Inflammatory Insights: Analysis of a Fecal Biomarker in Neurodegenerative and Gastrointestinal Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants, Data Collection, and Ethical Statement
2.2. Specimen Collection and Analytic Method Used for Fecal Calprotectin Detection
2.3. Used Reference Ranges
2.4. Statistical Analyses
3. Results
3.1. Socio-Demographic Characterization of Patients
3.2. Consideration of Fecal Calprotectin Levels in the Studied Groups
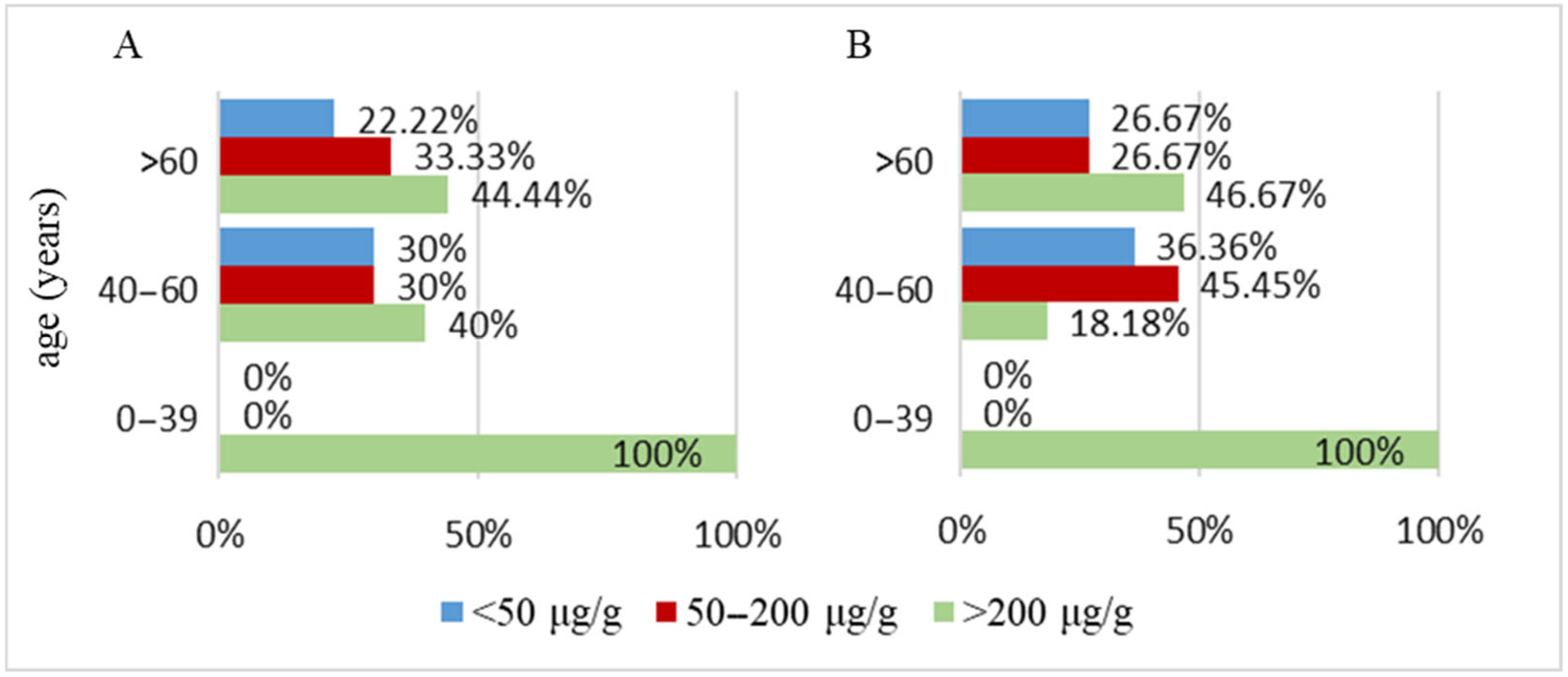
3.2.1. Parkinson’s Disease Cohort
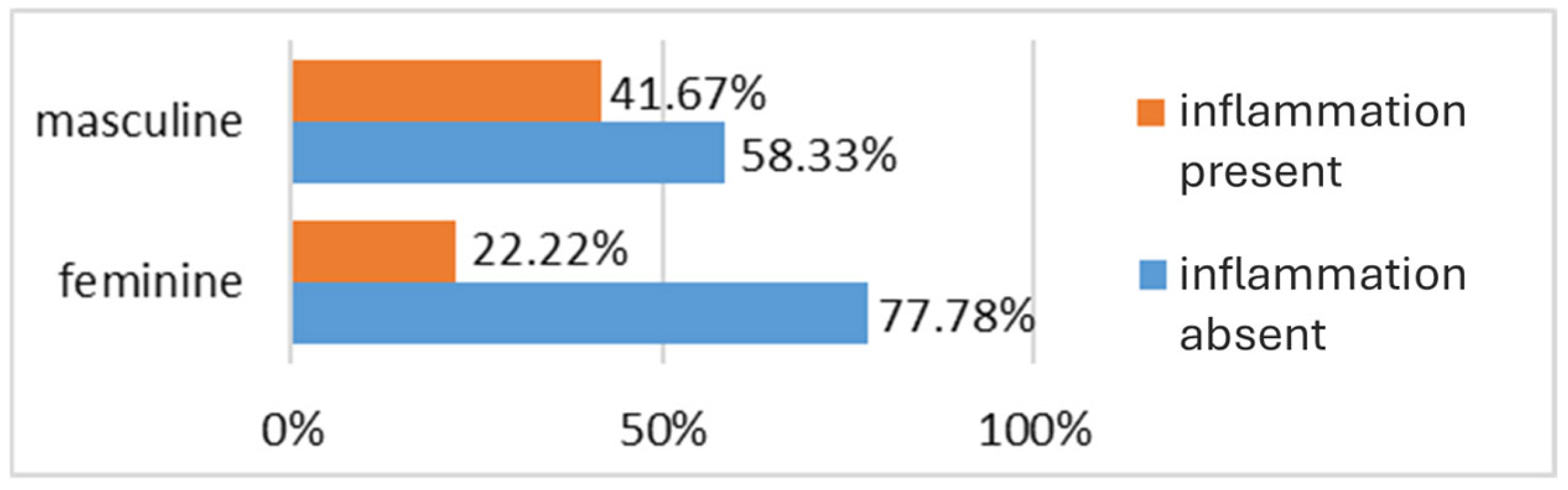
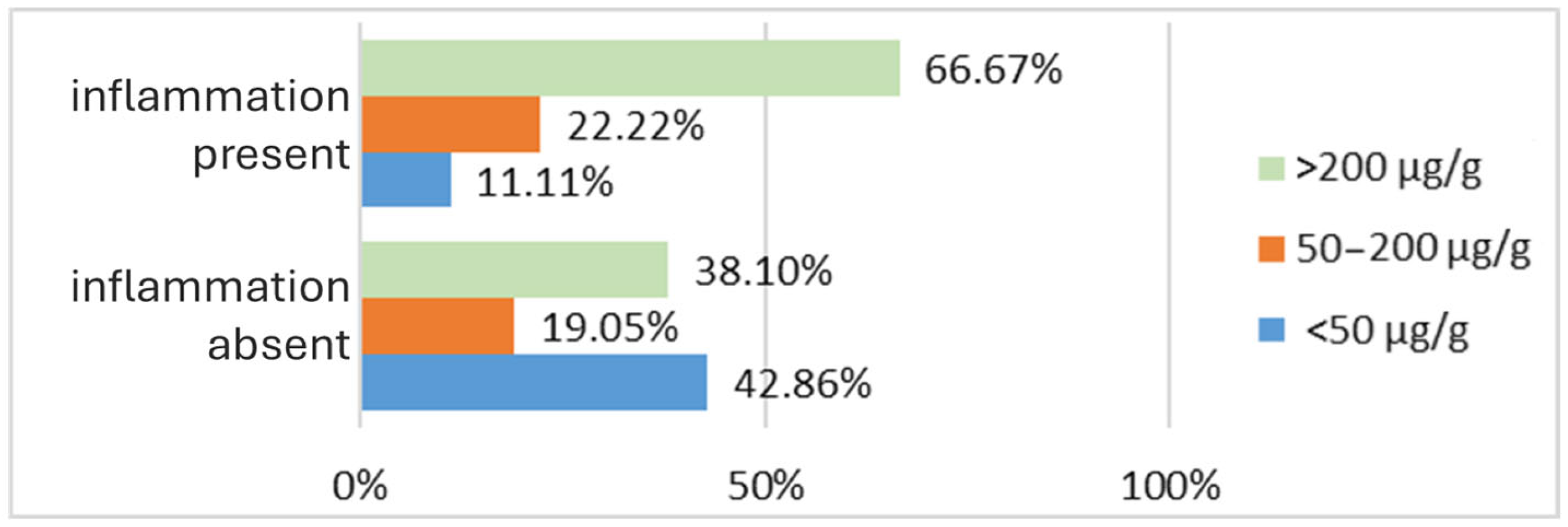
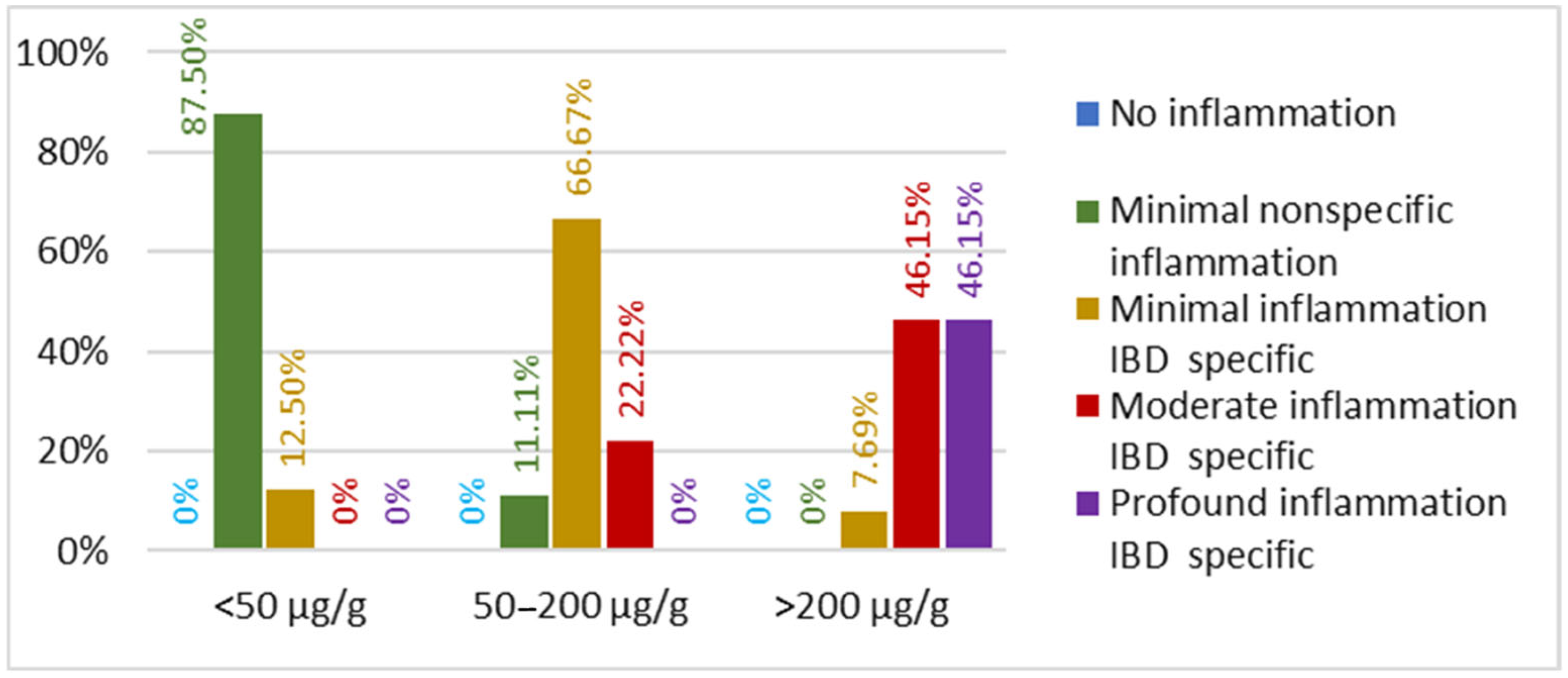
3.2.2. Inflammatory Bowel Disease Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| PD | Parkinson’s disease |
| GIT | Gastrointestinal tract |
| IBS | Irritable bowel syndrome |
| UC | Ulcerative colitis |
| CD | Crohn’s disease |
| PPI | Proton pump inhibitors |
| ASIR | Age-standardized incidence rate |
| EAPC | Estimated annual percentage change |
| HPA | Hypothalamic–pituitary–adrenal axis |
References
- Batiha, G.E.-S.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E. SIRT1 pathway in Parkinson’s disease: A faraway snapshot but so close. Inflammopharmacology 2023, 31, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Marsden, C.D. Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 672–681. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Xu, S.; Liu, Z.; Zhu, Y.; Zhang, X.; Xu, R. Sporadic Parkinson’s Disease Potential Risk Loci Identified in Han Ancestry of Chinese Mainland. Front. Aging Neurosci. 2021, 12, 603793. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Marta, D.; Dănău, A.; Lefter, A.; Tulbă, D.; Cozma, L.; Manole, E.; Gherghiceanu, M.; Ceafalan, L.C.; Popescu, B.O. Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson’s Disease. Front. Neurosci. 2021, 15, 689723. [Google Scholar] [CrossRef]
- Alrouji, M.; Al-Kuraishy, H.M.; Al-Buhadily, A.K.; Al-Gareeb, A.I.; Elekhnawy, E.; Batiha, G.E.-S. DPP-4 inhibitors and type 2 diabetes mellitus in Parkinson’s disease: A mutual relationship. Pharmacol. Rep. 2023, 75, 923–936. [Google Scholar] [CrossRef]
- Alrouji, M.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Saad, H.M.; Batiha, G.E.-S. A story of the potential effect of non-steroidal anti-inflammatory drugs (NSAIDs) in Parkinson’s disease: Beneficial or detrimental effects. Inflammopharmacology 2023, 31, 673–688. [Google Scholar] [CrossRef]
