Epidemiology of Dermatomyositis and Other Idiopathic Inflammatory Myopathies in Northern Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Outcome Variables
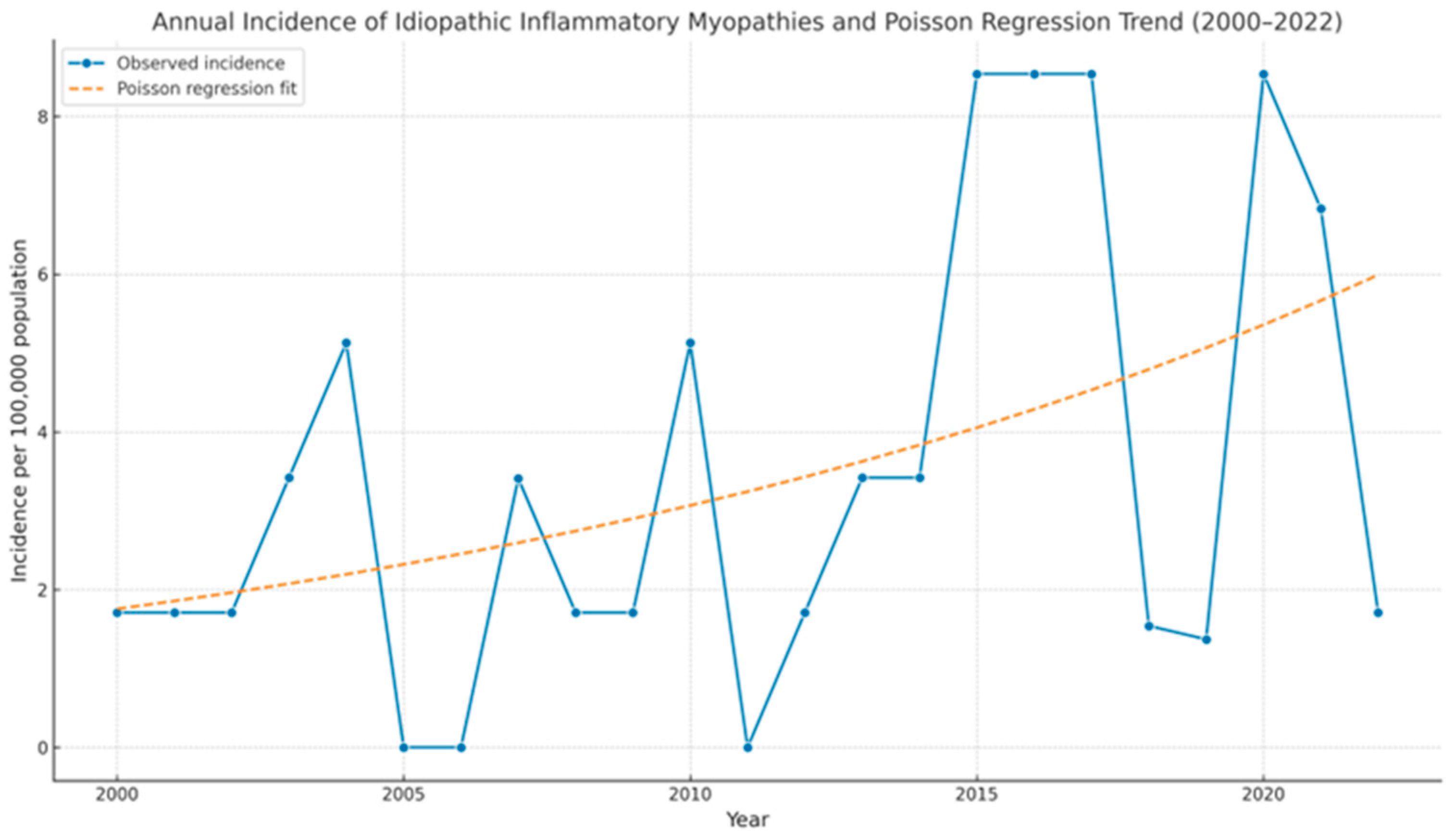
2.3. Statistical Analysis
2.4. Literature Review
3. Results
3.1. Dermatomyositis
3.1.1. Adult DM
3.1.2. Juvenile DM
3.2. Antisynthetase Syndrome
3.3. Immune-Mediated Necrotizing Myopathy
3.4. Polymyositis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plotz, P.H.; Rider, L.G.; Targoff, I.N.; Raben, N.; O’Hanlon, T.P.; Miller, F.W. NIH conference. Myositis: Immunologic contributions to understanding cause, pathogenesis, and therapy. Ann. Intern. Med. 1995, 122, 715–724. [Google Scholar] [CrossRef]
- Lundberg, I.E.; de Visser, M.; Werth, V.P. Classification of myositis. Nat. Rev. Rheumatol. 2018, 14, 269–278. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 2017, 69, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J. Current classification and management of inflammatory myopathies. J. Neuromuscul. Dis. 2018, 5, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Khoo, T.; Lilleker, J.B.; Thong, B.Y.H.; Leclair, V.; Lamb, J.A.; Chinoy, H. Epidemiology of the idiopathic inflammatory myopathies. Nat. Rev. Rheumatol. 2023, 19, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Polymyositis, dermatomyositis and inclusion-body myositis. N. Engl. J. Med. 1991, 325, 1487–1498. [Google Scholar] [CrossRef]
- Dobloug, C.; Garen, T.; Bitter, H.; Stjärne, J.; Stenseth, G.; Grøvle, L.; Sem, M.; Gran, J.T.; Molberg, Ø. Prevalence and clinical characteristics of adult polymyositis and dermatomyositis; data from a large and unselected Norwegian cohort. Ann. Rheum. Dis. 2015, 74, 1551–1556. [Google Scholar] [CrossRef]
- Bernatsky, S.; Joseph, L.; Pineau, C.A.; Bélisle, P.; Boivin, J.F.; Banerjee, D.; Clarke, A.E. Estimating the prevalence of polymyositis and dermatomyositis from administrative data: Age, sex and regional differences. Ann. Rheum. Dis. 2009, 68, 1192–1196. [Google Scholar] [CrossRef]
- Opinc, A.H.; Makowska, J.S. Antisynthetase syndrome—Much more than just a myopathy. Semin. Arthritis Rheum. 2021, 51, 72–83. [Google Scholar] [CrossRef]
