Hormonal Atrial Fibrillation: Pathophysiological Mechanisms That Trigger and Sustain the Arrhythmic Circuits
Abstract
1. Introduction
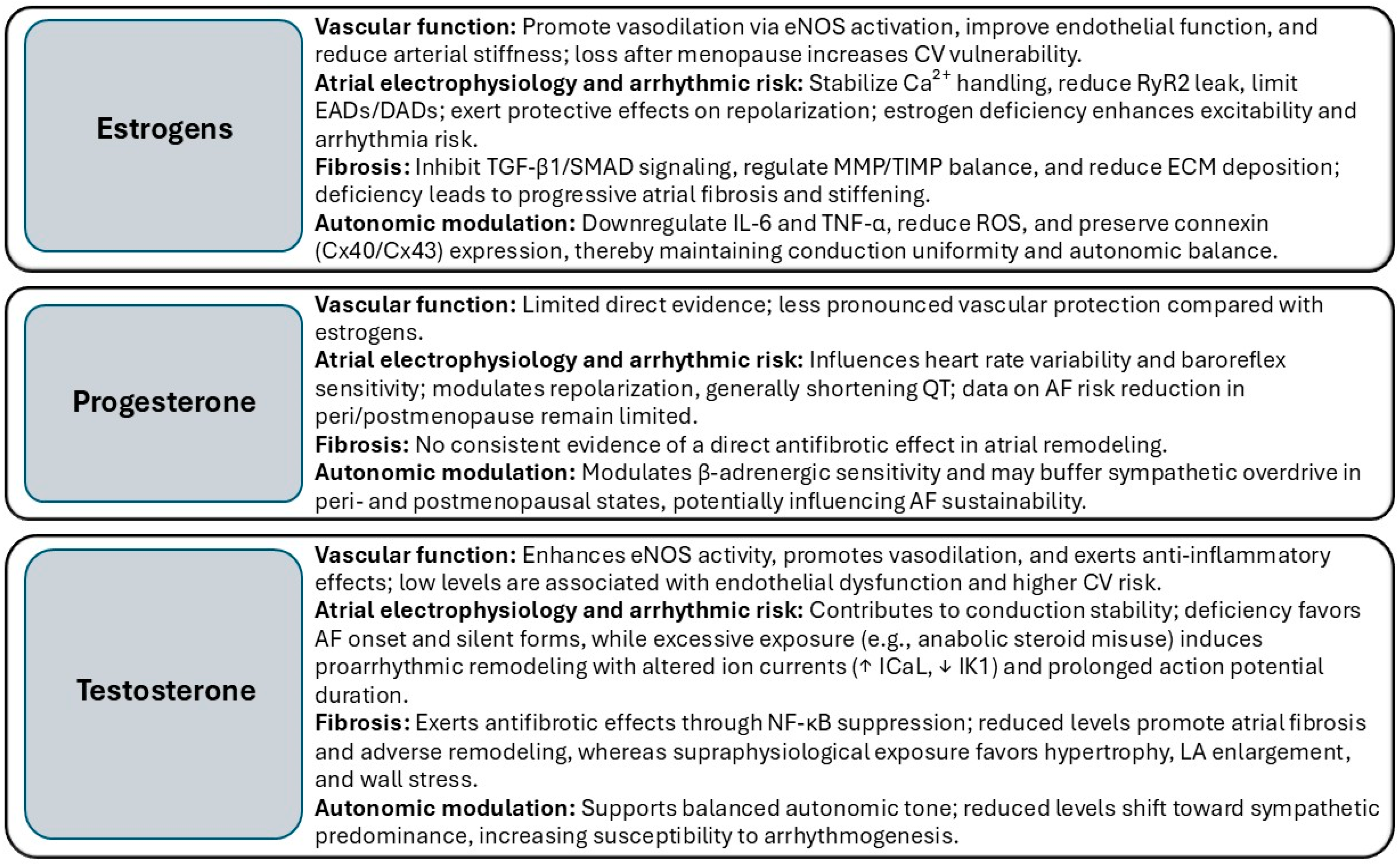
2. Sex Hormones in Regulating Cardiac Structure and Function
3. Hormonal Modulation of Atrial Substrate Remodeling and Atrial Fibrillation Onset
4. Remodeling Mechanisms Underlying the Progression of Atrial Fibrillation
| Arrhythmogenic Mechanisms | Hormonal Modulation | Hormone Deficiency Effects | Excess/Supraphysiological Exposure |
|---|---|---|---|
| Triggered Activity (EADs/DADs) | E2: stabilizes Ca2+ handling, ↓ RyR2 phosphorylation, ↓ SCR events; Progesterone: buffers β-adrenergic Ca2+ loading; Testosterone: maintains eNOS activity, suppresses NF-κB | ↑ Ca2+ leak, ↑ SCR events, more DADs, heightened adrenergic sensitivity | Testosterone excess: ↑ ICaL, ↓ IK1, ↑ β-adrenergic responsiveness, ↑ EADs/DADs |
| Re-entry Substrate | E2: preserves Cx40/Cx43 expression, distribution, phosphorylation; Testosterone: maintains gap junction organization | Disrupted connexin localization, conduction slowing, anisotropy → re-entry facilitation | Testosterone excess: LA enlargement, prolonged conduction delays |
| Fibrosis/Structural Remodeling | E2: inhibits TGF-β1/SMAD axis, regulates MMP/TIMP balance, ↓ ECM deposition; Testosterone: antifibrotic via NF-κB suppression | ↑ TGF-β activity, ↑ collagen synthesis, ECM accumulation, stiffening of atrial wall | Testosterone excess: hypertrophy, wall stress, fibrosis |
| Inflammation/Oxidative Stress | E2: ER-β–mediated ↓ IL-6/TNF-α, inhibits NADPH oxidase–derived ROS; Testosterone: anti-inflammatory via NF-κB inhibition | ↑ pro-inflammatory cytokines, ↑ ROS ↑ electrical instability | Testosterone excess: ↑ oxidative stress, pro-inflammatory signaling |
| Autonomic Modulation | Progesterone: modulates β-adrenergic sensitivity; Testosterone/E2: maintain autonomic balance | ↑ sympathetic tone, ↑ arrhythmia triggers | Testosterone excess: ↑ β-adrenergic responsiveness, vagal effects are context-dependent |
5. Clinical and Epidemiological Evidence on Hormonal Status in AF Onset and Progression
6. Hormone-Targeted Therapeutic Strategies in Atrial Fibrillation Management
| Treatment Strategy | Women (Postmenopause/Estrogen Deficiency) | Men (Gradual Testosterone Decline) | General Considerations |
|---|---|---|---|
| Rhythm control | Greater symptom burden, ↓ success with class III drugs, ↑ risk of TdP; ablation is less used and performed later | Higher efficacy when initiated early; risk of progression to persistent AF if delayed | Personalize strategy according to atrial remodeling and hormonal status |
| Rate control | More frequent digoxin use (linked to ↑ mortality); higher need for AV nodal ablation/pacemaker | More stable response to β-blockers | Revise algorithms to adapt to sex-specific physiology |
| Ablation | Lower success due to TGFβ -dependent fibrotic substrate; ↑ vascular complication risk | Higher utilization, often in early stages | Mapping beyond PV may be considered in postmenopausal women |
| Stroke prevention | Higher thromboembolic risk even at equivalent CHA2DS2-VA score; DOACs reduce disparity | Risk is more correlated with comorbidities | Monitor drug metabolism and hormonal changes |
| HRT | Potential to reduce atrial vulnerability; risk of proarrhythmia | Testosterone replacement may improve substrate, but with variable effects on arrhythmogenicity | Selective use after whole risk–benefit evaluation |
| Treatment | Efficacy | Safety |
|---|---|---|
| Anticoagulation (DOAC vs. VKA) | Comparable benefit in both sexes: ↓ stroke/SE | DOAC: ↓ major bleeding and ICH vs. VKA in both sexes |
| Catheter ablation (RF and Cryo techniques) | Slightly higher recurrence in women, especially in persistent AF; others report no major differences. | Slightly higher periprocedural complications in women (vascular/bleeding) no significant differences. |
| Catheter ablation—Pulsed Field Ablation (PFA) | No significant sex differences in 1-year freedom from AF/AT recurrence. | Similar safety profile overall; one analysis reported slightly higher acute complications in women, but absolute rates were low. |
| Surgical (Cox-Maze/surgical ablation) | Long-term outcomes are similar between sexes (SR maintenance, survival, QoL). | Comparable safety profile across sexes in historical and adjusted cohorts. |
7. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AI | Artificial intelligence |
| AR | Androgen receptor |
| CA | Catheter ablation |
| Cryo | Cryoballoon |
| CHA2DS2-VA | Congestive heart failure–Hypertension–Age–Diabetes–Stroke/Thromboembolism–Vascular disease–Age (65–74) score (without Sex) |
| CHA2DS2-VASc | Congestive heart failure–Hypertension–Age–Diabetes–Stroke/Thromboembolism–Vascular disease–Age (65–74)–Sex category score |