- Gonzalez-Latapi, P.; Bayram, E.; Litvan, I.; Marras, C. Cognitive impairment in Parkinson’s disease: Epidemiology, clinical profile, protective and risk factors. Behav. Sci. 2021, 11, 74. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Zaidalkiani, A.T.; Alexiou, A.; Papadakis, M.; Bahaa, M.M.; Al-Faraga, A.; Batiha, G.E.-S. Calprotectin in Parkinsonian disease: Anticipation and dedication. Ageing Res. Rev. 2024, 93, 102143. [Google Scholar] [CrossRef]
- Zhang, F.; Yue, L.; Fang, X.; Wang, G.; Li, C.; Sun, X.; Jia, X.; Yang, J.; Song, J.; Zhang, Y. Altered gut microbiota in Parkinson’s disease patients/healthy spouses and its association with clinical features. Park. Relat. Disord. 2020, 81, 84–88. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Le, W. Intestinal permeability, dysbiosis, inflammation and enteric glia cells: The intestinal etiology of Parkinson’s disease. Aging Dis. 2022, 13, 1381. [Google Scholar] [CrossRef]
- Inciarte-Mundo, J.; Frade-Sosa, B.; Sanmartí, R. From bench to bedside: Calprotectin (S100A8/S100A9) as a biomarker in rheumatoid arthritis. Front. Immunol. 2022, 13, 1001025. [Google Scholar] [CrossRef]
