther. 2010, 17, 1206–1213. [Google Scholar] [CrossRef]
- Guedan, S.; Calderon, H.; Posey, A.D., Jr.; Maus, M.V. Engineering and Design of Chimeric Antigen Receptors. Mol. Ther. Methods Clin. Dev. 2019, 12, 45–156. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhong, J.F.; Zhang, X. Engineering CAR-T Cells. Biomark. Res. 2017, 5, 22. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- El-Daly, S.M.; Hussein, J. Genetically Engineered CAR T-immune Cells for Cancer Therapy: Recent Clinical Developments, Challenges, and Future Directions. J. Appl. Biomed. 2019, 17, 11. [Google Scholar] [CrossRef]
- Mazinani, M.; Rahbarizadeh, F. CAR-T Cell Potency: From Structural Elements to Vector Backbone Components. Biomark. Res. 2022, 10, 70. [Google Scholar] [CrossRef]
- Jayaraman, J.; Mellody, M.P.; Hou, A.J.; Desai, R.P.; Fung, A.W.; Pham, A.H.T.; Chen, Y.Y.; Zhao, W. CAR-T Design: Elements and their Synergistic Function. EBioMedicine 2020, 58, 102931. [Google Scholar] [CrossRef] [PubMed]
- Weinkove, R.; George, P.; Dasyam, N.; McLellan, A.D. Selecting Costimulatory Domains for Chimeric Antigen Receptors: Functional and Clinical Considerations. Clin. Transl. Immunol. 2019, 8, e1049. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, Y.; Asakura, Y.; Utsunomiya, N.; Nakanishi, M.; Arata, Y.; Itoh, S.; Nagase, F.; Kurosawa, Y. Expression of Chimeric Receptor Composed of Immunoglobulin-Derived V Regions and T-Cell Receptor-Derived C Regions. Biochem. Biophys. Res. Commun. 1987, 149, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Thistlethwaite, F.C.; Gilham, D.E.; Guest, R.D.; Rothwell, D.G.; Pillai, M.; Burt, D.J.; Byatte, A.J.; Kirillova, N.; Valle, J.W.; Sharma, S.K.; et al. The Clinical Efficacy of First-generation Carcinoembryonic Antigen (CEACAM5)-specific CAR T Cells is Limited by Poor Persistence and Transient Pre-conditioning-dependent Respiratory Toxicity. Cancer Immunol. Immunother. 2017, 66, 1425–1436. [Google Scholar] [CrossRef]
- van der Stegen, S.J.; Hamieh, M.; Sadelain, M. The Pharmacology of Second-generation Chimeric Antigen Receptors. Nat. Rev. Drug Discov. 2015, 14, 499–509. [Google Scholar] [CrossRef]
- Huang, R.; Li, X.; He, Y.; Zhu, W.; Gao, L.; Liu, Y.; Gao, L.; Wen, Q.; Zhong, J.F.; Zhang, C.; et al. Recent Advances in CAR-T Cell Engineering. J. Hematol. Oncol. 2020, 13, 86. [Google Scholar] [CrossRef]
- Singh, N.; Frey, N.V.; Engels, B.; Barrett, D.M.; Shestova, O.; Ravikumar, P.; Cummins, K.D.; Lee, Y.G.; Pajarillo, R.; Chun, I.; et al. Antigen-independent Activation Enhances the Efficacy of 4-1BB-costimulated CD22 CAR T Cells. Nat. Med. 2021, 27, 842–850. [Google Scholar] [CrossRef]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef]
- Acuto, O.; Michel, F. CD28-Mediated Co-Stimulation: A Quantitative Support for TCR Signalling. Nat. Rev. Immunol. 2003, 3, 939–951. [Google Scholar] [CrossRef]
- Hutloff, A.; Dittrich, A.M.; Beier, K.C.; Eljaschewitsch, B.; Kraft, R.; Anagnostopoulos, I.; Kroczek, R.A. ICOS is an Inducible T-Cell Co-stimulator Structurally and Functionally Related to CD28. Nature 1999, 397, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Finney, H.M.; Akbar, A.N.; Lawson, A.D. Activation of Resting Human Primary T Cells With Chimeric Receptors: Costimulation From CD28, Inducible Costimulator, CD134, and CD137 in Series With Signals From the TCR Zeta Chain. J. Immunol. 2004, 172, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Croft, M. The Role of TNF Superfamily Members in T-cell Function and Diseases. Nat. Rev. Immunol. 2009, 9, 271–285. [Google Scholar] [CrossRef]
- Guedan, S.; Posey, A.D., Jr.; Shaw, C.; Wing, A.; Da, T.; Patel, P.R.; McGettigan, S.E.; Casado-Medrano, V.; Kawalekar, O.U.; Uribe-Herranz, M.; et al. Enhancing CAR T Cell Persistence Through ICOS and 4-1BB Costimulation. JCI Insight 2018, 3, e96976. [Google Scholar] [CrossRef]
- van den Berg, J.; Laubli, H.; Khanna, N.; Jeker, L.T.; Holbro, A. Basic Concepts and Indications of CAR T Cells. Hamostaseologie 2025, 45, 14–23. [Google Scholar] [CrossRef]
- Zhong, X.S.; Matsushita, M.; Plotkin, J.; Riviere, I.; Sadelain, M. Chimeric Antigen Receptors Combining 4-1BB and CD28 Signaling Domains Augment PI3kinase/AKT/Bcl-XL Activation and CD8+ T Cell-mediated Tumor Eradication. Mol. Ther. 2010, 18, 413–420. [Google Scholar] [CrossRef]
- Tang, L.; Pan, S.; Wei, X.; Xu, X.; Wei, Q. Arming CAR-T Cells with Cytokines and More: Innovations in the Fourth-generation CAR-T Development. Mol. Ther. 2023, 31, 3146–3162. [Google Scholar] [CrossRef]
- Boettcher, M.; Joechner, A.; Li, Z.; Yang, S.F.; Schlegel, P. Development of CAR T Cell Therapy in Children-A Comprehensive Overview. J. Clin. Med. 2022, 11, 2158. [Google Scholar] [CrossRef]
- Hawkins, E.R.; D’Souza, R.R.; Klampatsa, A. Armored CAR T-Cells: The Next Chapter in T-Cell Cancer Immunotherapy. Biologics 2021, 15, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Abate-Daga, D.; Davila, M.L. CAR Models: Next-Generation CAR Modifications for Enhanced T-Cell Function. Mol. Ther. Oncol. 2016, 3, 16014. [Google Scholar] [CrossRef]
- Khan, S.H.; Choi, Y.; Veena, M.; Lee, J.K.; Shin, D.S. Advances in CAR T Cell Therapy: Antigen Selection, Modifications, and Current Trials for Solid Tumors. Front. Immunol. 2024, 15, 1489827. [Google Scholar] [CrossRef]
- Peng, L.; Sferruzza, G.; Yang, L.; Zhou, L.; Chen, S. CAR-T and CAR-NK as Cellular Cancer Immunotherapy for Solid Tumors. Cell. Mol. Immunol. 2024, 21, 1089–1108. [Google Scholar] [CrossRef] [PubMed]
- Kloss, C.C.; Lee, J.; Zhang, A.; Chen, F.; Melenhorst, J.J.; Lacey, S.F.; Maus, M.V.; Fraietta, J.A.; Zhao, Y.; June, C.H. Dominant-Negative TGF-beta Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation And Augments Prostate Cancer Eradication. Mol. Ther. 2018, 26, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, T.; Li, X.; Zhang, H.; Li, Y.; Zhang, S.; Luo, S.; Zheng, T. Therapeutic Targets of Armored Chimeric Antigen Receptor T Cells Navigating the Tumor Microenvironment. Exp. Hematol. Oncol. 2024, 13, 96. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, X.; Xie, Z.; Qiu, J.; Yang, J.; Deng, Y.; Long, R.; Tang, G.; Zhang, C.; Zuo, J. Prospects and Challenges of CAR-T Cell Therapy Combined with ICIs. Front. Oncol. 2024, 14, 1368732. [Google Scholar] [CrossRef]
- Rafiq, S.; Yeku, O.O.; Jackson, H.J.; Purdon, T.J.; van Leeuwen, D.G.; Drakes, D.J.; Song, M.; Miele, M.M.; Li, Z.; Wang, P.; et al. Targeted Delivery of a PD-1-Blocking scFv by CAR-T Cells Enhances Anti-tumor Efficacy in vivo. Nat. Biotechnol. 2018, 36, 847–856. [Google Scholar] [CrossRef]
- Fang, J.; Ding, N.; Guo, X.; Sun, Y.; Zhang, Z.; Xie, B.; Li, Z.; Wang, H.; Mao, W.; Lin, Z.; et al. alphaPD-1-mesoCAR-T Cells Partially Inhibit the Growth of Advanced/Refractory Ovarian Cancer in a Patient Along With Daily Apatinib. J. Immunotherap. Cancer 2021, 9, e001162. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Abken, H. TRUCKs: The Fourth Generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Abrantes, R.; Duarte, H.O.; Gomes, C.; Walchli, S.; Reis, C.A. CAR-Ts: New Perspectives in Cancer Therapy. FEBS Lett. 2022, 596, 403–416. [Google Scholar] [CrossRef]
- Kagoya, Y.; Tanaka, S.; Guo, T.; Anczurowski, M.; Wang, C.H.; Saso, K.; Butler, M.O.; Minden, M.D.; Hirano, N. A Novel Chimeric Antigen Receptor Containing a JAK-STAT Signaling Domain Mediates Superior Antitumor Effects. Nat. Med. 2018, 24, 352–359. [Google Scholar] [CrossRef]
- Kim, D.W.; Cho, J.Y. Recent Advances in Allogeneic CAR-T Cells. Biomolecules 2020, 10, 263. [Google Scholar] [CrossRef]