| Cx40 | Connexin 40 |
| Cx43 | Connexin 43 |
| CV | Cardiovascular |
| DAD | Delayed afterdepolarization |
| DOAC | Direct oral anticoagulant |
| E2 | Estradiol |
| EAD | Early afterdepolarization |
| EAT | Epicardial adipose tissue |
| ECG | Electrocardiogram |
| ECM | Extracellular matrix |
| eNOS | Endothelial nitric oxide synthase |
| ER-α | Estrogen receptor alpha |
| ER-β | Estrogen receptor beta |
| GLP-1RA | Glucagon-like peptide-1 receptor agonist |
| HF | Heart failure |
| HFpEF | Heart failure with preserved ejection fraction |
| HRT | Hormone replacement therapy |
| ICaL | L-type calcium current |
| IK1 | Inward rectifier potassium current |
| IL-6 | Interleukin-6 |
| LA | Left atrium/left atrial |
| LVZ | Low-voltage zone |
| MANIFEST-PF | Multinational Survey on the Methods, Efficacy, and Safety on the Postapproval Clinical Use of Pulsed Field Ablation |
| MMP | Matrix metalloproteinase |
| MRI | Magnetic resonance imaging |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| PFA | Pulsed-field ablation |
| PVs | Pulmonary veins |
| QoL | Quality of life |
| RF | Radiofrequency |
| ROS | Reactive oxygen species |
| RyR2 | Ryanodine receptor 2 |
| SCR | Spontaneous calcium release |
| SGLT2 | Sodium–glucose cotransporter 2 |
| Smad3 | Mothers against decapentaplegic homolog 3 |
| TGF-β | Transforming growth factor beta |
| TIMPs | Tissue inhibitors of metalloproteinases |
| TNF-α | Tumor necrosis factor alpha |
References
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 Year Trends in Atrial Fibrillation Prevalence, Incidence, Risk Factors, and Mortality in the Framingham Heart Study: A Cohort Study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef]
- Peters, R.W.; Gold, M.R. The Influence of Gender on Arrhythmias. Cardiol. Rev. 2004, 12, 97–105. [Google Scholar] [CrossRef]
- Westerman, S.; Wenger, N. Gender Differences in Atrial Fibrillation: A Review of Epidemiology, Management, and Outcomes. CCR 2019, 15, 136–144. [Google Scholar] [CrossRef]
- Burgess, S.; Zaman, S.; Towns, C.; Coylewright, M.; Cader, F.A. The Under-Representation of Women in Cardiovascular Clinical Trials: State-of-the-Art Review and Ethical Considerations. Am. Heart J. 2025, 282, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.W.; Moser, C.B.; Murabito, J.M.; Sullivan, L.M.; Wang, N.; Ellinor, P.T.; Vasan, R.S.; Benjamin, E.J.; Coviello, A.D. Association of Sex Hormones, Aging, and Atrial Fibrillation in Men: The Framingham Heart Study. Circ. Arrhythmia Electrophysiol. 2014, 7, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Menazza, S.; Murphy, E. The Expanding Complexity of Estrogen Receptor Signaling in the Cardiovascular System. Circ. Res. 2016, 118, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.J.; Schroeder, M.E.; Aguado, B.A.; Anseth, K.S.; Leinwand, L.A. Matters of the Heart: Cellular Sex Differences. J. Mol. Cell. Cardiol. 2021, 160, 42–55. [Google Scholar] [CrossRef]
- Bukovsky, A.; Caudle, M.R.; Cekanova, M.; Fernando, R.I.; Wimalasena, J.; Foster, J.S.; Henley, D.C.; Elder, R.F. Placental Expression of Estrogen Receptor Beta and Its Hormone Binding Variant–Comparison with Estrogen Receptor Alpha and a Role for Estrogen Receptors in Asymmetric Division and Differentiation of Estrogen-Dependent Cells. Reprod. Biol. Endocrinol. 2003, 1, 36. [Google Scholar] [CrossRef]
- Murphy, E. Estrogen Signaling and Cardiovascular Disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef]
- Minson, C.T.; Halliwill, J.R.; Young, T.M.; Joyner, M.J. Influence of the Menstrual Cycle on Sympathetic Activity, Baroreflex Sensitivity, and Vascular Transduction in Young Women. Circulation 2000, 101, 862–868. [Google Scholar] [CrossRef]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and Women’s Cardiovascular Health: Is It Really an Obvious Relationship? Arch. Med. Sci. 2023, 19, 458–466. [Google Scholar] [CrossRef]
- Maas, A.H.E.M.; Rosano, G.; Cifkova, R.; Chieffo, A.; Van Dijken, D.; Hamoda, H.; Kunadian, V.; Laan, E.; Lambrinoudaki, I.; Maclaran, K.; et al. Cardiovascular Health after Menopause Transition, Pregnancy Disorders, and Other Gynaecologic Conditions: A Consensus Document from European Cardiologists, Gynaecologists, and Endocrinologists. Eur. Heart J. 2021, 42, 967–984. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.; Kaunitz, A.M.; Faubion, S.S.; Hayes, S.N.; Lau, E.S.; Pristera, N.; Scott, N.; Shifren, J.L.; Shufelt, C.L.; Stuenkel, C.A.; et al. Rethinking Menopausal Hormone Therapy: For Whom, What, When, and How Long? Circulation 2023, 147, 597–610. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef]
- Schafstedde, M.; Nordmeyer, S. The Role of Androgens in Pressure Overload Myocardial Hypertrophy. Front. Endocrinol. 2023, 14, 1112892. [Google Scholar] [CrossRef]
