Development and Validation of a Machine Learning Model to Predict Anti-Drug Antibody Formation During Infliximab Induction in Crohn’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Acquisition and Processing
2.3. Outcome Definition of ADA Status in CD Patients
2.4. Feature Selection
2.5. Model Development and Validation
2.6. Model Explainability
2.7. Statistical Analysis
3. Results
3.1. Description of Variables
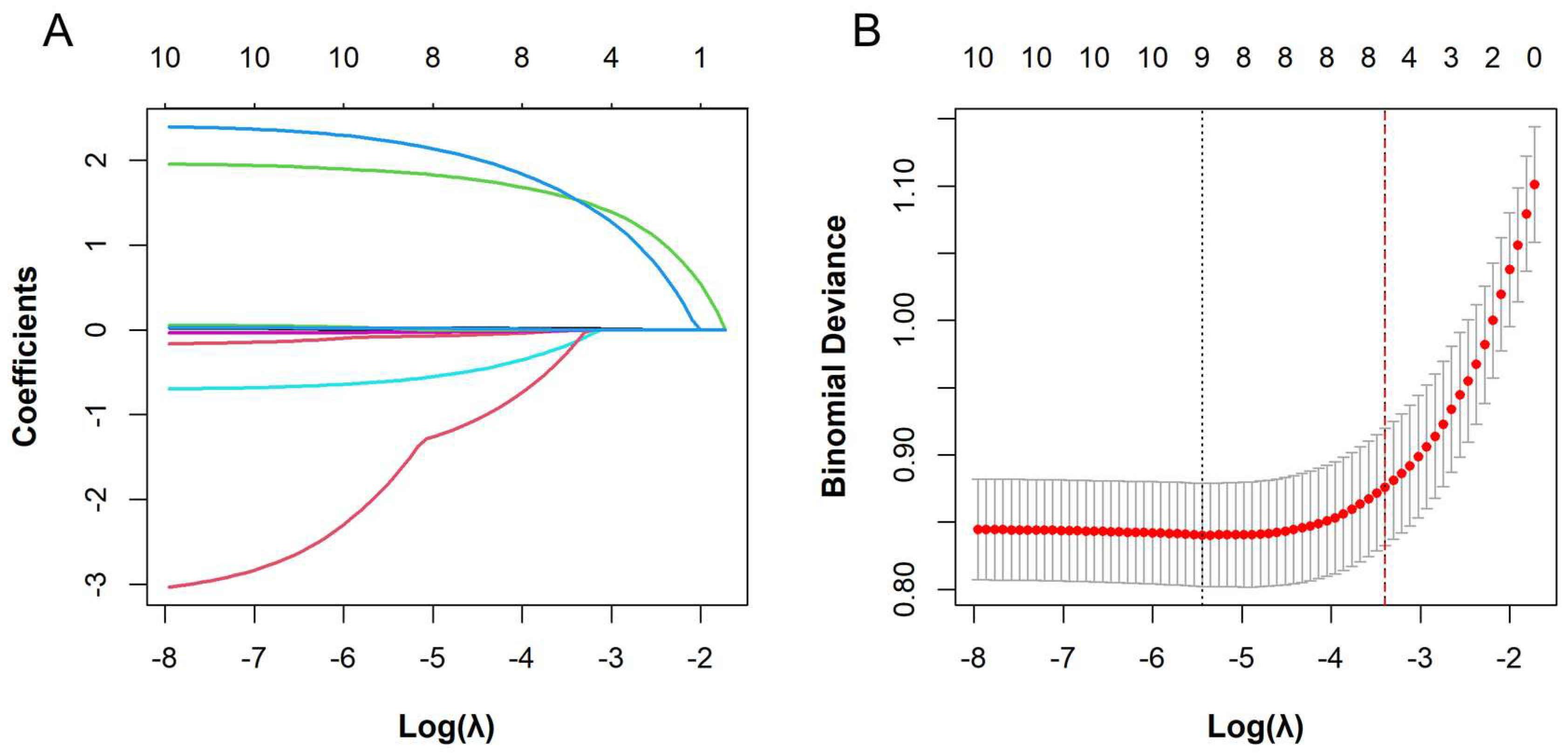
3.2. Variable Selection
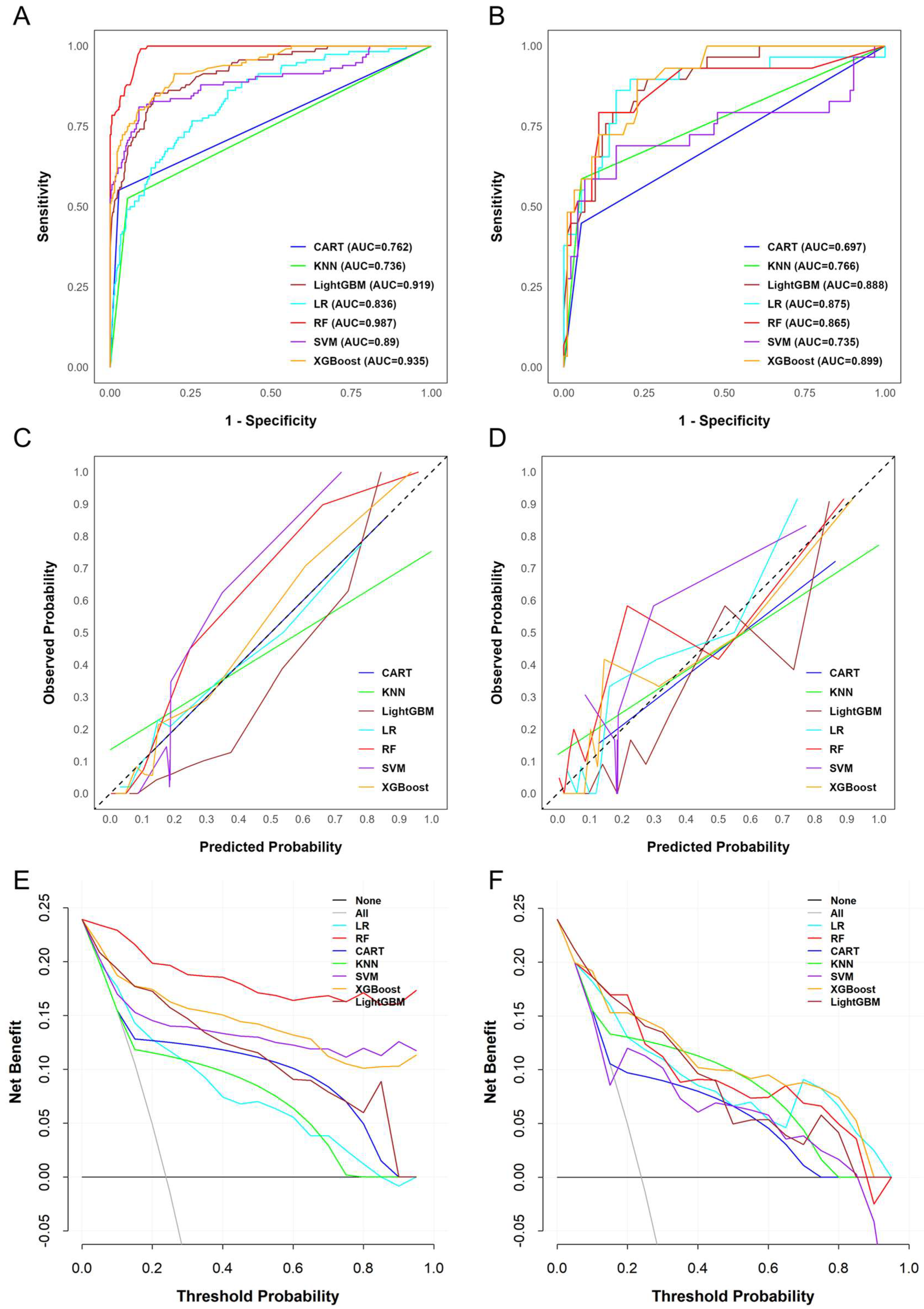
3.3. Assessment of Predictive Model Performance
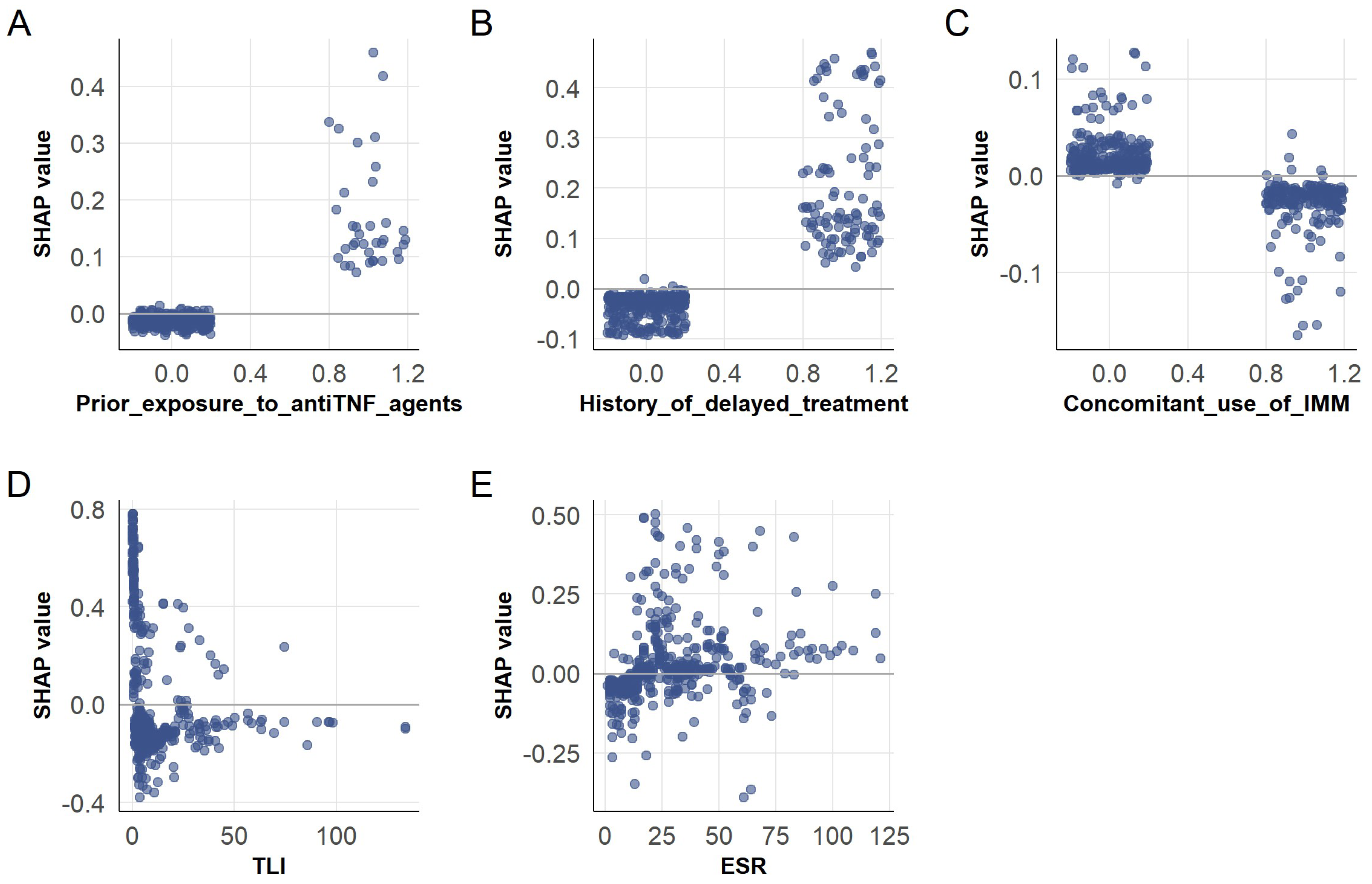
3.4. SHAP-Based Interpretation of ADA Prediction in the XGBoost Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO guidelines on therapeutics in Crohn’s disease: Medical treatment. J. Crohns Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, B.-L.; Mao, R.; Zhang, S.-H.; He, Y.; Zeng, Z.-R.; Ben-Horin, S.; Chen, M.-H. Systematic review with meta-analysis: Loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J. Gastroenterol. 2017, 52, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies. Front. Immunol. 2020, 11, 1951. [Google Scholar] [CrossRef] [PubMed]
- Brun, M.K.; Gehin, J.E.; Bjørlykke, K.H.; Warren, D.J.; Klaasen, R.A.; Sexton, J.; Sandanger, Ø.; Kvien, T.K.; Mørk, C.; Jahnsen, J.; et al. Clinical consequences of infliximab immunogenicity and the effect of proactive therapeutic drug monitoring: Exploratory analyses of the randomised, controlled NOR-DRUM trials. Lancet Rheumatol. 2024, 6, e226–e236. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Wagner, C.L.; Bala, M.; Mayer, L.; Travers, S.; Diamond, R.H.; Olson, A.; Bao, W.; Rutgeerts, P. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2004, 2, 542–553. [Google Scholar] [CrossRef]
- Stallhofer, J.; Guse, J.; Kesselmeier, M.; Grunert, P.C.; Lange, K.; Stalmann, R.; Eckardt, V.; Stallmach, A. Immunomodulator comedication promotes the reversal of anti-drug antibody-mediated loss of response to anti-TNF therapy in inflammatory bowel disease. Int. J. Colorectal. Dis. 2023, 38, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Q.; Zhao, J.; Liu, T.; Yao, J.; Peng, X.; Zhi, M.; Zhang, M. HLA-DQA1*05 correlates with increased risk of anti-drug antibody development and reduced response to infliximab in Chinese patients with Crohn’s disease. Gastroenterol. Rep. 2024, 12, goae074. [Google Scholar] [CrossRef]
