1. Introduction
Acute rheumatic fever (ARF) occurs as a short-term nonsuppurative sequela of pharyngitis caused by
Streptococcus pyogenes [
1]. Among Streptococci, which are classified according to the carbohydrate composition of cell wall antigens—such as polysaccharides, teichoic acids—as well as sequence variations in the
emm gene encoding the M protein, only certain strains of group A beta-hemolytic
Streptococcus have been linked to ARF [
2]. The immune-mediated mechanisms underlying this important disease have not yet been fully elucidated, and only a small number of specific
emm/M protein types can be considered pathogenic, i.e., potentially characterized by the possibility of inducing ARF, with differences between high- and low-income countries [
3]. The most significant ARF-related complication is rheumatic heart disease (RHD), which may cause substantial effects on the individual’s health, often resulting in chronic illness and even premature death [
4]. ARF and RHD cause a large worldwide burden of morbidity, but factors contributing to the specific involvement of the heart in ARF remain unclear. Indeed, RHD is still a major cause of cardiac failure in different countries due to poor rates of diagnosis and the lack of proper ARF treatment or prophylaxis [
3]. Despite the widespread application of the Jones criteria, carditis may result in either underdiagnosis or sometimes overdiagnosis. However, the only strategy available for effective ARF control is secondary prophylaxis with long-acting penicillin G-benzathine administered every three weeks [
4].
A definite reason for heart valve injuries occurring in ARF attacks is not understood, but 25-hydroxyvitamin D [25(OH)-vitamin D] deficiency has been associated with various cardiovascular risk factors and has been linked to a higher mortality following renin–angiotensin–aldosterone system activation, abnormal nitric oxide regulation, oxidative stress, and altered multiple inflammatory pathways [
5]. Several observational studies have found low vitamin D levels in patients with cardiovascular diseases compared to healthy individuals [
6].
The general aim of this retrospective study was to assess the clinical and laboratory characteristics of a single-centre cohort of patients with ARF hospitalized in our department over a 20-year period and to assess whether serum levels of vitamin D measured near the diagnosis of ARF were associated with the occurrence of RHD.
2. Patients and Methods
Out of 90 eligible pediatric patients in this study, all with a post-streptococcal illness, 11 were not considered because they were diagnosed with post-streptococcal arthritis, and 1 was excluded due to incomplete inpatient data. Patients considered in our evaluation were 78 and were all Caucasian, having an average age at ARF diagnosis of 10.6 ± 2.7 years (range 2.4–15 years); all of them were living in the central or southern Italy (at a latitude varying from 41°13′ N to 37°56′ N, with ultraviolet index of 1–3 in wintertime) and were managed at the Department of Life Sciences and Public Health of our University in Rome. All patients with ARF are regularly hospitalized in our context, and no case of suspected ARF is managed on an outpatient basis. At the time of the first clinical assessment, a questionnaire focusing on the eventual exposure to risk factors during the four weeks prior to illness, along with a general interview of the patients’ parents, was administered.
Diagnosis of ARF was established according to the modified Jones criteria, designed to identify the initial attack of ARF through a defined combination of major and minor manifestations with evidence of a previous group A
Streptococcus pyogenes infection. Indeed, a throat swab for group A
Streptococcus pyogenes was obtained from all patients in the cohort. The same criteria, supporting the use of Doppler echocardiography (Philips Medical, Andover, MA, USA; equipped with multifrequency S12-8, S8-3, and X5-1 transducers depending on the patient size) for the diagnosis of carditis as a major manifestation of ARF, were used to evaluate mitral and aortic valve involvement (regurgitation in at least two views, jet length ≥ 2 cm in at least one view, peak velocity > 3 m/s, confirmed via Doppler flow velocity evaluation) even in the absence of classic auscultatory findings [
7].