- Hiltensperger, M.; Krackhardt, A.M. Current and Future Concepts for the Generation and Application of Genetically Engineered CAR-T and TCR-T Cells. Front. Immunol. 2023, 14, 1121030. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Lu, S.; Ding, F.; Wang, X.; Zhu, C.; Wang, Y.; Wang, K. Molecular Understanding and Clinical Outcomes of CAR T Cell Therapy in the Treatment of Urological Tumors. Cell Death Dis. 2024, 15, 359. [Google Scholar] [CrossRef]
- Lu, L.; Xie, M.; Yang, B.; Zhao, W.B.; Cao, J. Enhancing the Safety of CAR-T Cell Therapy: Synthetic Genetic Switch for Spatiotemporal Control. Sci. Adv. 2024, 10, eadj6251. [Google Scholar] [CrossRef]
- Flugel, C.L.; Majzner, R.G.; Krenciute, G.; Dotti, G.; Riddell, S.R.; Wagner, D.L.; Abou-El-Enein, M. Overcoming On-target, Off-tumour Toxicity of CAR T Cell Therapy for Solid Tumours. Nat. Rev. Clin. Oncol. 2023, 20, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Guercio, M.; Manni, S.; Boffa, I.; Caruso, S.; Di Cecca, S.; Sinibaldi, M.; Abbaszadeh, Z.; Camera, A.; Ciccone, R.; Polito, V.A.; et al. Inclusion of the Inducible Caspase 9 Suicide Gene in CAR Construct Increases Safety of CAR.CD19 T Cell Therapy in B-Cell Malignancies. Front. Immunol. 2021, 12, 755639. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.H.; Williams, J.Z.; Lim, W.A. Engineering T Cells to Treat Cancer: The Convergence of Immuno-Oncology and Synthetic Biology. Annu. Rev. Cancer Biol. 2020, 4, 121–139. [Google Scholar] [CrossRef]
- Lee, Y.G.; Chu, H.; Lu, Y.; Leamon, C.P.; Srinivasarao, M.; Putt, K.S.; Low, P.S. Regulation of CAR T Cell-mediated Cytokine Release Syndrome-like Toxicity Using Low Molecular Weight Adapters. Nat. Comm. 2019, 10, 2681. [Google Scholar] [CrossRef]
- McCue, A.C.; Yao, Z.; Kuhlman, B. Advances in Modular Control of CAR-T Therapy with Adapter-mediated CARs. Adv. Drug Deliv. Rev. 2022, 187, 114358. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, K.; Lanitis, E.; Poussin, M.; Lynn, R.C.; Gavin, B.P.; Kelderman, S.; Yu, J.; Scholler, N.; Powell, D.J., Jr. A Universal Strategy for Adoptive Immunotherapy of Cancer Through Use of a Novel T-cell Antigen Receptor. Cancer Res. 2012, 72, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Dundas, C.M.; Demonte, D.; Park, S. Streptavidin-Biotin Technology: Improvements and Innovations in Chemical and Biological Applications. Appl. Microbiol. Biotechnol. 2013, 97, 9343–9353. [Google Scholar] [CrossRef]
- Zabel, M.; Tauber, P.A.; Pickl, W.F. The Making and Function of CAR Cells. Immunol. Lett. 2019, 212, 53–69. [Google Scholar] [CrossRef]
- Cartellieri, M.; Feldmann, A.; Koristka, S.; Arndt, C.; Loff, S.; Ehninger, A.; von Bonin, M.; Bejestani, E.P.; Ehninger, G.; Bachmann, M.P. Switching CAR T Cells on and off: A Novel Modular Platform for Retargeting of T Cells to AML Blasts. Blood Cancer J. 2016, 6, e458. [Google Scholar] [CrossRef]
- Bachmann, M. The UniCAR System: A Modular CAR T Cell Approach to Improve the Safety of CAR T Cells. Immunol. Lett. 2019, 211, 13–22. [Google Scholar] [CrossRef]
- Arndt, C.; Loureiro, L.R.; Feldmann, A.; Jureczek, J.; Bergmann, R.; Mathe, D.; Hegedus, N.; Berndt, N.; Koristka, S.; Mitwasi, N.; et al. UniCAR T Cell Immunotherapy Enables Efficient Elimination of Radioresistant Cancer Cells. OncoImmunology 2020, 9, 1743036. [Google Scholar] [CrossRef]
- Feldmann, A.; Arndt, C.; Bergmann, R.; Loff, S.; Cartellieri, M.; Bachmann, D.; Aliperta, R.; Hetzenecker, M.; Ludwig, F.; Albert, S.; et al. Retargeting of T Lymphocytes to PSCA- or PSMA Positive Prostate Cancer Cells Using the Novel Modular Chimeric Antigen Receptor Platform Technology “UniCAR”. Oncotarget 2017, 8, 31368–31385. [Google Scholar] [CrossRef]
- Cho, J.H.; Collins, J.J.; Wong, W.W. Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell 2018, 173, 1426–1438. [Google Scholar] [CrossRef]
- Reinke, A.W.; Grant, R.A.; Keating, A.E. A Synthetic Coiled-Coil Interactome Provides Heterospecific Modules for Molecular Engineering. J. Am. Chem. Soc. 2010, 132, 6025–6031. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y. Increasing T Cell Versatility with SUPRA CARs. Cell 2018, 173, 1316–1317. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Okuma, A.; Sofjan, K.; Lee, S.; Collins, J.J.; Wong, W.W. Engineering Advanced Logic and Distributed Computing in Human CAR Immune Cells. Nat. Comm. 2021, 12, 792. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, J.; Mu, W.; Zhou, J.; Zhu, L. Advances in Universal CAR-T Cell Therapy. Front. Immunol. 2021, 12, 744823. [Google Scholar] [CrossRef] [PubMed]
- Chekol Abebe, E.; Yibeltal Shiferaw, M.; Tadele Admasu, F.; Asmamaw Dejenie, T. Ciltacabtagene Autoleucel: The Second Anti-BCMA CAR T-cell Therapeutic Armamentarium of Relapsed or Refractory Multiple Myeloma. Front. Immunol. 2022, 13, 991092. [Google Scholar] [CrossRef] [PubMed]
- Bupha-Intr, O.; Haeusler, G.; Chee, L.; Thursky, K.; Slavin, M.; Teh, B. CAR-T Cell Therapy and Infection: A Review. Expert Rev. Anti-Infect. Ther. 2021, 19, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Breman, E. Engineering Strategies to Safely Drive CAR T-cells Into the Future. Front. Immunol. 2024, 15, 1411393. [Google Scholar] [CrossRef]
- Teoh, P.J.; Chng, W.J. CAR T-cell Therapy in Multiple Myeloma: More Room for Improvement. Blood Cancer J. 2021, 11, 84. [Google Scholar] [CrossRef]
- Chouhan, S.; Muhammad, N.; Usmani, D.; Khan, T.H.; Kumar, A. Molecular Sentinels: Unveiling the Role of Sirtuins in Prostate Cancer Progression. Int. J. Mol. Sci. 2024, 26, 183. [Google Scholar] [CrossRef]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-specific Membrane Antigen Cleavage of Vitamin B9 Stimulates Oncogenic Signaling Through Metabotropic Glutamate Receptors. J. Exp. Med. 2018, 215, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Rahn, K.A.; Slusher, B.S.; Kaplin, A.I. Glutamate in CNS Neurodegeneration and Cognition and its Regulation by GCPII Inhibition. Curr. Med. Chem. 2012, 19, 1335–1345. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate Specific Membrane Antigen Expression in Prostatic Intraepithelial Neoplasia and Adenocarcinoma: A Study of 184 Cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M., Jr.; Wang, C.Y.; Haas, G.P. Expression of Prostate-Specific Membrane Antigen in Normal and Malignant Human Tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D.W. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef]
- Puik, J.R.; Le, C.; Kazemier, G.; Oprea-Lager, D.E.; Swijnenburg, R.J.; Giovannetti, E.; Griffioen, A.W.; Huijbers, E.J. Prostate-specific Membrane Antigen as Target for Vasculature-directed Therapeutic Strategies in Solid Tumors. Crit. Rev. Oncol. Hematol. 2025, 205, 104556. [Google Scholar] [CrossRef]
- Christiansen, J.J.; Rajasekaran, S.A.; Inge, L.; Cheng, L.; Anilkumar, G.; Bander, N.H.; Rajasekaran, A.K. N-Glycosylation and Microtubule Integrity are Involved in Apical Targeting of Prostate-specific Membrane Antigen: Implications for Immunotherapy. Mol. Cancer Ther. 2005, 4, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Heynickx, N.; Herrmann, K.; Vermeulen, K.; Baatout, S.; Aerts, A. The Salivary Glands as a Dose Limiting Organ of PSMA-Targeted Radionuclide Therapy: A Review of the Lessons Learnt so Far. Nucl. Med. Biol. 2021, 98–99, 30–39. [Google Scholar] [CrossRef]
- Capasso, G.; Stefanucci, A.; Tolomeo, A. A Systematic Review on the Current Status of PSMA-targeted Imaging and Radioligand Therapy. Eur. J. Med. Chem. 2024, 263, 115966. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Eder, M. [(68)Ga]Ga-PSMA-11: The First FDA-Approved (68)Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14, 713. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Piflufolastat F 18: Diagnostic First Approval. Mol. Diagn. Ther. 2021, 25, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Flotufolastat F 18: Diagnostic First Approval. Mol. Diagn. Ther. 2023, 27, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Lutetium Lu 177 Vipivotide Tetraxetan: First Approval. Mol. Diagn. Ther. 2022, 26, 467–475. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Yuan, H.; Cai, P.; Wu, T.; Yang, Z.; Nie, J.; Zhang, W.; Huang, Z.; Liu, N.; et al. Development and Evaluation of Novel (68)Ga/(177)Lu-Labeled PSMA Inhibitors with Enhanced Pharmacokinetics and Tumor Imaging for Prostate Cancer. Mol. Pharm. 2025, 22, 1584–1597. [Google Scholar] [CrossRef]
