Public Health Screening for Cardiometabolic Risk: Lessons from Advanced Glycation End-Products and ABC Target Achievement in Dalmatian Adults with Type 2 Diabetes
Abstract
1. Introduction
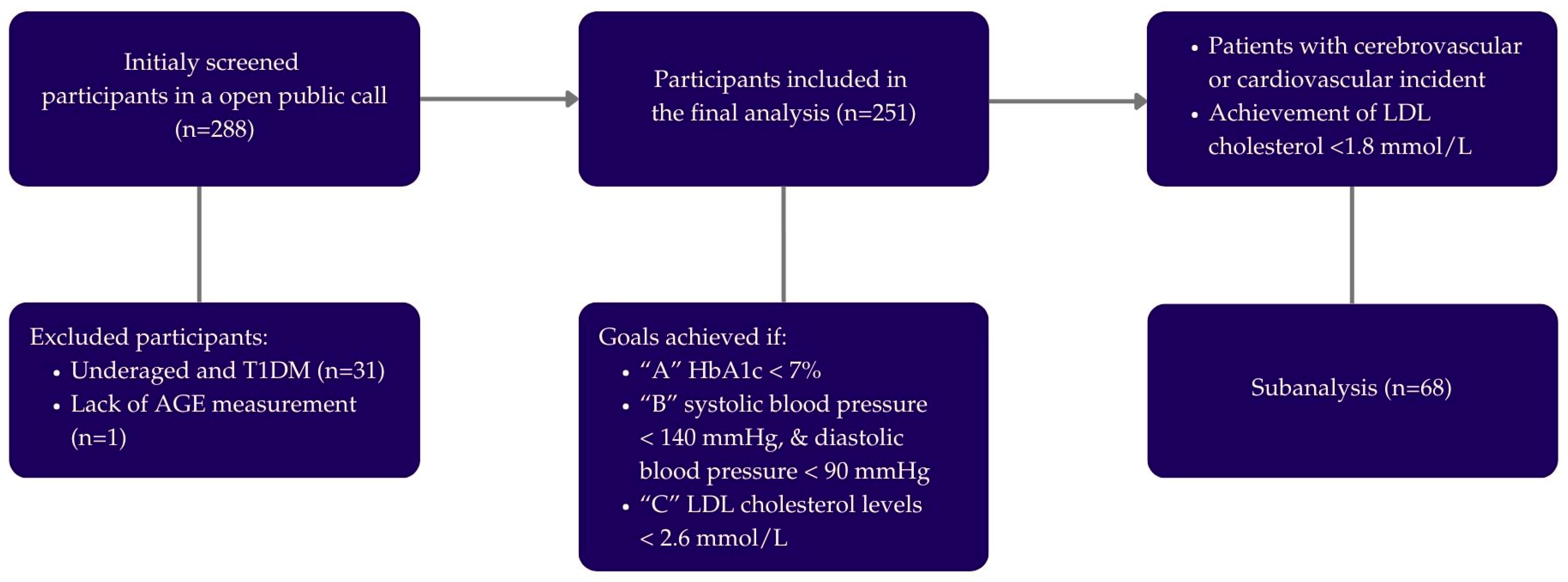
2. Materials and Methods
2.1. Study Design
2.2. Population
2.3. Medical History, Clinical and Laboratory Parameters
2.4. Body Composition and Anthropometry Measurements
2.5. Advanced Glycation End-Product Measurement
2.6. Statistical Analysis
3. Results
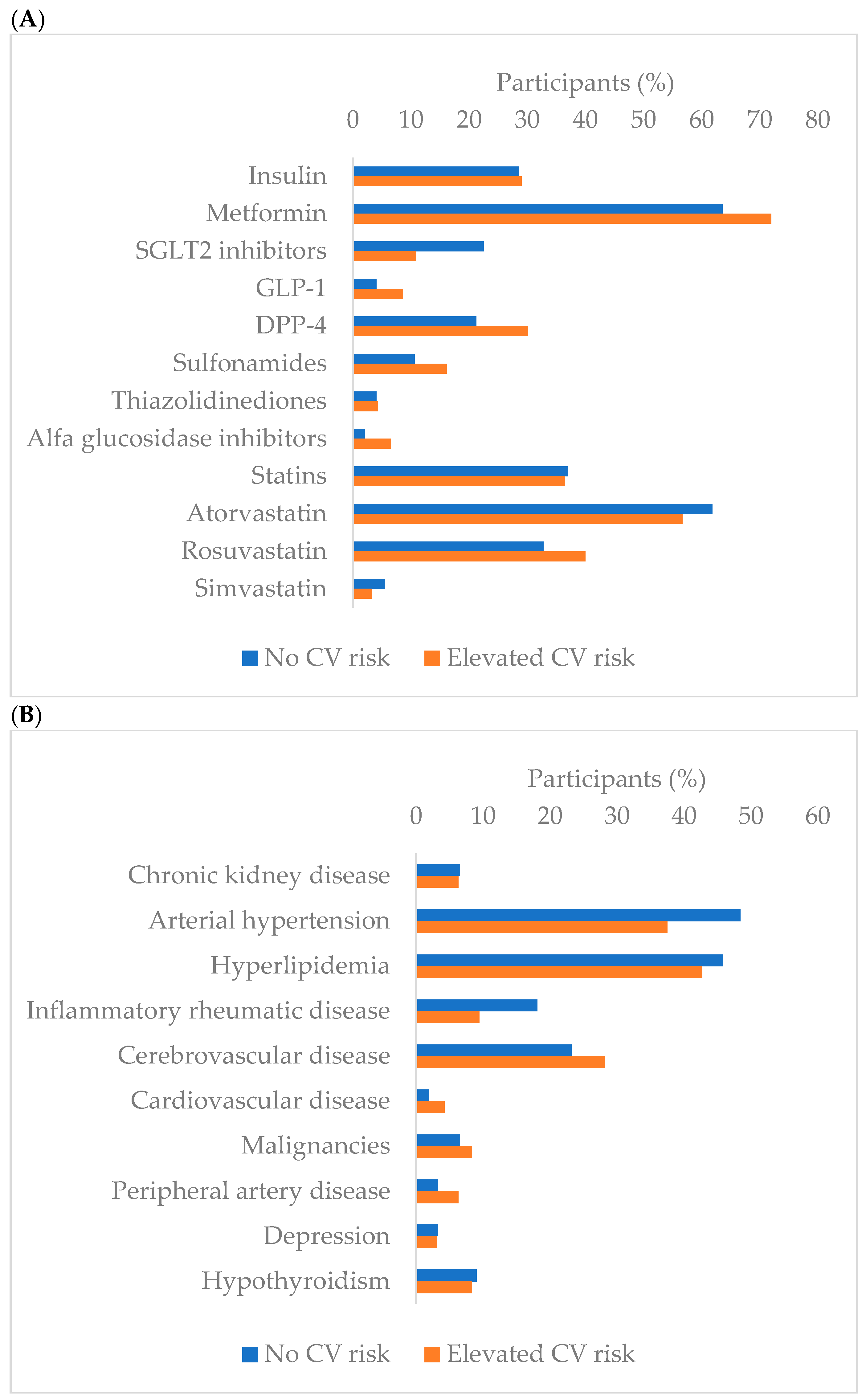
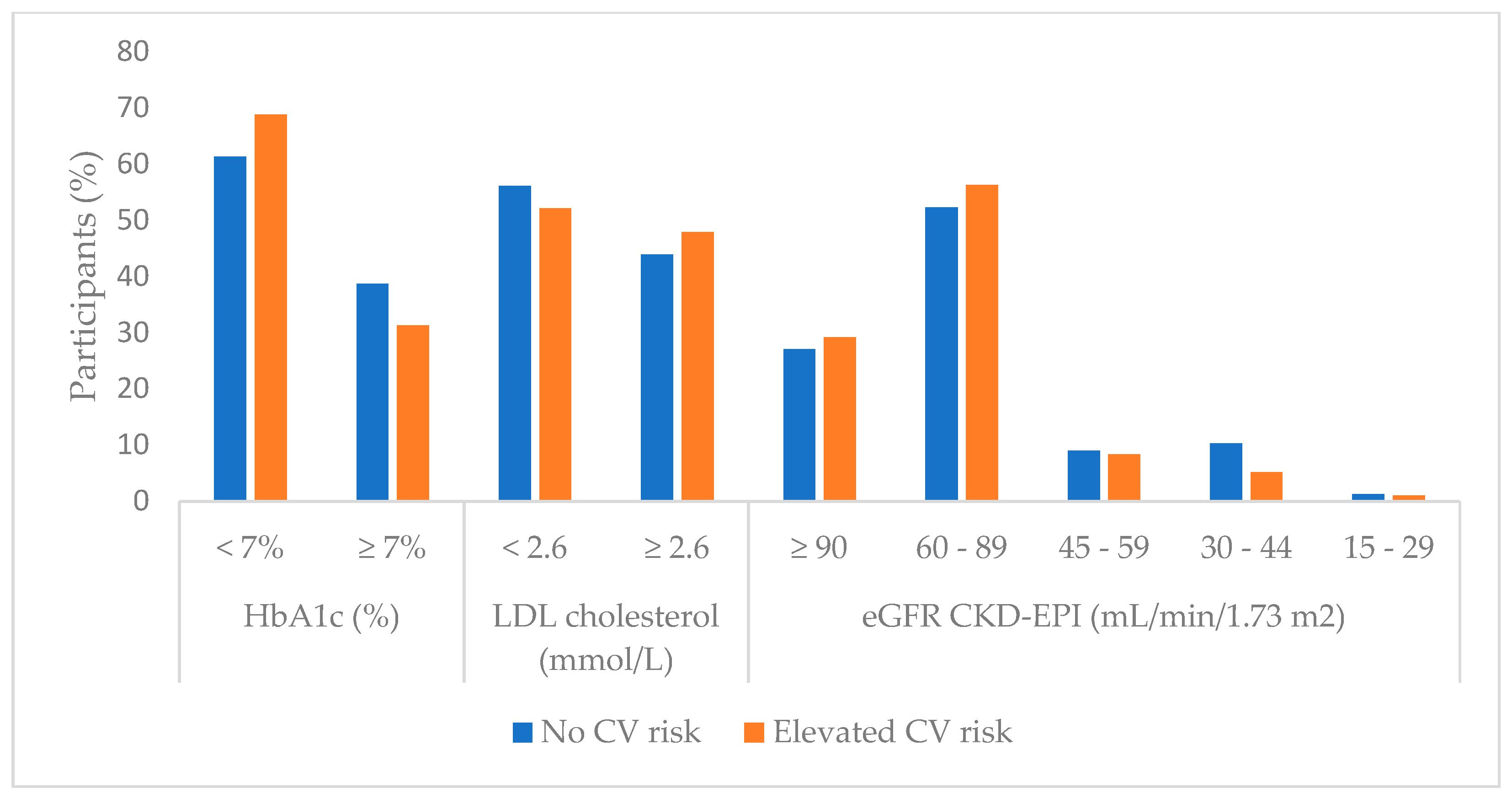
3.1. Laboratory Parameters Regarding Cardiovascular Risk Groups
3.2. Anthropometric and Body Composition Measurements Regarding Cardiovascular Risk Groups
3.3. ABC Group Analysis
3.4. Predictor Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, J.C.N.; Lim, L.L.; Wareham, N.J.; Shaw, J.E.; Orchard, T.J.; Zhang, P.; Lau, E.S.H.; Eliasson, B.; Kong, A.P.S.; Ezzati, M.; et al. The Lancet Commission on Diabetes: Using Data to Transform Diabetes Care and Patient Lives. Lancet 2020, 396, 2019–2082. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes Mellitus, the Fastest Growing Global Public Health Concern: Early Detection Should Be Focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Dianna, J. Magliano. In IDF Atlas 11th Edition 2025, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025. [Google Scholar]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Kaabi, J. Al Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- The Lancet. Diabetes: A Defining Disease of the 21st Century. Lancet 2023, 401, 2087. [Google Scholar] [CrossRef]
- Huang, X.; Wu, Y.; Ni, Y.; Xu, H.; He, Y. Global, Regional, and National Burden of Type 2 Diabetes Mellitus Caused by High BMI from 1990 to 2021, and Forecasts to 2045: Analysis from the Global Burden of Disease Study 2021. Front. Public Health 2025, 13, 1515797. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes-2014. Diabetes Care 2014, 37, S14–S80. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S52–S76. [Google Scholar] [CrossRef]
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, Å.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care 2023, 46, e151–e199. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998, 317, 713–720. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998, 317, 70–713. [Google Scholar] [CrossRef]
- Anwer, Z.; Sharma, P.K.; Garg, V.K.; Kumar, N.; Kumari, A. Hypertension Management in Diabetic Patients. Eur. Rev. Med. Pharmacol. Sci. 2011, 15. [Google Scholar]
- Turner, R.; Turner, R.C.; Holman, R.R.; Cull, C.A.; Stratton, I.M.; Matthews, D.R.; Frighi, V.; Manley, S.E.; Neil, A.; McElroy, H.; et al. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of Cholesterol-Lowering with Simvastatin in 5963 People with Diabetes: A Randomised Placebo-Controlled Trial. Lancet 2003, 361, 2005–2016. [Google Scholar] [CrossRef]
