Association Between Atopic Dermatitis and Colorectal Cancer Risk: A Korean Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval, Data Acquisition, and Study Framework
2.2. Outcome: Colorectal Cancer
2.3. Exposure: Atopic Dermatitis
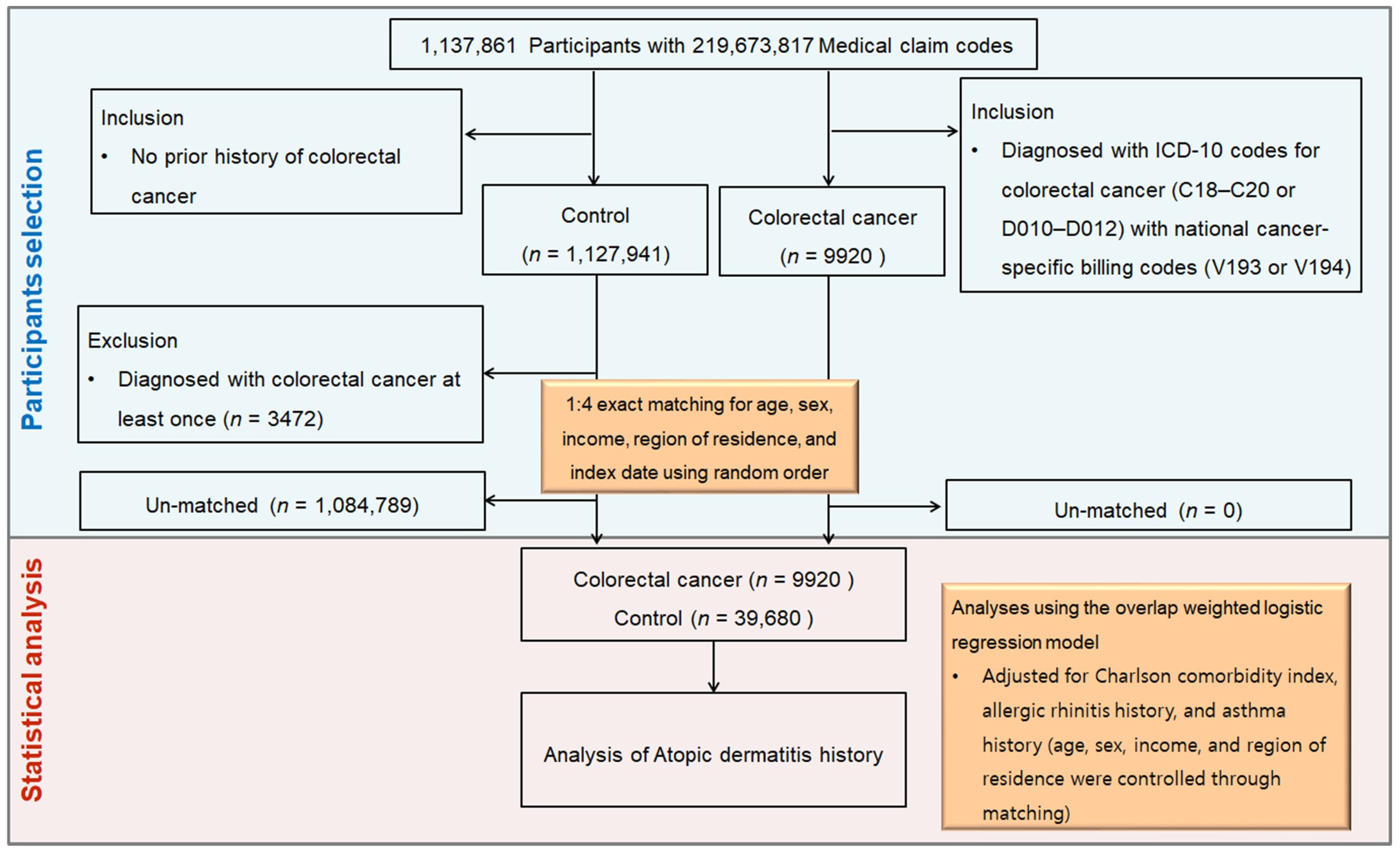
2.4. Participant Selection and Matching
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Relationship Between Colorectal Cancer and Atopic Dermatitis
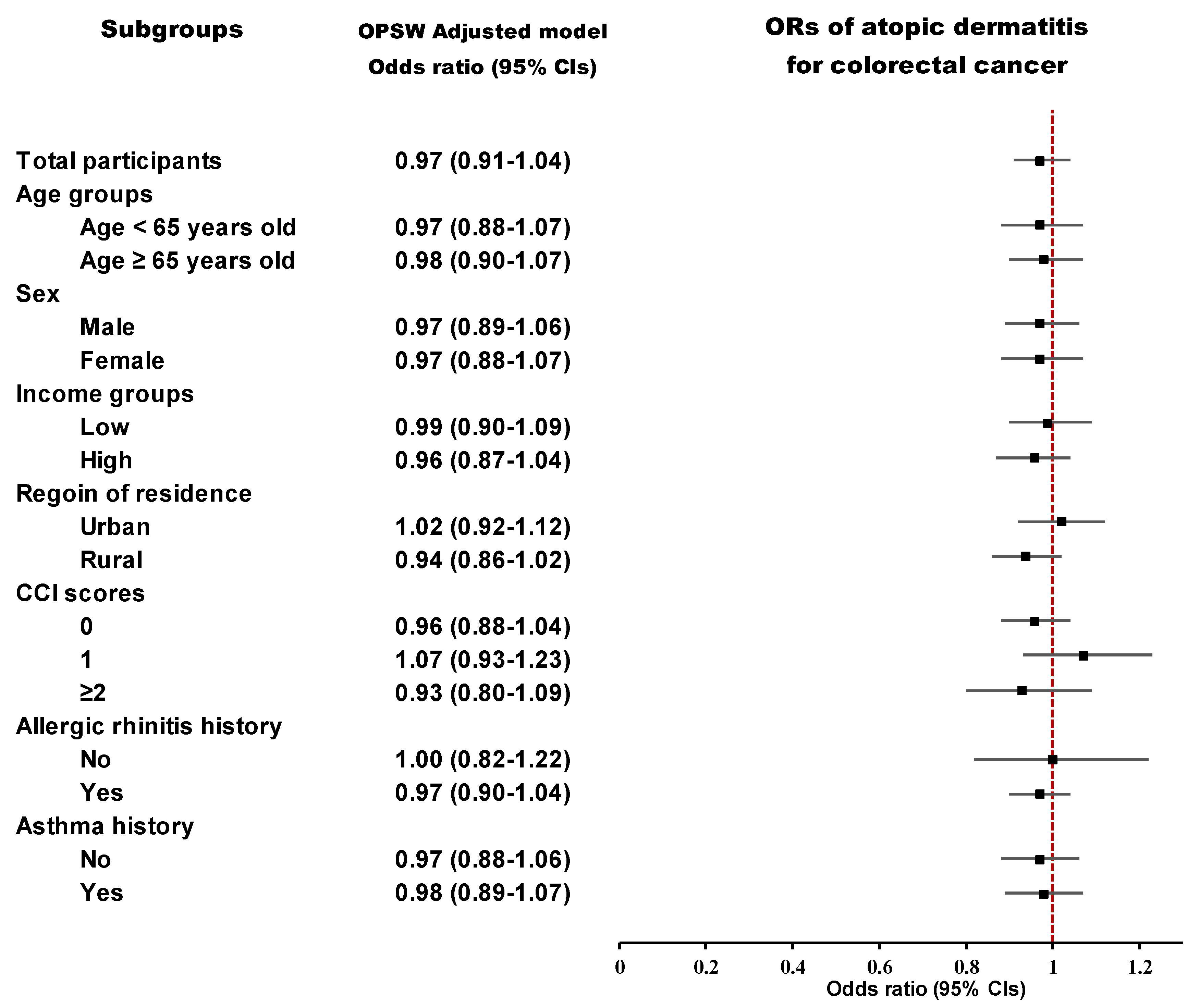
3.3. Subgroup Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2018. Cancer Res. Treat. 2021, 53, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef]
- Luk, D.; Hon, K.L.E.; Dizon, M.V.C.; Leong, K.F.; Tay, Y.K.; Koh, M.J.; Chandran, N.S.; Wananukul, S.; Chatproedprai, S.; Luger, T. Practical Recommendations for the Topical Treatment of Atopic Dermatitis in South and East Asia. Dermatol. Ther. 2021, 11, 275–291. [Google Scholar] [CrossRef]
- Eyerich, K.; Gooderham, M.J.; Silvestre, J.F.; Shumack, S.P.; Mendes-Bastos, P.; Aoki, V.; Ortoncelli, M.; Silverberg, J.I.; Teixeira, H.D.; Chen, S.H.; et al. Real-world clinical, psychosocial and economic burden of atopic dermatitis: Results from a multicountry study. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 340–353. [Google Scholar] [CrossRef]
- Egeberg, A.; Andersen, Y.M.; Gislason, G.H.; Skov, L.; Thyssen, J.P. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy 2017, 72, 783–791. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Halling, A.S.; Schmid-Grendelmeier, P.; Guttman-Yassky, E.; Silverberg, J.I. Comorbidities of atopic dermatitis-what does the evidence say? J. Allergy Clin. Immunol. 2023, 151, 1155–1162. [Google Scholar] [CrossRef]
- Wang, L.; Bierbrier, R.; Drucker, A.M.; Chan, A.W. Noncutaneous and Cutaneous Cancer Risk in Patients With Atopic Dermatitis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 158–171. [Google Scholar] [CrossRef]
- Bílek, O.; Munzarová, M.; Zahálková, M. Atopy and cancer. Neoplasma 1975, 22, 441–444. [Google Scholar]
- Vena, J.E.; Bona, J.R.; Byers, T.E.; Middleton, E., Jr.; Swanson, M.K.; Graham, S. Allergy-related diseases and cancer: An inverse association. Am. J. Epidemiol. 1985, 122, 66–74. [Google Scholar] [CrossRef]
