Preventive and Therapeutic Effects of Hydrogen-Generating Si-Based Agent on Pressure Ulcers in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
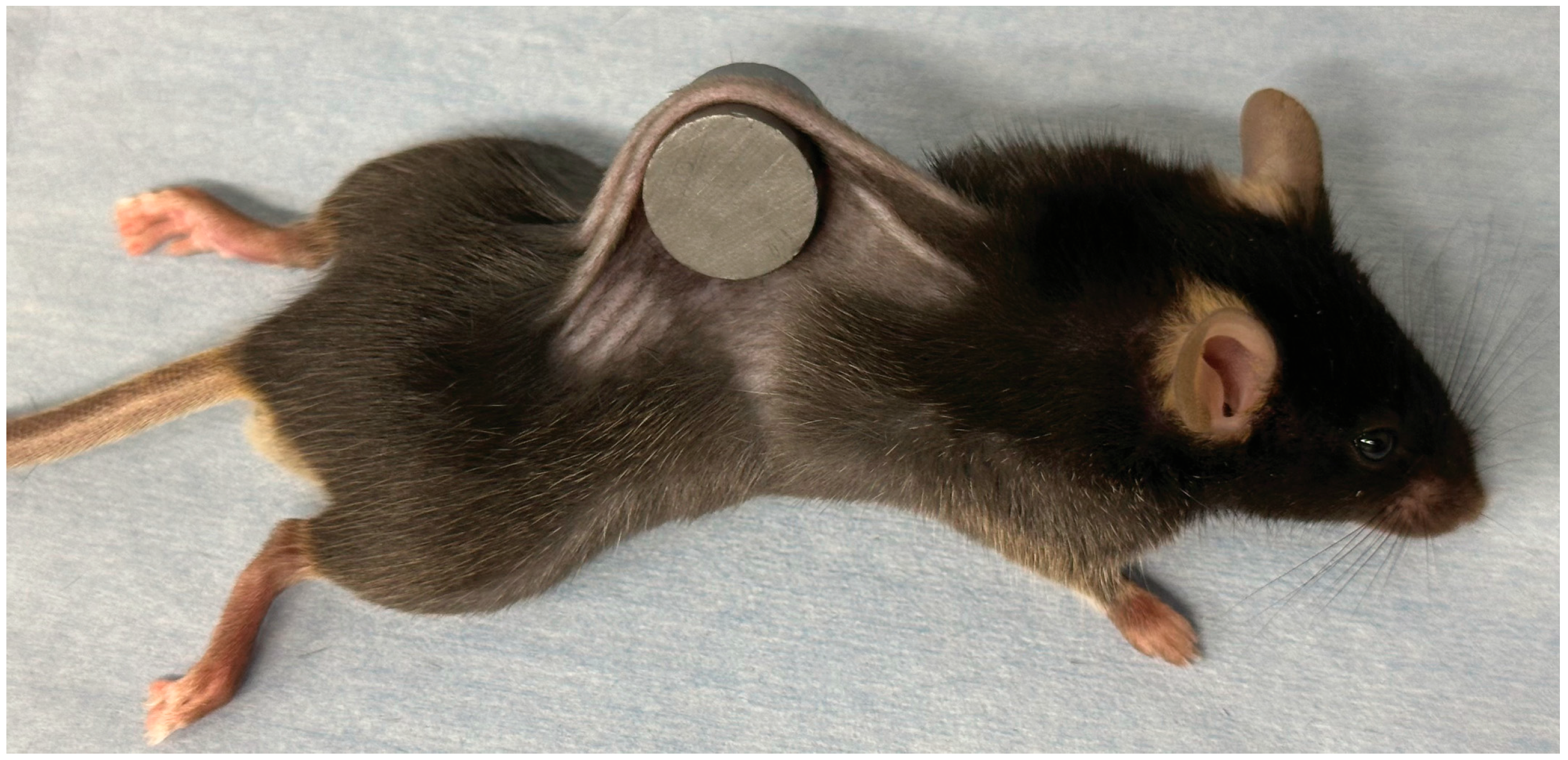
2.2. Study Design and Animal Pressure Injury Model
2.3. Histology
2.4. Apoptosis-Related Protein Measurements
2.5. Oxidative Stress Measurements
2.6. Inflammatory Cytokine Gene Expression Measurements
2.7. Statistics
3. Results
3.1. Macroscopic Findings
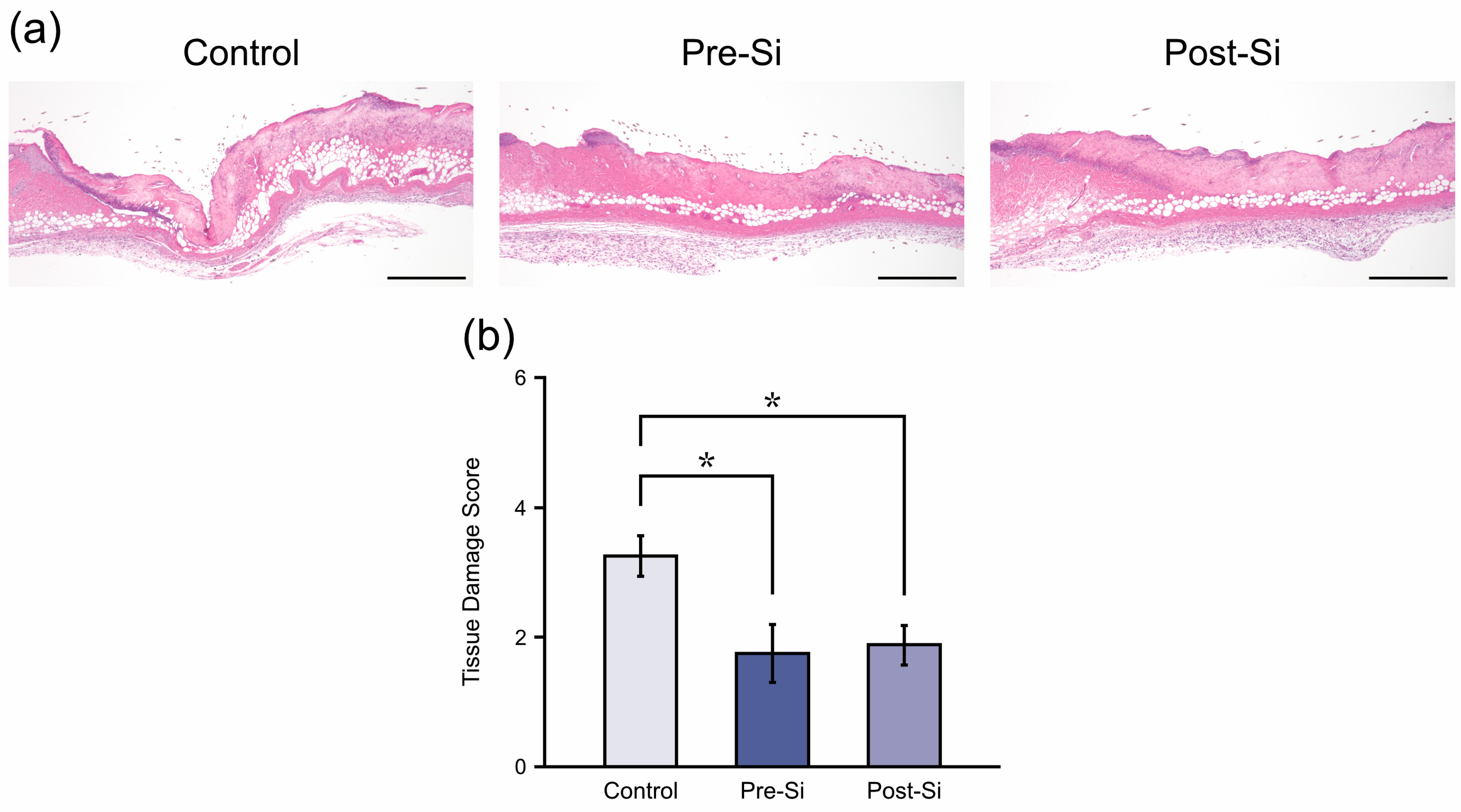
3.2. Histological Findings
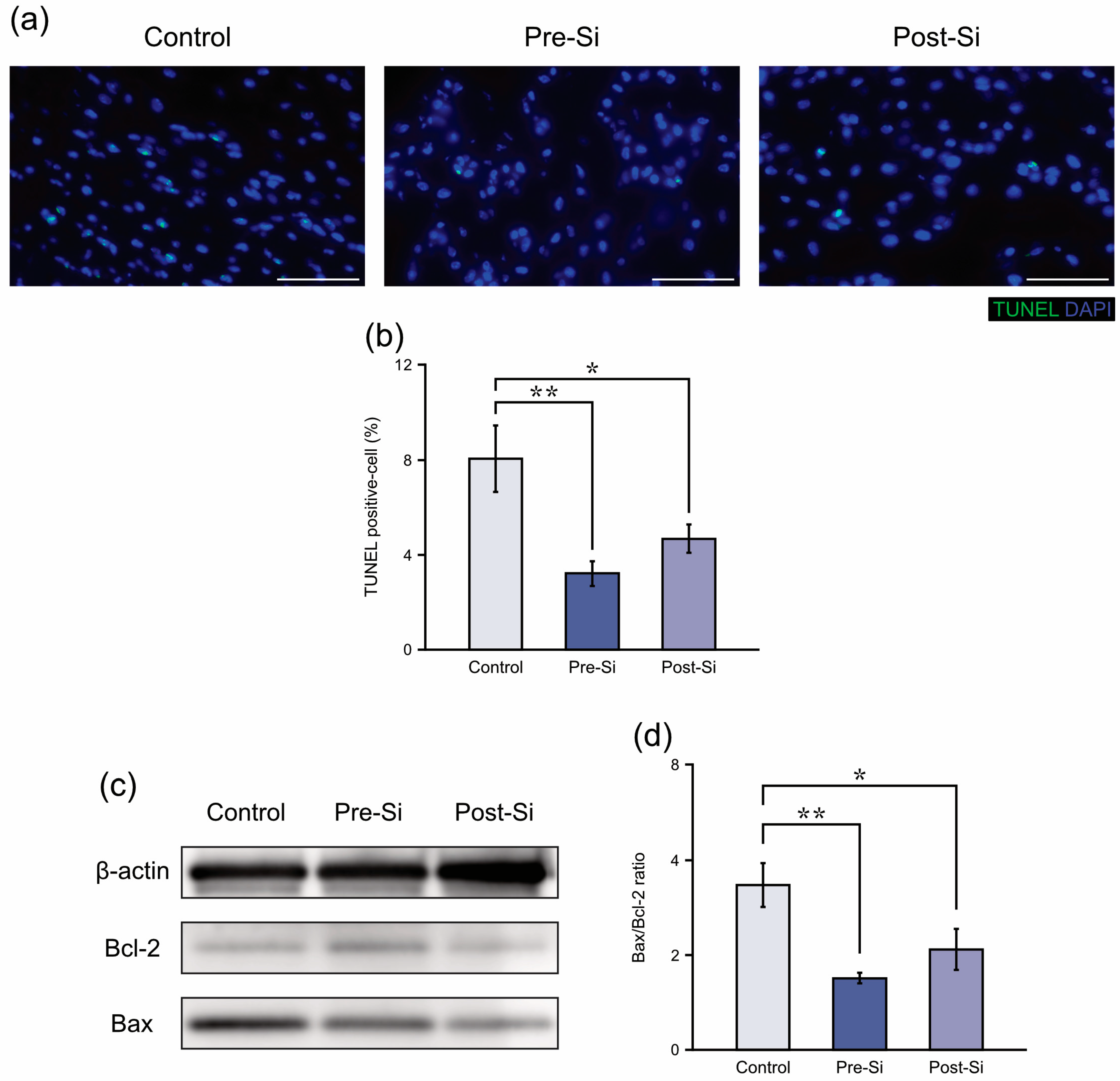
3.3. Apoptosis-Related Findings
3.4. Oxidative Stress Findings
3.5. Inflammation-Related Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Si | Silicon |
| IRI | ischemia/reperfusion injury |
| HE | hematoxylin and eosin |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| DAPI | 4′,6-diamidino-2-phenylindole |
| PVDF | PolyVinylidene DiFluoride |
| TBST | Tris Buffered Saline with Tween 20 |
| HRP | Horseradish Peroxidase |
| MDA | malondialdehyde |
| 8-OHdG | 8-hydroxy-2-deoxyguanosine |
| DNA | DeoxyriboNucleic Acid |
| RNA | RiboNucleic Acid |
| RT-qPCR | real-time quantitative reverse-transcription PCR |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| TNF-α | tumor necrosis factor alpha |
| ppm | parts per million |
References
- Hajhosseini, B.; Longaker, M.T.; Gurtner, G.C. Pressure Injury. Ann. Surg. 2020, 271, 671–679. [Google Scholar] [CrossRef]
- Gefen, A.; Brienza, D.M.; Cuddigan, J.; Haesler, E.; Kottner, J. Our contemporary understanding of the aetiology of pressure ulcers/pressure injuries. Int. Wound J. 2022, 19, 692–704. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef]
- Houwing, R.; Overgoor, M.; Kon, M.; Jansen, G.; van Asbeck, B.S.; Haalboom, J.R. Pressure-induced skin lesions in pigs: Reperfusion injury and the effects of vitamin E. J. Wound Care 2000, 9, 36–40. [Google Scholar] [CrossRef]