- Babcock, M.C.; DuBose, L.E.; Witten, T.L.; Stauffer, B.L.; Hildreth, K.L.; Schwartz, R.S.; Kohrt, W.M.; Moreau, K.L. Oxidative Stress and Inflammation Are Associated with Age-Related Endothelial Dysfunction in Men with Low Testosterone. J. Clin. Endocrinol. Metab. 2022, 107, e500–e514. [Google Scholar] [CrossRef]
- Oskui, P.M.; French, W.J.; Herring, M.J.; Mayeda, G.S.; Burstein, S.; Kloner, R.A. Testosterone and the Cardiovascular System: A Comprehensive Review of the Clinical Literature. JAHA 2013, 2, e000272. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Bhasin, S.; Flevaris, P.; Mitchell, L.M.; Basaria, S.; Boden, W.E.; Cunningham, G.R.; Granger, C.B.; Khera, M.; Thompson, I.M.; et al. Cardiovascular Safety of Testosterone-Replacement Therapy. N. Engl. J. Med. 2023, 389, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Sawhney, A.; Nebuwa, C.; Borra, V.; Deb, N.; Halder, A.; Rajak, K.; Jha, M.; Wajid, Z.; Thachil, R.; et al. Association between Testosterone Replacement Therapy and Cardiovascular Outcomes: A Meta-Analysis of 30 Randomized Controlled Trials. Prog. Cardiovasc. Dis. 2024, 85, 45–53. [Google Scholar] [CrossRef]
- Mason, F.E.; Liutkute, A.; Voigt, N. Testosterone and Atrial Fibrillation: Does the Dose Make the Poison? Cardiovasc. Res. 2025, 121, 1144–1145. [Google Scholar] [CrossRef]
- Ebong, I.A.; Appiah, D.; Mauricio, R.; Narang, N.; Honigberg, M.C.; Ilonze, O.J.; Aggarwal, N.R.; Zanni, M.V.; Mohammed, S.F.; Cho, L.; et al. Sex Hormones and Heart Failure Risk. JACC Adv. 2025, 4, 101650. [Google Scholar] [CrossRef] [PubMed]
- Curcio, A.; Torella, D.; Iaconetti, C.; Pasceri, E.; Sabatino, J.; Sorrentino, S.; Giampà, S.; Micieli, M.; Polimeni, A.; Henning, B.J.; et al. MicroRNA-1 Downregulation Increases Connexin 43 Displacement and Induces Ventricular Tachyarrhythmias in Rodent Hypertrophic Hearts. PLoS ONE 2013, 8, e70158. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Rahman, F.; Schnabel, R.B.; Yin, X.; Benjamin, E.J.; Christophersen, I.E. Atrial Fibrillation in Women: Epidemiology, Pathophysiology, Presentation, and Prognosis. Nat. Rev. Cardiol. 2016, 13, 321–332. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Smith, C.E.R.; Louch, W.E.; Morotti, S.; Dobrev, D.; Grandi, E.; Ni, H. Enhanced Ca2+-Driven Arrhythmogenic Events in Female Patients with Atrial Fibrillation. JACC Clin. Electrophysiol. 2024, 10, 2371–2391. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.P.V.; Sandberg, K. Estrogen Regulation of Tumor Necrosis Factor-α: A Missing Link Between Menopause and Cardiovascular Risk in Women? Hypertension 2005, 46, 21–22. [Google Scholar] [CrossRef]
- Ramos-Mondragón, R.; Lozhkin, A.; Vendrov, A.E.; Runge, M.S.; Isom, L.L.; Madamanchi, N.R. NADPH Oxidases and Oxidative Stress in the Pathogenesis of Atrial Fibrillation. Antioxidants 2023, 12, 1833. [Google Scholar] [CrossRef]
- Yildirir, A.; Kabakci, G.; Akgul, E.; Tokgozoglu, L.; Oto, A. Effects of Menstrual Cycle on Cardiac Autonomic Innervation As Assessed By Heart Rate Variability. Noninvasive Electrocardiol. 2001, 7, 60–63. [Google Scholar] [CrossRef]
- Schmalenberger, K.M.; Eisenlohr-Moul, T.A.; Jarczok, M.N.; Eckstein, M.; Schneider, E.; Brenner, I.G.; Duffy, K.; Schweizer, S.; Kiesner, J.; Thayer, J.F.; et al. Menstrual Cycle Changes in Vagally-Mediated Heart Rate Variability Are Associated with Progesterone: Evidence from Two Within-Person Studies. J. Clin. Med. 2020, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Hohnloser, S.H.; Marcus, F.I.; Mortara, A.; Nohara, R.; Bigger, J.T.; Camm, A.J.; Schwartz, P.J. Baroreflex Sensitivity and Heart Rate Variability in the Identification of Patients at Risk for Life-Threatening Arrhythmias: Implications for Clinical Trials. Circulation 2001, 103, 2072–2077. [Google Scholar] [CrossRef]
- Souza, H.C.D.; Tezini, G.C.S.V. Autonomic Cardiovascular Damage during Post-Menopause: The Role of Physical Training. Aging Dis. 2013, 4, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kurokawa, J.; Bai, C.-X.; Asada, K.; Xu, J.; Oren, R.V.; Zhu, Z.I.; Clancy, C.E.; Isobe, M.; Furukawa, T. Progesterone Regulates Cardiac Repolarization Through a Nongenomic Pathway: An In Vitro Patch-Clamp and Computational Modeling Study. Circulation 2007, 116, 2913–2922. [Google Scholar] [CrossRef]
- Wong, J.A.; Rexrode, K.M.; Sandhu, R.K.; Moorthy, M.V.; Conen, D.; Albert, C.M. Menopausal Age, Postmenopausal Hormone Therapy and Incident Atrial Fibrillation. Heart 2017, 103, 1954–1961. [Google Scholar] [CrossRef]
- Jin, H.; Qiu, W.-B.; Mei, Y.-F.; Wang, D.-M.; Li, Y.-G.; Tan, X.-R. Testosterone Alleviates Tumor Necrosis Factor-Alpha-Mediated Tissue Factor Pathway Inhibitor Downregulation via Suppression of Nuclear Factor-Kappa B in Endothelial Cells. Asian J. Androl. 2009, 11, 266–271. [Google Scholar] [CrossRef]