Exclusion criteria for participation to this study were comorbidities with immunodeficiency or other immune-mediated disorders, such as systemic juvenile idiopathic arthritis and blood diseases. Demographic data, month of the year at diagnosis, clinical signs, and laboratory variables at disease onset, including C-reactive protein (CRP) and anti-streptolysin O (ASO) titer, were retrieved from medical charts. Vitamin D assessment was part of the routine workup for all hospitalized patients in our department; its serum levels, measured using automated chemiluminescence immunoassay technology (Atellica Siemens HealthineersTM, Swedish Hospital, Chicago, IL, USA) within the first three days of hospitalization, were retrieved from medical charts; only five patients in the cohort underwent a second blood sampling within the first two weeks post-hospitalization. All patients diagnosed with ARF underwent Doppler echocardiography to assess any cardiac involvement, and echocardiography examinations were repeated as case by case required; these patients received regular long-term follow-up (the minimal follow-up duration for a minority of cases was 5 years, while others were assessed yearly with a complete cardiologic evaluation).
3. Ethics Approval
The Local Ethics Committee authorized different study protocols related to nutritional issues (including vitamin D) in patients with complex diseases, as well as rare conditions and rheumatologic disorders (approval code: 2105; approval date: 5 February 2019). The whole study was performed in line with the principles of the 1964 Declaration of Helsinki and its later amendments. All patients’ caregivers were informed about the aims of this retrospective evaluation, and all of them signed a written consent for both unrestricted access to patients’ medical charts and for the evaluation of children’s anonymized data.
4. Statistical Analysis
Data were presented as mean (m) ± standard deviation (SD) for normally distributed continuous variables, as median (M) and interquartile range (IQR) for not-normally distributed continuous variables, and as count (percentages) for categorical variables. The Student’s t-test and the Mann–Whitney U test were used to compare continuous variables (for normally and non-normally distributed variables, respectively), while the chi-square test or Fisher’s exact test (depending on group size) was applied for categorical data. Additionally, we performed a multinomial logistic regression, adjusting for sex and age and including all factors that showed a p-value less than 0.2 in the univariate analysis. Furthermore, we constructed a receiver operating characteristic (ROC) curve with 500 bootstrap repetitions to evaluate the diagnostic accuracy of serum vitamin D levels in predicting cardiac involvement in patients with ARF. Finally, we used Youden’s test to identify the optimal cut-off value of vitamin D for distinguishing patients with and without cardiac involvement, and we also calculated the sensitivity, specificity, and positive and negative predictive values for that threshold.
5. Results
Among the enrolled patients, 50% were males and 50% females. Fifty patients (64%) had a positive
Streptococcus pyogenes pharyngeal swab, while the mean ASO titer was 1433 ± 1171 IU/mL in the totality of patients (it is considered pathologically increased if >200 IU/mL). Thirty-nine patients of the cohort (50%) had arthritis in at least one joint, and the median number of involved joints was 0.5, with an interquartile range (IQR) of 0–2. Seventy percent of patients (55) reported arthralgia, and twenty-three percent (18) exhibited neurological symptoms (chorea) of varying severity. Cardiac involvement was observed in 66 patients (84.6% of the cohort). Among these, 63 patients (81%) displayed mitral regurgitation (mild in 41, moderate in 19, and severe in 3), while 45 (58%) showed aortic valve regurgitation (mild in 35, moderate in 7, and severe in 3). The severity of mitral and aortic regurgitation was assessed via echocardiography with specific metrics related to jet length ≥ 2 cm and peak velocity > 3 m/s. Of 66 patients with carditis, 24 (36.4%) had a single valve involvement, while 42 (63.6%) had both mitral and aortic valve involvement. Four patients (5%) presented signs of myocarditis, and four (5%) also had pericarditis. The most common electrocardiographic abnormality was an increased PR interval duration (first-degree atrioventricular block), which was found in 12 patients (15% of the cohort). Additionally, 15 patients (19%) showed left ventricular dilation on echocardiography, and 2 had severe heart disease requiring specific heart surgical intervention. As conceivable, a significantly higher proportion of patients with cardiac involvement required corticosteroid treatment compared to those without carditis (59%
versus 25%,
p = 0.05). The distribution of clinical manifestations of ARF in patients considered by this study is shown in
Table 1.