- Sener, G.; Sert, G.; Ozer Sehirli, A.; Arbak, S.; Gedik, N.; Ayanoglu-Dulger, G. Melatonin protects against pressure ulcer-induced oxidative injury of the skin and remote organs in rats. J. Pineal Res. 2006, 40, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Danigo, A.; Nasser, M.; Bessaguet, F.; Javellaud, J.; Oudart, N.; Achard, J.M.; Demiot, C. Candesartan restores pressure-induced vasodilation and prevents skin pressure ulcer formation in diabetic mice. Cardiovasc. Diabetol. 2015, 14, 26. [Google Scholar] [CrossRef]
- Donato-Trancoso, A.; Monte-Alto-Costa, A.; Romana-Souza, B. Olive oil-induced reduction of oxidative damage and inflammation promotes wound healing of pressure ulcers in mice. J. Dermatol. Sci. 2016, 83, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Romana-Souza, B.; Dos Santos, J.S.; Monte-Alto-Costa, A. Caffeic acid phenethyl ester promotes wound healing of mice pressure ulcers affecting NF-kappaB, NOS2 and NRF2 expression. Life Sci. 2018, 207, 158–165. [Google Scholar] [CrossRef]
- Bonham, C.A.; Rodrigues, M.; Galvez, M.; Trotsyuk, A.; Stern-Buchbinder, Z.; Inayathullah, M.; Rajadas, J.; Gurtner, G.C. Deferoxamine can prevent pressure ulcers and accelerate healing in aged mice. Wound Repair Regen. 2018, 26, 300–305. [Google Scholar] [CrossRef]
- Liu, J.; Rybakina, E.G.; Korneva, E.A.; Noda, M. Effects of Derinat on ischemia-reperfusion-induced pressure ulcer mouse model. J. Pharmacol. Sci. 2018, 138, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Elizondo, M.B.; Barenholz-Cohen, T.; Weihs, D. Sodium pyruvate pre-treatment prevents cell death due to localised, damaging mechanical strains in the context of pressure ulcers. Int. Wound J. 2019, 16, 1153–1163. [Google Scholar] [CrossRef]