- Badrising, U.A.; Maat-Schieman, M.; Van Duinen, S.G.; Breedveld, F.; van Doorn, P.; Van Engelen, B.; Van Den Hoogen, F.; Hoogendijk, J.; Howeler, C.; De Jager, A.; et al. Epidemiology of inclusion body myositis in the Netherlands: A nationwide study. Neurology 2000, 55, 1385–1387. [Google Scholar] [CrossRef]
- Dobloug, G.C.; Antal, E.A.; Sveberg, L.; Garen, T.; Bitter, H.; Stjärne, J.; Grøvle, L.; Gran, J.T.; Molberg, Ø. High prevalence of inclusion body myositis in Norway; a population-based clinical epidemiology study. Eur. J. Neurol. 2015, 22, 672-e41. [Google Scholar] [CrossRef]
- Greenberg, S.A. Inclusion body myositis: Clinical features and pathogenesis. Nat. Rev. Rheumatol. 2019, 15, 257–272. [Google Scholar] [CrossRef]
- Leff, R.L.; Burgess, S.H.; Miller, F.W.; Love, L.A.; Targoff, I.N.; Dalakas, M.C.; Joffe, M.M.; Plotz, P.H. Distinct seasonal patterns in the onset of adult idiopathic inflammatory myopathy in patients with anti-Jo-1 and anti-signal recognition particle autoantibodies. Arthritis Rheum. 1991, 34, 1391–1396. [Google Scholar] [CrossRef]
- Medsger, T.A.J.; Dawson, W.N.J.; Masi, A.T. The epidemiology of polymyositis. Am. J. Med. 1970, 48, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.; Buchbinder, R.; Jolley, D.; Dennett, X.; Buchanan, R. Incidence of inflammatory myopathies in Victoria, Australia, and evidence of spatial clustering. J. Rheumatol. 1999, 26, 1094–1100. [Google Scholar] [PubMed]
- Allenbach, Y.; Benveniste, O.; Stenzel, W.; Boyer, O. Immune-mediated necrotizing myopathy: Clinical features and pathogenesis. Nat. Rev. Rheumatol. 2020, 16, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Connors, G.R.; Christopher-Stine, L.; Oddis, C.V.; Danoff, S.K. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years? Chest 2010, 138, 1464–1474. [Google Scholar] [CrossRef]
- Bendewald, M.J.; Wetter, D.A.; Li, X.; Davis, M.P.D. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: A population-based study in Olmsted County, Minnesota. Arch. Dermatol. 2010, 146, 26–30. [Google Scholar] [CrossRef]