By dividing patients based on the presence (or absence) of carditis, univariate analysis revealed no significant differences in age, sex, and other ARF signs (fever, arthritis, arthralgia, chorea, and/or skin lesions). Serum vitamin D levels were significantly lower in patients with ARF and cardiac involvement compared to those without (18 ± 6
versus 38 ± 8 ng/mL,
p < 0.001). In addition, the proportion of patients with normal serum vitamin D levels was significantly higher in those without cardiac involvement (92%) compared to those with carditis (3%,
p < 0.001). No differences were found between the two groups regarding the period of diagnosis. Notably, 33 patients (42% of the cohort) had their vitamin D level measured during spring or summer season (from May to October), while the remaining were tested during the wintertime. Specifically, 29 patients with carditis (44% of the cohort) were diagnosed during winter, compared to 4 patients without cardiac involvement (33.3% of the cohort,
p = 0.54). To account for any potential confounding factors, we performed a multivariate analysis using logistic regression, adjusting for sex, age, and period of diagnosis (spring/summer or fall/winter), and including all the variables that showed a
p-value ≤ 0.2 in the univariate analysis. The results of the logistic regression are shown in
Table 2, revealing a wide confidence interval, which may be related to our model instability, likely explained by the relatively limited sample size of patients.
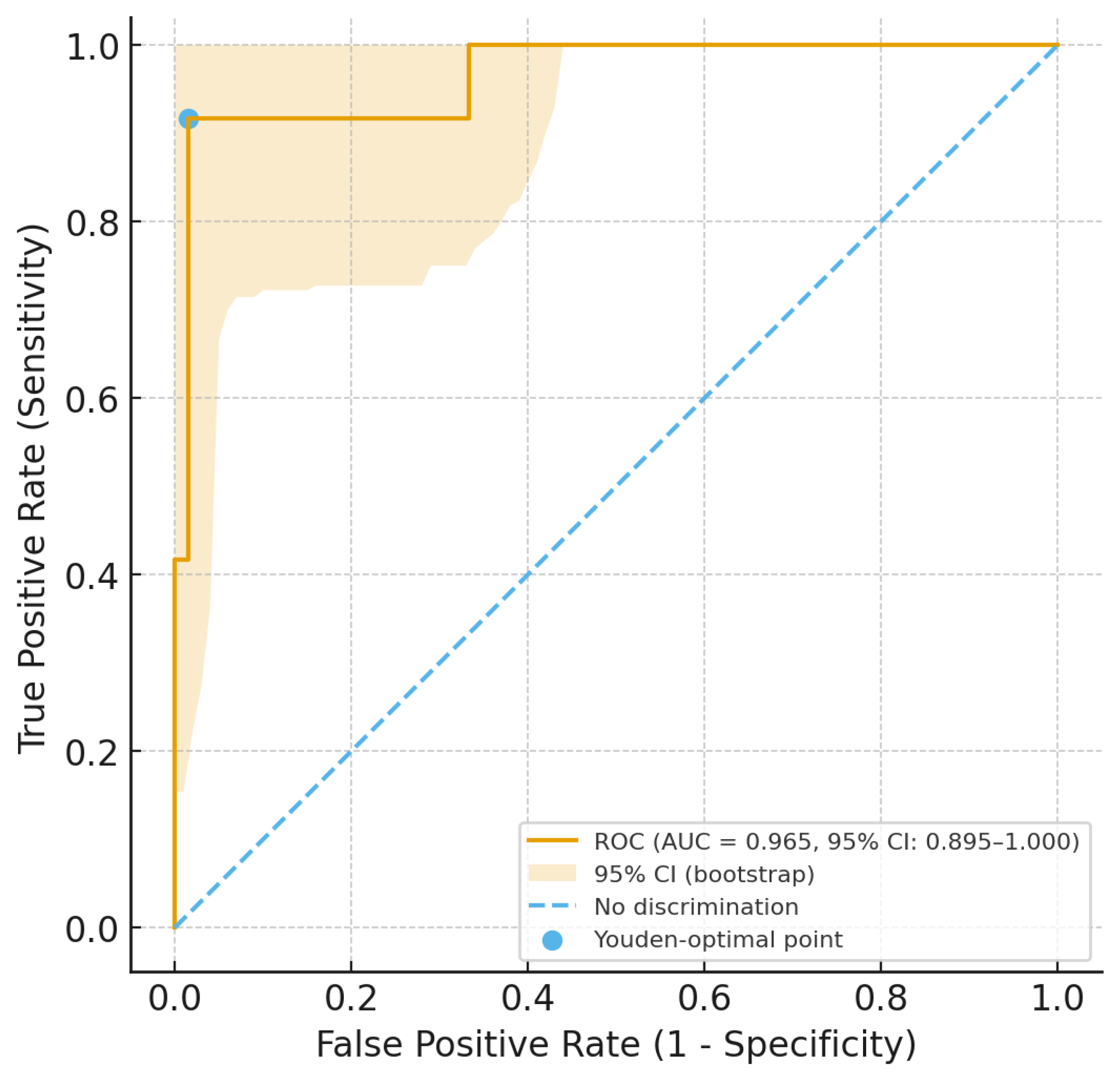
Serum levels of 25(OH)-vitamin D less than 30 ng/mL, assessed near the time of ARF diagnosis, were strongly associated with the presence of cardiac involvement. Concurrently, we also used vitamin D levels and the presence of cardiac involvement to generate a ROC curve with 5000 bootstrap replications to evaluate diagnostic accuracy and obtain the 95% confidence intervals (CIs). The accuracy evaluated as the area under the ROC curve was 0.965 (95% CI: 0.894–1.000), with a standard error of 0.028 and
p < 0.001 (see
Figure 1).