- Ricciuto, A.; Griffiths, A.M. Clinical value of fecal calprotectin. Crit. Rev. Clin. Lab. Sci. 2019, 56, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.A.; Park, J.H.; Mansouri, D.; Combet, E.; Horgan, P.G.; McMillan, D.C.; Roxburgh, C.S. The role of faecal calprotectin in diagnosis and staging of colorectal neoplasia: A systematic review and meta-analysis. BMC Gastroenterol. 2022, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A.; Koszewicz, M.; Panek-Jeziorna, M.; Koziorowska-Gawron, E.; Budrewicz, S. Fecal Calprotectin as a Marker of the Gut Immune System Activation Is Elevated in Parkinson’s Disease. Front. Neurosci. 2019, 13, 992. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Lobbestael, E.; Vermeire, S.; Sabino, J.; Cleynen, I. Inflammatory bowel disease and Parkinson’s disease: Common pathophysiological links. Gut 2021, 70, 408–417. [Google Scholar] [CrossRef]
- Heinzel, S.; Berg, D.; Gasser, T.; Chen, H.; Yao, C.; Postuma, R.B.; the MDS Task Force on the Definition of Parkinson’s Disease. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2019, 34, 1464–1470. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Gil-Nagel, A.; Gracia, L.M.; Gomez, J.B.; Martinez-Sarries, J.; Bermejo, F.; The cooperative multicentric group. Unified Parkinson’s disease rating scale characteristics and structure. Mov. Disord. 1994, 9, 76–83. [Google Scholar] [CrossRef]