- Bolender, C.M.; Jimenez, A.; Clarke, J.T.; Willson, T.M.; Stevens, V.W.; Rhoads, J.L.W. Incidence of dermatomyositis in a nationwide cohort study of US veterans. JAMA Dermatol. 2022, 158, 1321–1323. [Google Scholar] [CrossRef]
- Kronzer, V.L.; Kimbrough, B.A.; Crowson, C.S.; Davis, J.M., 3rd; Holmqvist, M.; Ernste, F.C. Incidence, prevalence, and mortality of dermatomyositis: A population-based cohort study. Arthritis Care Res. 2023, 75, 348–355. [Google Scholar] [CrossRef]
- Balci, M.A.; Donmez, S.; Saritas, F.; Bas, V.; Pamuk, O.N. The epidemiology of dermatomyositis in northwestern Thrace region in Turkey: Epidemiology of dermatomyositis in Turkey. Rheumatol. Int. 2017, 37, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Mendez, E.P.; Lipton, R.; Ramsey-Goldman, R.; Roettcher, P.; Bowyer, S.; Dyer, A.; Pachman, L.M.; NIAMS Juvenile DM Registry Physician Referral Group. US incidence of juvenile dermatomyositis, 1995–1998: Results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003, 49, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Concannon, A.; Han, D.Y. Incidence, severity and clinical manifestations of juvenile dermatomyositis among Maori and Pacific Island compared to European children. J. Paediatr. Child Health 2021, 57, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Moegle, C.; Severac, F.; Lipsker, D. Epidemiology of juvenile dermatomyositis in Alsace. Br. J. Dermatol. 2020, 182, 1307–1308. [Google Scholar] [CrossRef]
- Enders, F.B.; Hofer, M. Juvenile dermatomyositis in Western Switzerland. Pediatr. Rheumatol. 2011, 9 (Suppl. 1), P52. [Google Scholar] [CrossRef]
- Symmons, D.P.; Sills, J.A.; Davis, S.M. The incidence of juvenile dermatomyositis: Results from a nation-wide study. Br. J. Rheumatol. 1995, 34, 732–736. [Google Scholar] [CrossRef]
- Coffey, C.; Wang, L.; Duong, S.; Hulshizer, C.; Crowson, C.; Ryu, J.; Ernste, F. Incidence of Antisynthetase Syndrome and Risk of Malignancy in a Population-based Cohort (1998-2019) [abstract]. Arthritis Rheumatol. 2021, 73 (Suppl. S9). Available online: https://acrabstracts.org/abstract/incidence-of-antisynthetase-syndrome-and-risk-of-malignancy-in-a-population-based-cohort-1998-2019/ (accessed on 30 January 2023).