- Wang, H.; Rothenbacher, D.; Löw, M.; Stegmaier, C.; Brenner, H.; Diepgen, T.L. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int. J. Cancer 2006, 119, 695–701. [Google Scholar] [CrossRef]
- Jacenik, D.; Karagiannidis, I.; Beswick, E.J. Th2 cells inhibit growth of colon and pancreas cancers by promoting anti-tumorigenic responses from macrophages and eosinophils. Br. J. Cancer 2023, 128, 387–397. [Google Scholar] [CrossRef]
- Bai, R.; Zheng, Y.; Dai, X. Atopic dermatitis: Diagnosis, molecular pathogenesis, and therapeutics. Mol. Biomed. 2025, 6, 71. [Google Scholar] [CrossRef]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef]
- Ojanperä, A.; Sirniö, P.; Elomaa, H.; Äijälä, V.K.; Karjalainen, H.; Tapiainen, V.V.; Kastinen, M.; Kehusmaa, A.; Rahkola, O.; Pohjanen, V.M.; et al. Significance of Th1 and Th2 Cell Densities and Th1/Th2 Cytokine Profiles in Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Perez, D.; Prados-Lopez, B.; Galvez, J.; Leon, J.; Carazo, A. Eosinophils in Colorectal Cancer: Emerging Insights into Anti-Tumoral Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 6098. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.Y.; Lai, P.Y.; Hu, J.M.; Hsu, C.H.; Chen, Y.C.; Tian, Y.F.; You, S.L.; Hsiao, C.W.; Chou, Y.C.; Sun, C.A. Association between atopic dermatitis and colorectal cancer risk: A nationwide cohort study. Medicine 2020, 99, e18530. [Google Scholar] [CrossRef]
- Ma, W.; Yang, J.; Li, P.; Lu, X.; Cai, J. Association between allergic conditions and colorectal cancer risk/mortality: A meta-analysis of prospective studies. Sci. Rep. 2017, 7, 5589. [Google Scholar] [CrossRef]
- Tambe, N.A.; Wilkens, L.R.; Wan, P.; Stram, D.O.; Gilliland, F.; Park, S.L.; Cozen, W.; Martínez-Maza, O.; Le Marchand, L.; Henderson, B.E.; et al. Atopic Allergic Conditions and Colorectal Cancer Risk in the Multiethnic Cohort Study. Am. J. Epidemiol. 2015, 181, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Negri, E.; Bosetti, C.; La Vecchia, C.; Levi, F.; Tomei, F.; Franceschi, S. Allergy and other selected diseases and risk of colorectal cancer. Eur. J. Cancer 1999, 35, 1838–1841. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.Q.; Huang, Z.M.; Zeng, R.Q.; Luo, Y.H.; Xie, Z.X.; Chen, Y.Z.; Chen, P.Z.; Luo, T.Y.; Sun, B.; Cheng, Z.J. Association between Atopic Dermatitis and Colorectal Cancer: TET2 as a Shared Gene Signature and Prognostic Biomarker. J. Cancer 2024, 15, 1414–1428. [Google Scholar] [CrossRef]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kang, H.S.; Kim, J.H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Nam, E.S.; Min, K.W.; Choi, H.G. Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals 2021, 14, 1283. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef]
- Lee, S.W.; Acharya, K.P. Propensity score matching for causal inference and reducing the confounding effects: Statistical standard and guideline of Life Cycle Committee. Life Cycle 2022, 2, e18. [Google Scholar] [CrossRef]
- Alderson, M. Mortality from Malignant Disease in Patients with Asthma. Lancet 1974, 304, 1475–1477. [Google Scholar] [CrossRef]
- Santillan, A.A.; Camargo, C.A., Jr.; Colditz, G.A. A meta-analysis of asthma and risk of lung cancer (United States). Cancer Causes Control 2003, 14, 327–334. [Google Scholar] [CrossRef]
- McWhorter, W.P. Allergy and risk of cancer. A prospective study using NHANESI followup data. Cancer 1988, 62, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Lindelöf, B.; Granath, F.; Tengvall-Linder, M.; Ekbom, A. Allergy and cancer. Allergy 2005, 60, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Diepgen, T.L. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy 2005, 60, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Pontikas, A.; Antonatos, C.; Evangelou, E.; Vasilopoulos, Y. Candidate Gene Association Studies in Atopic Dermatitis in Participants of European and Asian Ancestry: A Systematic Review and Meta-Analysis. Genes 2023, 14, 1456. [Google Scholar] [CrossRef]
- Lawson, L.P.; Parameswaran, S.; Panganiban, R.A.; Constantine, G.M.; Weirauch, M.T.; Kottyan, L.C. Update on the genetics of allergic diseases. J. Allergy Clin. Immunol. 2025, 155, 1738–1752. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Q.; Lin, Y.; Lin, S.; Gao, J.; Chen, S. m5C regulator-mediated methylation modification phenotypes characterized by distinct tumor microenvironment immune heterogenicity in colorectal cancer. Sci. Rep. 2023, 13, 11950. [Google Scholar] [CrossRef]