- Sobral, M.C.; Mota, S.I.; Oliveira, P.J.; Urbano, A.M.; Paulo, A. Two Targets, One Mission: Heterobivalent Metal-Based Radiopharmaceuticals for Prostate Cancer Imaging and Therapy. ChemMedChem 2025, 20, e2500128. [Google Scholar] [CrossRef]
- Previti, S.; Bodin, S.; Remond, E.; Vimont, D.; Hindie, E.; Morgat, C.; Cavelier, F. Rational Design of NT-PSMA Heterobivalent Probes for Prostate Cancer Theranostics. RSC Med. Chem. 2024, 15, 4153–4158. [Google Scholar] [CrossRef]
- Bodin, S.; Previti, S.; Jestin, E.; Remond, E.; Vimont, D.; Lamare, F.; Ait-Arsa, I.; Hindie, E.; Cavelier, F.; Morgat, C. Design and Synthesis of (68)Ga-Labeled Peptide-Based Heterodimers for Dual Targeting of NTS1 and GRPR. ChemMedChem 2025, 20, e202400843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Z.; Huang, Y.; Li, C.; Li, Y.; Peng, Y.; Sheng, Z.; Liang, Y. A novel Androgen-Independent Radiotracer with Dual Targeting of NTSR1 and PSMA for PET/CT Imaging of Prostate Cancer. Eur. J. Med. Chem. 2025, 282, 117050. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, Y.; Hussein Mer, A.; Fattah Maran, B.; Omidvar, L.; Misamogooe, F.; Amirkhani, Z.; Javaheri Haghighi, N.; Bagheri, N.; Keshtkaran, Z.; Rezaei, B.; et al. Clinical and Preclinical Advances in PSMA-Directed Antibody-Drug Conjugates (ADCs): Current Status and Hope for the Future. Bioorg. Chem. 2024, 153, 107803. [Google Scholar] [CrossRef]
- Maher, J.; Brentjens, R.J.; Gunset, G.; Riviere, I.; Sadelain, M. Human T-lymphocyte Cytotoxicity and Proliferation Directed by a Single Chimeric TCRzeta /CD28 Receptor. Nat. Biotechnol. 2002, 20, 70–75. [Google Scholar] [CrossRef]
- Slovin, S.F.; Wang, X.; Hullings, M.; Arauz, G.; Bartido, S.; Lewis, J.S.; Schöder, H.; Zanzonico, P.; Scher, H.I.; Sadelain, M.; et al. Chimeric Antigen Receptor (CAR+) Modified T Cells Targeting Prostate-specific Membrane Antigen (PSMA) in Patients (pts) with Castrate Metastatic Prostate Cancer (CMPC). J. Clin. Oncol. 2013, 31, 72. [Google Scholar] [CrossRef]
- Ma, Q.; Safar, M.; Holmes, E.; Wang, Y.; Boynton, A.L.; Junghans, R.P. Anti-prostate Specific Membrane Antigen Designer T Cells for Prostate Cancer Therapy. Prostate 2004, 61, 12–25. [Google Scholar] [CrossRef]
- Gade, T.P.; Hassen, W.; Santos, E.; Gunset, G.; Saudemont, A.; Gong, M.C.; Brentjens, R.; Zhong, X.S.; Stephan, M.; Stefanski, J.; et al. Targeted Elimination of Prostate Cancer by Genetically Directed Human T Lymphocytes. Cancer Res. 2005, 65, 9080–9088. [Google Scholar] [CrossRef]
- Junghans, R.P.; Ma, Q.; Rathore, R.; Gomes, E.M.; Bais, A.J.; Lo, A.S.; Abedi, M.; Davies, R.A.; Cabral, H.J.; Al-Homsi, A.S.; et al. Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: Possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate 2016, 76, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Gomes, E.M.; Lo, A.S.; Junghans, R.P. Advanced Generation Anti-prostate Specific Membrane Antigen Designer T Cells for Prostate Cancer Immunotherapy. Prostate 2014, 74, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Alzubi, J.; Dettmer-Monaco, V.; Kuehle, J.; Thorausch, N.; Seidl, M.; Taromi, S.; Schamel, W.; Zeiser, R.; Abken, H.; Cathomen, T.; et al. PSMA-Directed CAR T Cells Combined with Low-Dose Docetaxel Treatment Induce Tumor Regression in a Prostate Cancer Xenograft Model. Mol. Ther. Oncol. 2020, 18, 226–235. [Google Scholar] [CrossRef]
- Zuccolotto, G.; Fracasso, G.; Merlo, A.; Montagner, I.M.; Rondina, M.; Bobisse, S.; Figini, M.; Cingarlini, S.; Colombatti, M.; Zanovello, P.; et al. PSMA-specific CAR-engineered T Cells Eradicate Disseminated Prostate Cancer in Preclinical Models. PLoS ONE 2014, 9, e109427. [Google Scholar] [CrossRef] [PubMed]
- Zuccolotto, G.; Penna, A.; Fracasso, G.; Carpanese, D.; Montagner, I.M.; Dalla Santa, S.; Rosato, A. PSMA-Specific CAR-Engineered T Cells for Prostate Cancer: CD28 Outperforms Combined CD28-4-1BB “Super-Stimulation”. Front. Oncol. 2021, 11, 708073. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Travis, M.A.; Sheppard, D. TGF-beta Activation and Function in Immunity. Annu. Rev. Immunol. 2014, 32, 51–82. [Google Scholar] [CrossRef]
- Dahmani, A.; Delisle, J.S. TGF-beta in T Cell Biology: Implications for Cancer Immunotherapy. Cancers 2018, 10, 194. [Google Scholar] [CrossRef]
- Wieser, R.; Attisano, L.; Wrana, J.L.; Massague, J. Signaling Activity of Transforming Growth Factor Beta Type II Receptors Lacking Specific Domains in the Cytoplasmic Region. Mol. Cell. Biol. 1993, 13, 7239–7247. [Google Scholar] [CrossRef]
- Gorelik, L.; Flavell, R.A. Immune-mediated Eradication of Tumors Through the Blockade of Transforming Growth Factor-beta Signaling in T Cells. Nat. Med. 2001, 7, 1118–1122. [Google Scholar] [CrossRef]
- Zhang, Q.; Helfand, B.T.; Carneiro, B.A.; Qin, W.; Yang, X.J.; Lee, C.; Zhang, W.; Giles, F.J.; Cristofanilli, M.; Kuzel, T.M. Efficacy Against Human Prostate Cancer by Prostate-specific Membrane Antigen-specific, Transforming Growth Factor-beta Insensitive Genetically Targeted CD8(+) T-cells Derived from Patients with Metastatic Castrate-resistant Disease. Eur. Urol. 2018, 73, 648–652. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03089203 (accessed on 25 March 2025).
- Narayan, V.; Barber-Rotenberg, J.S.; Jung, I.Y.; Lacey, S.F.; Rech, A.J.; Davis, M.M.; Hwang, W.T.; Lal, P.; Carpenter, E.L.; Maude, S.L.; et al. PSMA-targeting TGFbeta-insensitive Armored CAR T Cells in Metastatic Castration-resistant Prostate Cancer: A Phase 1 Trial. Nat. Med. 2022, 28, 724–734. [Google Scholar] [CrossRef]
- Gladney, W.; Vultur, A.; Schweizer, M.; Fraietta, J.; Rech, A.; June, C.; O’Rourke, M.; Roberts, A.; Patel, H.; Rosen, J.; et al. Analyses of Severe Immune-mediated Toxicity in Patients with Advanced mCRPC Treated with a PSMA-targeted Armored CAR T-cells. J. Immunotherap. Cancer 2022, 10, A353. [Google Scholar] [CrossRef]
- Carabasi, M.H.; McKean, M.; Stein, M.N.; Schweizer, M.T.; Luke, J.J.; Narayan, V.; Pachynski, R.K.; Parikh, R.A.; Zhang, J.; Fountaine, T.J.; et al. PSMA Targeted Armored Chimeric Antigen Receptor (CAR) T-cells in Patients with Advanced mCRPC: A Phase I Experience. J. Clin. Oncol. 2021, 39, 2534. [Google Scholar] [CrossRef]
- McKean, M.; Carabasi, M.H.; Stein, M.N.; Schweizer, M.T.; Luke, J.J.; Narayan, V.; Parikh, R.A.; Pachynski, R.K.; Zhang, J.; Peddareddigari, V.G.R.; et al. Safety and Early Efficacy Results from a Phase 1, Multicenter Trial of PSMA-targeted Armored CAR T Cells in Patients with Advanced mCRPC. J. Clin. Oncol. 2022, 40, 94. [Google Scholar] [CrossRef]
- Weimin, S.; Abula, A.; Qianghong, D.; Wenguang, W. Chimeric Cytokine Receptor Enhancing PSMA-CAR-T Cell-mediated Prostate Cancer Regression. Cancer Biol. Ther. 2020, 21, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Melchionda, F.; Fry, T.J.; Milliron, M.J.; McKirdy, M.A.; Tagaya, Y.; Mackall, C.L. Adjuvant IL-7 or IL-15 Overcomes Immunodominance and Improves Survival of the CD8+ Memory Cell Pool. J. Clin. Investig. 2005, 115, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Sukumaran, S.; Bajgain, P.; Watanabe, N.; Heslop, H.E.; Rooney, C.M.; Brenner, M.K.; Fisher, W.E.; Leen, A.M.; Vera, J.F. Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer. Mol. Ther. 2017, 25, 249–258. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05489991 (accessed on 7 April 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06046040 (accessed on 27 March 2025).