- Felice, K.J.; North, W.A. Inclusion body myositis in Connecticut: Observations in 35 patients during an 8-year period. Medicine 2001, 80, 320–327. [Google Scholar] [CrossRef]
- Shelly, S.; Mielke, M.M.; Mandrekar, J.; Milone, M.; Ernste, F.C.; Naddaf, E.; Liewluck, T. Epidemiology and natural history of inclusion body myositis: A 40-year population-based study. Neurology 2021, 96, e2653–e2661. [Google Scholar] [CrossRef]
- Phillips, B.A.; Zilko, P.J.; Mastaglia, F.L. Prevalence of sporadic inclusion body myositis in Western Australia. Muscle Nerve 2000, 23, 970–972. [Google Scholar] [CrossRef]
- Lefter, S.; Hardiman, O.; Ryan, A.M. A population-based epidemiologic study of adult neuromuscular disease in the Republic of Ireland. Neurology 2017, 88, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, U.; Pullerits, R.; Lindberg, C.; Oldfors, A. Epidemiology, survival, and clinical characteristics of inclusion body myositis. Ann. Neurol. 2022, 92, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.; Mielke, M.M.; Paul, P.; Milone, M.; Tracy, J.A.; Mills, J.R.; Klein, C.J.; Ernste, F.C.; Mandrekar, J.; Liewluck, T. Incidence and prevalence of immune-mediated necrotizing myopathy in adults in Olmsted County, Minnesota. Muscle Nerve 2022, 65, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Peña, D.; Ocejo-Vinyals, J.G.; Mazariegos-Cano, J.; Pelayo-Negro, A.L.; Remuzgo-Martínez, S.; Genre, F.; García-Dorta, A.; Renuncio-García, M.; Martínez-Taboada, V.M.; García-Ibarbia, C.; et al. Epidemiological and genetic features of anti-3-hydroxy-3-methylglutaryl-CoA reductase necrotizing myopathy: Single-center experience and literature review. Eur. J. Intern. Med. 2022, 101, 86–92. [Google Scholar] [CrossRef]
- Araki, S.; Uchino, M.; Kumamoto, T. Prevalence studies of multiple sclerosis, myasthenia gravis, and myopathies in Kumamoto district, Japan. Neuroepidemiology 1987, 6, 120–129. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; el-Mangoush, M.A.; Gerryo, S.E. Descriptive epidemiology of selected neuromuscular disorders in Benghazi, Libya. Acta Neurol. Scand. 1987, 75, 95–100. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Primers 2021, 7, 86. [Google Scholar] [CrossRef]
- Hallowell, R.W.; Paik, J.J. Myositis-associated interstitial lung disease: A comprehensive approach to diagnosis and management. Clin. Exp. Rheumatol. 2022, 40, 373–383. [Google Scholar] [CrossRef]

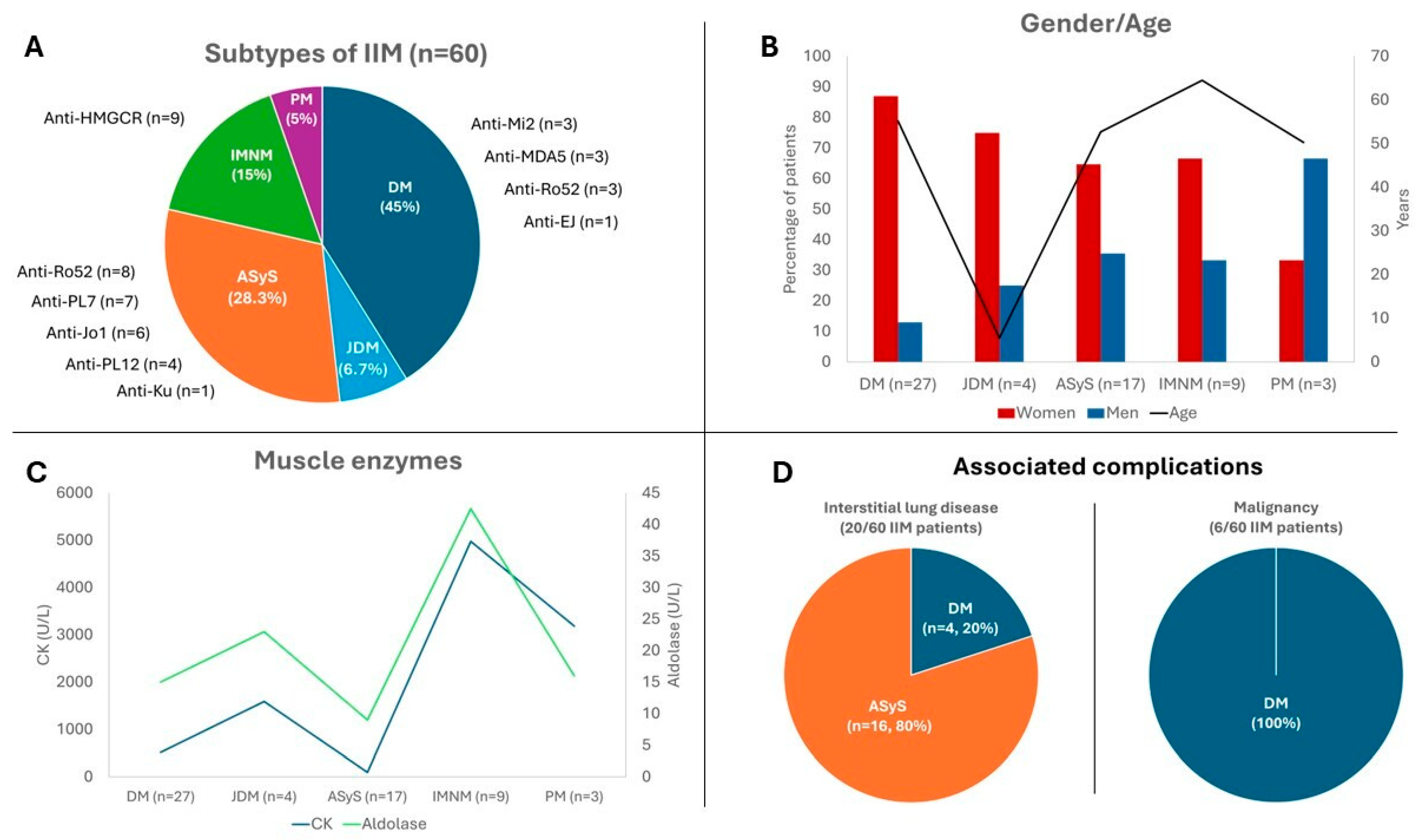
