1. Introduction
Cervical cancer ranks as the fourth most prevalent malignant tumor among women globally, posing a significant public health challenge that affects women’s well-being [
1]. Data from 2020 reveal approximately 604,127 new cases of cervical cancer and 341,831 related deaths, underscoring the disease’s substantial burden [
2]. Histologically, cervical cancer is classified primarily according to pathomorphologic characteristics and can be categorized into subtypes such as squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma. Cervical squamous cell carcinoma (CSCC) constitutes roughly 70% to 80% of all cervical cancer cases, making it the most prevalent pathological type [
3]. Persistent infection with high-risk human papillomavirus (HPV) is identified as the primary cause of CSCC. Such persistent HPV infection can lead to cervical squamous intraepithelial lesions, which are further classified into low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL) based on the depth and extent of the lesions within the cervical tissue [
4]. Epidemiological studies indicate that approximately 60% of LSIL cases resolve spontaneously. However, some cases progress to invasive carcinoma due to persistent HPV infection, advancing to HSIL [
5]. HSIL, a precancerous lesion of cervical cancer, is often associated with persistent infection by high-risk HPV types and may eventually develop into squamous cell carcinoma of the cervix if not promptly addressed. Currently, the treatment of early-stage cervical cancer relies on surgery, but some patients remain at risk of recurrence. Therefore, gaining a deeper understanding of the mechanisms underlying cervical cancer (CC) development and identifying key therapeutic targets are crucial.
Progesterone receptor membrane component 1 (PGRMC1) is a key member of the Membrane-Associated Progesterone Receptor (MAPR) family, encoded by the PGRMC1 gene located on the X chromosome. This protein features a cytochrome b5-like heme/sterol binding domain and plays a pivotal role in numerous cellular and tissue functions, including cytochrome P450 metabolism, heme homeostasis, carcinogenesis, drug resistance, regulation of female reproduction, and neural function regulation [
6]. As a type of membrane receptor, PGRMC1 swiftly responds to progesterone signaling and modulates intracellular signaling processes, notably through the activation of the Ras signaling pathway, among other mechanisms [
7]. PGRMC1 has demonstrated a pro-carcinogenic effect in lung, colon, and thyroid cancers, where it can promote tumorigenesis and progression. Notably, in the plasma of lung cancer patients, PGRMC1 expression is significantly elevated, making it a promising new therapeutic target for lung cancer [
8]. In breast cancer, PGRMC1 is markedly overexpressed and has been identified as a novel biomarker for characterizing estrogen receptor status and tissue hypoxia [
9]. Additionally, in ovarian cancer, stable transfection with PGRMC1-specific siRNA significantly inhibits the growth of transplanted tumors, highlighting its potential as a therapeutic target [
10]. However, the role of PGRMC1 in the development and progression of cervical cancer, especially cervical intraepithelial neoplasia (CIN), has not been fully investigated.
In this study, we intended to collect clinical tissue samples and utilize a cervical cancer cell line (HeLa) in vivo and in vitro to study the expression of PGRMC1 in cervical squamous intraepithelial lesion and its possible mechanism. We found that PGRMC1 binds to and regulates VIM phosphorylation to promote cervical squamous intraepithelial lesion progression. Our study expands the understanding of PGRMC1’s role in cervical carcinogenesis and provides an experimental basis for targeted therapy of cervical cancer.
2. Materials and Methods
2.1. Clinical Tissue Samples
The clinical specimens used in this study were obtained from the remaining portion of HPV-positive patients’ cervix biopsies sent for pathology at Jiangning Hospital in Nanjing, China, and were pathologically graded according to the colposcopic Reid scoring criteria [
11]. Finally, they were divided into four groups, including the Cervicitis group, the LSIL group, the HSIL group, and the CSCC group. All clinical samples were obtained from patients who underwent colposcopy at the Obstetrics and Gynecology outpatient clinic of Nanjing Jiangning Hospital from September 2022 to December 2024, and the specimens obtained were the remaining portions of the subjects’ pathology slides. All subjects signed an informed consent form, and the project was examined and approved by the Ethics Committee of Nanjing Jiangning Hospital (Ethics Approval Number: No. 2021-03-020-K01). Patient characteristics was described in
Table 1.
2.2. Cell Culture, Passaging, and Grouping
HeLa cells and SiHa cells were purchased from HyCyte (#TCH-C326, Suzhou, China) and were cultured in DMEM (Biochannel, Nanjing, China) supplemented with 10% fetal bovine serum (FBS, Biochannel, China) in a humidified atmosphere containing 5% CO
2 at 37 °C. In the experiment, PGRMC1 siRNA or control siRNA were constructed by tsingke (Beijing, China) and then they were transfected into HeLa cells using Lipomaster 3000 Transfection Reagent (# TL301-01, Vazyme, Nanjing, China). The siRNA sequences were listed in
Table 2.