- Calcinotto, A.; Spataro, C.; Zagato, E.; Di Mitri, D.; Gil, V.; Crespo, M.; De Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 Secreted by Myeloid Cells Drives Castration-resistant Prostate Cancer. Nature 2018, 559, 363–369. [Google Scholar] [CrossRef]
- Wang, D.; Shao, Y.; Zhang, X.; Lu, G.; Liu, B. IL-23 and PSMA-targeted Duo-CAR T Cells in Prostate Cancer Eradication in a Preclinical Model. J. Transl. Med. 2020, 18, 23. [Google Scholar] [CrossRef]
- Alexander, E.; Leong, K.W. Discovery of Nanobodies: A Comprehensive Review of their Applications and Potential Over the Past Five Years. J. Nanobiotechnol. 2024, 22, 661. [Google Scholar] [CrossRef]
- Safarzadeh Kozani, P.; Naseri, A.; Mirarefin, S.M.J.; Salem, F.; Nikbakht, M.; Evazi Bakhshi, S.; Safarzadeh Kozani, P. Nanobody-based CAR-T Cells for Cancer Immunotherapy. Biomark. Res. 2022, 10, 24. [Google Scholar] [CrossRef]
- Hassani, M.; Hajari Taheri, F.; Sharifzadeh, Z.; Arashkia, A.; Hadjati, J.; van Weerden, W.M.; Modarressi, M.H.; Abolhassani, M. Construction of a Chimeric Antigen Receptor Bearing a Nanobody Against Prostate a Specific Membrane Antigen in Prostate Cancer. J. Cell. Biochem. 2019, 120, 10787–10795. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Qin, J.; Sun, Y.; Xiong, H.; Lin, B.; Liu, K.; Tan, B.; Zhang, C.; Huang, C.; et al. LIGHT/TNFSF14 Promotes CAR-T Cell Trafficking and Cytotoxicity Through Reversing Immunosuppressive Tumor Microenvironment. Mol. Ther. 2023, 31, 2575–2590. [Google Scholar] [CrossRef]
- Martinez-Usatorre, A.; De Palma, M. A LIGHTning Strike to the Metastatic Niche. Cell Rep. 2020, 30, 599–601. [Google Scholar] [CrossRef]
- Skeate, J.G.; Otsmaa, M.E.; Prins, R.; Fernandez, D.J.; Da Silva, D.M.; Kast, W.M. TNFSF14: LIGHTing the Way for Effective Cancer Immunotherapy. Front. Immunol. 2020, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04053062 (accessed on 11 May 2025).
- UroToday.com. Available online: https://www.urotoday.com/conference-highlights/asco-gu-2022/asco-gu-2022-prostate-cancer/135359-asco-gu-2022-phase-1-study-of-p-psma-101-car-t-cells-in-patients-with-metastatic-castration-resistant-prostate-cancer-mcrpc.html (accessed on 23 May 2025).
- Slovin, S.F.; Dorff, T.B.; Falchook, G.S.; Wei, X.X.; Gao, X.; McKay, R.R.; Oh, D.Y.; Wibmer, A.G.; Spear, M.A.; McCaigue, J.; et al. Phase 1 Study of P-PSMA-101 CAR-T Cells in Patients with Metastatic Castration-resistant Prostate Cancer (mCRPC). J. Clin. Oncol. 2022, 40, 98. [Google Scholar] [CrossRef]
- Serganova, I.; Moroz, E.; Cohen, I.; Moroz, M.; Mane, M.; Zurita, J.; Shenker, L.; Ponomarev, V.; Blasberg, R. Enhancement of PSMA-Directed CAR Adoptive Immunotherapy by PD-1/PD-L1 Blockade. Mol. Ther. Oncolytics 2017, 4, 41–54. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04768608 (accessed on 4 June 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05732948 (accessed on 9 May 2025).
- Jiao, C.; Zvonkov, E.; Lai, X.; Zhang, R.; Liu, Y.; Qin, Y.; Savchenko, V.; Gabeeva, N.; Chung, T.H.; Sheng, L.; et al. 4SCAR2.0: A Multi-CAR-T Therapy Regimen for the Treatment of Relapsed/refractory B Cell Lymphomas. Blood Cancer J. 2021, 11, 59. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04429451 (accessed on 6 May 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05437315 (accessed on 2 June 2025).
- Bhat, A.M.; Mohapatra, B.C.; Luan, H.; Mushtaq, I.; Chakraborty, S.; Kumar, S.; Wu, W.; Nolan, B.; Dutta, S.; Storck, M.D.; et al. GD2 and its Biosynthetic Enzyme GD3 Synthase Promote Tumorigenesis in Prostate Cancer by Regulating Cancer Stem Cell Behavior. Sci. Rep. 2024, 14, 13523. [Google Scholar] [CrossRef]
- Avencell Website. Available online: https://avencell.com/science/universal-switchable-car/ (accessed on 11 June 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04633148 (accessed on 28 May 2025).
- Nova Therapeutics Website. Available online: https://www.novat-llc.com/ (accessed on 12 June 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05656573 (accessed on 29 May 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05354375 (accessed on 30 May 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06228404 (accessed on 31 May 2025).
- Raff, A.B.; Gray, A.; Kast, W.M. Prostate Stem Cell Antigen: A Prospective Therapeutic and Diagnostic Target. Cancer Lett. 2009, 277, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Saeki, N.; Gu, J.; Yoshida, T.; Wu, X. Prostate Stem Cell Antigen: A Jekyll and Hyde Molecule? Clin. Cancer Res. 2010, 16, 3533–3538. [Google Scholar] [CrossRef]
- Loertscher, R.; Lavery, P. The Role of Glycosyl Phosphatidyl Inositol (GPI)-anchored Cell Surface Proteins in T-cell Activation. Transpl. Immunol. 2002, 9, 93–96. [Google Scholar] [CrossRef]
- Rege, T.A.; Hagood, J.S. Thy-1, a Versatile Modulator of Signaling Affecting Cellular Adhesion, Proliferation, Survival, and Cytokine/growth Factor Responses. Biochim. Biophys. Acta 2006, 1763, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Treister, A.; Sagi-Assif, O.; Meer, M.I.; Smorodinsky, N.; Anavi, R.; Golan, I.; Meshel, T.; Kahana, O.; Eshel, R.; Katz, B.-Z.; et al. Expression of Ly-6, a Marker for Highly Malignant Murine Tumor Cells, is Regulated by Growth Conditions and Stress. Int. J. Cancer 1998, 77, 306–313. [Google Scholar] [CrossRef]
- Bamezai, A.; Rock, K.L. Overexpressed Ly-6A.2 Mediates Cell-Cell Adhesion by Binding a Ligand Expressed on Lymphoid Cells. Proc. Natl. Acad. Sci. USA 1995, 92, 4294–4298. [Google Scholar] [CrossRef]
- Gu, Z.; Thomas, G.; Yamashiro, J.; Shintaku, I.P.; Dorey, F.; Raitano, A.; Witte, O.N.; Said, J.W.; Loda, M.; Reiter, R.E. Prostate Stem Cell Antigen (PSCA) Expression Increases with High Gleason Score, Advanced Stage and Bone Metastasis in Prostate Cancer. Oncogene 2000, 19, 1288–1296. [Google Scholar] [CrossRef]
- Han, K.R.; Seligson, D.B.; Liu, X.; Horvath, S.; Shintaku, P.I.; Thomas, G.V.; Said, J.W.; Reiter, R.E. Prostate Stem Cell Antigen Expression is Associated with Gleason Score, Seminal Vesicle Invasion and Capsular Invasion in Prostate Cancer. J. Urol. 2004, 171, 1117–1121. [Google Scholar] [CrossRef]
- Lam, J.S.; Yamashiro, J.; Shintaku, I.P.; Vessella, R.L.; Jenkins, R.B.; Horvath, S.; Said, J.W.; Reiter, R.E. Prostate Stem Cell Antigen is Overexpressed in Prostate Cancer Metastases. Clin. Cancer Res. 2005, 11, 2591–2596. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Z.; Liu, Y.; Si, T.; Yu, H.; Li, B.; Tian, W. Prostate Stem Cell Antigen and Cancer Risk, Mechanisms and Therapeutic Implications. Expert Rev. Anticancer Ther. 2014, 14, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Priceman, S.J.; Gerdts, E.A.; Tilakawardane, D.; Kennewick, K.T.; Murad, J.P.; Park, A.K.; Jeang, B.; Yamaguchi, Y.; Yang, X.; Urak, R.; et al. Co-stimulatory Signaling Determines Tumor Antigen Sensitivity and Persistence of CAR T Cells Targeting PSCA+ Metastatic Prostate Cancer. Oncoimmunology 2018, 7, e1380764. [Google Scholar] [CrossRef]
- Murad, J.P.; Tilakawardane, D.; Park, A.K.; Lopez, L.S.; Young, C.A.; Gibson, J.; Yamaguchi, Y.; Lee, H.J.; Kennewick, K.T.; Gittins, B.J.; et al. Pre-conditioning Modifies the TME to Enhance Solid Tumor CAR T Cell Efficacy and Endogenous Protective Immunity. Mol. Ther. 2021, 29, 2335–2349. [Google Scholar] [CrossRef]
- Dorff, T.B.; Blanchard, M.S.; Adkins, L.N.; Luebbert, L.; Leggett, N.; Shishido, S.N.; Macias, A.; Del Real, M.M.; Dhapola, G.; Egelston, C.; et al. PSCA-CAR T Cell Therapy in Metastatic Castration-resistant Prostate Cancer: A Phase 1 Trial. Nat. Med. 2024, 30, 1636–1644. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05805371 (accessed on 31 March 2025).