- Nakamura, H.; Sekiguchi, A.; Ogawa, Y.; Kawamura, T.; Akai, R.; Iwawaki, T.; Makiguchi, T.; Yokoo, S.; Ishikawa, O.; Motegi, S.I. Zinc deficiency exacerbates pressure ulcers by increasing oxidative stress and ATP in the skin. J. Dermatol. Sci. 2019, 95, 62–69. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Lee, E.; Choi, S.; Moon, J.; An, H.; Ji, E.; Na, J.; Kim, B. Phospholipid fraction has protective effect by improving inflammation and skin barrier function in ischemia-reperfusion injury in mice. All Life 2020, 13, 623–633. [Google Scholar] [CrossRef]
- Inoue, Y.; Uchiyama, A.; Sekiguchi, A.; Yamazaki, S.; Fujiwara, C.; Yokoyama, Y.; Ogino, S.; Torii, R.; Hosoi, M.; Akai, R.; et al. Protective effect of dimethyl fumarate for the development of pressure ulcers after cutaneous ischemia-reperfusion injury. Wound Repair Regen. 2020, 28, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ham, S.; Jung, B.K.; Park, J.W.; Kim, J.; Lee, J.H. Effect of Baicalin on Wound Healing in a Mouse Model of Pressure Ulcers. Int. J. Mol. Sci. 2022, 24, 329. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, J.; Teng, Y.; Fan, Z.; Jia, M.; Teng, H.; Zhuge, L. Tryptanthrin promotes pressure ulcers healing in mice by inhibiting macrophage-mediated inflammation via cGAS/STING pathways. Int. Immunopharmacol. 2024, 130, 111687. [Google Scholar] [CrossRef] [PubMed]
- Desneves, K.J.; Todorovic, B.E.; Cassar, A.; Crowe, T.C. Treatment with supplementary arginine, vitamin C and zinc in patients with pressure ulcers: A randomised controlled trial. Clin. Nutr. 2005, 24, 979–987. [Google Scholar] [CrossRef]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef]
- Schoofs, H.; Schmit, J.; Rink, L. Zinc Toxicity: Understanding the Limits. Molecules 2024, 29, 3130. [Google Scholar] [CrossRef]
- Sunkara, V.; Pelkowski, T.D.; Dreyfus, D.; Satoskar, A. Acute Kidney Disease Due to Excessive Vitamin C Ingestion and Remote Roux-en-Y Gastric Bypass Surgery Superimposed on CKD. Am. J. Kidney Dis. 2015, 66, 721–724. [Google Scholar] [CrossRef]