- Kotze, L.M.; Nisihara, R.M.; Marion, S.B.; Cavassani, M.F.; Kotze, P.G. Fecal calprotectin: Levels for the etiological diagnosis in Brazilian patients with gastrointestinal symptoms. Arq. Gastroenterol. 2015, 52, 50–54. [Google Scholar] [CrossRef]
- Abraham, B.P.; Kane, S. Fecal markers: Calprotectin and lactoferrin. Gastroenterol. Clin. N. Am. 2012, 41, 483–495. [Google Scholar] [CrossRef]
- Synevo. Calprotectina în Materii Fecale. Synevo România. Available online: https://www.synevo.ro/shop/calprotectina-in-materii-fecale/ (accessed on 4 September 2025).
- Augustin, A.; Guennec, A.L.; Umamahesan, C.; Kendler-Rhodes, A.; Tucker, R.M.; Chekmeneva, E.; Takis, P.; Lewis, M.; Balasubramanian, K.; DeSouza, N.; et al. Faecal metabolite deficit, gut inflammation and diet in Parkinson’s disease: Integrative analysis indicates inflammatory response syndrome. Clin. Transl. Med. 2023, 13, e1152. [Google Scholar] [CrossRef]
- Bucur, V.M. Probleme Actuale ale Varstei a Treia; Editura Eurostampa: Timisoara, Romania, 2001; pp. 11–12. [Google Scholar]
- Breaz, A. Vârsta a Treia, azi. Cluj: Ed Eikon. Available online: https://www.researchgate.net/profile/Maria_Breaz2/publication/313429197_CARTEA_I-_REAVIZUITA/data/589a59eeaca2721f0db12710/CARTEA-I-REAVIZUITA.docx (accessed on 9 October 2024).