- Foster, A.E.; Mahendravada, A.; Shinners, N.P.; Chang, W.C.; Crisostomo, J.; Lu, A.; Khalil, M.; Morschl, E.; Shaw, J.L.; Saha, S.; et al. Regulated Expansion and Survival of Chimeric Antigen Receptor-Modified T Cells Using Small Molecule-Dependent Inducible MyD88/CD40. Mol. Ther. 2017, 25, 2176–2188. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Gerken, C.; Nguyen, P.; Krenciute, G.; Spencer, D.M.; Gottschalk, S. Inducible Activation of MyD88 and CD40 in CAR T Cells Results in Controllable and Potent Antitumor Activity in Preclinical Solid Tumor Models. Cancer Discov. 2017, 7, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Collinson-Pautz, M.R.; Morschl, E.; Lu, A.; Szymanski, S.P.; Zhang, M.; Brandt, M.E.; Chang, W.C.; Sharp, K.L.; Toler, S.M.; et al. Two-Dimensional Regulation of CAR-T Cell Therapy with Orthogonal Switches. Mol. Ther. Oncolytics 2019, 12, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.N.; Dumbrava, E.E.; Teply, B.A.; Gergis, U.S.; Guiterrez, M.E.; Reshef, R.; Subudhi, S.K.; Jacquemont, C.F.; Senesac, J.H.; Bayle, J.H.; et al. PSCA-targeted BPX-601 CAR T Cells with Pharmacological Activation by Rimiducid in Metastatic Pancreatic and Prostate Cancer: A Phase 1 Dose Escalation Trial. Nat. Comm. 2024, 15, 10743. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT02744287 (accessed on 25 April 2025).
- Rocha, S.M.; Socorro, S.; Passarinha, L.A.; Maia, C.J. Comprehensive Landscape of STEAP Family Members Expression in Human Cancers: Unraveling the Potential Usefulness in Clinical Practice Using Integrated Bioinformatics Analysis. Data 2022, 7, 64. [Google Scholar] [CrossRef]
- Chen, W.J.; Wu, H.T.; Li, C.L.; Lin, Y.K.; Fang, Z.X.; Lin, W.T.; Liu, J. Regulatory Roles of Six-Transmembrane Epithelial Antigen of the Prostate Family Members in the Occurrence and Development of Malignant Tumors. Front. Cell Dev. Biol. 2021, 9, 752426. [Google Scholar] [CrossRef]
- Oosterheert, W.; Gros, P. Cryo-Electron Microscopy Structure and Potential Enzymatic Function of Human Six-transmembrane Epithelial Antigen of the Prostate 1 (STEAP1). J. Biol. Chem. 2020, 295, 9502–9512. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K.; Arihara, Y.; Hayasaka, N.; Murase, K.; Iyama, S.; Kobune, M.; Miyanishi, K.; Kato, J. Six-transmembrane Epithelial Antigen of the Prostate 1 Protects Against Increased Oxidative Stress via a Nuclear Erythroid 2-related Factor Pathway in Colorectal Cancer. Cancer Gene Ther. 2019, 26, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, T.G.; Diebold, I.; Esposito, I.; Plehm, S.; Hauer, K.; Thiel, U.; da Silva-Buttkus, P.; Neff, F.; Unland, R.; Muller-Tidow, C.; et al. STEAP1 is Associated with the Invasive and Oxidative Stress Phenotype of Ewing Tumors. Mol. Cancer Res. 2012, 10, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Whiteland, H.; Spencer-Harty, S.; Morgan, C.; Kynaston, H.; Thomas, D.H.; Bose, P.; Fenn, N.; Lewis, P.; Jenkins, S.; Doak, S.H. A Role for STEAP2 in Prostate Cancer Progression. Clin. Exp. Metastasis 2014, 31, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tao, Y.; Zhang, Z.; Guo, X.; An, P.; Shen, Y.; Wu, Q.; Yu, Y.; Wang, F. Metalloreductase Steap3 Coordinates the Regulation of Iron Homeostasis and Inflammatory Responses. Haematologica 2012, 97, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Hubert, R.S.; Vivanco, I.; Chen, E.; Rastegar, S.; Leong, K.; Mitchell, S.C.; Madraswala, R.; Zhou, Y.; Kuo, J.; Raitano, A.B.; et al. STEAP: A Prostate-Specific Cell-Surface Antigen Highly Expressed in Human Prostate Tumors. Proc. Natl. Acad. Sci. USA 1999, 96, 14523–14528. [Google Scholar] [CrossRef]
- Gomes, I.M.; Rocha, S.M.; Gaspar, C.; Alvelos, M.I.; Santos, C.R.; Socorro, S.; Maia, C.J. Knockdown of STEAP1 Inhibits Cell Growth and Induces Apoptosis in LNCaP Prostate Cancer Cells Counteracting the Effect of Androgens. Med. Oncol. 2018, 35, 40. [Google Scholar] [CrossRef]
- Huo, S.F.; Shang, W.L.; Yu, M.; Ren, X.P.; Wen, H.X.; Chai, C.Y.; Sun, L.; Hui, K.; Liu, L.H.; Wei, S.H.; et al. STEAP1 Facilitates Metastasis and Epithelial-Mesenchymal Transition of Lung Adenocarcinoma via the JAK2/STAT3 Signaling Pathway. Biosci. Rep. 2020, 40, BSR20193169. [Google Scholar] [CrossRef]
- Gomes, I.M.; Arinto, P.; Lopes, C.; Santos, C.R.; Maia, C.J. STEAP1 is Overexpressed in Prostate Cancer and Prostatic Intraepithelial Neoplasia Lesions, and it is Positively Associated with Gleason Score. Urol. Oncol. 2014, 32, e23–e53. [Google Scholar] [CrossRef]
- Xu, M.; Evans, L.; Bizzaro, C.L.; Quaglia, F.; Verrillo, C.E.; Li, L.; Stieglmaier, J.; Schiewer, M.J.; Languino, L.R.; Kelly, W.K. STEAP1-4 (Six-Transmembrane Epithelial Antigen of the Prostate 1-4) and Their Clinical Implications for Prostate Cancer. Cancers 2022, 14, 4034. [Google Scholar] [CrossRef]
- Bhatia, V.; Kamat, N.V.; Pariva, T.E.; Wu, L.T.; Tsao, A.; Sasaki, K.; Sun, H.; Javier, G.; Nutt, S.; Coleman, I.; et al. Targeting Advanced Prostate Cancer with STEAP1 Chimeric Antigen Receptor T Cell and Tumor-localized IL-12 Immunotherapy. Nat. Comm. 2023, 14, 2041. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06236139 (accessed on 8 April 2025).
- Porkka, K.P.; Helenius, M.A.; Visakorpi, T. Cloning and Characterization of a Novel Six-transmembrane Protein STEAP2, Expressed in Normal and Malignant Prostate. Lab. Investig. 2002, 82, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, K.S.; Elbi, C.; Korkmaz, C.G.; Loda, M.; Hager, G.L.; Saatcioglu, F. Molecular Cloning and Characterization of STAMP1, a Highly Prostate-Specific Six Transmembrane Protein that is Overexpressed in Prostate Cancer. J. Biol. Chem. 2002, 277, 36689–36696. [Google Scholar] [CrossRef] [PubMed]
- Burnell, S.E.A.; Spencer-Harty, S.; Howarth, S.; Bodger, O.; Kynaston, H.; Morgan, C.; Doak, S.H. Utilisation of the STEAP Protein Family in a Diagnostic Setting May Provide a More Comprehensive Prognosis of Prostate Cancer. PLoS ONE 2019, 14, e0220456. [Google Scholar] [CrossRef]
- Hasegawa, H.; Li, C.; Alba, B.M.; Penny, D.M.; Xia, Z.; Dayao, M.R.; Li, P.; Zhang, J.; Zhou, J.; Lim, D.; et al. Membrane Cholesterol Modulates STEAP2 Conformation During Dynamic Intracellular Trafficking Processes Leading to Broad Subcellular Distribution. Exp. Cell Res. 2018, 370, 208–226. [Google Scholar] [CrossRef]
- Zanvit, P.; van Dyk, D.; Fazenbaker, C.; McGlinchey, K.; Luo, W.; Pezold, J.M.; Meekin, J.; Chang, C.Y.; Carrasco, R.A.; Breen, S.; et al. Antitumor Activity of AZD0754, a dnTGFbetaRII-armored, STEAP2-targeted CAR-T Cell Therapy, in Prostate Cancer. J. Clin. Investig. 2023, 133, e169655. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06267729 (accessed on 10 June 2025).