- Williams, R.; Dauleh, M.; Abendroth, C.; Kaur, G. Acute Kidney Injury From Biopsy-Proven Renal Oxalosis From Excessive Intake of Vitamin C Leading to End-Stage Kidney Disease. Cureus 2022, 14, e33061. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, M.T.; Gong, G.H. L-arginine overdose is a potential risk factor for myocardial injury in patients with type 2 diabetes. World J. Diabetes 2025, 16, 104409. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kato, S.; Matsuoka, D.; Tanaka, H.; Miwa, N. Hydrogen water intake via tube-feeding for patients with pressure ulcer and its reconstructive effects on normal human skin cells in vitro. Med. Gas Res. 2013, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wang, G.Z.; Tang, L.Y.; Su, H.L.; Chen, H.Y.; Liao, W.Q.; Xu, J.H. Hydrogen gas inhalation protects against cutaneous ischaemia/reperfusion injury in a mouse model of pressure ulcer. J. Cell. Mol. Med. 2018, 22, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Imamura, R.; Koyama, Y.; Kondo, M.; Kobayashi, H.; Nonomura, N.; Shimada, S. Renoprotective and neuroprotective effects of enteric hydrogen generation from Si-based agent. Sci. Rep. 2020, 10, 5859. [Google Scholar] [CrossRef]
- Koyama, Y.; Kobayashi, Y.; Kobayashi, H.; Shimada, S. Diverse Possibilities of Si-Based Agent, a Unique New Antioxidant. Antioxidants 2023, 12, 1061. [Google Scholar] [CrossRef]
- Kawamura, M.; Imamura, R.; Kobayashi, Y.; Taniguchi, A.; Nakazawa, S.; Kato, T.; Namba-Hamano, T.; Abe, T.; Uemura, M.; Kobayashi, H.; et al. Oral Administration of Si-Based Agent Attenuates Oxidative Stress and Ischemia-Reperfusion Injury in a Rat Model: A Novel Hydrogen Administration Method. Front Med. 2020, 7, 95. [Google Scholar] [CrossRef]
- Otani, N.; Tomita, K.; Kobayashi, Y.; Kuroda, K.; Koyama, Y.; Kobayashi, H.; Kubo, T. Hydrogen-generating Si-based agent protects against skin flap ischemia-reperfusion injury in rats. Sci. Rep. 2022, 12, 6168. [Google Scholar] [CrossRef]