- Jia, G.; Sowers, J.R. Hypertension in Diabetes: An Update of Basic Mechanisms and Clinical Disease. Hypertension 2021, 78, 1197–1205. [Google Scholar] [CrossRef]
- Ohishi, M. Hypertension with Diabetes Mellitus: Physiology and Pathology Review-Article. Hypertens. Res. 2018, 41, 389–393. [Google Scholar] [CrossRef]
- Ferrannini, E.; Cushman, W.C. Diabetes and Hypertension: The Bad Companions. Lancet 2012, 380, 601–610. [Google Scholar] [CrossRef]
- Emdin, C.A.; Rahimi, K.; Neal, B.; Callender, T.; Perkovic, V.; Patel, A. Blood Pressure Lowering in Type 2 Diabetes: A Systematic Review and Meta-Analysis. JAMA 2015, 313, 603–615. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Borén, J. New Insights into the Pathophysiology of Dyslipidemia in Type 2 Diabetes. Atherosclerosis 2015, 239, 483–495. [Google Scholar] [CrossRef]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-Rich Lipoproteins and Their Remnants: Metabolic Insights, Role in Atherosclerotic Cardiovascular Disease, and Emerging Therapeutic Strategies-a Consensus Statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef] [PubMed]
- Eilat-Tsanani, S.; Margalit, A.; Golan, L.N. Occurrence of Comorbidities in Newly Diagnosed Type 2 Diabetes Patients and Their Impact after 11 Years’ Follow-Up. Sci. Rep. 2021, 11, 11071. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tiwari, D.; Kathrikolly, T.; Vyawahare, A.; Sharma, B.; Ganla, M.; Maheshkumar, K.; Vijayakumar, V.; Saboo, B.; Kadam, N.S. Interplay Between the American Diabetes Association’s ABC Targets for Diabetes, Insulin Resistance Indices, and Dyslipidemia in Indian Type 2 Diabetes Patients. Cureus 2024, 16, e60268. [Google Scholar] [CrossRef] [PubMed]
- Larry, M.; Alizadeh, S.; Naderi, S.; Salekani, B.; Mansournia, M.A.; Rabizadeh, S.; Esteghamati, A.; Nakhjavani, M. Inadequate Achievement of ABC Goals (HbA1c, Blood Pressure, LDL-C) among Patients with Type 2 Diabetes in an Iranian Population, 2012–2017. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 619–625. [Google Scholar] [CrossRef]
- Mengstie, M.A.; Chekol Abebe, E.; Behaile Teklemariam, A.; Tilahun Mulu, A.; Agidew, M.M.; Teshome Azezew, M.; Zewde, E.A.; Agegnehu Teshome, A. Endogenous Advanced Glycation End Products in the Pathogenesis of Chronic Diabetic Complications. Front. Mol. Biosci. 2022, 9, 1002710. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Salvayre, R.; Augé, N.; Pamplona, R.; Portero-Otín, M. Hyperglycemia and Glycation in Diabetic Complications. Antioxid. Redox Signal 2009, 11, 3071–3109. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef]
- Chawla, D.; Bansal, S.; Banerjee, B.D.; Madhu, S.V.; Kalra, O.P.; Tripathi, A.K. Role of Advanced Glycation End Product (AGE)-Induced Receptor (RAGE) Expression in Diabetic Vascular Complications. Microvasc. Res. 2014, 95, 1–6. [Google Scholar] [CrossRef]
- Da Moura Semedo, C.; Webb, M.; Waller, H.; Khunti, K.; Davies, M. Skin Autofluorescence, a Non-Invasive Marker of Advanced Glycation End Products: Clinical Relevance and Limitations. Postgrad. Med. J. 2017, 93, 289–294. [Google Scholar] [CrossRef]