Therefore, we applied the Youden index, and the optimal vitamin D cut-off value for diagnosing cardiac involvement in ARF was found to be 32.5 ng/mL; this cut-off showed a sensitivity of 98.5 (95% CI: 91.84–99.96), a specificity of 91.7 (95% CI: 61.52–99.79), a positive predictive value of 98.5 (95% CI: 90.86–99.76), and a negative predictive value of 91.7 (95% CI: 60.98–98.73).
We divided patients with RHD into two groups to assess whether vitamin D levels were correlated with the severity of cardiac involvement according to echocardiography findings. The first group included patients with mild valvular involvement, while the second included those having moderate-to-severe involvement of heart valves. We chose to combine patients with moderate and moderate/severe manifestations due to the limited number of patients with severe involvement. Patients were classified into the moderate/severe group if echocardiographic findings showed at least one cardiac valve with moderate or severe regurgitation or if they had heart failure with reduced ejection fraction. All patients who did not meet these criteria were assigned to the mild cardiac involvement group. As a result, the presence of arthritis and vitamin D levels between 20 and 30 ng/dL were associated with mild RHD, whereas vitamin D levels below 20 ng/mL were linked to moderate and severe forms of RHD. Male sex and lower mean serum levels of vitamin D demonstrated a trend (not statistically significant) towards an association with moderate-to-severe carditis (see
Table 3). We also performed multiple logistic regression analysis to correct these results for age, sex, and season in which ARF was diagnosed. This analysis confirmed that the presence of arthritis was associated with milder forms of RHD; conversely, vitamin D levels lower than 20 ng/mL were significantly associated with moderate and severe forms of carditis (
Table 4).
6. Discussion
The present study reveals that vitamin D levels are statistically and independently associated with the development of cardiac involvement in patients with ARF, and that a vitamin D cut-off of 32.5 ng/mL exhibits considerable diagnostic accuracy to suggest the occurrence of RHD, with high sensitivity and specificity. Interestingly, very low levels of vitamin D (˂20 ng/mL) were associated with severe RHD.