- van der Have, M.; Oldenburg, B.; Kaptein, A.A.; Jansen, J.M.; Scheffer, R.C.; van Tuyl, B.A.; van der Meulen-de Jong, A.E.; Pierik, M.; Siersema, P.D.; van Oijen, M.G.; et al. Non-adherence to anti-TNF therapy is associated with illness perceptions and clinical outcomes in outpatients with inflammatory bowel disease: Results from a prospective multicentre study. J. Crohns Colitis 2016, 10, 549–555. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mould, D.; Smeekes, O.; Ashruf, Y.; Kuin, S.; Strik, A.; van den Brink, G.R.; D’Haens, G.R. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23, 650–660. [Google Scholar] [CrossRef]
- Brun, M.K.; Goll, G.L.; Jørgensen, K.K.; Sexton, J.; Gehin, J.E.; Sandanger, Ø.; Olsen, I.C.; Klaasen, R.A.; Warren, D.J.; Mørk, C.; et al. Risk factors for anti-drug antibody formation to infliximab: Secondary analyses of a randomised controlled trial. J. Intern. Med. 2022, 292, 477–491. [Google Scholar] [CrossRef]
- Brun, M.K.; Bjørlykke, K.H.; Viken, M.K.; Stenvik, G.-E.; Klaasen, R.A.; Gehin, J.E.; Warren, D.J.; Sexton, J.; Sandanger, Ø.; Kvien, T.K.; et al. HLA-DQ2 is associated with anti-drug antibody formation to infliximab in patients with immune-mediated inflammatory diseases. J. Intern. Med. 2023, 293, 648–655. [Google Scholar] [CrossRef]
- Rajula, H.S.R.; Verlato, G.; Manchia, M.; Antonucci, N.; Fanos, V. Comparison of conventional statistical methods with machine learning in medicine: Diagnosis, drug development, and treatment. Medicina 2020, 56, 455. [Google Scholar] [CrossRef]
- Cai, W.; Wu, X.; Guo, K.; Chen, Y.; Shi, Y.; Lin, X. Deep-learning, radiomics and clinic based fusion models for predicting response to infliximab in Crohn’s disease patients: A multicentre, retrospective study. J. Inflamm. Res. 2024, 17, 7639–7651. [Google Scholar] [CrossRef]
- Qiu, Y.; Hu, S.; Chao, K.; Huang, L.; Huang, Z.; Mao, R.; Su, F.; Zhang, C.; Lin, X.; Cao, Q.; et al. Developing a machine-learning prediction model for infliximab response in Crohn’s disease: Integrating clinical characteristics and longitudinal laboratory trends. Inflamm. Bowel Dis. 2025, 31, 1334–1343. [Google Scholar] [CrossRef]