- Hangai, M.; Takebe, N.; Honma, H.; Sasaki, A.; Chida, A.; Nakano, R.; Togashi, H.; Nakagawa, R.; Oda, T.; Matsui, M.; et al. Association of Advanced Glycation End Products with Coronary Artery Calcification in Japanese Subjects with Type 2 Diabetes as Assessed by Skin Autofluorescence. J. Atheroscler. Thromb. 2016, 23, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, T.; Liu, C.; Hu, H.; Dai, F.; Xia, L.; Zhang, Q. Effectiveness of Early Advanced Glycation End Product Accumulation Testing in the Diagnosis of Diabetes: A Health Risk Factor Analysis Using the Body Mass Index as a Moderator. Front. Endocrinol. 2022, 12, 766778. [Google Scholar] [CrossRef] [PubMed]
- Turk, Z.; Mesić, R.; Benko, B. Comparison of Advanced Glycation Endproducts on Haemoglobin (Hb-AGE) and Haemoglobin A(1c) for the Assessment of Diabetic Control. Clinica Chimica Acta 1998, 277, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.B.; Chu, N.F.; Syu, J.T.; Hsieh, A.T.; Hung, Y.R. Advanced Glycation End Products (AGEs) in Relation to Atherosclerotic Lipid Profiles in Middle-Aged and Elderly Diabetic Patients. Lipids Health Dis. 2011, 10, 228. [Google Scholar] [CrossRef]
- Rezaei, M.; Rabizadeh, S.; Mirahmad, M.; Hajmiri, M.S.; Nakhjavani, M.; Hemmatabadi, M.; Shirzad, N. The Association between Advanced Glycation End Products (AGEs) and ABC (Hemoglobin A1C, Blood Pressure, and Low-Density Lipoprotein Cholesterol) Control Parameters among Patients with Type 2 Diabetes Mellitus. Diabetol. Metab. Syndr. 2022, 14, 122. [Google Scholar] [CrossRef]
- Mallipattu, S.K.; Uribarri, J. Advanced glycation end product accumulation: A new enemy to target in chronic kidney disease? Curr. Opin. Nephrol. Hypertens. 2014, 23, 547–554. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Naskret, D.; Zozulinska-Ziolkiewicz, D.; Pilacinski, S.; Uruska, A.; Grzelka, A.; Wegner, M.; Wierusz-Wysocka, B. Skin autofluorescence is associated with carotid intima-media thickness, diabetic microangiopathy, and long-lasting metabolic control in type 1 diabetic patients. Results from Poznan Prospective Study. Microvasc Res. 2015, 98, 62–67. [Google Scholar] [CrossRef]
- Radić, J.; Vučković, M.; Đogaš, H.; Grubić, M.; Belančić, A.; Tandara, L.; Šolić Šegvić, L.; Novak, I.; Radić, M. Beyond Blood Sugar: Low Awareness of Kidney Disease among Type 2 Diabetes Mellitus Patients in Dalmatia—Insights from the First Open Public Call. Medicina 2024, 60, 1643. [Google Scholar] [CrossRef]
- Radić, J.; Belančić, A.; Đogaš, H.; Vučković, M.; Đogaš, T.; Tandara, L.; Grubić, M.; Šolić Šegvić, L.; Novak, I.; Radić, M. The Power of Movement: Linking Physical Activity with Nutritional Health and Blood Sugar Balance in a Dalmatian Type 2 Diabetic Population. Nutrients 2025, 17, 187. [Google Scholar] [CrossRef]
- MedCalc MedCalc Statistical Software. Available online: https://www.medcalc.org/en/ (accessed on 6 June 2022).