The occurrence of RHD is common in countries with socioeconomic disadvantage, household crowding, or poor access to primary health care services as a consequence of undertreated pharyngeal infections caused by group A
Streptococcus pyogenes [
8,
9]. Global estimates suggest that millions of individuals may develop RHD, with a potential risk of death above 1.4 million per year [
10]. The mitral valve is most widely involved in RHD, but aortic valve involvement occurs in ¼ of cases as well [
11]. A broader knowledge about risk factors and pathophysiological mechanisms underlying RHD should help optimize prophylaxis strategies for both ARF and RHD.
Indeed, improvements in living conditions and the employment of antimicrobial drugs are thought to have virtually eliminated ARF in most high-income countries [
12,
13]. Several aspects of nutrition may also contribute to the risk of ARF, including overall nutritional status with intake of micronutrients [
14]. In particular, the serum levels of 25(OH)-vitamin D have been related to ARF in the medical literature, even though the general incidence of both streptococcal infections and ARF shows seasonal peaks during winter and spring months, when 25(OH)-vitamin D levels are expected to be lower because of the lack of sunlight exposure [
15].
A host of epidemiological studies supports the association between vitamin D deficiency and the severity of different immune-mediated diseases such as ARF. For instance, poor nutritional status with hypovitaminosis D has been found in Nepalese populations, who had higher rates of RHD compared to healthy controls [
16]. Yusuf et al. found significantly lower serum levels of 25(OH)-vitamin D in 55 Indian patients with RHD having mild to moderately calcified valves (median of 20.4 ng/mL) and in 55 patients with severely calcified valves (median of 11.4 ng/mL) compared to those with less severely damaged valves [
17].
Vitamin D, known for its essential role in calcium and bone homeostasis, has multiple effects that go beyond the skeleton, including regulation of immunity pathways and modulation of autoimmune processes. Unfortunately, vitamin D deficiency is a global health problem, particularly in children, and several reports have revealed suboptimal serum supply of 25(OH)-vitamin D in children with IgA vasculitis, periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis syndrome, and Kawasaki disease [
18,
19,
20]. At the intersection of rheumatology and cardiovascular medicine, there is a growing awareness that recognizes how individuals with autoimmune and autoinflammatory conditions have a much higher likelihood of developing heart diseases [
21]. Even children with hereditary periodic fever syndromes are prone to display heterogeneous heart complications [
22,
23], though the most relevant childhood condition associated with acquired cardiovascular abnormalities in children living in high-income countries is Kawasaki disease [
24], requiring prompt treatment with intravenous immunoglobulin to decrease disease-associated inflammatory burden and prevent the formation of a structural damage within coronary arteries [
25].
Cumulative reports show that low vitamin D status, along with genetic and environmental factors, may be involved in the pathogenesis of different immune-mediated disorders, and specifically of RHD. Onan et al. prospectively found lower levels of 25(OH)-vitamin D levels, measured with high-performance liquid chromatography, in 77% of a cohort of Turkish children with RHD compared to age-matched healthy controls having innocent murmurs over a 15-month period, with nonsignificant correlation with carditis severity [
26]. We also know that the production of vascular endothelial growth factor is regulated by vitamin D, and that endothelial cell dysfunction may have a role in heart valve remodeling and in initiating the process of RHD [
27,
28]. Furthermore, an Arabian study identified an association between the GC2 polymorphism of the vitamin D-binding protein (VDBP) and ARF [
29]. VDBP belongs to the albumin family and is essential for intracellular transport of vitamin D to various cell types, including macrophages and B lymphocytes [
30].
VDBP gene polymorphisms have also been associated with an increased risk of mitral and aortic valve calcifications in Iranian and Mexican children with RHD [
31,
32].