- Schöler, D.; Kostev, K.; Peters, M.; Zamfir, C.; Wolk, A.; Roderburg, C.; Loosen, S.H. Machine learning can predict the probability of biologic therapy in patients with inflammatory bowel disease. J. Clin. Med. 2022, 11, 4586. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Niu, R.; Xiong, S.; He, J.; Wang, Y.; Zhang, P.; Su, F.; Liu, Z.; Zhou, L.; et al. Multi-omics biomarkers for predicting efficacy of biologic and small-molecule therapies in adults with inflammatory bowel disease: A systematic review. United Eur. Gastroenterol. J. 2025, 13, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Aden, K.; Blase, J.I.; Baran, N.; Bordoni, D.; Tran, F.; Conrad, C.; Avalos, D.; Jaeckel, C.; Scherer, M.; et al. Longitudinal multi-omics analysis identifies early blood-based predictors of anti-TNF therapy response in inflammatory bowel disease. Genome Med. 2022, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Sazonovs, A.; Kennedy, N.A.; Moutsianas, L.; Heap, G.A.; Rice, D.L.; Reppell, M.; Bewshea, C.M.; Chanchlani, N.; Walker, G.J.; Perry, M.H.; et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology 2019, 158, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.J.; Plichta, D.; Hogstrom, L.; Borren, N.Z.; Lau, H.; Gregory, S.M.; Tan, W.; Khalili, H.; Clish, C.; Vlamakis, H.; et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe 2021, 29, 1294–1304.e1294. [Google Scholar] [CrossRef]
- Yang, T.; Feng, J.; Yao, R.; Feng, Q.; Shen, J. CT-based pancreatic radiomics predicts secondary loss of response to infliximab in biologically naïve patients with Crohn’s disease. Insights Imaging 2024, 15, 69–82. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Zhou, Z.; Zhong, Y.; Zhang, R.; Shen, X.; Huang, L.; He, W.; Lin, J.; Fang, J.; et al. CT-based radiomics signature of visceral adipose tissue and bowel lesions for identifying patients with Crohn’s disease resistant to infliximab. Insights Imaging 2024, 15, 28–42. [Google Scholar] [CrossRef]
- Con, D.; van Langenberg, D.R.; Vasudevan, A. Deep learning vs conventional learning algorithms for clinical prediction in Crohn’s disease: A proof-of-concept study. World J. Gastroenterol. 2021, 27, 6476–6488. [Google Scholar] [CrossRef]