2.3. Antibody Preparation
Primary antibodies against PGRMC1 (# A5619), VIM (# A19607), ACTB (# AC0260), MMP2 (# A19080), HA-Tag (# AE124), DDDDK-Tag (# AE121), VIM-S39 (# AP0806), VIM-S56 (# AP0804), VIM-S83 (# AP0799) as well as secondary antibody goat anti-rabbit IgG (# AS014) were from Abclonal (Wuhan, China). Primary antibodies against MMP9 (# HA722106) was purchased from HUABIO (Hangzhou, China).
2.4. Bioinformatics Analysis
Prognostic survival data were obtained from the Kaplan–Meier Plotter database (
www.kmplot.com). Based on the mean expression value of PGRMC1 in the cervical cancer tissue microarray, the samples were divided into two groups (high expression group and low expression group). The overall survival (OS), distant metastasis free survival (DMFS), post progression survival (PPS), and recurrence free survival (RFS) of patients with cervical cancer were analyzed to study their correlation with the expression of PGRMC1. Data on PGRMC1 expression and the correlation of various clinicopathological parameters of PGRMC1 with cervical cancer were obtained from the UALCAN database (
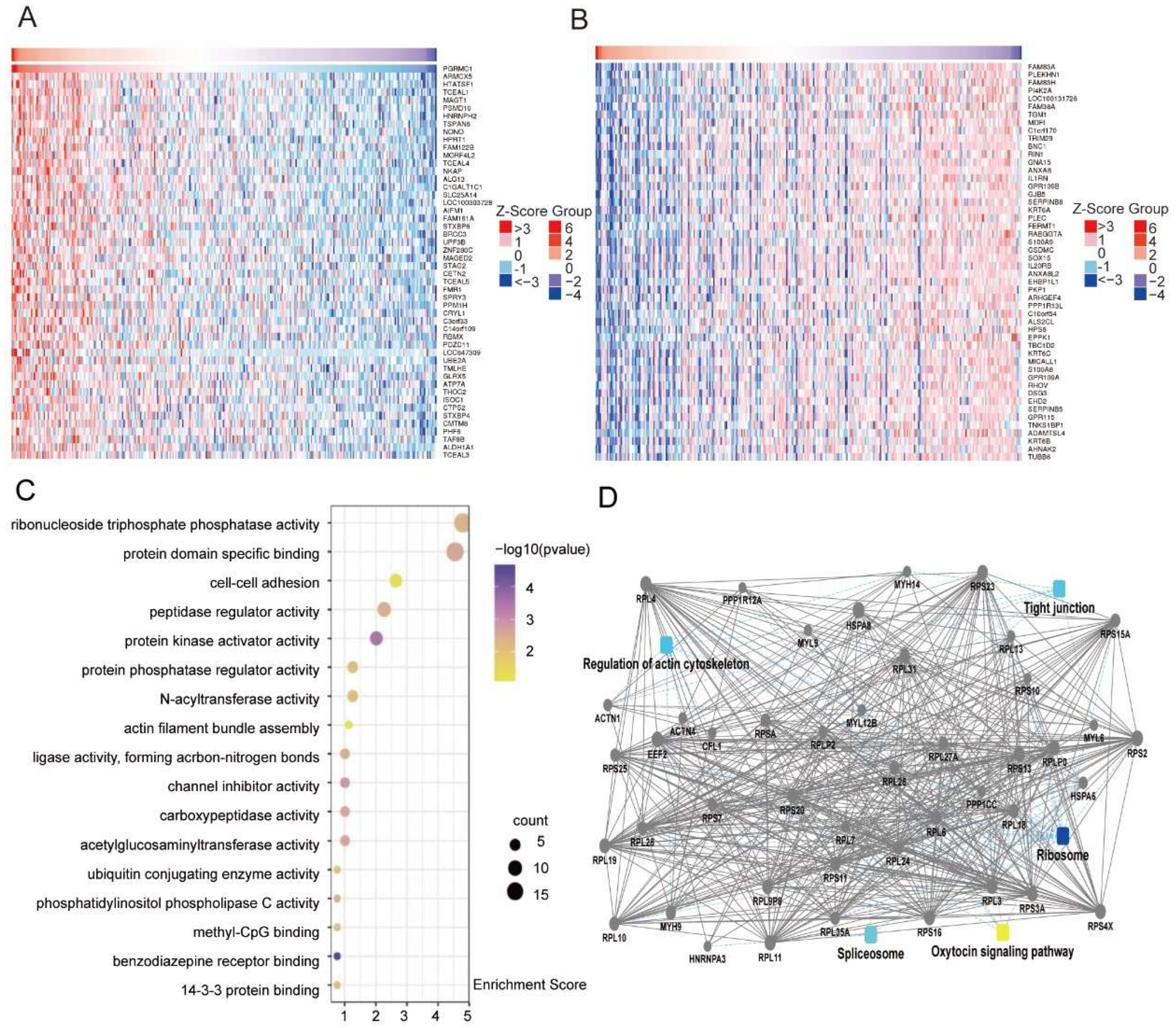
www.ualcan.path.uab.edu). Mining of genes co-expressed with PGRMC1 in cervical cancer was performed using data from the LinkedOmics database, followed by enrichment analysis using the Micro Bioinformatics Platform (
www.bioinformatics.com.cn). We defined
p < 0.05 and FDR < 0.25 as a significantly enriched gene set, and obtained 24,776 genes co-expressed with PGRMC1 in cervical cancer. The data were presented in the form of heat maps, showing the top 50 genes with positive or negative correlations.