However, our study presents several limitations. First, although patient histories were rigorously reviewed by a panel of experienced clinicians using the ARF-revised Jones criteria for diagnosis, any adjustment for potential confounding factors contributing to hypovitaminosis D, such as children’s nutritional habits, was not performed. In addition, epidemiological variations in ARF incidence were not considered, and several well-known risk factors for vitamin D deficiency in children, including limited outdoor activity (which reduces sunlight exposure) and obesity, were not accounted for. Furthermore, the retrospective design of this study, the exact timing of vitamin D assessment in relation to hospitalization, the absence of non-white children in our study, and the relatively low number of ARF cases over the past two decades (due to the single-centre nature of this study) may have influenced the final results and hindered the generalizability of our findings.
7. Conclusions
The development of RHD in subsets of pediatric patients with ARF is not definitively explained. This study provides a freeze image of vitamin D status at the time of ARF diagnosis, without considering specific time points. Interestingly, hypovitaminosis D was found as an independent factor associated with both RHD development and RHD severity in a single-centre cohort of children with ARF over two decades. Further work is warranted to define the role of vitamin D in ARF and RHD pathophysiology and to establish the potential existence of temporal relationships between vitamin D status and disease onset, activity, and treatment response. Such studies should involve larger cohorts of patients with a standardized assessment of potential confounding factors such as diet, sun exposure, ethnicity, and medications.
Author Contributions
Conceptualization: D.R. and M.C.; methodology and data curation: D.R., G.P. and M.C.; writing original draft preparation: D.R.; review and editing: M.C.; cardiologic assessments and critical evaluation of the paper: G.D.R., A.B.D. and C.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Local Ethical Committee of the Università Cattolica Sacro Cuore, Rome (Italy), authorized study protocols related to nutritional issues in patients with complex and rare diseases as well as rheumatologic diseases (approval code: 2105; approval date: 5 February 2019).
Informed Consent Statement
A formal written informed consent was obtained from all caregivers of participants included in the study to publish this paper.
Data Availability Statement
No datasets were generated or analyzed during the current study.
Acknowledgments
The authors thank the families of children participating in this retrospective study. The authors did not receive support from any organization for the submitted work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ARF | acute rheumatic fever |
| RHD | rheumatic heart disease |
| ROC | receiver operating characteristic |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
References
- Zhang, J.; Jia, S.; Chen, Y.; Han, J.; Zhang, H.; Jiang, W. Recent Advances on the Prevention and Management of Rheumatic Heart Disease. Glob. Hear. 2025, 20, 17. [Google Scholar] [CrossRef]
- de Crombrugghe, G.; Baroux, N.; Botteaux, A.; Moreland, N.J.; A Williamson, D.; Steer, A.C.; Smeesters, P.R. The Limitations of the Rheumatogenic Concept for Group A Streptococcus: Systematic Review and Genetic Analysis. Clin. Infect. Dis. 2019, 70, 1453–1460. [Google Scholar] [CrossRef]