- IBDGoCM, A. Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). J. Dig. Dis. 2021, 22, 298–317. [Google Scholar] [CrossRef] [PubMed]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Vermeire, S.; Noman, M.; Van Assche, G.; Baert, F.; D’Haens, G.; Rutgeerts, P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007, 56, 1226–1231. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Khanna, R.; Levesque, B.G.; Stitt, L.; Zou, G.Y.; Singh, S.; Lockton, S.; Hauenstein, S.; Ohrmund, L.; Greenberg, G.R.; et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 2015, 64, 1539–1545. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Ferrante, M.; Van Assche, G.; Ballet, V.; Compernolle, G.; Van Steen, K.; Simoens, S.; Rutgeerts, P.; Gils, A.; Vermeire, S. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015, 148, 1320–1329.e1323. [Google Scholar] [CrossRef]
- Bots, S.J.; Parker, C.E.; Brandse, J.F.; Löwenberg, M.; Feagan, B.G.; Sandborn, W.J.; Jairath, V.; D’Haens, G.; Vande Casteele, N. Anti-drug antibody formation against biologic agents in inflammatory bowel disease: A systematic review and meta-analysis. BioDrugs 2021, 35, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Moraes, L.; Magnusson, M.K.; Öhman, L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand. J. Gastroenterol. 2018, 53, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of inflammatory bowel disease: Innate immune system. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Adedokun, O.J.; Gasink, C.; Gao, L.-L.; Cornillie, F.J.; D’Haens, G.R.; Rutgeerts, P.J.; Reinisch, W.; Sandborn, W.J.; Hanauer, S.B. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: A post hoc analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1525–1532. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Heap, G.A.; Green, H.D.; Hamilton, B.; Bewshea, C.; Walker, G.J.; Thomas, A.; Nice, R.; Perry, M.H.; Bouri, S.; et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: A prospective, multicentre, cohort study. Lancet Gastroenterol. Hepatol. 2019, 4, 341–353. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Diamond, R.H.; Wagner, C.L.; Fasanmade, A.A.; Olson, A.D.; Marano, C.W.; Johanns, J.; Lang, Y.; Sandborn, W.J. Clinical trial: Benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment. Pharmacol. Ther. 2009, 30, 210–226. [Google Scholar] [CrossRef]