- Shimada, M.; Koyama, Y.; Kobayashi, Y.; Matsumoto, Y.; Kobayashi, H.; Shimada, S. Si-based agent alleviated small bowel ischemia-reperfusion injury through antioxidant effects. Sci. Rep. 2024, 14, 4141. [Google Scholar] [CrossRef] [PubMed]
- Otani, N.; Tomita, K.; Kobayashi, Y.; Kuroda, K.; Kobayashi, H.; Kubo, T. Hydrogen-Generating Silicon-Based Agent Improves Fat Graft Survival in Rats. Plast. Reconstr. Surg. 2024, 154, 90e–99e. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Koyama, Y.; Kobayashi, Y.; Hirota, I.; Sun, Y.; Ohtsu, I.; Imai, H.; Yoshioka, Y.; Yanagawa, H.; Sumi, T.; Kobayashi, H.; et al. A new therapy against ulcerative colitis via the intestine and brain using the Si-based agent. Sci. Rep. 2022, 12, 9634. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Koyama, Y.; Kobayashi, Y.; Kobayashi, H.; Shimada, S. Effect of the new silicon-based agent on the symptoms of interstitial pneumonitis. Sci. Rep. 2023, 13, 5707. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Demiot, C.; Sarrazy, V.; Javellaud, J.; Gourloi, L.; Botelle, L.; Oudart, N.; Achard, J.M. Erythropoietin restores C-fiber function and prevents pressure ulcer formation in diabetic mice. J. Investig. Dermatol. 2011, 131, 2316–2322. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxid. Med. Cell. Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef]
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.; Sato, B.; Li, X.K. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485. [Google Scholar] [CrossRef]
- Yang, F.; Lei, Y.; Liu, R.; Luo, X.; Li, J.; Zeng, F.; Lu, S.; Huang, X.; Lan, Y. Hydrogen: Potential Applications in Solid Organ Transplantation. Oxid. Med. Cell. Longev. 2021, 2021, 6659310. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Liu, C.; Wei, J.; Wang, X.; Zhao, Q.; Lv, J.; Tan, Z.; Xin, Y.; Jiang, X. Radiation-induced skin reactions: Oxidative damage mechanism and antioxidant protection. Front. Cell Dev. Biol. 2024, 12, 1480571. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, H.; Li, X.; Dong, J.; Wang, S.; Hao, J.; Liang, G. Role of oxidative stress in post-burn wound healing. Burn. Trauma 2025, 13, tkaf040. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Scharffetter-Kochanek, K. Oxidative stress in chronic venous leg ulcers. Wound Repair Regen. 2005, 13, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.; Zhao, S.H.; Qian, L.R.; Li, B.L.; Ni, J.; Cai, J.M. Hydrogen protects rats from dermatitis caused by local radiation. J. Dermatol. Treat. 2014, 25, 182–188. [Google Scholar] [CrossRef]
- Watanabe, S.; Fujita, M.; Ishihara, M.; Tachibana, S.; Yamamoto, Y.; Kaji, T.; Kawauchi, T.; Kanatani, Y. Protective effect of inhalation of hydrogen gas on radiation-induced dermatitis and skin injury in rats. J. Radiat. Res. 2014, 55, 1107–1113. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otani, N.; Oue, T.; Kobayashi, Y.; Kobayashi, H.; Tomita, K.; Kubo, T. Preventive and Therapeutic Effects of Hydrogen-Generating Si-Based Agent on Pressure Ulcers in Mice. Biomedicines 2025, 13, 2475. https://doi.org/10.3390/biomedicines13102475
Otani N, Oue T, Kobayashi Y, Kobayashi H, Tomita K, Kubo T. Preventive and Therapeutic Effects of Hydrogen-Generating Si-Based Agent on Pressure Ulcers in Mice. Biomedicines. 2025; 13(10):2475. https://doi.org/10.3390/biomedicines13102475
Chicago/Turabian StyleOtani, Naoya, Takaki Oue, Yuki Kobayashi, Hikaru Kobayashi, Koichi Tomita, and Tateki Kubo. 2025. "Preventive and Therapeutic Effects of Hydrogen-Generating Si-Based Agent on Pressure Ulcers in Mice" Biomedicines 13, no. 10: 2475. https://doi.org/10.3390/biomedicines13102475
APA StyleOtani, N., Oue, T., Kobayashi, Y., Kobayashi, H., Tomita, K., & Kubo, T. (2025). Preventive and Therapeutic Effects of Hydrogen-Generating Si-Based Agent on Pressure Ulcers in Mice. Biomedicines, 13(10), 2475. https://doi.org/10.3390/biomedicines13102475