- IBM Corp. IBM SPSS Statistics for Windows, Version 26.0. 2019; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Hegab, Z. Role of Advanced Glycation End Products in Cardiovascular Disease. World J. Cardiol. 2012, 4, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, S.; Zhou, Y.; Tang, X.; Ye, N.; Huang, W.; Tang, X.; Jiang, B.; Pan, Y. The Associations between Skin Advanced Glycation End-Products and Framingham Cardiovascular Risk in Different Age Groups. Front. Cardiovasc. Med. 2025, 12, 1491643. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Cáceres-Acosta, M.F. Blood Pressure Control and Impact on Cardiovascular Events in Patients with Type 2 Diabetes Mellitus: A Critical Analysis of the Literature. Clínica Investig. Arterioscler. 2019, 31, 31–47. [Google Scholar] [CrossRef]
- Seng, L.L.; Kiat, T.P.H.; Bee, Y.M.; Jafar, T.H. Real-World Systolic and Diastolic Blood Pressure Levels and Cardiovascular Mortality in Patients With Type 2 Diabetes—Results From a Large Registry Cohort in Asia. J. Am. Heart Assoc. 2023, 12, e9008. [Google Scholar] [CrossRef]
- Dozio, E.; Caldiroli, L.; Molinari, P.; Castellano, G.; Delfrate, N.W.; Romanelli, M.M.C.; Vettoretti, S. Accelerated AGEing: The Impact of Advanced Glycation End Products on the Prognosis of Chronic Kidney Disease. Antioxidants 2023, 12, 584. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Worldwide Burden of LDL Cholesterol: Implications in Cardiovascular Disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 241–244. [Google Scholar] [CrossRef]
- Burger, P.M.; Dorresteijn, J.A.N.; Koudstaal, S.; Holtrop, J.; Kastelein, J.J.P.; Jukema, J.W.; Ridker, P.M.; Mosterd, A.; Visseren, F.L.J. Course of the Effects of LDL-Cholesterol Reduction on Cardiovascular Risk over Time: A Meta-Analysis of 60 Randomized Controlled Trials. Atherosclerosis 2024, 396, 118540. [Google Scholar] [CrossRef]
- Kusunoki, M.; Hisano, F.; Matsuda, S.I.; Kusunoki, A.; Abe, T.; Tsutsumi, K.; Miyata, T. Effects of SGLT2 Inhibitors and DPP-4 Inhibitors on Advanced Glycation End Products. Drug Res. 2023, 74, 77–80. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Cameron, A.J.; Magliano, D.J.; Shaw, J.E.; Zimmet, P.Z.; Carstensen, B.; Alberti, K.G.M.; Tuomilehto, J.; Barr, E.L.M.; Pauvaday, V.K.; Kowlessur, S.; et al. The Influence of Hip Circumference on the Relationship between Abdominal Obesity and Mortality. Int. J. Epidemiol. 2012, 41, 484–494. [Google Scholar] [CrossRef]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of Body Mass Index with Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018, 3, 280–287. [Google Scholar] [CrossRef]