- Ni, J.; Cozzi, P.J.; Duan, W.; Shigdar, S.; Graham, P.H.; John, K.H.; Li, Y. Role of the EpCAM (CD326) in Prostate Cancer Metastasis and Progression. Cancer Metastasis Rev. 2012, 31, 779–791. [Google Scholar] [CrossRef]
- Poczatek, R.B.; Myers, R.B.; Manne, U.; Oelschlager, D.K.; Weiss, H.L.; Bostwick, D.G.; Grizzle, W.E. Ep-Cam Levels in Prostatic Adenocarcinoma and Prostatic Intraepithelial Neoplasia. J. Urol. 1999, 162, 1462–1466. [Google Scholar] [CrossRef]
- Zellweger, T.; Ninck, C.; Bloch, M.; Mirlacher, M.; Koivisto, P.A.; Helin, H.J.; Mihatsch, M.J.; Gasser, T.C.; Bubendorf, L. Expression Patterns of Potential Therapeutic Targets in Prostate Cancer. Int. J. Cancer 2005, 113, 619–628. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer Stem Cells in Solid Tumours: Accumulating Evidence and Unresolved Questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, Y.; Ma, W.; Zhang, S.; Zhang, Y.Q. Adoptive T-Cell Therapy of Prostate Cancer Targeting the Cancer Stem Cell Antigen EpCAM. BMC Immunol. 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Qin, D.; Li, D.; Zhang, B.; Chen, Y.; Liao, X.; Li, X.; Alexander, P.B.; Wang, Y.; Li, Q.J. Potential Lung Attack and Lethality Generated by EpCAM-specific CAR-T Cells in Immunocompetent Mouse Models. Oncoimmunology 2020, 9, 1806009. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03013712 (accessed on 29 March 2025).
- Emami, N.; Scorilas, A.; Soosaipillai, A.; Earle, T.; Mullen, B.; Diamandis, E.P. Association Between Kallikrein-related Peptidases (KLKs) and Macroscopic Indicators of Semen Analysis: Their Relation to Sperm Motility. Biol. Chem. 2009, 390, 921–929. [Google Scholar] [CrossRef]
- Shang, Z.; Niu, Y.; Cai, Q.; Chen, J.; Tian, J.; Yeh, S.; Lai, K.P.; Chang, C. Human Kallikrein 2 (KLK2) Promotes Prostate Cancer Cell Growth via Function as a Modulator to Promote the ARA70-enhanced Androgen Receptor Transactivation. Tumor Biol. 2014, 35, 1881–1890. [Google Scholar] [CrossRef]
- Paniagua-Herranz, L.; Moreno, I.; Nieto-Jimenez, C.; Garcia-Lorenzo, E.; Diaz-Tejeiro, C.; Sanvicente, A.; Doger, B.; Pedregal, M.; Ramon, J.; Bartolome, J.; et al. Genomic and Immunologic Correlates in Prostate Cancer with High Expression of KLK2. Int. J. Mol. Sci. 2024, 25, 2222. [Google Scholar] [CrossRef] [PubMed]
- Khosroabadi, G.; Yousefnia, S. Mechanistic and Diagnostic Roles of Kallikrein Related Peptidases 2 (KLK2) in Prostate Cancer. J. Pers. Med. 2023, 8, 5–12. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05022849 (accessed on 13 June 2025).
- Zong, J.; Li, Y.R. iPSC Technology Revolutionizes CAR-T Cell Therapy for Cancer Treatment. Bioengineering 2025, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.; Garcia, A.; Chang, C.-W.; Yang, B.-H.; Ibitokou, S.; Pride, C.; Markov, S.; Liao, A.; Pribadi, M.; Pan, Y.; et al. Off-the-shelf iPSC-derived CAR-T Cells Targeting KLK2 Demonstrate Prolonged Tumor Control and Survival in Xenograft Models of Prostate Cancer. J. Immunotherap. Cancer 2022, 10, A343. [Google Scholar] [CrossRef]
- Flem-Karlsen, K.; Fodstad, O.; Tan, M.; Nunes-Xavier, C.E. B7-H3 in Cancer—Beyond Immune Regulation. Trends Cancer 2018, 4, 401–404. [Google Scholar] [CrossRef]
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, O.; Tan, M. New Frontiers in Immune Checkpoint B7-H3 (CD276) Research and Drug Development. Mol. Cancer 2023, 22, 43. [Google Scholar] [CrossRef]
- Benzon, B.; Zhao, S.G.; Haffner, M.C.; Takhar, M.; Erho, N.; Yousefi, K.; Hurley, P.; Bishop, J.L.; Tosoian, J.; Ghabili, K.; et al. Correlation of B7-H3 with Androgen Receptor, Immune Pathways and Poor Outcome in Prostate Cancer: An Expression-based Analysis. Prostate Cancer Prostatic Dis. 2017, 20, 28–35. [Google Scholar] [CrossRef]
- Li, S.; Zhang, M.; Wang, M.; Wang, H.; Wu, H.; Mao, L.; Zhang, M.; Li, H.; Zheng, J.; Ma, P.; et al. B7-H3 Specific CAR-T Cells Exhibit Potent Activity Against Prostate Cancer. Cell Death Dis. 2023, 9, 147. [Google Scholar] [CrossRef]
- Barden, J.A.; Yuksei, A.; Pedersen, J.; Danieletto, S.; Delprado, W. Non-Functional P2X7: A Novel and Ubiquitous Target in Human Cancer. J. Clin. Cell Immunol. 2014, 05, 237. [Google Scholar] [CrossRef]
- Barden, J.A.; Gidley-Baird, A.; Teh, L.C.; Rajasekariah, G.H.; Pedersen, J.; Christensen, N.I.; Spielman, D.; Ashley, D.M. Therapeutic Targeting of the Cancer-specific Cell Surface Biomarker nfP2X7. J. Clin. Cell Immunol. 2016, 7, 432. [Google Scholar] [CrossRef]
- Gilbert, S.M.; Oliphant, C.J.; Hassan, S.; Peille, A.L.; Bronsert, P.; Falzoni, S.; Di Virgilio, F.; McNulty, S.; Lara, R. ATP in the Tumour Microenvironment Drives Expression of nfP2X(7), a Key Mediator of Cancer Cell Survival. Oncogene 2019, 38, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Bandara, V.; Foeng, J.; Gundsambuu, B.; Norton, T.S.; Napoli, S.; McPeake, D.J.; Tyllis, T.S.; Rohani-Rad, E.; Abbott, C.; Mills, S.J.; et al. Pre-clinical Validation of a Pan-cancer CAR-T Cell Immunotherapy Targeting nfP2X7. Nat. Comm. 2023, 14, 5546. [Google Scholar] [CrossRef] [PubMed]
- Raulet, D.H.; Gasser, S.; Gowen, B.G.; Deng, W.; Jung, H. Regulation of Ligands for the NKG2D Activating Receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef]
- Sentman, C.L.; Meehan, K.R. NKG2D CARs as Cell Therapy for Cancer. Cancer J. 2014, 20, 156–159. [Google Scholar] [CrossRef]