- Balan, S.; Krishna, M.P.; Sasidharan, A.; Mithun, C. Acute rheumatic fever and Post-streptococcal reactive arthritis. Best Pr. Res. Clin. Rheumatol. 2025, 39, 102067. [Google Scholar] [CrossRef]
- Hirani, K.; Rwebembera, J.; Webb, R.; Beaton, A.; Kado, J.; Carapetis, J.; Bowen, A. Acute rheumatic fever. Lancet 2025, 405, 2164–2178. [Google Scholar] [CrossRef]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef]
- Skaaby, T. The relationship of vitamin D status to risk of cardiovascular disease and mortality. Dan Med. J. 2015, 62, 2. [Google Scholar]
- Gewitz, M.H.; Baltimore, R.S.; Tani, L.Y.; A Sable, C.; Shulman, S.T.; Carapetis, J.; Remenyi, B.; A Taubert, K.; Bolger, A.F.; Beerman, L.; et al. Revision of the Jones Criteria for the Diagnosis of Acute Rheumatic Fever in the Era of Doppler Echocardiography. Circulation 2015, 131, 1806–1818. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, G.; Guilherme, L. Acute rheumatic fever. Lancet 2018, 392, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Guo, D.; Yu, D. A mini review of the pathogenesis of acute rheumatic fever and rheumatic heart disease. Front. Cell. Infect. Microbiol. 2025, 15, 1447149. [Google Scholar] [CrossRef] [PubMed]
- Talwar, K.K.; Gupta, A. Predictors of mortality in chronic rheumatic heart disease. Indian J. Med Res. 2016, 144, 311–313. [Google Scholar] [CrossRef]
- Nkomo, V.T. Epidemiology and prevention of valvular heart diseases and infective endocarditis in Africa. Heart 2006, 93, 1510–1519. [Google Scholar] [CrossRef]
- Mutarelli, A.; Nogueira, G.P.G.; Pantaleao, A.N.; Nogueira, A.; Giavina-Bianchi, B.; Fonseca, I.M.G.; Nascimento, B.R.; Dutra, W.O.; Levine, R.A.; Nunes, M.C.P.; et al. Prevalence of Rheumatic Heart Disease in First-degree Relatives of Index-cases: A Systematic Review and Meta-Analysis. Glob. Hear. 2025, 20, 24. [Google Scholar] [CrossRef]
- Lupieri, A.; Jha, P.K.; Nizet, V.; Dutra, W.O.; Nunes, M.C.P.; Levine, R.A.; Aikawa, E. Rheumatic heart valve disease: Navigating the challenges of an overlooked autoimmune disorder. Front. Cardiovasc. Med. 2025, 12, 1537104. [Google Scholar] [CrossRef]
- Baker, M.G.; Gurney, J.; Oliver, J.; Moreland, N.J.; A Williamson, D.; Pierse, N.; Wilson, N.; Merriman, T.R.; Percival, T.; Murray, C.; et al. Risk Factors for Acute Rheumatic Fever: Literature Review and Protocol for a Case-Control Study in New Zealand. Int. J. Environ. Res. Public Heal. 2019, 16, 4515. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Aydin, A.; Demir, T.; Kosger, P.; Ozdemir, G.; Ucar, B.; Kilic, Z. Acute rheumatic fever: A single center experience with 193 clinical cases. Minerva Pediatr. 2016, 68, 134–142. [Google Scholar]
- Thorup, L.; Hamann, S.A.; Tripathee, A.; Koirala, B.; Gyawali, B.; Neupane, D.; Mota, C.C.; Kallestrup, P.; Hjortdal, V.E. Evaluating Vitamin D levels in Rheumatic Heart Disease patients and matched controls: A case-control study from Nepal. PLoS ONE 2020, 15, e0237924. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, J.; P, J.; Mukhopadhyay, S.; Vignesh, V.; Tyagi, S. Evaluation of serum 25-hydroxyvitamin D levels in calcific rheumatic mitral stenosis—A cross sectional study. Indian Hear. J. 2018, 70, 206–213. [Google Scholar] [CrossRef]
- Rigante, D.; Guerriero, C.; Silvaroli, S.; Paradiso, F.V.; Sodero, G.; Laferrera, F.; Franceschi, F.; Candelli, M. Predictors of Gastrointestinal Involvement in Children with IgA Vasculitis: Results from a Single-Center Cohort Observational Study. Children 2024, 11, 215. [Google Scholar] [CrossRef]