- Tsuneda, T.; Yamashita, T.; Kato, T.; Sekiguchi, A.; Sagara, K.; Sawada, H.; Aizawa, T.; Fu, L.; Fujiki, A.; Inoue, H. Deficiency of Testosterone Associates with the Substrate of Atrial Fibrillation in the Rat Model. Cardiovasc. Electrophysiol. 2009, 20, 1055–1060. [Google Scholar] [CrossRef]
- Zeller, T.; Schnabel, R.B.; Appelbaum, S.; Ojeda, F.; Berisha, F.; Schulte-Steinberg, B.; Brueckmann, B.-E.; Kuulasmaa, K.; Jousilahti, P.; Blankenberg, S.; et al. Low Testosterone Levels Are Predictive for Incident Atrial Fibrillation and Ischaemic Stroke in Men, but Protective in Women–Results from the FINRISK Study. Eur. J. Prev. Cardiolog. 2018, 25, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Andelova, K.; Egan Benova, T.; Szeiffova Bacova, B.; Sykora, M.; Prado, N.J.; Diez, E.R.; Hlivak, P.; Tribulova, N. Cardiac Connexin-43 Hemichannels and Pannexin1 Channels: Provocative Antiarrhythmic Targets. Int. J. Mol. Sci. 2020, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Yeap, B.B.; Ball, J.; Clayton-Chubb, D.; Hussain, S.M.; Brodtmann, A.; Tonkin, A.M.; Neumann, J.T.; Schneider, H.G.; Fitzgerald, S.; et al. Testosterone and the Risk of Incident Atrial Fibrillation in Older Men: Further Analysis of the ASPREE Study. eClinicalMedicine 2024, 72, 102611. [Google Scholar] [CrossRef]
- Sharma, R.; Oni, O.A.; Gupta, K.; Sharma, M.; Sharma, R.; Singh, V.; Parashara, D.; Kamalakar, S.; Dawn, B.; Chen, G.; et al. Normalization of Testosterone Levels After Testosterone Replacement Therapy Is Associated with Decreased Incidence of Atrial Fibrillation. JAHA 2017, 6, e004880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.-M.; Wang, Y.-Z.; Zhang, Y.-Y.; Jin, X.-X.; Zhao, Y.; Wang, J.; Sun, Y.-L.; Xue, G.-L.; Li, P.-H.; et al. Increment of Late Sodium Currents in the Left Atrial Myocytes and Its Potential Contribution to Increased Susceptibility of Atrial Fibrillation in Castrated Male Mice. Heart Rhythm. 2017, 14, 1073–1080. [Google Scholar] [CrossRef]
- Curcio, A.; Scalise, R.; Indolfi, C. Pathophysiology of Atrial Fibrillation and Approach to Therapy in Subjects Less than 60 Years Old. Int. J. Mol. Sci. 2024, 25, 758. [Google Scholar] [CrossRef]
- Heijman, J.; Guichard, J.-B.; Dobrev, D.; Nattel, S. Translational Challenges in Atrial Fibrillation. Circ. Res. 2018, 122, 752–773. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Korantzopoulos, P.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 26, 209. [Google Scholar] [CrossRef]
- Pang, Z.; Ren, Y.; Yao, Z. Interactions between Atrial Fibrosis and Inflammation in Atrial Fibrillation. Front. Cardiovasc. Med. 2025, 12, 1578148. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; O’Mahony, F.; Lubahn, D.; Levin, E.R. Estrogen Receptor-β Prevents Cardiac Fibrosis. Mol. Endocrinol. 2010, 24, 2152–2165. [Google Scholar] [CrossRef]
- Gramley, F.; Lorenzen, J.; Koellensperger, E.; Kettering, K.; Weiss, C.; Munzel, T. Atrial Fibrosis and Atrial Fibrillation: The Role of the TGF-Β1 Signaling Pathway. Int. J. Cardiol. 2010, 143, 405–413. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Li, Y.; Li, P.; Zhang, Y.; Xu, G.; Sun, K. Estrogen Inhibits TGF-β1-stimulated Cardiac Fibroblast Differentiation and Collagen Synthesis by Promoting Cdc42. Mol. Med. Rep. 2024, 30, 123. [Google Scholar] [CrossRef]
- Linssen, P.B.C.; Brunner-La Rocca, H.-P.; Schalkwijk, C.G.; Beulens, J.W.J.; Elders, P.J.M.; Van Der Heijden, A.A.; Slieker, R.C.; Stehouwer, C.D.A.; Henry, R.M.A. Serum Matrix Metalloproteinases and Left Atrial Remodeling—The Hoorn Study. Int. J. Mol. Sci. 2020, 21, 4944. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, S.; Dworatzek, E.; Fritschka, S.; Pham, T.H.; Regitz-Zagrosek, V. 17β-Estradiol Inhibits Matrix Metalloproteinase-2 Transcription via MAP Kinase in Fibroblasts. Cardiovasc. Res. 2010, 85, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, O.; Ertem, F.U.; Vatankulu, M.A.; Erdogan, E.; Tasal, A.; Kucukbuzcu, S.; Goktekin, O. Novel Fibro-Inflammation Markers in Assessing Left Atrial Remodeling in Non-Valvular Atrial Fibrillation. Med. Sci. Monit. 2014, 20, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Voloshenyuk, T.G.; Gardner, J.D. Estrogen Improves TIMP-MMP Balance and Collagen Distribution in Volume-Overloaded Hearts of Ovariectomized Females. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R683–R693. [Google Scholar] [CrossRef]
- Rodríguez-Sinovas, A.; Sánchez, J.A.; Valls-Lacalle, L.; Consegal, M.; Ferreira-González, I. Connexins in the Heart: Regulation, Function and Involvement in Cardiac Disease. Int. J. Mol. Sci. 2021, 22, 4413. [Google Scholar] [CrossRef]