- He, C.; Zhou, Y.; Li, Z.; Farooq, M.A.; Ajmal, I.; Zhang, H.; Zhang, L.; Tao, L.; Yao, J.; Du, B.; et al. Co-Expression of IL-7 Improves NKG2D-Based CAR T Cell Therapy on Prostate Cancer by Enhancing the Expansion and Inhibiting the Apoptosis and Exhaustion. Cancers 2020, 12, 1969. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Wang, Q.; Lal, P.; Carroll, A.M.; de la Llera-Moya, M.; Xu, X.; Greene, M.I. Suppression of Human Prostate Tumor Growth by a Unique Prostate-specific Monoclonal Antibody F77 Targeting a Glycolipid Marker. Proc. Natl. Acad. Sci. USA 2010, 107, 732–737. [Google Scholar] [CrossRef]
- Grover, P.; Nunez-Cruz, S.; Leferovich, J.; Wentz, T.; Bagchi, A.; Milone, M.C.; Greene, M.I. F77 Antigen is a Promising Target for Adoptive T Cell Therapy of Prostate Cancer. Biochem. Biophys. Res. Commun. 2023, 680, 51–60. [Google Scholar] [CrossRef]
- Wolf, P.; Alzubi, J.; Gratzke, C.; Cathomen, T. The Potential of CAR T Cell Therapy for Prostate Cancer. Nat. Rev. Urol. 2021, 18, 556–571. [Google Scholar] [CrossRef]
- Cao, Z.; Pu, C.; Jiang, X.; Han, G.; Shen, X.; Wang, W.; Ding, W.; Huang, Z.; Huang, X.; Jia, B.; et al. Novel PAP-targeted CAR-T Therapy Enhances Antitumor Efficacy Through CoupledCAR Approach. J. Immunother. Cancer 2025, 13, e011238. [Google Scholar] [CrossRef]
- Arndt, C.; Bergmann, R.; Striese, F.; Merkel, K.; Mathe, D.; Loureiro, L.R.; Mitwasi, N.; Kegler, A.; Fasslrinner, F.; Gonzalez Soto, K.E.; et al. Development and Functional Characterization of a Versatile Radio-/Immunotheranostic Tool for Prostate Cancer Management. Cancers 2022, 14, 1996. [Google Scholar] [CrossRef]
- Loureiro, L.R.; Pike, S.; Wuest, M.; Bergman, C.N.; KR, J.O.; Bergmann, R.; Feldmann, A.; Wuest, F.; Bachmann, M. Tackling Prostate Cancer with Theranostic E5B9-Bombesin Target Modules (TMs): From Imaging to Treatment with UniCAR T-Cells. Int. J. Mol. Sci. 2025, 26, 2686. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Zhu, Y.; Fang, Y.; Lyu, Z.; Yang, L. Emerging Trends in Clinical Allogeneic CAR Cell Therapy. Med 2025, 6, 100677. [Google Scholar] [CrossRef] [PubMed]
- Van der Vreken, A.; Vanderkerken, K.; De Bruyne, E.; De Veirman, K.; Breckpot, K.; Menu, E. Fueling CARs: Metabolic Strategies to Enhance CAR T-Cell Therapy. Exp. Hematol. Oncol. 2024, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Foeng, J.; Comerford, I.; McColl, S.R. Harnessing the Chemokine System to Home CAR-T Cells into Solid Tumors. Cell Rep. Med. 2022, 3, 100543. [Google Scholar] [CrossRef]
- Bansal, D.; Reimers, M.A.; Knoche, E.M.; Pachynski, R.K. Immunotherapy and Immunotherapy Combinations in Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 334. [Google Scholar] [CrossRef]
- Hovhannisyan, L.; Riether, C.; Aebersold, D.M.; Medova, M.; Zimmer, Y. CAR T Cell-based Immunotherapy and Radiation Therapy: Potential, Promises and Risks. Mol. Cancer 2023, 22, 82. [Google Scholar] [CrossRef]

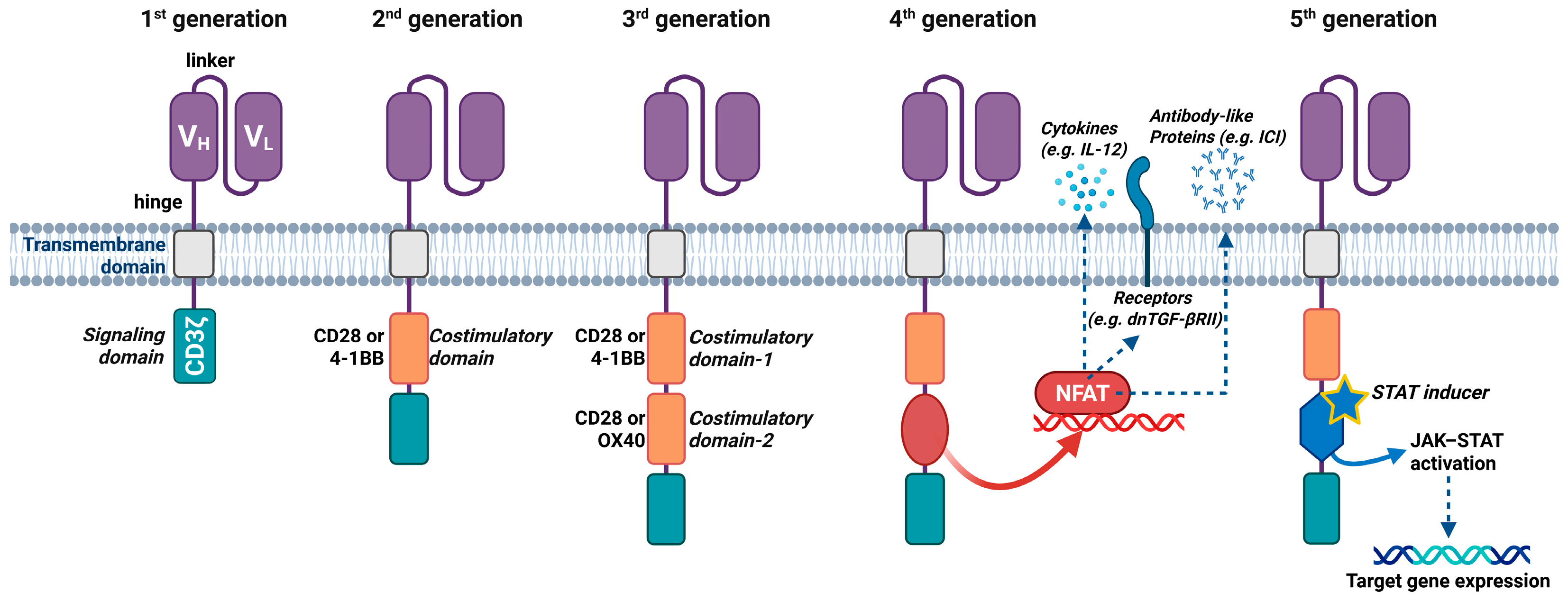

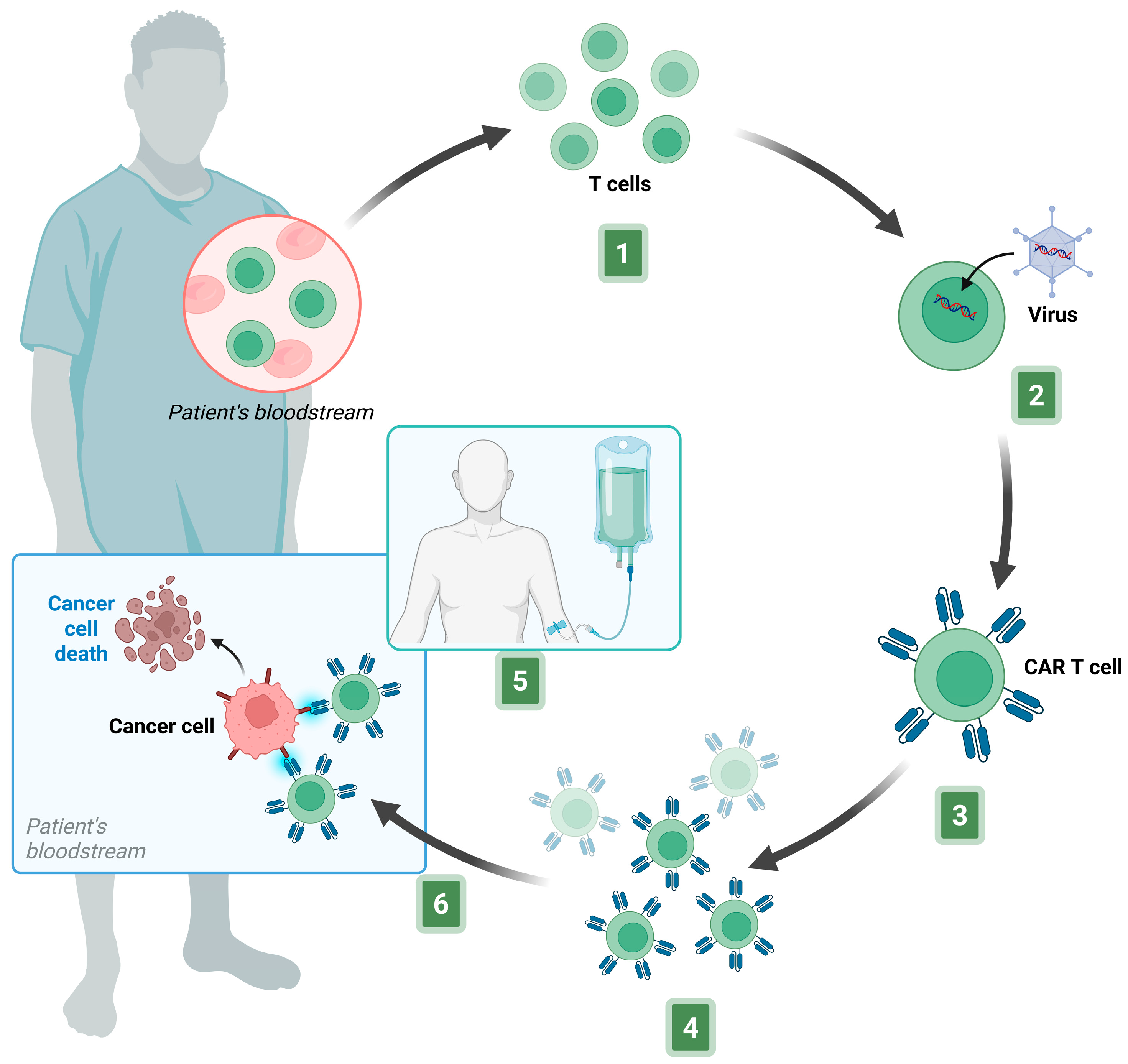
| Target Antigen | Expression Profile | Advantages | Disadvantages/Limitations | Clinical Status |
|---|---|---|---|---|
| PSMA | Highly overexpressed in primary and metastatic PCa; low expression in normal prostate, kidneys, salivary glands, and small intestine. | Highly validated antigen; extensive preclinical/clinical data; strong tumor specificity; suitability for radiopharmaceutical development. | On-target/off-tumor toxicity (salivary glands, kidneys); antigen heterogeneity; immune escape. | Multiple phase I/II trials |
| PSCA | Overexpressed in 90% of PCa; also expressed in other tumors; low expression in normal prostate, bladder and pancreas. | Coexpression with PSMA allows dual targeting; promising preclinical safety. | Severe immune-mediated adverse events (i.e., CRS). | Phase I trials |
| STEAP1 | Expressed in prostate, respiratory tract and CNS Markedly overexpressed in PCa and mCRPC. | Well-conserved across tumor stages; effective in combination with IL-12. | Limited clinical data | Phase I/II |
| STEAP2 | Expressed in prostate, brain, pancreas, ovary; upregulated in PCa. | Increased potency in hostile TME through armored CAR-T | Limited clinical data; moderate expression in non-prostatic tissues. | Phase I/II |
| EpCAM | Broadly expressed in epithelial tumors including PCa; limited expression in normal epithelia. | Accessible surface antigen. | High risk of on-target/off-tumor effects | Early-phase studies |
| KLK2 | Restricted to prostate epithelium and PCa; androgen-regulated enzyme. | Prostate-restricted; relevance to cancer progression. | Expression heterogeneity in advanced PCa. | Phase I |
| B7-H3 | Overexpressed in PCa and many solid tumors; low in normal tissue. | Excellent immunotherapeutic target; favorable tumor-to-normal expression ratio. | Limited clinical validation in PCa; cross-reactivity with vasculature. | Preclinical. |
| nfP2X7 | Aberrantly expressed on a broad range of malignancies | Unique cancer-specific epitope; not expressed in healthy tissues. | Early discovery stage; limited expression data in Protein Atlas. | Preclinical. |
| NKG2DL | Upregulated by stress, chronic inflammation, irradiation, or certain drug; expressed in PCa and TME | Broadly expressed on tumors and immunosuppressive cells | Expression on normal cells “on-target, off-tumor” toxicity risks. | Preclinical. |