- de la Calle-Fabregat, C.; Calafell-Segura, J.; Gardet, M.; Dunsmore, G.; Mulder, K.; Ciudad, L.; Silvin, A.; Moreno-Càceres, J.; Corbí, Á.L.; Muñoz-Pinedo, C.; et al. NF-κB and TET2 promote macrophage reprogramming in hypoxia that overrides the immunosuppressive effects of the tumor microenvironment. Sci. Adv. 2024, 10, eadq5226. [Google Scholar] [CrossRef]
- Wan, J.; Shin, D.B.; Syed, M.N.; Abuabara, K.; Lemeshow, A.R.; Fuxench, Z.C.C.; Gelfand, J.M. Malignancy risk in patients with atopic dermatitis: A population-based cohort study. Br. J. Dermatol. 2023, 189, 53–61, Erratum in Br. J. Dermatol. 2024, 190, e49. https://doi.org/10.1093/bjd/ljad072. [Google Scholar] [CrossRef]
- Li, Y.; Su, J.; Luo, D.; Duan, Y.; Huang, Z.; He, M.; Tao, J.; Xiao, S.; Xiao, Y.; Chen, X.; et al. Processed Food and Atopic Dermatitis: A Pooled Analysis of Three Cross-Sectional Studies in Chinese Adults. Front. Nutr. 2021, 8, 754663. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Huncharek, M.; Muscat, J.; Kupelnick, B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: A meta-analysis of 26,335 cases from 60 observational studies. Nutr. Cancer 2009, 61, 47–69. [Google Scholar] [CrossRef]
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; La Vecchia, C.; et al. Alcohol drinking and colorectal cancer risk: An overall and dose–response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef]
| Characteristics | Before PS Overlap Weighting Adjustment | After PS Overlap Weighting Adjustment | |||||
|---|---|---|---|---|---|---|---|
| Colorectal Cancer | Control | Standardized Difference | Colorectal Cancer | Control | Standardized Difference | ||
| Age (n, %) | 0.00 | 0.00 | |||||
| 0–4 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | |||
| 5–9 | N/A | N/A | N/A | N/A | |||
| 10–14 | 3 (0.03) | 12 (0.03) | 2 (0.03) | 2 (0.03) | |||
| 15–19 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | |||
| 20–24 | 8 (0.08) | 32 (0.08) | 6 (0.08) | 6 (0.08) | |||
| 25–29 | 26 (0.26) | 104 (0.26) | 21 (0.26) | 21 (0.26) | |||
| 30–34 | 94 (0.95) | 376 (0.95) | 75 (0.95) | 75 (0.95) | |||
| 35–39 | 180 (1.81) | 720 (1.81) | 144 (1.81) | 144 (1.81) | |||