- Ruiz, H.H.; Ramasamy, R.; Schmidt, A.M. Advanced Glycation End Products: Building on the Concept of the “Common Soil” in Metabolic Disease. Endocrinology 2020, 161, bqz006. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; De Michele, G.; Fimiani, F.; Acerbo, V.; Scherillo, G.; Signore, G.; Rotolo, F.P.; Scialla, F.; Raucci, G.; Panico, D.; et al. Visceral Adipose Tissue and Residual Cardiovascular Risk: A Pathological Link and New Therapeutic Options. Front. Cardiovasc. Med. 2023, 10, 1187735. [Google Scholar] [CrossRef] [PubMed]
- IDF. IDF Global Clinical Practice Recommendations for Managing Type 2 Diabetes; IDF: Brussels, Belgium, 2025. [Google Scholar]
- van Waateringe, R.P.; Slagter, S.N.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Lutgers, H.L.; Wolffenbuttel, B.H.R. Lifestyle and Clinical Determinants of Skin Autofluorescence in a Population-Based Cohort Study. Eur. J. Clin. Invest. 2016, 46, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.J.; Van De Zande, S.C.; Mulder, D.J. Skin Autofluorescence as Tool for Cardiovascular and Diabetes Risk Prediction. Curr. Opin. Nephrol. Hypertens. 2022, 31, 522–526. [Google Scholar] [CrossRef]
- Giugliano, D.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Esposito, K. Glycemic Control, Preexisting Cardiovascular Disease, and Risk of Major Cardiovascular Events in Patients with Type 2 Diabetes Mellitus: Systematic Review With Meta-Analysis of Cardiovascular Outcome Trials and Intensive Glucose Control Trials. J. Am. Heart Assoc. 2019, 8, e012356. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Haas, M.J. Cardioprotective Antihyperglycemic Drugs Ameliorate Endoplasmic Reticulum Stress. Am. J. Physiol. Cell Physiol. 2024, 326, C89–C94. [Google Scholar] [CrossRef]
- Balogh, D.B.; Wagner, L.J.; Fekete, A. An Overview of the Cardioprotective Effects of Novel Antidiabetic Classes: Focus on Inflammation, Oxidative Stress, and Fibrosis. Int. J. Mol. Sci. 2023, 24, 7789. [Google Scholar] [CrossRef]
- Yamagishi, S.; Nakamura, K.; Matsui, T.; Inoue, H. A Novel Pleiotropic Effect of Atorvastatin on Advanced Glycation End Product (AGE)-Related Disorders. Med. Hypotheses 2007, 69, 338–340. [Google Scholar] [CrossRef]
- Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients 2022, 14, 4588. [Google Scholar] [CrossRef] [PubMed]
- Fender, A.C.; Dobrev, D. Evolving Insights into the Pleiotropic Cardioprotective Mechanisms of SGLT2 Inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Strong, J. The Pleiotropic Effects of Sodium–Glucose Cotransporter-2 Inhibitors: Beyond the Glycemic Benefit. Diabetes Ther. 2019, 10, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Bain, S.; Hicks, D.; Newland Jones, P.; Patel, D.C.; Evans, M.; Fernando, K.; James, J.; Milne, N.; Viljoen, A.; et al. SGLT2 Inhibitors: Cardiovascular Benefits Beyond HbA1c—Translating Evidence into Practice. Diabetes Therapy 2019, 10, 1595–1622. [Google Scholar] [CrossRef]