- Ben-Shatach, Z.; Ziv-Baran, T.; Fudim, E.; Yavzori, M.; Picard, O.; Levartovsky, A.; Selinger, L.; Weiss, B.; Kopylov, U.; Eliakim, R.; et al. Delaying an infliximab infusion by more than 3 days is associated with a significant reduction in trough levels but not with clinical worsening. Ther. Adv. Gastroenterol. 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Chen, S.; Liu, X. Delayed infliximab treatment affects the outcomes of patients with Crohn’s disease during the COVID-19 epidemic in China: A propensity score-matched analysis. Front. Med. 2021, 8, 819557. [Google Scholar] [CrossRef] [PubMed]
- Vande Casteele, N.; Abreu, M.T.; Flier, S.; Papamichael, K.; Rieder, F.; Silverberg, M.S.; Khanna, R.; Okada, L.; Yang, L.; Jain, A.; et al. Patients with low drug levels or antibodies to a prior anti-tumor necrosis factor are more likely to develop antibodies to a subsequent anti-tumor necrosis factor. Clin. Gastroenterol. Hepatol. 2022, 20, 465–467. [Google Scholar] [CrossRef]
- Qiu, Y.; Mao, R.; Chen, B.-L.; Zhang, S.-H.; Guo, J.; He, Y.; Zeng, Z.-R.; Ben-Horin, S.; Chen, M.-H. Effects of combination therapy with immunomodulators on trough levels and antibodies against tumor necrosis factor antagonists in patients with inflammatory bowel disease: A meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Stray, N.; Sauar, J.; Vatn, M.H.; Moum, B. C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008, 57, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Iaculli, E.; Agostini, M.; Biancone, L.; Fiorani, C.; Di Vizia, A.; Montagnese, F.; Sibio, S.; Manzelli, A.; Tesauro, M.; Rufini, A.; et al. C-reactive protein levels in the perioperative period as a predictive marker of endoscopic recurrence after ileo-colonic resection for Crohn’s disease. Cell Death Discov. 2016, 2, 16032. [Google Scholar] [CrossRef]
- Moran, C.J.; Kaplan, J.L.; Winter, H.S. Genetic variation affects C-reactive protein elevations in Crohn’s disease. Inflamm. Bowel Dis. 2018, 24, 2048–2052. [Google Scholar] [CrossRef]
- Thalmaier, D.; Dambacher, J.; Seiderer, J.; Konrad, A.; Schachinger, V.; Pfennig, S.; Otte, J.M.; Crispin, A.; Göke, B.; Ochsenkühn, T.; et al. The +1059G/C polymorphism in the C-reactive protein (CRP) gene is associated with involvement of the terminal ileum and decreased serum CRP levels in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2006, 24, 1105–1115. [Google Scholar] [CrossRef]