- Oyamada, M.; Takebe, K.; Oyamada, Y. Regulation of Connexin Expression by Transcription Factors and Epigenetic Mechanisms. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 118–133. [Google Scholar] [CrossRef]
- Ai, X.; Yan, J.; Pogwizd, S.M. Serine-Threonine Protein Phosphatase Regulation of Cx43 Dephosphorylation in Arrhythmogenic Disorders. Cell. Signal. 2021, 86, 110070. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Lee, T.-I.; Chen, Y.-C.; Kao, Y.-H.; Lu, Y.-Y.; Lin, Y.-K.; Chen, S.-A.; Chen, Y.-J. Testosterone Replacement Increases Aged Pulmonary Vein and Left Atrium Arrhythmogenesis with Enhanced Adrenergic Activity. Int. J. Cardiol. 2014, 176, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Thibault, S.; Ton, A.-T.; Huynh, F.; Fiset, C. Connexin Lateralization Contributes to Male Susceptibility to Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 10696. [Google Scholar] [CrossRef] [PubMed]
- Akçakoyun, M.; Alizade, E.; Gündoğdu, R.; Bulut, M.; Tabakcı, M.M.; Açar, G.; Avcı, A.; Şimşek, Z.; Fidan, S.; Demir, S.; et al. Long-Term Anabolic Androgenic Steroid Use Is Associated with Increased Atrial Electromechanical Delay in Male Bodybuilders. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Magnussen, C.; Ojeda, F.M.; Wild, P.S.; Sörensen, N.; Rostock, T.; Hoffmann, B.A.; Prochaska, J.; Lackner, K.J.; Beutel, M.E.; Blettner, M.; et al. Atrial Fibrillation Manifestations Risk Factors and Sex Differences in a Population-Based Cohort (From the Gutenberg Health Study). Am. J. Cardiol. 2018, 122, 76–82. [Google Scholar] [CrossRef]
- Piccini, J.P.; Simon, D.N.; Steinberg, B.A.; Thomas, L.; Allen, L.A.; Fonarow, G.C.; Gersh, B.; Hylek, E.; Kowey, P.R.; Reiffel, J.A.; et al. Differences in Clinical and Functional Outcomes of Atrial Fibrillation in Women and Men: Two-Year Results from the ORBIT-AF Registry. JAMA Cardiol. 2016, 1, 282. [Google Scholar] [CrossRef]
- Tan, J.L.; Johnson, L.; Dziubinski, M.; Napiorkowski, N.; Witkowska, O.; Slusarczyk, M.E.; Healey, J.S.; Russo, A.M. Sex Differences in Presentation of Atrial Fibrillation: Findings from 30-Day Ambulatory Monitoring in Real-World Practice. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 22, 100208. [Google Scholar] [CrossRef]
- Blum, S.; Muff, C.; Aeschbacher, S.; Ammann, P.; Erne, P.; Moschovitis, G.; Di Valentino, M.; Shah, D.; Schläpfer, J.; Fischer, A.; et al. Prospective Assessment of Sex-Related Differences in Symptom Status and Health Perception Among Patients with Atrial Fibrillation. JAHA 2017, 6, e005401. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Zeljko, H.M.; Bogossian, H.; Ciudin, R.; Costa, F.; Heijman, J.; Kochhaeuser, S.; Manola, S.; Scherr, D.; Sohal, M.; et al. Atrial Fibrillation-related Stroke in Women: Evidence and Inequalities in Epidemiology, Mechanisms, Clinical Presentation, and Management. Clin. Cardiol. 2020, 43, 14–23. [Google Scholar] [CrossRef]
- Mills, M.T.; Bucci, T.; Calvert, P.; Gupta, D.; Lip, G.Y.H. Temporal Trends in the Association Between Female Sex and Ischemic Stroke Among Patients with Atrial Fibrillation. JAHA 2025, 14, e040325. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Norby, F.L.; Gottesman, R.F.; Mosley, T.H.; Soliman, E.Z.; Agarwal, S.K.; Loehr, L.R.; Folsom, A.R.; Coresh, J.; Alonso, A. Association of Atrial Fibrillation with Cognitive Decline and Dementia Over 20 Years: The ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). JAHA 2018, 7, e007301. [Google Scholar] [CrossRef] [PubMed]
- Field, T.S.; Weijs, B.; Curcio, A.; Giustozzi, M.; Sudikas, S.; Katholing, A.; Wallenhorst, C.; Weitz, J.I.; Cohen, A.T.; Martinez, C. Incident Atrial Fibrillation, Dementia and the Role of Anticoagulation: A Population-Based Cohort Study. Thromb. Haemost. 2019, 119, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Chen, J.; Wang, H.-T.; Chang, Y.-T.; Chong, S.-Z.; Hsueh, S.; Chung, C.-M.; Lin, Y.-S. Sex Difference in the Risk of Dementia in Patients with Atrial Fibrillation. Diagnostics 2021, 11, 760. [Google Scholar] [CrossRef]
- Golive, A.; May, H.T.; Bair, T.L.; Jacobs, V.; Crandall, B.G.; Cutler, M.J.; Day, J.D.; Mallender, C.; Osborn, J.S.; Weiss, J.P.; et al. The Impact of Gender on Atrial Fibrillation Incidence and Progression to Dementia. Am. J. Cardiol. 2018, 122, 1489–1495. [Google Scholar] [CrossRef]
- Wu, V.C.-C.; Wu, M.; Aboyans, V.; Chang, S.-H.; Chen, S.-W.; Chen, M.-C.; Wang, C.-L.; Hsieh, I.-C.; Chu, P.-H.; Lin, Y.-S. Female Sex as a Risk Factor for Ischaemic Stroke Varies with Age in Patients with Atrial Fibrillation. Heart 2020, 106, 534–540. [Google Scholar] [CrossRef]
- Mikkelsen, A.P.; Lindhardsen, J.; Lip, G.Y.H.; Gislason, G.H.; Torp-Pedersen, C.; Olesen, J.B. Female Sex as a Risk Factor for Stroke in Atrial Fibrillation: A Nationwide Cohort Study. J. Thromb. Haemost. 2012, 10, 1745–1751. [Google Scholar] [CrossRef]