- Talari, K.; Goyal, M. Retrospective Studies—Utility and Caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S128–S145. [Google Scholar] [CrossRef]
- Mach, F.; Koskinas, K.C.; Roeters van Lennep, J.E.; Tokgözoğlu, L.; Badimon, L.; Baigent, C.; Benn, M.; Binder, C.J.; Catapano, A.L.; De Backer, G.G.; et al. 2025 Focused Update of the 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2025. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; Hanssen, H.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
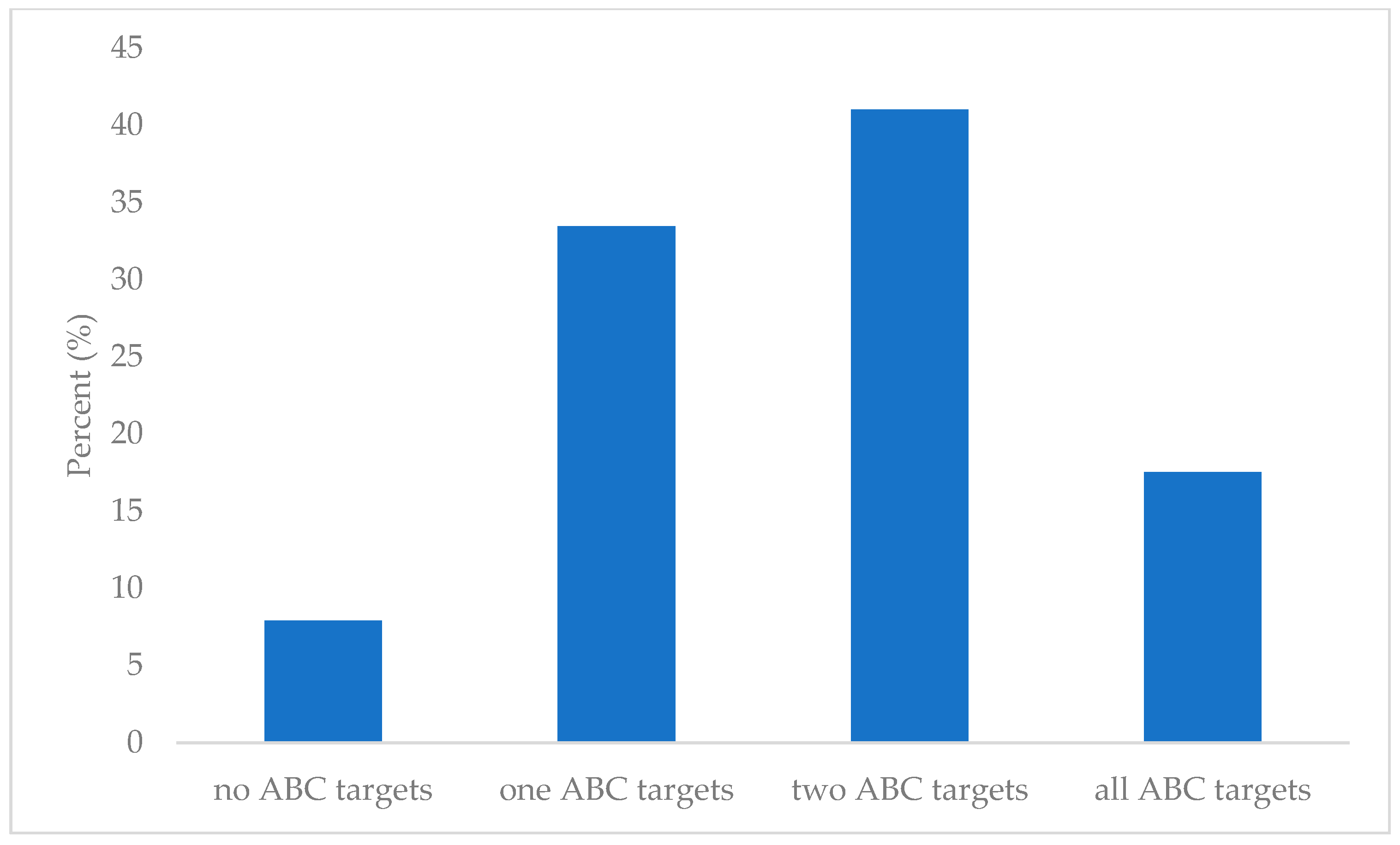
| General Data | No CV Risk (n = 155) | Elevated CV Risk (n = 96) | Total (n = 251) | p | |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 68 (61–74) | 67 (60–73) | 67 (60–73) | 0.34 † | |
| Disease duration of DMT2 1 (years), median (IQR) | 11 (6–20) | 10 (6–20) | 10 (6–20) | 0.69 † | |
| Sex, n (%) | |||||
| Men | 60 (39) | 61 (64) | 121 (48) | <0.001 * | |
| Women | 95 (61) | 35 (36) | 130 (52) | ||
| pSBP 1 (mmHg), median (IQR) | 138 (125–152) | 140 (127.3–152) | 138 (125–152) | 0.34 † | |
| pDBP 1 (mmHg), median (IQR) | 81 (75–87) | 85 (76.3–91) | 81 (75–88) | 0.02 † | |
| Arterial BP, n (%) | |||||
| <140/90 mmHg | 77 (49.7) | 47 (49) | 124 (49.4) | 0.91 * | |
| ≥140/90 mmHg | 78 (50.3) | 49 (51) | 127 (50.6) | ||
| Endocrinology examination, n (%) | 106 (68.4) | 71 (74) | 177 (70.5) | 0.35 * | |
| Nephrology examination, n (%) | 54 (34.8) | 26 (27.1) | 80 (31.9) | 0.20 * | |
| Regular nephrology examination, n (%) | 19 (12.3) | 12 (12.5) | 31 (12.4) | 0.96 * | |
| Smoking, n (%) | 28 (18.1) | 16 (16.7) | 44 (17.5) | 0.78 * | |
| Duration of smoking, median (IQR) | 30 (25–40) | 38 (30–47) | 30 (25–44) | 0.77 † | |
| Former smokers, n (%) | 54 (34.8) | 32 (33.3) | 86 (34.3) | 0.81 * | |
| Time without smoking (years), median (IQR) | 15 (6–23) | 15 (11–29) | 15 (8–25) | 0.69 † | |
| No CV Risk (n = 155) | Elevated CV Risk (n = 96) | Total (n = 251) | p * | ||
|---|---|---|---|---|---|
| BMI 1 (kg/m2), median (IQR) | 27.4 (23.8–30.6) | 27.8 (24.4–32.9) | 27.3 (22.2–31.6) | 0.007 | |
| <25 kg/m2, n (%) | 48 (31.6) | 26 (27.7) | 74 (30.1) | 0.67 † | |
| ≥25 kg/m2 and <30 kg/m2, n (%) | 60 (39.5) | 36 (38.3) | 96 (39) | ||
| ≥30 kg/m2, n (%) | 44 (28.9) | 32 (34) | 76 (30.9) | ||
| HC 1 (cm), median (IQR) | 104.5 (99–113) | 109 (102.5–115) | 108 (99–113) | 0.04 | |
| No Goals Achieved (n = 20) | One Goal Achieved (n = 84) | Two Goals Achieved (n = 103) | All Goals Achieved (n = 44) | p | ||
|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 64 (59–68) | 68 (61–74) | 68 (59–74) | 67 (63–71) | 0.34 † | |
| Sex, n (%) | ||||||
| Men | 11 (55) | 50 (48.5) | 42 (50) | 18 (40.9) | 0.70 | |
| Women | 9 (45) | 53 (51.5) | 42 (50) | 26 (59.1) | ||
| Disease duration of DMT2 (years), median (IQR) | 18 (11–23) | 10 (4–20) | 12 (6–20) | 10 (7–20) | 0.07 † | |