- Romero-Cara, P.; Torres-Moreno, D.; Pedregosa, J.; Vílchez, J.A.; García-Simón, M.S.; Ruiz-Merino, G.; Morán-Sanchez, S.; Conesa-Zamora, P. A FCGR3A polymorphism predicts anti-drug antibodies in chronic inflammatory bowel disease patients treated with anti-TNF. Int. J. Med. Sci. 2018, 15, 10–15. [Google Scholar] [CrossRef]
- Alatawi, H.; Mosli, M.; Saadah, O.I.; Annese, V.; Al-Hindi, R.; Alatawy, M.; Al-Amrah, H.; Alshehri, D.; Bahieldin, A.; Edris, S. Attributes of intestinal microbiota composition and their correlation with clinical primary non-response to anti-TNF-α agents in inflammatory bowel disease patients. Bosn. J. Basic Med. Sci. 2022, 22, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Payrovnaziri, S.N.; Chen, Z.; Rengifo-Moreno, P.; Miller, T.; Bian, J.; Chen, J.H.; Liu, X.; He, Z. Explainable artificial intelligence models using real-world electronic health record data: A systematic scoping review. J. Am. Med. Inform. Assoc. 2020, 27, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Ning, Y.; Ong, M.E.H. Explainable artificial intelligence in emergency medicine: An overview. Clin. Exp. Emerg. Med. 2023, 10, 354–362. [Google Scholar] [CrossRef]
- Raygoza Garay, J.A.; Turpin, W.; Lee, S.H.; Smith, M.I.; Goethel, A.; Griffiths, A.M.; Moayyedi, P.; Espin-Garcia, O.; Abreu, M.; Aumais, G.L.; et al. Gut microbiome composition is associated with future onset of Crohn’s disease in healthy first-degree relatives. Gastroenterology 2023, 165, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Liu, Y.; Pei, Q.; Ning, X.; Zou, Y.; Liu, L.; Song, L.; Guo, C.; Sun, Y.; Deng, K.; et al. Long short-term memory network for development and simulation of warfarin dosing model based on time series anticoagulant data. Front. Cardiovasc. Med. 2022, 9, 881111. [Google Scholar] [CrossRef]

| Variables | Total (n = 485) | ADA Negative (n = 369) | ADA Positive (n = 116) | p Value |
|---|---|---|---|---|
| Sex, n (%) | 0.187 | |||
| Male, n (%) | 375 (77.32%) | 291 (78.86%) | 84 (72.41%) | |
| Female, n (%) | 110 (22.68%) | 78 (21.14%) | 32 (27.59%) | |
| BMI (kg/m2), M (Q1, Q3) | 19.22 (17.44, 20.90) | 19.47 (17.74, 20.96) | 18.46 (17.08, 20.47) | 0.013 |