2.5. Total RNA Isolation and Real-Time Fluorescence Quantitative PCR Analysis
Total RNA was extracted by TRIzol (# 15596018, Invitrogen, Carlsbad, CA, USA) and was then reverse transcribed into cDNA using HiScript II One Step RT-PCR Kit (# P611-01, Vazyme, Nanjing, China). The cDNA was obtained and subjected to qPCR experiments with ChamQ SYBR qPCR Master Mix (# Q341-02, Vazyme, China). The primers were listed in
Table 3.
2.6. Western Blot Analysis (WB)
HeLa cells were lysed using RIPA buffer, supplemented with a protease inhibitor, to prepare protein samples. Subsequently, these protein samples were separated using a precast gel (Model # M00669, GenScript, Nanjing, China) and then transferred onto PVDF membranes. The membranes were blocked and incubated overnight at 4 °C with the primary antibody, diluted at a ratio of 1:1000. The following day, the membrane was rinsed three times with Western washing solution (Product # P0023C6, Beyotime, Shanghai, China). Next, the secondary antibody (diluted at 1:5000) was added and incubated on a shaker at room temperature for 1 h. The membranes were then treated with ECL luminescent solution (Product # P0018M, Beyotime, Shanghai, China), and the bands were visualized using a chemiluminescent imaging system.
2.7. Immunofluorescence Staining
The cells were grown on coverslips in a 12-well plate with a cell coverage rate of about 50%. After treatment, the cells were immobilized with 4% paraformaldehyde for 10 min, permeabilized with 0.2% TritonX-100 at room temperature for 10 min, and followed by a block with 5% BSA for 30 min. Then the 12-well plate was added the primary antibody (used at 1:100 dilution) for incubation in a wet dark box at room temperature. After incubation, primary antibody was removed. The cells were washed with PBS twice and incubated with secondary antibody for 1 h. After incubation, secondary antibody was removed. The cells were washed with PBS twice and incubated with DAPI (# D3571, Invitrogen, USA) for nuclear staining. Images were captured by confocal microscopy and visualized with ZEN software 2.3 version.