| Adult DM (n = 27) | Juvenile DM (n = 4) | ASyS (n = 17) | IMNM (n = 9) | PM (n = 3) | |
|---|---|---|---|---|---|
| Demographic features | |||||
| Sex (female/male), n (%) | 23 (87%)/4 (13%) | 3 (75)/1 (25) | 11 (64.7)/6 (35.3) | 6 (66.6)/3 (33.3) | 1 (33.3)/2 (66.6) |
| Age at diagnosis, mean ± SD | 55.2 ± 17.9 | 5.5 ± 1.5 | 52.7 ± 11.7 | 64.5 ± 7 | 50.3 ± 11.0 |
| Duration of symptoms after diagnosis (months), mean ±SD | 0 [0–4.8] | 0 [0–2] | 6 [6–12] | 4 [3–5] | 18.0 [15–21] |
| Dyslipidemia, n (%) | 9 (33.3) | 0 | 4 (23.5) | 9 | 1 (33.3) |
| Statin exposure, n (%) | 4 (14.8) | 0 | 4 (23.5) | 9 | 1 (33.3) |
| Arterial Hypertension, n (%) | 7 (25.9) | 0 | 1 (5.9) | 7 | 1 (33.3) |
| Type 2 DM, n (%) | 4 (14.8) | 0 | 0 | 7 | 0 |
| Clinical manifestations | |||||
| Muscle weakness, n (%) | 21 (77.8) | 4 (100) | 4 (28.6) | 9 (100) | 2 (66.6) |
| Myalgia, n (%) | 9 (33.3) | 2 (50) | 4 (28.6) | 4 (44.4) | 2 (66.6) |
| Dysphagia, n (%) | 11 (40.7) | 3 (75) | 2 (11.8) | 3 (33.3) | 0 |
| Skin rash, n (%) | 26 (96.3) | 4 (100) | 3 (17.6) | 0 | 0 |
| Gottron’s papules, n (%) | 22 (81.5) | 2 (50) | 1 (5.6) | 0 | 0 |
| Gottron’s sign, n (%) | 22 (81.5) | 2 (50) | 0 | 0 | 0 |
| Heliothrope rash, n (%) | 8 (29.6) | 2 (50) | 0 | 0 | 0 |
| Raynaud phenomenon, n (%) | 3 (11.1) | 0 | 9 (53) | 0 | 1 (66.6) |
| Malignancy, n (%) | 6 (22.2) | 0 | 0 | 0 | 0 |
| Interstitial Lung Disease, n (%) | 4 (14.8) | 0 | 16 (94) | 0 | 0 |
| Arthritis, n (%) | 7 (25.9) | 0 | 5 (29.4) | 0 | 0 |
| Calcinosis, n (%) | 4 (14.8) | 2 (50) | 0 | 0 | 0 |
| Laboratory tests | |||||
| CK, median [IQR] | 516 [186–1217] | 1591 [946–2027] | 100 [40–885] | 4977 [3273–9271] | 3158 [1807–3629] |
| Aldolase, median [IQR] | 15 [10–23] | 23 [21.5–24] | 9 [9–16] | 42.5 [26.5–63] | 16 [11–53] |
| Anti-MDA5, n (%) | 3 (10) | 0 | 0 | 0 | 0 |
| Anti-Mi2, n (%) | 3 (10) | 0 | 0 | 0 | 0 |
| Anti-TIF-Y, n (%) | 0 | 0 | 0 | 0 | 0 |
| Anti-JO1, n (%) | 0 | 0 | 6 (35.3) | 0 | 0 |
| Anti-PL7, n (%) | 0 | 0 | 7 (41.2) | 0 | 0 |
| Anti-PL12, n (%) | 0 | 0 | 4 (23.5) | 0 | 0 |
| Anti-EJ, n (%) | 1 (3.3) | 0 | 0 | 0 | 0 |
| Anti-HMGCR/SRP, n (%) | 0 | 0 | 0 | 9 (100)/0 | 0 |
| Anti-RO52, n (%) | 3 (10) | 0 | 8 (47.0) | 0 | 0 |
| Anti-Ku, n (%) | 0 | 0 | 1 (5.9) | 0 | 0 |
| Anti-PM-SCL100, n (%) | 0 | 0 | 0 | 0 | 1 (33.3) |
| Imaging procedures | |||||
| Inflammatory findings on MRI, n/N (%) | 9/11 (82) | 1/1 (100) | 10/10 (100) | 4/4 (100) | 2/3 (66.6) |