| Age at onset (yr), M (Q1, Q3) | 25.00 (20.00, 30.00) | 25.00 (20.00, 30.00) | 25.00 (21.00, 30.00) | 0.409 |
| Age at initiation of IFX (yr), M (Q1, Q3) | 28.00 (23.00, 34.00) | 28.00 (23.00, 33.00) | 29.50 (24.00, 36.00) | 0.044 |
| Disease duration (yr), M (Q1, Q3) | 2.00 (1.00, 5.00) | 2.00 (1.00, 5.00) | 2.00 (1.00, 7.00) | 0.148 |
| Age at diagnosis, n (%) | 0.336 | |||
| <16 | 29 (5.98%) | 24 (6.50%) | 5 (4.31%) | |
| 16–40 | 426 (87.84%) | 325 (88.08%) | 101 (87.07%) | |
| >40 | 30 (6.19%) | 20 (5.42%) | 10 (8.62%) | |
| Location at diagnosis, n (%) | 0.249 | |||
| L1 | 53 (10.93%) | 46 (12.47%) | 7 (6.03%) | |
| L2 | 20 (4.12%) | 16 (4.34%) | 4 (3.45%) | |
| L3 | 375 (77.32%) | 280 (75.88%) | 95 (81.90%) | |
| L4 | 37 (7.63%) | 27 (7.32%) | 10 (8.62%) | |
| Behavior at diagnosis, n (%) | 0.860 | |||
| B1 | 275 (56.70%) | 210 (56.91%) | 65 (56.03%) | |
| B2 | 80 (16.49%) | 59 (15.99%) | 21 (18.10%) | |
| B3 | 130 (26.80%) | 100 (27.10%) | 30 (25.86%) | |
| CDAI, n (%) | 0.338 | |||
| remission | 100 (20.62%) | 74 (20.05%) | 26 (22.41%) | |
| mild | 248 (51.13%) | 193 (52.30%) | 55 (47.41%) | |
| moderate | 124 (25.57%) | 90 (24.39%) | 34 (29.31%) | |
| severe | 13 (2.68%) | 12 (3.25%) | 1 (0.86%) | |
| Perianal disease, n (%) | 0.530 | |||
| No | 166 (34.23%) | 123 (33.33%) | 43 (37.07%) | |
| Yes | 319 (65.77%) | 246 (66.67%) | 73 (62.93%) | |
| EIM, n (%) | 0.497 | |||
| No | 405 (83.51%) | 311 (84.28%) | 94 (81.03%) | |
| Yes | 80 (16.49%) | 58 (15.72%) | 22 (18.97%) | |
| Complications, n (%) | 1.000 | |||
| No | 242 (49.90%) | 184 (49.86%) | 58 (50.00%) | |
| Yes | 243 (50.10%) | 185 (50.14%) | 58 (50.00%) | |
| History of intestinal surgery, n (%) | 0.201 | |||
| No | 343 (70.72%) | 255 (69.11%) | 88 (75.86%) | |
| Yes | 142 (29.28%) | 114 (30.89%) | 28 (24.14%) | |
| History of delayed treatment, n (%) | <0.001 | |||
| No | 382 (78.76%) | 326 (88.35%) | 56 (48.28%) | |
| Yes | 103 (21.24%) | 43 (11.65%) | 60 (51.72%) | |
| Prior exposure to anti-TNF agents, n (%) | <0.001 | |||
| No | 449 (92.58%) | 359 (97.29%) | 90 (77.59%) | |
| Yes | 36 (7.42%) | 10 (2.71%) | 26 (22.41%) | |
| Concomitant use of IMM, n (%) | 0.039 | |||
| No | 284 (58.56%) | 206 (55.83%) | 78 (67.24%) | |
| Yes | 201 (41.44%) | 163 (44.17%) | 38 (32.76%) | |