2.8. Cell Scratching Experiment
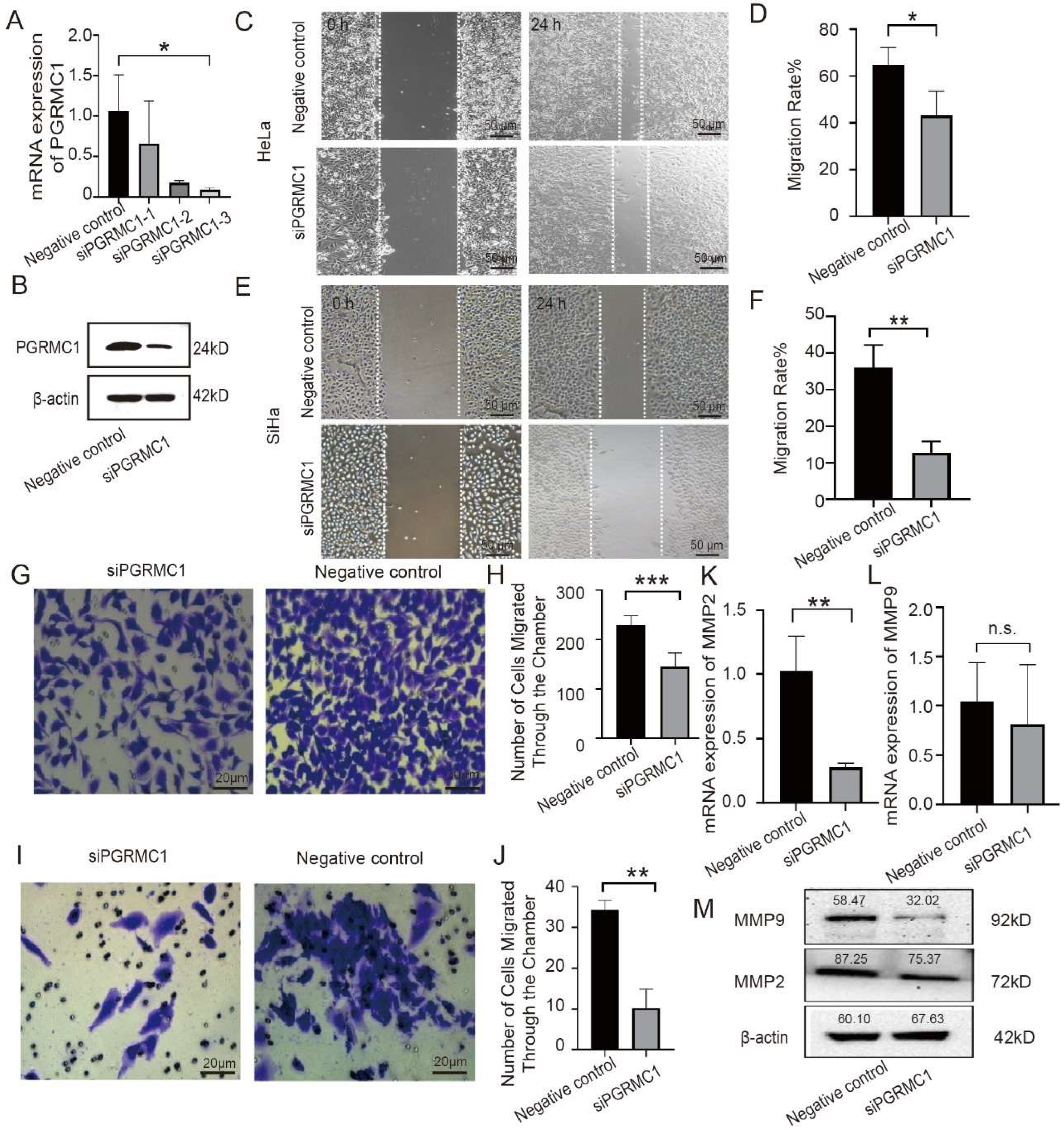
HeLa cells or SiHa cells (used at a density of 6 × 104 cells per well) were grown in 6-well culture plate, of which 5 parallel horizontal lines were drawn on the back. According to the pre-marked line, uniform scratches were made vertically against the plate cover of the wells. The marking line on the bottom of the plate was erased, and the scratched area was photographed under an inverted microscope to ensure that the scratches were centered, the edges were perpendicular and the background conditions were consistent in all wells, and the samples were taken and photographed at the time points of 0 h (immediately after the scratches), 6 h, 12 h, and 24 h, respectively. Mark the scratch edges using image J software 1.53t version, and then calculate the area between the two cell edges at 0 h and 24 h. Convert it into the gray values and calculate the migration rates of cells in different groups.
2.9. Transwell Cell Invasion Assay
HeLa cells were grown in a 6-well culture plate at a concentration of 2 × 105 cells per well. The next day, the cells were transfected with PGRMC1 siRNA or negative siRNA for 24 h, and then the cells were digested and resuspended. For invasion experiments, matrigel matrix gel (Corning, NY, USA) was diluted with ice-cold serum-free medium, and was uniformly encapsulated in the basement membrane of the upper chamber of the Transwell (Corning, NY, USA). The cell suspension was added into the upper chamber of the pre-coated matrigel chambers, and the complete medium was added to the lower chamber. For migration assays, cell suspensions were added to Transwell chambers without matrigel matrix gel. The chambers were taken out at indicated time, washed and fixed in methanol for 20 min. After a crystal violet staining, the upper layer cells were wiped off and the cell number was counted under the microscope. The invasive ability is reflected by comparing the number of cells passing through the chambers in different groups.