| 40–44 | 359 (3.62) | 1436 (3.62) | 287 (3.62) | 287 (3.62) | |||
| 45–49 | 578 (5.83) | 2312 (5.83) | 461 (5.82) | 461 (5.82) | |||
| 50–54 | 968 (9.76) | 3872 (9.76) | 773 (9.76) | 773 (9.76) | |||
| 55–59 | 1242 (12.52) | 4968 (12.52) | 991 (12.51) | 991 (12.51) | |||
| 60–64 | 1393 (14.04) | 5572 (14.04) | 1111 (14.02) | 1111 (14.02) | |||
| 65–69 | 1488 (15.00) | 5952 (15.00) | 1187 (14.98) | 1187 (14.98) | |||
| 70–74 | 1471 (14.83) | 5884 (14.83) | 1175 (14.84) | 1175 (14.84) | |||
| 75–79 | 1060 (10.69) | 4240 (10.69) | 847 (10.69) | 847 (10.69) | |||
| 80–84 | 672 (6.77) | 2688 (6.77) | 539 (6.80) | 539 (6.80) | |||
| 85+ | 376 (3.79) | 1504 (3.79) | 301 (3.80) | 301 (3.80) | |||
| Sex (n, %) | 0.00 | 0.00 | |||||
| Male | 5933 (59.81) | 23,732 (59.81) | 4736 (59.80) | 4736 (59.80) | |||
| Female | 3987 (40.19) | 15,948 (40.19) | 3184 (40.20) | 3184 (40.20) | |||
| Income (n, %) | 0.00 | 0.00 | |||||
| 1 (lowest) | 1990 (20.06) | 7960 (20.06) | 1589 (20.06) | 1589 (20.06) | |||
| 2 | 1253 (12.63) | 5012 (12.63) | 1000 (12.62) | 1000 (12.62) | |||
| 3 | 1562 (15.75) | 6248 (15.75) | 1246 (15.74) | 1247 (15.74) | |||
| 4 | 2059 (20.76) | 8236 (20.76) | 1644 (20.76) | 1644 (20.76) | |||
| 5 (highest) | 3056 (30.81) | 12,224 (30.81) | 2441 (30.82) | 2441 (30.82) | |||
| Region of residence (n, %) | 0.00 | 0.00 | |||||
| Urban | 4447 (44.83) | 17,788 (44.83) | 3550 (44.82) | 3550 (44.82) | |||
| Rural | 5473 (55.17) | 21,892 (55.17) | 4370 (55.18) | 4370 (55.18) | |||
| CCI score (Mean, SD) | 3.60 (2.44) | 0.99 (1.65) | 0.09 | 3.58 (2.18) | 1.07 (0.77) | 0.00 | |
| Allergic rhinitis history (n, %) | 7722 (77.84) | 31,987 (80.61) | 0.07 | 6212 (78.43) | 6212 (78.43) | 0.00 | |
| Asthma history (n, %) | 3951 (39.83) | 16,362 (41.23) | 0.03 | 3176 (40.11) | 3176 (40.11) | 0.00 | |
| Atopic dermatitis (n, %) | 783 (7.89) | 3284 (8.28) | 0.01 | 628 (7.93) | 645 (8.14) | 0.01 | |
| Characteristics | N of Colorectal Cancer | N of Control | Odd Ratios for Colorectal Cancer (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude | p | Overlap Weighted Model † | p | ||
| Total participants (n = 49,600) | |||||||