- Rigante, D.; Manna, R.; Candelli, M. Exploring the significance of vitamin D insufficiency in the periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome: A single-center retrospective assessment during the decade 2014–2024. Intern. Emerg. Med. 2025, 20, 1721–1729. [Google Scholar] [CrossRef]
- Rigante, D.; De Rosa, G.; Delogu, A.B.; Rotunno, G.; Cianci, R.; Di Pangrazio, C.; Sodero, G.; Basile, U.; Candelli, M. Hypovitaminosis D and Leukocytosis to Predict Cardiovascular Abnormalities in Children with Kawasaki Disease: Insights from a Single-Center Retrospective Observational Cohort Study. Diagnostics 2024, 14, 1228. [Google Scholar] [CrossRef] [PubMed]
- Issa, F.; Abdulla, M.; Retnowati, F.D.; Al-Khawaga, H.; Alhiraky, H.; Al-Harbi, K.M.; Al-Haidose, A.; Maayah, Z.H.; Abdallah, A.M. Cardio-Rheumatic Diseases: Inflammasomes Behaving Badly. Int. J. Mol. Sci. 2025, 26, 3520. [Google Scholar] [CrossRef]
- Rigante, D.; Frediani, B.; Galeazzi, M.; Cantarini, L. From the Mediterranean to the Sea of Japan: The Transcontinental Odyssey of Autoinflammatory Diseases. BioMed Res. Int. 2013, 2013, 485103. [Google Scholar] [CrossRef] [PubMed]
- Rigante, D.; Cantarini, L.; Imazio, M.; Lucherini, O.M.; Sacco, E.; Galeazzi, M.; Brizi, M.G.; Brucato, A. Autoinflammatory diseases and cardiovascular manifestations. Ann. Med. 2011, 43, 341–346. [Google Scholar] [CrossRef]
- De Rosa, G.; Pardeo, M.; Rigante, D. Current recommendations for the pharmacologic therapy in Kawasaki syndrome and management of its cardiovascular complications. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 301–308. [Google Scholar]
- Rigante, D.; Valentini, P.; Rizzo, D.; Leo, A.; De Rosa, G.; Onesimo, R.; De Nisco, A.; Angelone, D.F.; Compagnone, A.; Delogu, A.B. Responsiveness to intravenous immunoglobulins and occurrence of coronary artery abnormalities in a single-center cohort of Italian patients with Kawasaki syndrome. Rheumatol. Int. 2010, 30, 841–846. [Google Scholar] [CrossRef]
- Onan, S.H. Evaluation of vitamin D levels in patients with acute rheumatic fever. Anatol. J. Cardiol. 2017, 18, 75–76. [Google Scholar] [CrossRef]
- Sarkar, S.; Chopra, S.; Rohit, M.K.; Banerjee, D.; Chakraborti, A. Vitamin D regulates the production of vascular endothelial growth factor: A triggering cause in the pathogenesis of rheumatic heart disease? Med. Hypotheses 2016, 95, 62–66. [Google Scholar] [CrossRef]
- Jamali, N.; Sorenson, C.M.; Sheibani, N. Vitamin D and regulation of vascular cell function. Am. J. Physiol. Circ. Physiol. 2018, 314, H753–H765. [Google Scholar] [CrossRef] [PubMed]
- Bahr, G.M.; Eales, L.J.; Nye, K.E.; Majeed, H.A.; Yousof, A.M.; Behbehani, K.; Rook, G.A. An association between Gc (vitamin D-binding protein) alleles and susceptibility to rheumatic fever. Immunology 1989, 67, 126–128. [Google Scholar] [PubMed]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Kiani, A.; Mohamadi-Nori, E.; Vaisi-Raygani, A.; Tanhapour, M.; Elahi-Rad, S.; Bahrehmand, F.; Rahimi, Z.; Pourmotabbed, T. Vitamin D-binding protein and vitamin D receptor genotypes and 25-hydroxyvitamin D levels are associated with development of aortic and mitral valve calcification and coronary artery diseases. Mol. Biol. Rep. 2019, 46, 5225–5236. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ballesteros, A.I.; Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Parra-Rojas, I.; de la Cruz-Mosso, U. Association of Vitamin D Metabolism Gene Polymorphisms with Autoimmunity: Evidence in Population Genetic Studies. Int. J. Mol. Sci. 2020, 21, 9626. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).