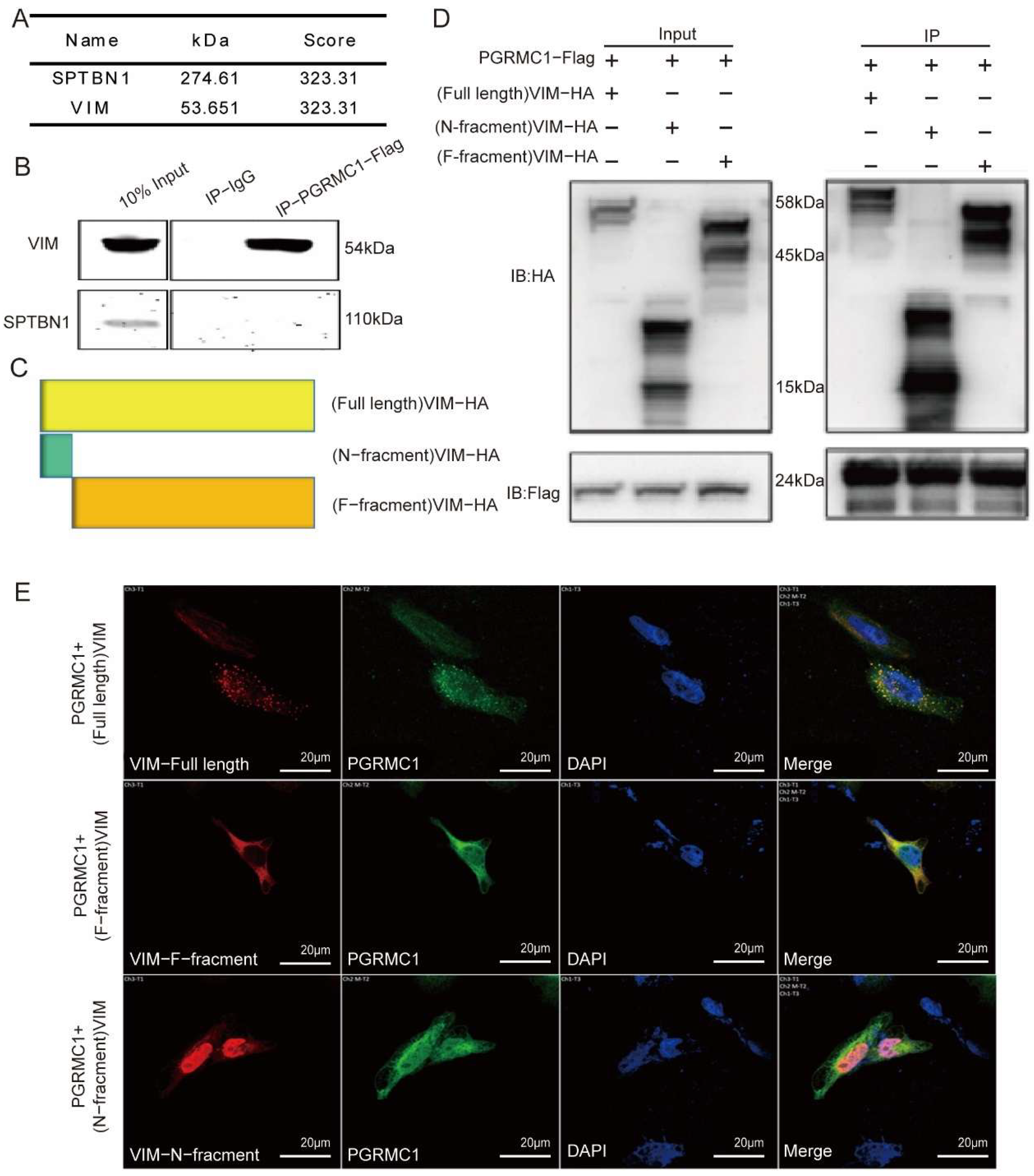
2.10. Coimmunoprecipitation (Co-IP)
HEK293T cells were co-transfected with FLAG-tagged PGRMC1 plasmid and HA-tagged VIM or truncated proteins plasmid for 48 h. Then the samples were washed three times with ice-cold PBS and lysed with IP buffer (RIPA Buffer with protease inhibitor and phosphatase inhibitor) in an ice bath for 30 min. The lysate was centrifuged at 12,000× g for 10 min at 4 °C, and the supernatant was collected and incubated overnight with Anti-DYKDDDDK (Flag) Affinity Gel (# HAK21024, HUABIO, Hangzhou, China) at 4 °C. The gel was washed more than three times, added 5 × SDS-PAGE Loading Buffer and boiled at 100 °C for 10 min. The samples were then assayed by WB validation.
2.11. Statistical Analysis
All data are presented as the mean ± standard deviation (SD). Student’s t-test was employed for comparisons between two independent sample groups. For single-factor comparisons among multiple groups, a one-way analysis of variance (ANOVA) was utilized, while a two-way ANOVA was used for two-factor comparisons among multiple groups. A p-value of less than 0.05 was considered statistically significant.
4. Discussion
In this experiment, employing a series of bioinformatics analyses and molecular-protein level investigations, we discovered that PGRMC1 inhibits the depolymerization of VIM by binding to its Ser-39 phosphorylation site, thereby preventing VIM phosphorylation. This mechanism subsequently enhances the migratory and invasive capabilities of HeLa cells. Consequently, our findings indicate that PGRMC1 exerts a promotional effect on cervical intraepithelial neoplasia, establishing it as a significant poor prognostic factor for cervical cancer due to its role in facilitating tumor migration and invasion.