- Clua-Espuny, J.L.; Panisello-Tafalla, A.; Lucas-Noll, J.; Muria-Subirats, E.; Forcadell-Arenas, T.; Carrera-Ortiz, J.M.; Molto-Balado, P.; Clua-Queralt, J.; Fusté-Anguera, I.; Reverte-Vilarroya, S. Stroke Risk Stratification in Incident Atrial Fibrillation: A Sex-Specific Evaluation of CHA2DS2-VA and CHA2DS2-VASc. JCDD 2025, 12, 259. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Laroche, C.; Boriani, G.; Cimaglia, P.; Dan, G.-A.; Santini, M.; Kalarus, Z.; Rasmussen, L.H.; Popescu, M.I.; Tica, O.; et al. Sex-Related Differences in Presentation, Treatment, and Outcome of Patients with Atrial Fibrillation in Europe: A Report from the Euro Observational Research Programme Pilot Survey on Atrial Fibrillation. EP Eur. 2015, 17, 24–31. [Google Scholar] [CrossRef]
- Rienstra, M.; Van Veldhuisen, D.J.; Hagens, V.E.; Ranchor, A.V.; Veeger, N.J.G.M.; Crijns, H.J.G.M.; Van Gelder, I.C. Gender-Related Differences in Rhythm Control Treatment in Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2005, 46, 1298–1306. [Google Scholar] [CrossRef]
- Essebag, V. Sex Differences in the Relationship Between Amiodarone Use and the Need for Permanent Pacing in Patients with Atrial Fibrillation. Arch. Intern. Med. 2007, 167, 1648. [Google Scholar] [CrossRef]
- Nikitin, N.P.; Witte, K.K.A.; Thackray, S.D.R.; Goodge, L.J.; Clark, A.L.; Cleland, J.G.F. Effect of Age and Sex on Left Atrial Morphology and Function. Eur. Heart J.-Cardiovasc. Imaging 2003, 4, 36–42. [Google Scholar] [CrossRef]
- Odening, K.E.; Deiß, S.; Dilling-Boer, D.; Didenko, M.; Eriksson, U.; Nedios, S.; Ng, F.S.; Roca Luque, I.; Sanchez Borque, P.; Vernooy, K.; et al. Mechanisms of Sex Differences in Atrial Fibrillation: Role of Hormones and Differences in Electrophysiology, Structure, Function, and Remodelling. EP Eur. 2019, 21, 366–376. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Pecen, L.; Ojeda, F.M.; Lucerna, M.; Rzayeva, N.; Blankenberg, S.; Darius, H.; Kotecha, D.; Caterina, R.D.; Kirchhof, P. Gender Differences in Clinical Presentation and 1-Year Outcomes in Atrial Fibrillation. Heart 2017, 103, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Gurevitz, O.T.; Varadachari, C.J.; Ammash, N.M.; Malouf, J.F.; Rosales, A.G.; Herges, R.M.; Bruce, C.J.; Somers, V.K.; Hammill, S.C.; Gersh, B.J.; et al. The Effect of Patient Sex on Recurrence of Atrial Fibrillation Following Successful Direct Current Cardioversion. Am. Heart J. 2006, 152, e9–e155. [Google Scholar] [CrossRef] [PubMed]
- Kassim, N.A.; Althouse, A.D.; Qin, D.; Leef, G.; Saba, S. Gender Differences in Management and Clinical Outcomes of Atrial Fibrillation Patients. J. Cardiol. 2017, 69, 195–200. [Google Scholar] [CrossRef]
- Calvert, P.; Mills, M.T.; Xydis, P.; Essa, H.; Ding, W.Y.; Koniari, I.; Farinha, J.M.; Harding, M.; Mahida, S.; Snowdon, R.; et al. Cost, Efficiency, and Outcomes of Pulsed Field Ablation vs Thermal Ablation for Atrial Fibrillation: A Real-World Study. Heart Rhythm. 2024, 21, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Duarte, F.; Silva-Teixeira, R.; Aguiar-Neves, I.; Almeida, J.G.; Fonseca, P.; Monteiro, A.V.; Oliveira, M.; Gonçalves, H.; Ribeiro, J.; Caramelo, F.; et al. Sex Differences in Atrial Remodeling and Atrial Fibrillation Recurrence after Catheter Ablation. Heart Rhythm. 2025, 22, e563–e571. [Google Scholar] [CrossRef]
- Vandenberk, B.; Chew, D.S.; Parkash, R.; Gillis, A.M. Sex and Racial Disparities in Catheter Ablation. Heart Rhythm O2 2022, 3, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhu, W.; Yang, H.; Dong, Q.; Fei, Y.; Li, X.; Li, S.; Han, B. Different Impact of Female Gender on the Outcome of Catheter Ablation between Paroxysmal and Persistent Atrial Fibrillation. BMC Cardiovasc. Disord. 2025, 25, 364. [Google Scholar] [CrossRef]
- Al-Sadawi, M.; Aslam, F.; Gier, C.; Aleem, S.; Ijaz, H.; Jacobs, R.; Cao, K.; Alsaiqali, M.; Singh, A. Effect of Gender on Atrial Fibrillation Ablation Outcomes Using a Propensity Score–Matched Analysis. Heart Rhythm O2 2023, 4, 309–316. [Google Scholar] [CrossRef]
- Sugumar, H.; Nanayakkara, S.; Chieng, D.; Wong, G.R.; Parameswaran, R.; Anderson, R.D.; Al-Kaisey, A.; Nalliah, C.J.; Azzopardi, S.; Prabhu, S.; et al. Arrhythmia Recurrence Is More Common in Females Undergoing Multiple Catheter Ablation Procedures for Persistent Atrial Fibrillation: Time to Close the Gender Gap. Heart Rhythm. 2020, 17, 692–698. [Google Scholar] [CrossRef]
- Yunus, F.N.; Perino, A.C.; Holmes, D.N.; Matsouaka, R.A.; Curtis, A.B.; Ellenbogen, K.A.; Frankel, D.S.; Knight, B.P.; Russo, A.M.; Lewis, W.R.; et al. Sex Differences in Ablation Strategy, Lesion Sets, and Complications of Catheter Ablation for Atrial Fibrillation: An Analysis from the GWTG-AFIB Registry. Circ. Arrhythmia Electrophysiol. 2021, 14, e009790. [Google Scholar] [CrossRef]