| AD | 783/9920 (7.9) | 3284/39,680 (8.3) | 0.95 (0.88–1.03) | 0.214 | 0.97 (0.91–1.04) | 0.387 | |
| Control | 9137/9920 (92.1) | 36,396/39,680 (91.7) | 1 | 1 | |||
| Age < 65 years old (n = 24,265) | |||||||
| AD | 338/4853 (7.0) | 1425/19,412 (7.3) | 0.94 (0.84–1.07) | 0.367 | 0.97 (0.88–1.07) | 0.543 | |
| Control | 4515/4853 (93.0) | 17,987/19,412 (92.7) | 1 | 1 | |||
| Age ≥ 65 years old (n = 25,335) | |||||||
| AD | 445/5067 (8.8) | 1859/20,268 (9.2) | 0.95 (0.86–1.06) | 0.394 | 0.98 (0.90–1.07) | 0.609 | |
| Control | 4622/5067 (91.2) | 18,409/20,268 (90.8) | 1 | 1 | |||
| Male (n = 29,665) | |||||||
| AD | 441/5933 (7.4) | 1838/23,732 (7.7) | 0.96 (0.86–1.07) | 0.425 | 0.97 (0.89–1.06) | 0.561 | |
| Control | 5492/5933 (92.6) | 21,894/23,732 (92.3) | 1 | 1 | |||
| Female (n = 19,935) | |||||||
| AD | 342/3987 (8.6) | 1446/15,948 (9.1) | 0.94 (0.83–1.06) | 0.334 | 0.97 (0.88–1.07) | 0.502 | |
| Control | 3645/3987 (91.4) | 14,502/15,948 (90.9) | 1 | 1 | |||
| Low income group (n = 24,025) | |||||||
| AD | 361/4805 (7.5) | 1485/19,220 (7.7) | 0.97 (0.86–1.09) | 0.622 | 0.99 (0.90–1.09) | 0.859 | |
| Control | 4444/4805 (92.5) | 17,735/19,220 (92.3) | 1 | 1 | |||
| High income group (n = 25,575) | |||||||
| AD | 422/5115 (8.3) | 1799/20,460 (8.8) | 0.93 (0.83–1.04) | 0.218 | 0.96 (0.87–1.04) | 0.313 | |
| Control | 4693/5115 (91.7) | 18,661/20,460 (91.2) | 1 | 1 | |||
| Urban resident (n = 22,235) | |||||||
| AD | 370/4447 (8.3) | 1493/17,788 (8.4) | 0.99 (0.88–1.12) | 0.875 | 1.02 (0.92–1.12) | 0.756 | |
| Control | 4077/4447 (91.7) | 16,295/17,788 (91.6) | 1 | 1 | |||
| Rural resident (n = 27,365) | |||||||
| AD | 413/5473 (7.5) | 1791/21,892 (8.2) | 0.92 (0.82–1.02) | 0.123 | 0.94 (0.86–1.02) | 0.138 | |
| Control | 5060/5473 (92.5) | 20,101/21,892 (91.8) | 1 | 1 | |||
| CCI scores = 0 (n = 30,415) | |||||||
| AD | 424/5448 (7.8) | 2051/24,967 (8.2) | 0.94 (0.85–1.05) | 0.291 | 0.96 (0.88–1.04) | 0.31 | |
| Control | 5024/5448 (92.2) | 22,916/24,967 (91.8) | 1 | 1 | |||
| CCI scores = 1 (n = 10,617) | |||||||
| AD | 216/2600 (8.3) | 664/8017 (8.3) | 1.00 (0.85–1.18) | 0.968 | 1.07 (0.93–1.23) | 0.361 | |
| Control | 2384/2600 (91.7) | 7353/8017 (91.7) | 1 | 1 | |||
| CCI scores ≥ 2 (n = 8568) | |||||||