| pSBP 1 (mmHg), median (IQR) | 149 (144–164) | 150 (137–160) | 135 (125–145) | 122 (114–133) | <0.001 †‡ | |
| pDBP 1 (mmHg), median (IQR) | 86 (93–90) | 87 (81–94) | 80 (74–86) | 76 (72–84) | <0.001 †‡ | |
| BMI 1 (kg/m2), median (IQR) | 27.5 (23.7–31.3) | 27.4 (23.9–31.8) | 27.9 (24.7–30.8) | 27.0 (23.0–30.5) | 0.85 † | |
| WHR 1, median (IQR) | 0.96 (0.90–0.99) | 0.94 (0.87–0.98) | 0.93 (0.87–0.99) | 0.92 (0.88–0.98) | 0.72 † | |
| eGFR CKD-EPI 1 (mL/min/1.73 m2), median (IQR) | 74.1 (54.4–90.2) | 76.3 (65.7–93.9) | 78 (65.7–90.9) | 75.9 (60.7–88.2) | 0.48 † | |
| HbA1c 1 (%), median (IQR) | 7.5 (7.3–8.0) | 8 (6.4–8.1) | 6.5 (6.0–7.0) | 6.2 (5.8–6.7) | <0.001 †‡ | |
| AGE 1, median (IQR) | 2.4 (1.9–2.9) | 2.2 (1.9–2.7) | 2.2 (2.0–2.6) | 2.3 (1.9–2.9) | 0.71 † | |
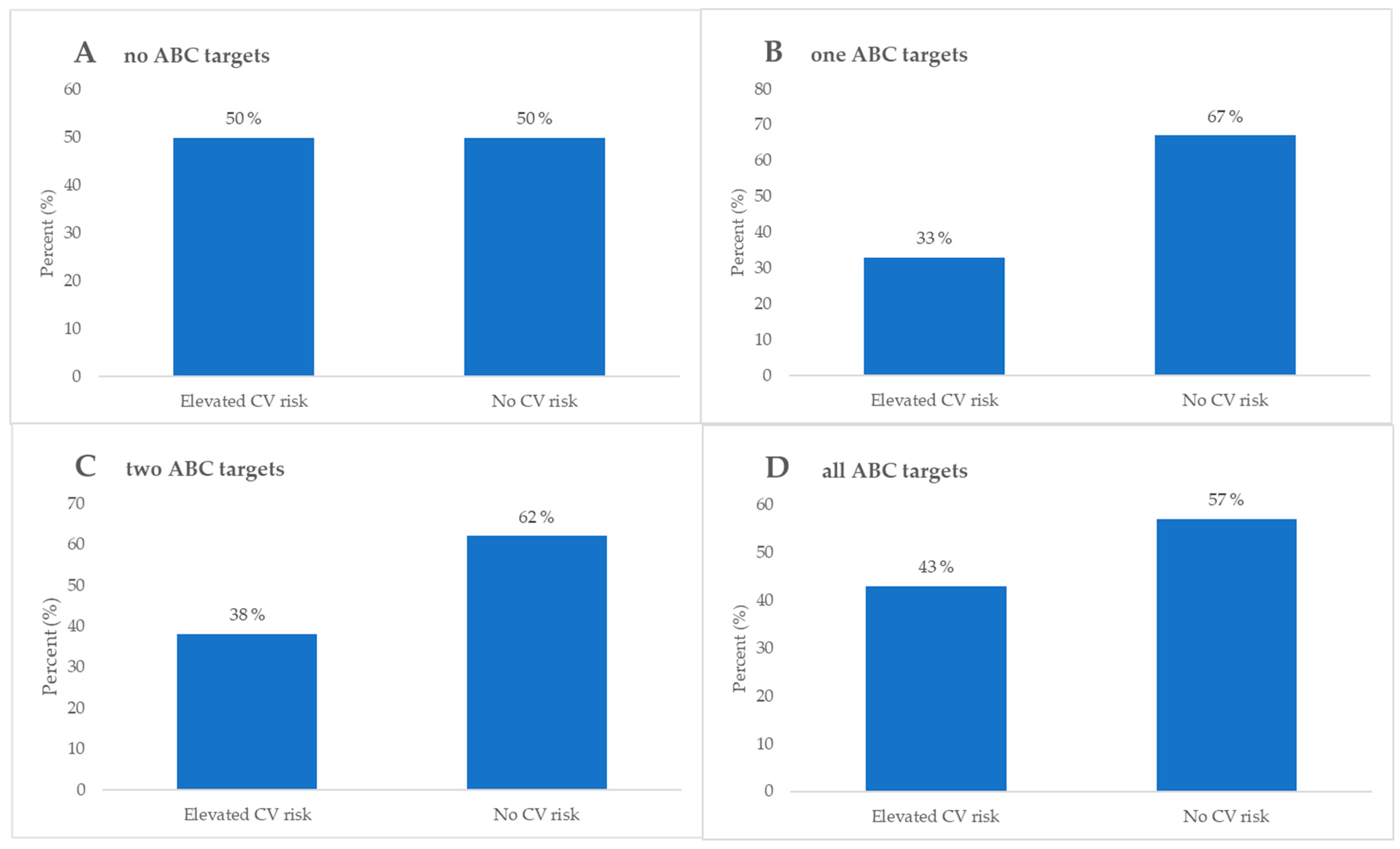
| Elevated CV 1 risk, n (%) | 10 (50) | 56 (67) | 64 (62) | 25 (57) | 0.48 * | |
| No CV 1 risk, n (%) | 10 (50) | 28 (33) | 39 (38) | 19 (43) | ||
| Total cholesterol (mmol/L), median (IQR) | 5.7 (5.2–6.3) | 5.2 (4.5–5.9) | 4.4 (3.7–5.1) | 3.75 (3.35–4.35) | <0.001 †‡ | |
| HDL 1 cholesterol (mmol/L), median (IQR) | 1.35 (1.2–1.5) | 1.5 (1.3–1.7) | 1.5 (1.2–1.7) | 1.4 (1.1–1.6) | 0.69 † | |
| LDL 1 cholesterol (mmol/L), median (IQR) | 3.3 (2.9–3.7) | 2.9 (2.4–3.5) | 2.1 (1.7–2.9) | 1.8 (1.4–2.0) | <0.001 †‡ | |
| Triglycerides (mmol/L), median (IQR) | 1.9 (1.35–2.45) | 1.4 (1.05–2.1) | 1.3 (0.9–2.0) | 1.3 (0.85–1.75) | 0.05 † | |
| Albuminuria (mg/L), median (IQR) | 5.5 (4–12) | 5 (2–17) | 5.0 (2.0–13) | 5.5 (52–9.5) | 0.86 † | |
| No Goals Achieved (n = 7) | One Goal Achieved (n = 20) | Two Goals Achieved (n = 26) | All Goals Achieved (n = 15) | p† | ||
|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 72 (63–77) | 71 (62.8–76) | 66 (57.8–71.3) | 70 (62–73) | 0.28 | |
| Sex, n (%) | ||||||
| Men | 5 (71.4) | 9 (45) | 17 (65.4) | 9 (60) | 0.51 * | |
| Women | 2 (28.6) | 11 (55) | 9 (34.6) | 6 (40) | ||
| Disease duration of DMT2 1 (years), median (IQR) | 20 (10–26) | 10.5 (10–26.5) | 10 (3.8–19.3) | 14 (7.5–30) | 0.28 | |
| pSBP 1 (mmHg), median (IQR) | 143 (131–146) | 140.5 (127.3–155.8) | 138 (130.5–149.3) | 129 (121–145) | 0.31 | |
| pDBP 1 (mmHg), median (IQR) | 85 (78–88) | 78 (74.3–84.3) | 79 (73.8–88) | 80 (74–84) | 0.42 | |
| BMI 1 (kg/m2), median (IQR) | 31.5 (26.7–34.9) | 28.4 (24.2–30) | 27.9 (23.7–31.5) | 25.7 (22.7–28.1) | 0.15 | |
| WHR 1, median (IQR) | 1 (1–1.1) | 0.9 (0.9–1) | 0.9 (0.9–1) | 1 (0.9–1) | 0.14 | |
| eGFR CKD-EPI 1 (mL/min/1.73 m2), median (IQR) | 72.5 (54.1–75.3) | 69.3 (60.5–82.5) | 65 (57.1–82.9) | 75.4 (62.8–84.3) | 0.87 | |
| HbA1c 1 (%), median (IQR) | 7.8 (7.3–9.4) | 6.6 (5.8–7.9) | 6.9 (6.2–7.4) | 6.4 (5.8–6.7) | 0.003 ‡ | |
| AGE 1, median (IQR) | 2.8 (2.7–3.1) | 2.3 (2–2.6) | 2.2 (1.9–2.6) | 2.2 (2–3.1) | 0.17 | |
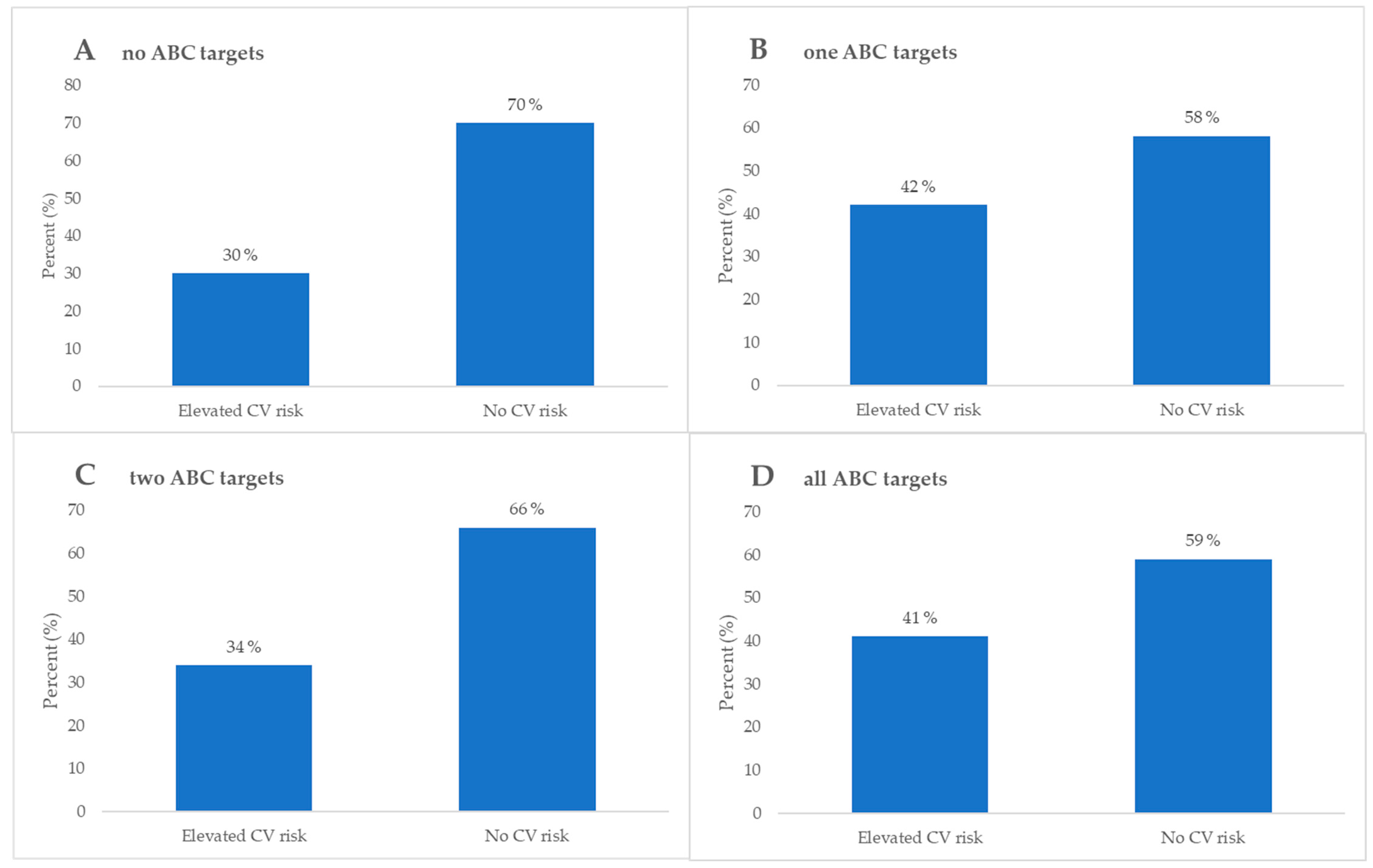