| Dosage (mg/kg), M (Q1, Q3) | 5.71 (5.17, 6.25) | 5.66 (5.17, 6.12) | 5.88 (5.19, 6.38) | 0.053 |
| TLI (ug/mL), M (Q1, Q3) | 4.68 (2.02, 11.72) | 6.20 (3.23, 13.66) | 0.79 (0.40, 3.97) | <0.001 |
| ESR (mm/h), M (Q1, Q3) | 22.00 (11.00, 38.00) | 19.00 (8.00, 35.00) | 28.50 (20.00, 47.50) | <0.001 |
| Ca (mmol/L), M (Q1, Q3) | 2.29 (2.21, 2.38) | 2.30 (2.22, 2.39) | 2.25 (2.19, 2.33) | 0.003 |
| ALB (g/L), Mean ± SD | 39.83 ± 5.10 | 40.23 ± 5.02 | 38.57 ± 5.14 | 0.003 |
| Variables | β | SE | Wald χ2 | OR (95% CI) | p Value |
|---|---|---|---|---|---|
| Prior exposure to anti-TNF agents | 2.406 | 0.457 | 27.682 | 11.091 (4.673, 28.415) | <0.001 |
| History of delayed treatment | 1.935 | 0.277 | 48.943 | 6.926 (4.049, 12.005) | <0.001 |
| Concomitant use of IMM | −0.709 | 0.275 | 6.649 | 0.492 (0.283, 0.836) | 0.010 |
| TLI | −0.037 | 0.013 | 8.184 | 0.964 (0.937, 0.986) | 0.004 |
| ESR | 0.019 | 0.006 | 8.814 | 1.019 (1.008, 1.030) | <0.001 |
| BMI | −0.09 | 0.048 | 3.470 | 0.914 (0.830, 1.003) | 0.062 |
| Ca | −1.779 | 0.978 | 3.311 | 0.169 (0.024, 1.112) | 0.069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Song, J.; Zheng, Z.; Peng, X.; Li, X.; Wu, W. Development and Validation of a Machine Learning Model to Predict Anti-Drug Antibody Formation During Infliximab Induction in Crohn’s Disease. Biomedicines 2025, 13, 2464. https://doi.org/10.3390/biomedicines13102464
Wang Y, Song J, Zheng Z, Peng X, Li X, Wu W. Development and Validation of a Machine Learning Model to Predict Anti-Drug Antibody Formation During Infliximab Induction in Crohn’s Disease. Biomedicines. 2025; 13(10):2464. https://doi.org/10.3390/biomedicines13102464
Chicago/Turabian StyleWang, Yiting, Jialin Song, Zhuoling Zheng, Xiang Peng, Xiaoyan Li, and Wenjiao Wu. 2025. "Development and Validation of a Machine Learning Model to Predict Anti-Drug Antibody Formation During Infliximab Induction in Crohn’s Disease" Biomedicines 13, no. 10: 2464. https://doi.org/10.3390/biomedicines13102464
APA StyleWang, Y., Song, J., Zheng, Z., Peng, X., Li, X., & Wu, W. (2025). Development and Validation of a Machine Learning Model to Predict Anti-Drug Antibody Formation During Infliximab Induction in Crohn’s Disease. Biomedicines, 13(10), 2464. https://doi.org/10.3390/biomedicines13102464