| AD | 143/1872 (7.6) | 569/6696 (8.5) | 0.89 (0.74–1.08) | 0.234 | 0.93 (0.80–1.09) | 0.382 | |
| Control | 1729/1872 (92.4) | 6127/6696 (91.5) | 1 | 1 | |||
| Non-allergic rhinitis history (n = 9891) | |||||||
| AD | 92/2198 (4.2) | 315/7693 (4.1) | 1.02 (0.81–1.30) | 0.849 | 1.00 (0.82–1.22) | 0.968 | |
| Control | 2106/2198 (95.8) | 7378/7693 (95.9) | 1 | 1 | |||
| Allergic rhinitis history (n = 39,709) | |||||||
| AD | 691/7722 (8.9) | 2969/31,987 (9.3) | 0.96 (0.88–1.05) | 0.368 | 0.97 (0.90–1.04) | 0.339 | |
| Control | 7031/7722 (91.1) | 29,018/31,987 (90.7) | 1 | 1 | |||
| Non-asthma history (n = 29,287) | |||||||
| AD | 396/5969 (6.6) | 1624/23,318 (7.0) | 0.95 (0.85–1.06) | 0.369 | 0.97 (0.88–1.06) | 0.473 | |
| Control | 5573/5969 (93.4) | 21,694/23,318 (93.0) | 1 | 1 | |||
| Asthma history (n = 20,313) | |||||||
| AD | 387/3951 (9.8) | 1660/16,362 (10.1) | 0.96 (0.86–1.08) | 0.515 | 0.98 (0.89–1.07) | 0.59 | |
| Control | 3564/3951 (90.2) | 14,702/16,362 (89.9) | 1 | 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.S.; Han, K.M.; Kim, J.-H.; Kim, J.H.; Choi, H.G.; Yoo, D.M.; Park, H.Y.; Kim, N.Y.; Kwon, M.J. Association Between Atopic Dermatitis and Colorectal Cancer Risk: A Korean Population-Based Study. Biomedicines 2025, 13, 2538. https://doi.org/10.3390/biomedicines13102538
Kang HS, Han KM, Kim J-H, Kim JH, Choi HG, Yoo DM, Park HY, Kim NY, Kwon MJ. Association Between Atopic Dermatitis and Colorectal Cancer Risk: A Korean Population-Based Study. Biomedicines. 2025; 13(10):2538. https://doi.org/10.3390/biomedicines13102538
Chicago/Turabian StyleKang, Ho Suk, Kyeong Min Han, Joo-Hee Kim, Ji Hee Kim, Hyo Geun Choi, Dae Myoung Yoo, Ha Young Park, Nan Young Kim, and Mi Jung Kwon. 2025. "Association Between Atopic Dermatitis and Colorectal Cancer Risk: A Korean Population-Based Study" Biomedicines 13, no. 10: 2538. https://doi.org/10.3390/biomedicines13102538
APA StyleKang, H. S., Han, K. M., Kim, J.-H., Kim, J. H., Choi, H. G., Yoo, D. M., Park, H. Y., Kim, N. Y., & Kwon, M. J. (2025). Association Between Atopic Dermatitis and Colorectal Cancer Risk: A Korean Population-Based Study. Biomedicines, 13(10), 2538. https://doi.org/10.3390/biomedicines13102538