Vimentin (VIM), a critical member of the type III intermediate filament protein family, is a highly conserved and multifunctional cytoskeletal protein essential for maintaining cellular morphology, transmitting mechanical stress, and facilitating signal transduction. Encoded by one of the largest gene families of intermediate filament proteins, VIM is a key structural component of the cytoskeleton. It plays a pivotal role in tissue fibrosis, wound healing, and is extensively involved in physiological processes such as inflammatory responses and lipid metabolism [
12]. Recent studies have indicated that VIM plays a pivotal role in tumorigenesis, development, and metastasis, emerging as a novel therapeutic target for various tumors [
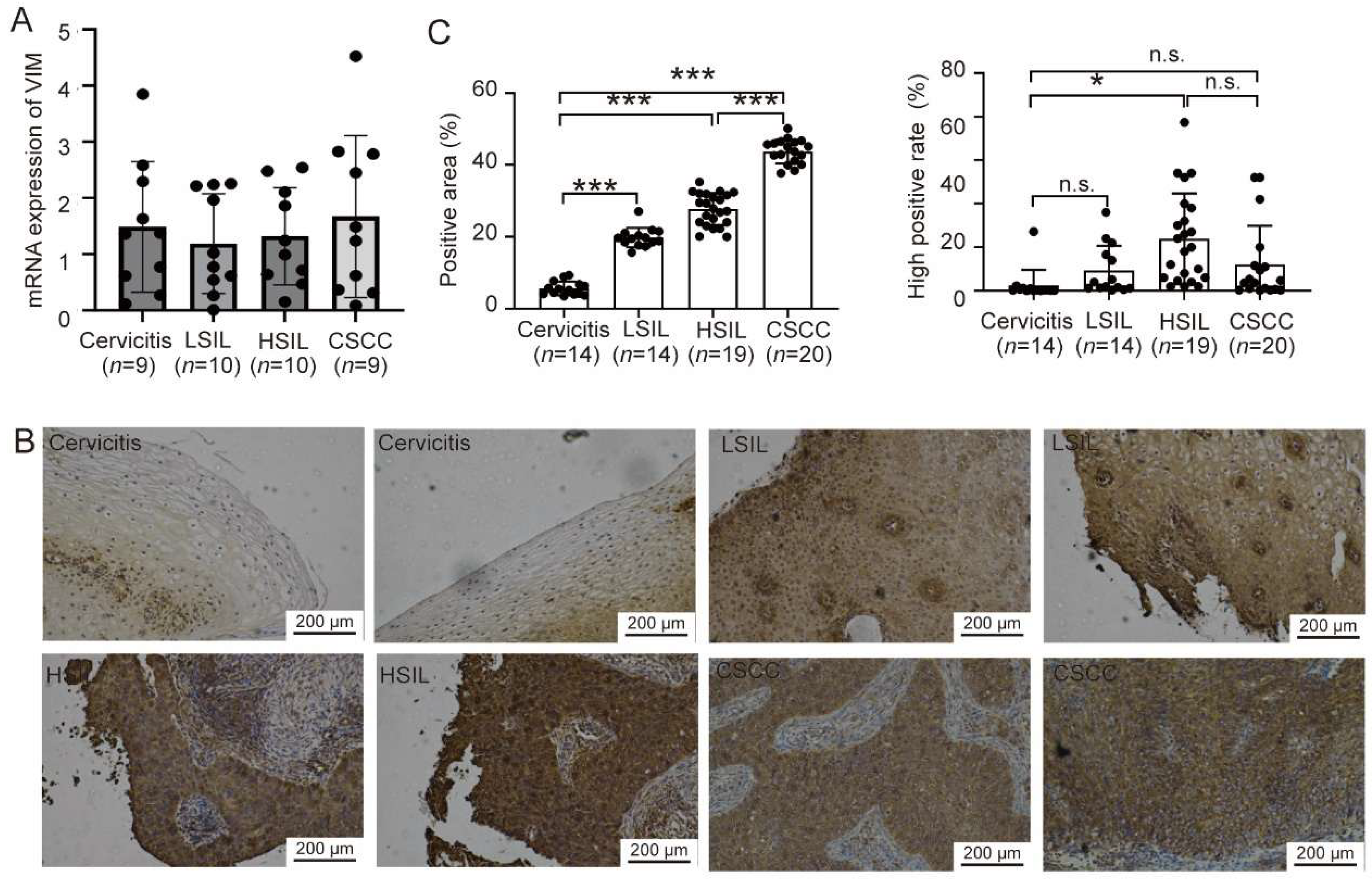
13]. Within the realm of gynecologic oncology research, investigations into VIM expression and its function are relatively nascent. Existing research has demonstrated that VIM expression levels are notably higher in cervical cancer tissues compared to those in chronic cervicitis, low-grade cervical intraepithelial neoplasia (CIN), and high-grade CIN. Additionally, the methylation degree of its promoter region increases, suggesting that VIM could serve as a significant molecular marker for the progression and infiltration of cervical cancer [
14]. In our experiment, through immunohistochemical analysis of collected typical clinical samples, we observed that VIM expression levels gradually increased and exhibited a significant trend during the process of cervical intraepithelial neoplasia. This finding also suggests that the elevation of VIM may serve as a potential indicator for cervical cancer.