- Kaiser, D.W.; Fan, J.; Schmitt, S.; Than, C.T.; Ullal, A.J.; Piccini, J.P.; Heidenreich, P.A.; Turakhia, M.P. Gender Differences in Clinical Outcomes After Catheter Ablation of Atrial Fibrillation. JACC Clin. Electrophysiol. 2016, 2, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.T.; Lip, G.Y.H.; Luther, V.; Gupta, D. Sex-Based Differences in Symptomatology in the First Month Following Atrial Fibrillation Catheter Ablation. Cardiovasc. Electrophysiol. 2025, 36, 2271–2278. [Google Scholar] [CrossRef]
- Zeitler, E.P.; Li, Y.; Silverstein, A.P.; Russo, A.M.; Poole, J.E.; Daniels, M.R.; Al-Khalidi, H.R.; Lee, K.L.; Bahnson, T.D.; Anstrom, K.J.; et al. Effects of Ablation Versus Drug Therapy on Quality of Life by Sex in Atrial Fibrillation: Results from the CABANA Trial. JAHA 2023, 12, e027871. [Google Scholar] [CrossRef]
- Yao, R.J.R.; Macle, L.; Deyell, M.W.; Tang, L.; Hawkins, N.M.; Sedlak, T.; Nault, I.; Verma, A.; Khairy, P.; Andrade, J.G. Impact of Female Sex on Clinical Presentation and Ablation Outcomes in the CIRCA-DOSE Study. JACC Clin. Electrophysiol. 2020, 6, 945–954. [Google Scholar] [CrossRef]
- Du Fay De Lavallaz, J.; Clerc, O.; Pudenz, C.; Illigens, B.; Kühne, M. Sex-specific Efficacy and Safety of Cryoballoon versus Radiofrequency Ablation for Atrial Fibrillation: A Systematic Review and Meta-analysis. Cardiovasc. Electrophysiol. 2019, 30, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Chun, K.R.J.; Metzner, A.; Ouyang, F.; Schlüter, M.; Elvan, A.; Braegelmann, K.M.; Kueffer, F.J.; et al. Impact of Female Sex on Clinical Outcomes in the FIRE AND ICE Trial of Catheter Ablation for Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2018, 11, e006204. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Neuzil, P.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Clinical Outcomes by Sex After Pulsed Field Ablation of Atrial Fibrillation. JAMA Cardiol. 2023, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Pancholy, S.B.; Sharma, P.S.; Pancholy, D.S.; Patel, T.M.; Callans, D.J.; Marchlinski, F.E. Meta-Analysis of Gender Differences in Residual Stroke Risk and Major Bleeding in Patients with Nonvalvular Atrial Fibrillation Treated with Oral Anticoagulants. Am. J. Cardiol. 2014, 113, 485–490. [Google Scholar] [CrossRef]
- Curcio, A.; Anselmino, M.; Di Biase, L.; Migliore, F.; Nigro, G.; Rapacciuolo, A.; Sergi, D.; Tomasi, L.; Pedrinelli, R.; Mercuro, G.; et al. The Gray Areas of Oral Anticoagulation for Prevention of Thromboembolic Events in Atrial Fibrillation Patients. J. Cardiovasc. Med. 2023, 24 (Suppl. 2), e97–e105. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.M.; Tremmel, J.A.; Lansberg, M.G.; Fan, J.; Askari, M.; Turakhia, M.P. Sex Differences in Oral Anticoagulation and Outcomes of Stroke and Intracranial Bleeding in Newly Diagnosed Atrial Fibrillation. JAHA 2020, 9, e015689. [Google Scholar] [CrossRef]
- Weberndörfer, V.; Beinart, R.; Ricciardi, D.; Ector, J.; Mahfoud, M.; Szeplaki, G.; Hemels, M.; DAS-CAM participants 2017/2018. Sex Differences in Rate and Rhythm Control for Atrial Fibrillation. EP Eur. 2019, 21, 690–697. [Google Scholar] [CrossRef]
- Russo, A.M.; Zeitler, E.P.; Giczewska, A.; Silverstein, A.P.; Al-Khalidi, H.R.; Cha, Y.-M.; Monahan, K.H.; Bahnson, T.D.; Mark, D.B.; Packer, D.L.; et al. Association Between Sex and Treatment Outcomes of Atrial Fibrillation Ablation Versus Drug Therapy: Results from the CABANA Trial. Circulation 2021, 143, 661–672. [Google Scholar] [CrossRef]
- Henry, L.; Hunt, S.; Holmes, S.D.; Martin, L.M.; Ad, N. Are There Gender Differences in Outcomes after the Cox-Maze Procedure for Atrial Fibrillation? Innovations 2013, 8, 190–198. [Google Scholar] [CrossRef]
- Linde, C.; Bongiorni, M.G.; Birgersdotter-Green, U.; Curtis, A.B.; Deisenhofer, I.; Furokawa, T.; Gillis, A.M.; Haugaa, K.H.; Lip, G.Y.H.; Van Gelder, I.; et al. Sex Differences in Cardiac Arrhythmia: A Consensus Document of the European Heart Rhythm Association, Endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. EP Eur. 2018, 20, 1565–1565ao. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Haung, Y.-B.; Kuo, H.-F.; Tang, W.-H.; Hsu, P.-C.; Su, H.-M.; Lin, T.-H.; Chu, C.-S.; Jhuo, S.-J.; Lee, K.-T.; et al. Hormone Replacement Therapy and Risk of Atrial Fibrillation in Taiwanese Menopause Women: A Nationwide Cohort Study. Sci. Rep. 2016, 6, 24132. [Google Scholar] [CrossRef]
- Apostolakis, S.; Sullivan, R.M.; Olshansky, B.; Lip, G.Y.H. Hormone Replacement Therapy and Adverse Outcomes in Women with Atrial Fibrillation: An Analysis from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management Trial. Stroke 2014, 45, 3076–3079. [Google Scholar] [CrossRef]