| Elevated CV 1 risk, n (%) | 7 (100) | 13 (65) | 21 (81) | 12 (80) | 0.29 * | |
| No CV 1 risk, n (%) | 0 (0) | 7 (35) | 5 (19) | 3 (20) | ||
| Total cholesterol (mmol/L), median (IQR) | 5.2 (4.9–6.1) | 5.0 (4.2–5.9) | 3.9 (3.5–4.3) | 3.4 (3–3.6) | <0.001 ║ | |
| HDL 1 cholesterol (mmol/L), median (IQR) | 1.4 (1.3–1.8) | 1.5 (1.1–1.6) | 1.4 (1.1–1.6) | 1.4 (1.3–1.6) | 0.97 | |
| LDL 1 cholesterol (mmol/L), median (IQR) | 3.0 (2.9–3.5) | 2.8 (2.2–3.3) | 1.8 (1.4–2.1) | 1.4 (1–1.5) | <0.001 ║ | |
| Triglycerides (mmol/L), median (IQR) | 1.8 (0.9–2.1) | 1.6 (1.1–2.2) | 1.3 (0.9–1.7) | 1.2 (0.9–1.6) | 0.18 | |
| Albuminuria (mg/L), median (IQR) | 8 (5–17) | 10.5 (2–44.5) | 5.5 (2–13.5) | 5 (2–6) | 0.14 | |
| ß | p Value | 95% CI | Regression Module | |
|---|---|---|---|---|
| AGE * | ||||
| GLP1 * | 0.31 | 0.04 | 0.006–0.619 | R2 = 0.018; R2adj = 0.013 F(1, 222) = 4.03; p = 0.04 Cohen’s f2 = 0.01 |
| Constant | 2.31 | <0.001 | 2.24–2.38 | |
| Number of ABC targets in all participants | ||||
| Sulfonamides | −0.39 | 0.02 | −0.72–−0.05 | R2 = 0.023; R2 adj = 0.018 F(1, 222) = 5.15; p = 0.02 Cohen’s f2 = 0.02 |
| Constant | 1.73 | <0.001 | 1.61–1.85 | |
| Number of ABC targets in participants with prior CV or CBV incidents * | ||||
| SGLT2 * | 0.69 | 0.02 | 0.10–1.29 | R2 = 0.073; R2adj = 0.057 F(2, 57) = 4.71; p = 0.01 Cohen’s f2 = 0.08 |
| BMI * | −0.05 | 0.03 | −0.09–−0.003 | |
| Constant | 2.88 | <0.001 | 1.65–4.09 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radić, J.; Vučković, M.; Đogaš, H.; Ødeverp, A.; Grubić, M.; Radić, M. Public Health Screening for Cardiometabolic Risk: Lessons from Advanced Glycation End-Products and ABC Target Achievement in Dalmatian Adults with Type 2 Diabetes. Biomedicines 2025, 13, 2418. https://doi.org/10.3390/biomedicines13102418
Radić J, Vučković M, Đogaš H, Ødeverp A, Grubić M, Radić M. Public Health Screening for Cardiometabolic Risk: Lessons from Advanced Glycation End-Products and ABC Target Achievement in Dalmatian Adults with Type 2 Diabetes. Biomedicines. 2025; 13(10):2418. https://doi.org/10.3390/biomedicines13102418
Chicago/Turabian StyleRadić, Josipa, Marijana Vučković, Hana Đogaš, Anders Ødeverp, Marina Grubić, and Mislav Radić. 2025. "Public Health Screening for Cardiometabolic Risk: Lessons from Advanced Glycation End-Products and ABC Target Achievement in Dalmatian Adults with Type 2 Diabetes" Biomedicines 13, no. 10: 2418. https://doi.org/10.3390/biomedicines13102418
APA StyleRadić, J., Vučković, M., Đogaš, H., Ødeverp, A., Grubić, M., & Radić, M. (2025). Public Health Screening for Cardiometabolic Risk: Lessons from Advanced Glycation End-Products and ABC Target Achievement in Dalmatian Adults with Type 2 Diabetes. Biomedicines, 13(10), 2418. https://doi.org/10.3390/biomedicines13102418