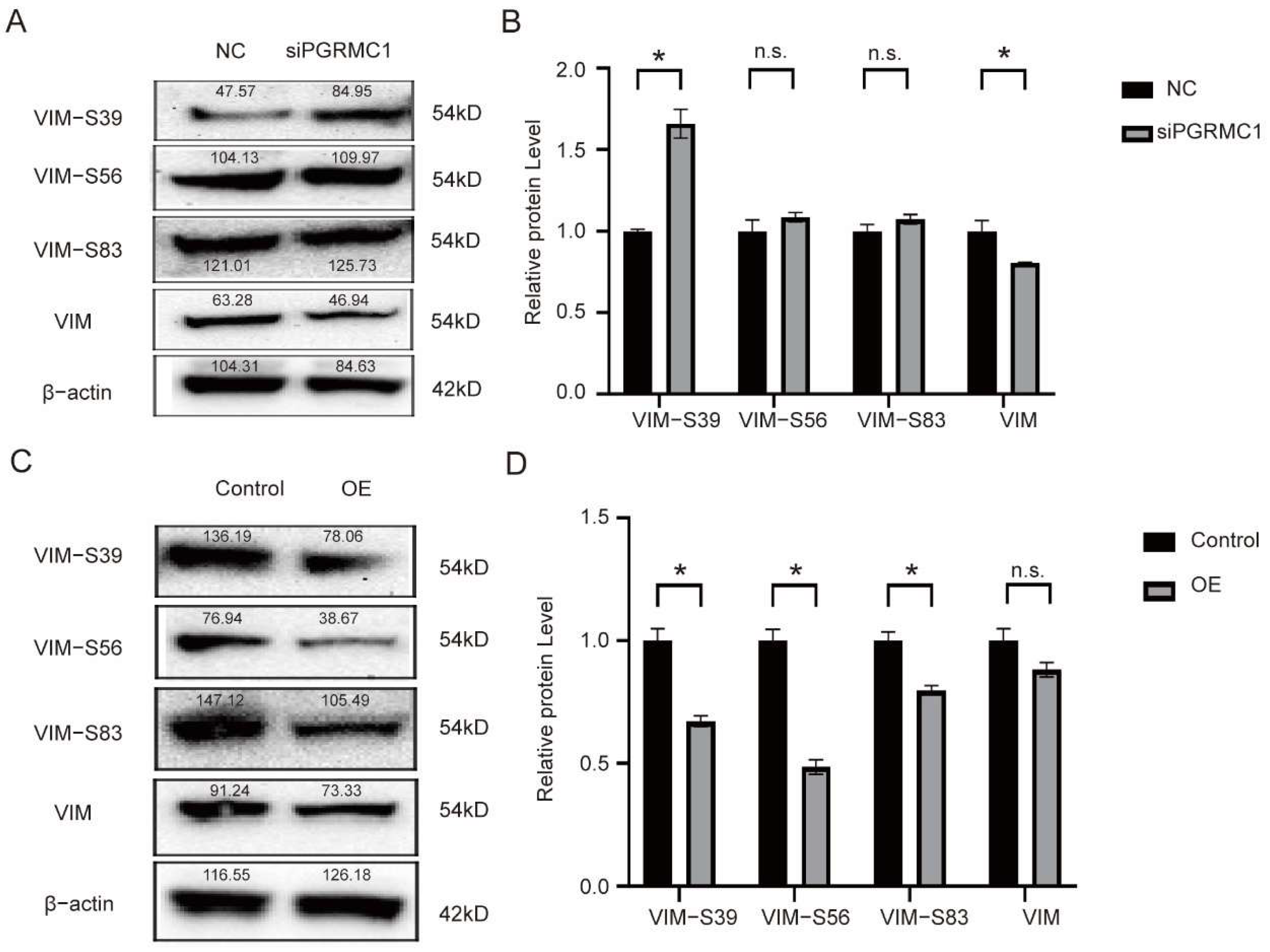
At the molecular level, the biological function of Vimentin (VIM) is predominantly regulated through phosphorylation modifications. Key phosphorylation sites identified to date include Ser39, Ser56, and Ser83, among others. VIM features multiple phosphorylation sites, each modulated by distinct kinases, primarily protein kinase C (PKC), cell-cycle-dependent kinase 1 (CDK1), mitogen-activated protein kinase (MAPK), casein kinase 2 (CK2), and others. Phosphorylation at Ser39 is intricately linked to the in vivo assembly of VIM intermediate filaments. This modification induces the depolymerization of VIM fibers, thereby remodeling the cytoskeletal structure and altering the cell’s morphology and mechanical properties. VIM-Ser56 undergoes phosphorylation by PKC and PAK, leading to the rearrangement of the intermediate filament network. This rearrangement provides a structural foundation for cell deformation and migration [
15]. The VIM-Ser83 site is phosphorylated by CDK1, which triggers the rearrangement and depolymerization of the VIM fiber network. This process is crucial for the cell’s entry into mitosis, chromosome segregation, and cell division [
16]. Conversely, the VIM-S39 site is phosphorylated through AKT-mediated pathways and is recognized as a phosphorylation site specific for certain kinases. This site can bind to various phosphorylated kinases, activating protein phosphorylation and thereby enhancing the migratory and invasive capabilities of cancer cells [
17]. In an organoid co-culture model of colorectal cancer, an upregulation in the phosphorylation level of VIM is considered a marker of epithelial–mesenchymal transition (EMT) and is associated with the invasive phenotype induced by stromal cells. This model simulates signal transduction within the tumor microenvironment, promoting the migration and invasion of tumor cells [
18]. Phosphorylation proteomics data reveal that in FAT1-mutated small cell lung cancer, 24 phosphorylation sites of VIM exhibit significant increases. These sites are primarily involved in cytoskeletal recombination and cell motility processes, indicating a close relationship between VIM phosphorylation and the metastatic potential of small cell lung cancer [
19]. The VIM plays a pivotal role in promoting the directional migration of cells in organoids by regulating cytoskeletal stability. In breast cancer cells, overexpression of Vimentin enhances cell hardness, motility, and directional migration ability, reorients microtubule polarity, and increases the EMT phenotype. These findings suggest that Vimentin maintains the mechanical integrity of EMT cancer cells by regulating cytoskeletal structure, thereby facilitating tumor metastasis. As an intermediate filament protein within the cytoskeleton, Vimentin interacts with stress fibers, influencing their dynamic flow and facilitating the transport of endocytic vesicles. This interaction plays a crucial role in the process of macrophages degrading the extracellular matrix (ECM), a process closely associated with tumor invasion [
20].