| F77 | Expressed on PCa cells; absent in normal tissue. | Highly tumor-specific; potential marker for aggressive PCa. | Limited validation; target epitope heterogeneity. | Preclinical. |
| NCT Number | Target | CAR Construct | Intervention * | Tumor Type | Phase | N° of Patients | Outcome Measures | Status |
|---|---|---|---|---|---|---|---|---|
| NCT01140373 | PSMA | scFv(J591)–CD28–CD3ζ | 2nd-gen CAR-T cells | CMPC | I | 13 | DLT, CTC, PSA response, T-cell expansion/persistence | Active, not recruiting |
| NCT00664196 | PSMA | scFv(3D8)–CD3ζ | 1st-gen CAR-T cells IL–2 | CRPC | I | 18 | AEs, MTD, Tumor response, PK, PD | Suspended |
| NCT03089203 | PSMA | scFv(J591)–4-1BB–CD3ζ–T2A–dnTGFβRII | CART–PSMA–TGFβRDN cells +/− standard LD regimen | CRPC | I | 23 | TRAE, ORR (RECIST), PSA response, OS, PFS | Active, not recruiting |
| NCT04227275 | PSMA | scFv(J591)–4-1BB–CD3ζ–T2A–dnTGFβRII | CART–PSMA–TGFβRDN cells anakinra | mCRPC | I | 16 | DLT, AEs, ORR (PSA response), T-cell expansion/persistence | Stopped |
| NCT05489991 | PSMA | scFv(J591)–CD2–CD3ζ–T2A–dnTGFBR–T2A–PD1–CD28 | TmPSMA–02 CAR-T cells | mCRPC | I/II | 1 | AEs, SAEs, RP2D, MTD, DLT, ORR (RECIST) | Stopped |
| NCT06046040 | PSMA | scFv(J591)–CD2–CD3ζ–T2A–dnTGFBR–T2A–PD1–CD28 | TmPSMA–02 CAR-T cells | mCRPC | I | 18 | AEs, DLT; MTD | Recruiting |
| NCT04053062 | PSMA | scFv(J591)–4-1BB–CD3ζ–T2A–LIGHT-VTP | LIGHT–PSMA CAR-T cells | CRPC | I | 12 | AEs | Suspended |
| NCT04249947 | PSMA | Undisclosed CAR | P–PSMA–101 CAR-T cells rimiducid | mCRPC | I | 40 | AEs, DLT, ORR (RECIST) | Terminated |
| NCT04768608 | PSMA | Undisclosed CAR | PD1–PSMA–CART cells | CRPC | I | 3 | AEs, PSA response, ORR, T-cells expansion/persistence | Completed |
| NCT05732948 | PSMA PSCA | Undisclosed CAR | PD–1 Silent PSMA/PSCA CAR-T cells | mCRPC | I | 12 | AEs, DLT | Recruiting |
| NCT04429451 | PSMA | anti-PSMAscFv–CD28–CD27–CD3ζ–T2A–iCas9 | 4SCAR–PSMA T–cells rimiducid | PCa | I/II | 100 | AEs, DLT, ORR, PFS, OS, T-cells expansion/persistence | Recruiting |
| NCT05437315 | PSMA GD2 | anti–GD2/PSMAscFvs–CD28–CD27–CD3ζ–T2A–iCas9 | bi–4SCAR–GD2/PSMA T–cells rimiducid | PCa | I/II | 60 | AEs, DLT, ORR, PFS, OS, T-cells expansion/persistence | Recruiting |
| NCT04633148 | PSMA | Undisclosed CAR | UniCAR02–T TMpPSMA | CRPC | I | 16 | AEs, DLT, MTD, RP2D, ORR, DOR, PFS, OS, PSA response, CTC | Terminated |
| NCT05656573 | PSMA | Undisclosed CAR | NT–1921 | mCRPC | I | 20 | AEs, PFS, OS, CTC, cytokine profile, PSA response, T-cells expansion/persistence | Recruiting |
| NCT05354375 | PSMA | Undisclosed CAR | PSMA–CAR-T cells | CRPC | I | 20 | AEs, ORR, PFS, OS | Recruiting |
| NCT06228404 | PSMA | Undisclosed CAR | PSMA–CAR-T cells | refractory CRPC | I | 18 | DLT, PSA response, ORR | Recruiting |
| NCT Number | Target | CAR Construct | Intervention * | Tumor Type | Phase | N° of Patients | Outcome Measures | Status |
|---|---|---|---|---|---|---|---|---|
| NCT03873805 | PSCA | scFv(hA11)–4-1BB–CD3ζ | PSCA CAR-T cells | mCRPC | I | 14 | AEs, DLT, RP2D, PFS, OS, cytokine profile, T-cells expansion/persistence | Active, not recruiting |
| NCT05805371 | PSCA | scFv(hA11)–4-1BB–CD3ζ | PSCA CAR-T cells +/− radiation | mCRPC | Ib | 21 | AEs, DLT, RP2D, PFS, OS, PSA response, cytokine profile, T-cells expansion/persistence | Recruiting |
| NCT02744287 | PSCA | iMC–T2A–scFv(hA11)–CD3ζ | BPX-601 rimiducid | mCRPC | I II | 151 | AEs, DLT, MTD, RP2D, PD, ORR (RECIST) | Suspended |
| NCT06236139 | STEAP1 | scFv(mAb120.545)–4-1BB–CD3ζ | STEAP1 CAR-T cells, enzalutamide | mCRPC | I II | 48 | AEs, ORR (RECIST) PSA response, TTR, DOR, PFS, OS | Recruiting |
| NCT06267729 | STEAP2 | scFv(40A3)–4-1BB–CD3ζ–T2A–dnTGFβRII | AZD0754 | mPCa | I II | 60 | AEs, DLT, PSA response, ORR, TTR, DOR, PFS, OS | Recruiting |
| NCT03013712 | EpCAM | Undisclosed CAR | EpCAM CAR-T cells | PCa | I II | 60 | AEs, ORR (RECIST), OS | Unknown status |
| NCT05022849 | KLK2 | Undisclosed CAR | JNJ–75229414 +/− Bridging therapy with anti-AR agents/docetaxel/radiotherapy | mCRPC | I | 15 | AEs, DLT, ORR, TTR, DOR, T–cell expansion/persistence | Active, not recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrò, M.L.; Ettari, R.; Di Chio, C.; De Luca, F.; Previti, S.; Zappalà, M. CAR-T Cell Therapy for Prostate Cancer: Current Advances and Future Perspectives. Biomedicines 2025, 13, 2545. https://doi.org/10.3390/biomedicines13102545
Calabrò ML, Ettari R, Di Chio C, De Luca F, Previti S, Zappalà M. CAR-T Cell Therapy for Prostate Cancer: Current Advances and Future Perspectives. Biomedicines. 2025; 13(10):2545. https://doi.org/10.3390/biomedicines13102545
Chicago/Turabian StyleCalabrò, Maria Luisa, Roberta Ettari, Carla Di Chio, Fabiola De Luca, Santo Previti, and Maria Zappalà. 2025. "CAR-T Cell Therapy for Prostate Cancer: Current Advances and Future Perspectives" Biomedicines 13, no. 10: 2545. https://doi.org/10.3390/biomedicines13102545
APA StyleCalabrò, M. L., Ettari, R., Di Chio, C., De Luca, F., Previti, S., & Zappalà, M. (2025). CAR-T Cell Therapy for Prostate Cancer: Current Advances and Future Perspectives. Biomedicines, 13(10), 2545. https://doi.org/10.3390/biomedicines13102545