| Biopsy | |||||
| Muscle biopsy, n (%) | 9 (33.3) | 2 (50) | 10 (58.8) | 9 (100) | 1 (33.3) |
| Adult DM (n = 27) | Juvenile DM (n = 4) | ASyS (n = 17) | IMNM (n = 9) | PM (n = 3) | |
|---|---|---|---|---|---|
| Intravenous corticosteroids, n (%) | 15 (55.6) | 1 (25) | 5 (29.4) | 0 (0) | 0 (0) |
| Oral corticosteroids, n (%) | 27 (100) | 4 (100) | 17 (100) | 7 (77.8) | 3 (100) |
| Hydroxychloroquine, n (%) | 13 (48.1) | 0 (0) | 0 (0) | 0(0) | 0 (0) |
| Azathioprine, n (%) | 14 (51.9) | 0 (0) | 8 (58.8) | 3 (33.3) | 2 (66.6) |
| Methotrexate, n (%) | 10 (37) | 4 (100) | 4 (23.5) | 2 (22.2) | 2 (66.6) |
| Mycophenolate, n (%) | 0 (0) | 0 (0) | 9 (52.9) | 0 (0) | 0 (0) |
| Cyclophosphamide, n (%) | 0 (0) | 0 (0) | 4 (23.5) | 0 (0) | 0 (0) |
| Rituximab, n (%) | 11 (40.7) | 0 (0) | 13 (76.5) | 4 (44.4) | 0 (0) |
| Intravenous immune-globulins, n (%) | 17 (72.7) | 2 (50) | 1 (5.9) | 6 (66.6) | 1 (33.3) |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Author, Year (Ref) | Country, Region | Study Period | Diagnosis Criteria | Number of Cases | Prevalence | Incidence (Per 100,000 People Except Where Noted) |
| Adult DM | ||||||
| Bendewald M et al., 2010 [18] | USA | 1976–2007 | Adapted from Gerami et al. | 20 DM | 21.4 | 0.96 |
| Bolender C et al. 2022 [19] | USA | 2005–2019 | Coded registration | 679 DM | 0.8 | |
| Kronzer et al. 2023 [20] | USA | 1995–2019 | EULAR/ACR 2017 | 29 DM | 13.0 | 1.1 |
| Balci M et al. 2017 [21] | Turkey | 2004–2014 | Bohan and Peter | 23 DM | 3.2 | 0.37 |
| Present study, 2025 | Spain | 2000–2022 | EULAR/ACR 2017 | 27 DM | 9.0 | 0.36 |
| Juvenile DM | ||||||
| Mendez et al. 2003. [22] | USA | 1994–1999 | Bohan and Peter | 395 JDM | 0.32 | |
| Concannon C et al. 2021. [23] | New Zealand | 2000–2020 | Bohan and Peter | 31 JDM | 0.24 | |
| Moegle C et al. 2020. [24] | France | 2000–2015 | Bohan and Peter or EULAR/ACR 2017 | 16 JDM | 3.78 | 0.27 |
| Enders et al. 2011 [25] | Switzerland | 1997–2010 | Expertise Criteria | 13 JDM | 0.35 | |
| Symmons et al. 1995 [26] | UK | 1992–1993 | Bohan and Peter | 48 JDM | 0.19 | |
| Present study, 2025 | Spain | 2000–2022 | EULAR/ACR 2017 criteria | 4 JDM | 1.0 | 0.006 |
| (b) | ||||||
| Author, Year (Ref) | Country, Region | Study Period | Diagnosis Criteria | Number of Cases | Prevalence | Incidence |
| ASyS | ||||||
| Coffey et al. 2021 [27] | USA | 1998–2019 | Salmon’s Criteria | 13 ASyS | 9.0 | 0.56 |