PGRMC1 exhibits high expression levels in breast and ovarian cancer tissues, where it is closely associated with malignant biological behaviors, including tumor cell proliferation, migration, and invasion. In breast cancer cells, PGRMC1 interacts with estrogen receptor α (ERα), activating the ERα signaling pathway and thereby promoting cancer cell proliferation. Similarly, in ovarian cancer, PGRMC1 enhances cell proliferation and migration through the activation of the ERK1/2 signaling pathway. Given its pivotal role, PGRMC1 emerges as a promising drug target. As a targetable protein and a component of a multi-protein complex, it binds progesterone, other steroids, and various drug compounds. Its ability to bind heme further underscores its potential in drug development. Designing drugs that specifically target PGRMC1 could inhibit tumor cell survival and proliferation [
21]. However, the development of targeted drugs against PGRMC1 presents significant challenges. Despite its attractiveness as a therapeutic target, PGRMC1’s complex interactions with other proteins and diverse functions complicate the design and development process. To date, no mature PGRMC1-targeted therapeutic drugs have received approval for clinical use. Additionally, research on the upstream and downstream regulatory factors of PGRMC1 in tumor cells remains limited, and its specific signal transduction network in hormone replacement therapy has not been fully elucidated. In our Western blot experiments, we successfully demonstrated that PGRMC1 influences the phosphorylation level at the S39 site by binding to the VIM-39 domain, thereby mediating protein–protein interactions. We hypothesize that the binding of PGRMC1 to the VIM-S39 domain may exert an antagonistic effect, promoting tumor cell migration and invasion, as well as the development of cervical intraepithelial neoplasia, by occupying the phosphorylation activation site on VIM and inhibiting phosphorylation at the VIM-S39 site. Nevertheless, the precise mechanisms underlying these processes remain to be thoroughly investigated.
There are some limitations of this study to point. In the bioinformatics analysis section, although we performed prognostic survival analysis basing on public databases (the TCGA database) and pointed that PGRMC1 is a poor prognostic factor; however, we do not have independent cohorts to verify this result. Furthermore, the clinical sample size remains relatively small. In the Co-IP experiments, although we demonstrated binding between PGRMC1 and VIM, but whether PGRMC1 directly binds to VIM is unclear. Future studies will be made up for these limitations and to clarify the underlying function of PGRMC1 in CIN-CSCC transition.
To sum up, our study finds that PGRMC1 expression is elevated in the process of CIN-CSCC transformation, and the specific mechanism is that PGRMC1 binds to VIM protein, inhibits the phosphorylation level of VIM (Ser-39) site to maintain VIM assembly, and interferes with PGRMC1 expression to inhibit cervical cancer cell migration and invasion. This study provides a new target for the intervention and treatment of cervical squamous carcinoma, which has important clinical significance.