- Blackwell, K.; Blackwell, M.; Blackwell, T. Testosterone Replacement Therapy and Cardiovascular Disease: Balancing Safety and Risks in Hypogonadal Men. Curr. Cardiol. Rep. 2023, 25, 1157–1163. [Google Scholar] [CrossRef]
- Olié, V.; Canonico, M.; Scarabin, P.-Y. Postmenopausal Hormone Therapy and Venous Thromboembolism. Thromb. Res. 2011, 127, S26–S29. [Google Scholar] [CrossRef]
- Marzak, H.; Ringele, R.; Matsushita, K.; Marchandot, B.; Fitouchi, S.; Cardi, T.; Kanso, M.; Schatz, A.; Hammann, J.; Ohlmann, P.; et al. Impact of Gender on Left Atrial Low-Voltage Zones in Patients with Persistent Atrial Fibrillation: Results of a Voltage-Guided Ablation. Front. Cardiovasc. Med. 2023, 10, 1229345. [Google Scholar] [CrossRef]
- Park, H.; Kwon, O.-S.; Shim, J.; Kim, D.; Park, J.-W.; Kim, Y.-G.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Choi, J.-I.; et al. Artificial Intelligence-Estimated Electrocardiographic Sex as a Recurrence Predictor after Atrial Fibrillation Catheter Ablation. Eur. Heart J.-Digit. Health 2025, 6, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, N.D.; Curcio, A.; Nodari, S.; Parati, G.; Carugo, S.; Molinari, M.; Acquistapace, F.; Gensini, G.; Molinari, G.; Working Group on Telecardiology, Informatics of the Italian Society of Cardiology. The Italian Society of Cardiology and Working Group on Telecardiology and Informatics 2023 Updated Position Paper on Telemedicine and Artificial Intelligence in Cardiovascular Disease. J. Cardiovasc. Med. 2023, 24 (Suppl. 2), e168–e177. [Google Scholar] [CrossRef] [PubMed]
- Curcio, A.; Quarta, R. Transesophageal Echocardiography before Atrial Fibrillation Ablation: To Do or Not to Do? Pol. Heart J. 2024, 82, 477–479. [Google Scholar] [CrossRef]
- Kumagai, K.; Nakashima, H.; Urata, H.; Gondo, N.; Arakawa, K.; Saku, K. Effects of Angiotensin II Type 1 Receptor Antagonist on Electrical and Structural Remodeling in Atrial Fibrillation. J. Am. Coll. Cardiol. 2003, 41, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Popovic, D.S.; Pamporis, K.; Theofilis, P.; Nasoufidou, A.; Stachteas, P.; Samaras, A.; Tzikas, A.; Giannakoulas, G.; et al. Effects of Mineralocorticoid Receptor Antagonists on New-Onset or Recurrent Atrial Fibrillation: A Bayesian and Frequentist Network Meta-Analysis of Randomized Trials. Curr. Probl. Cardiol. 2024, 49, 102742. [Google Scholar] [CrossRef]
- Paw, M.; Kusiak, A.A.; Nit, K.; Litewka, J.J.; Piejko, M.; Wnuk, D.; Sarna, M.; Fic, K.; Stopa, K.B.; Hammad, R.; et al. Hypoxia Enhances Anti-Fibrotic Properties of Extracellular Vesicles Derived from hiPSCs via the miR302b-3p/TGFβ/SMAD2 Axis. BMC Med. 2023, 21, 412. [Google Scholar] [CrossRef]
- Mohammad, Z.; Ahmad, J.; Sultan, A.; Penagaluri, A.; Morin, D.; Dominic, P. Effect of Sacubitril–Valsartan on the Incidence of Atrial Fibrillation: A Meta-analysis. Cardiovasc. Electrophysiol. 2023, 34, 1037–1042. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-Analysis. Adv. Ther. 2024, 41, 3749–3756. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Maeda, M.; Oba, K.; Maimaituxun, G.; Arasaki, O.; Yagi, S.; Kusunose, K.; Soeki, T.; Yamada, H.; Fukuda, D.; et al. Sex Differences in the Association between Epicardial Adipose Tissue Volume and Left Atrial Volume Index. BMC Cardiovasc. Disord. 2024, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Kidess, G.G.; Hamza, M.; Goru, R.; Basit, J.; Alraiyes, M.; Alraies, M.C. The Impact of Sodium-Glucose Cotransporter-2 Inhibitors on Atrial Fibrillation Burden in Diabetic Patients. Am. J. Cardiol. 2025, 246, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bikou, O.; Thomas, D.; Trappe, K.; Lugenbiel, P.; Kelemen, K.; Koch, M.; Soucek, R.; Voss, F.; Becker, R.; Katus, H.A.; et al. Connexin 43 Gene Therapy Prevents Persistent Atrial Fibrillation in a Porcine Model. Cardiovasc. Res. 2011, 92, 218–225. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, L.R.; Celeste, A.; Curcio, A. Hormonal Atrial Fibrillation: Pathophysiological Mechanisms That Trigger and Sustain the Arrhythmic Circuits. Biomedicines 2025, 13, 2466. https://doi.org/10.3390/biomedicines13102466
Romano LR, Celeste A, Curcio A. Hormonal Atrial Fibrillation: Pathophysiological Mechanisms That Trigger and Sustain the Arrhythmic Circuits. Biomedicines. 2025; 13(10):2466. https://doi.org/10.3390/biomedicines13102466
Chicago/Turabian StyleRomano, Letizia Rosa, Aldo Celeste, and Antonio Curcio. 2025. "Hormonal Atrial Fibrillation: Pathophysiological Mechanisms That Trigger and Sustain the Arrhythmic Circuits" Biomedicines 13, no. 10: 2466. https://doi.org/10.3390/biomedicines13102466
APA StyleRomano, L. R., Celeste, A., & Curcio, A. (2025). Hormonal Atrial Fibrillation: Pathophysiological Mechanisms That Trigger and Sustain the Arrhythmic Circuits. Biomedicines, 13(10), 2466. https://doi.org/10.3390/biomedicines13102466