| Present Study, 2025 | Spain | 2000–2022 | Connor’s Criteria | 17 ASyS | 5.0 | 0.26 |
| IBM | ||||||
| Felice et al. 2001 [28] | USA | 1992–2000 | Griggs Criteria | 35 IBM | 2.9 | |
| Shelly et al. 2021 [29] | USA | 2010–2019 | ENMC 2011 | 21 IBM | 19.2 | 0.47 |
| Phillips et al. 2000 [30] | Australia | 1988–1998 | Griggs Criteria | 17 IBM | 3.53 | |
| Lefter et al. 2017 [31] | Ireland | 1990–2013 | MRC | 149 IBM | 11.7 | |
| Badrising et al. 2000 [10] | Netherlands | 1982–1999 | ENCM | 128 IBM | 3.2 | 0.25 |
| Dobloug et al. 2015 [11] | Norway | 2003–2012 | ENMC 2011 | 95 IBM | 3.3 | 0.2–0–6 |
| Lindgren et al. 2022 [32] | Sweden | 1985–2017 | ENMC 2011 | 128 IBM | 3.2 | 0.25 |
| Present Study, 2025 | Spain | 2000–2022 | ENMC | 0 IBM | ||
| IMNM | ||||||
| Shelly S et al. 2022 [33] | USA | 1999–2019 | ENMC Criteria | 7 IMNM | 1.9 | 0.59 |
| Prieto-Peña et al. 2022 [34] | Spain | 2016–2021 | ENMC Criteria | 8 IMNM | 3.0 | 0.6 |
| Present Study 2025 | Spain | 2000–2022 | ENMC Criteria | 9 IMNM | 3.0 | 0.14 |
| PM | ||||||
| Araki et al. 1987 [35] | Japan | 1977–1982 | Walton-Adams Criteria | 27 PM | 5.0 | |
| Radhakrishnan et al. 1987 [36] | Libya | 1983–1985 | DeVere and Bradley Criteria | 13 PM | 0.84 | |
| Present Study 2025 | Spain | 2000–2022 | EULAR/ACR 2017 Criteria | 3 PM | 1.0 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrales-Selaya, C.; Prieto-Peña, D.; Martínez-López, D.; Benavides-Villanueva, F.; Blanco, R. Epidemiology of Dermatomyositis and Other Idiopathic Inflammatory Myopathies in Northern Spain. Biomedicines 2025, 13, 2537. https://doi.org/10.3390/biomedicines13102537
Corrales-Selaya C, Prieto-Peña D, Martínez-López D, Benavides-Villanueva F, Blanco R. Epidemiology of Dermatomyositis and Other Idiopathic Inflammatory Myopathies in Northern Spain. Biomedicines. 2025; 13(10):2537. https://doi.org/10.3390/biomedicines13102537
Chicago/Turabian StyleCorrales-Selaya, Cristina, Diana Prieto-Peña, David Martínez-López, Fabricio Benavides-Villanueva, and Ricardo Blanco. 2025. "Epidemiology of Dermatomyositis and Other Idiopathic Inflammatory Myopathies in Northern Spain" Biomedicines 13, no. 10: 2537. https://doi.org/10.3390/biomedicines13102537
APA StyleCorrales-Selaya, C., Prieto-Peña, D., Martínez-López, D., Benavides-Villanueva, F., & Blanco, R. (2025). Epidemiology of Dermatomyositis and Other Idiopathic Inflammatory Myopathies in Northern Spain. Biomedicines, 13(10), 2537. https://doi.org/10.3390/biomedicines13102537