- Zhu, Y.; Yuan, M.; Liu, Y.; Yang, F.; Chen, W.-Z.; Xu, Z.-Z.; Xiang, Z.-B.; Xu, R.-S. Association between inflammatory bowel diseases and Parkinson’s disease: Systematic review and meta-analysis. Neural Regen. Res. 2022, 17, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef] [PubMed]
- Orozco, J.L.; Valderrama-Chaparro, J.A.; Pinilla-Monsalve, G.D.; Molina-Echeverry, M.I.; Pérez Castaño, A.M.; Ariza-Araújo, Y.; Prada, S.I.; Takeuchi, Y. Parkinson’s disease prevalence, age distribution and staging in Colombia. Neurol. Int. 2020, 12, 8401. [Google Scholar] [CrossRef]
- Parkinson’s Foundation. Statistics. Parkinson’s Foundation. Available online: https://www.parkinson.org/understanding-parkinsons/statistics (accessed on 6 January 2025).
- Parkinson’s U.K. The Incidence and Prevalence of Parkinson’s in the UK: Results from the Clinical Practice Research Datalink (Reference Report). 2017. Available online: https://www.parkinsons.org.uk/sites/default/files/2018-01/Prevalence%20%20Incidence%20Report%20Latest_Public_2.pdf (accessed on 6 January 2025).
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for agerelated diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Heinzel, S.; Jureczek, J.; Kainulainen, V.; Nieminen, A.I.; Suenkel, U.; von Thaler, A.K.; Kaleta, C.; Eschweiler, G.W.; Brockmann, K.; Aho, V.T.E.; et al. Elevated fecal calprotectin is associated with gut microbial dysbiosis, altered serum markers and clinical outcomes in older individuals. Sci. Rep. 2024, 14, 13513. [Google Scholar] [CrossRef]
- Schwiertz, A.; Spiegel, J.; Dillmann, U.; Grundmann, D.; Bürmann, J.; Faßbender, K.; Schäfer, K.-H.; Unger, M.M. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Park. Relat. Disord. 2018, 50, 104–107. [Google Scholar] [CrossRef]
- Suryono; Kido, J.; Hayashi, N.; Kataoka, M.; Shinohara, Y.; Nagata, T. Norepinephrine stimulates calprotectin expression in human monocytic cells. J. Periodontal Res. 2006, 41, 159–164. [Google Scholar] [CrossRef]
- Karling, P.; Norrback, K.-f.; Adolfsson, R.; Danielsson, Å. Gastrointestinal symptoms are associated with hypothalamic-pituitary-adrenal axis suppression in healthy individuals Scand. J. Gastroenterol. 2007, 42, 1294–1301. [Google Scholar]
- Ibrahimagic, O.C.; Jakubovic, A.C.; Smajlovic, D.; Dostovic, Z.; Kunic, S.; Iljazovic, A. Psychological stress and changes of hypothalamic-pituitary-adrenal axis in patients with “de novo” Parkinson’s disease. Med. Arch. 2016, 70, 445. [Google Scholar] [CrossRef]
- Naimov, O.; Matmurodov, R.; Abdukodirov, E. Gastrointestinal disturbances in various forms of parkinsonism. J. Neurol. Sci. 2019, 405, 187–188. [Google Scholar] [CrossRef]
- Dumitru, A.; Matei, E.; Cozaru, G.C.; Chisoi, A.; Alexandrescu, L.; Popescu, R.C.; Butcaru, M.P.; Dumitru, E.; Rugină, S.; Tocia, C. Endotoxin Inflammatory Action on Cells by Dysregulated-Immunological-Barrier-Linked ROS-Apoptosis Mechanisms in Gut–Liver Axis. Int. J. Mol. Sci. 2024, 25, 2472. [Google Scholar] [CrossRef] [PubMed]
- Rolli-Derkinderen, M.; Leclair-Visonneau, L.; Bourreille, A.; Coron, E.; Neunlist, M.; Derkinderen, P. Is Parkinson’s disease a chronic low-grade inflammatory bowel disease? J. Neurol. 2019, 267, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; Bruley des Varannes, S.; Naveilhan, P.; Nguyen, J.-M.; et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013, 50, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Metta, V.; Leta, V.; Mrudula, K.R.; Prashanth, L.; Goyal, V.; Borgohain, R.; Chung-Faye, G.; Chaudhuri, K.R. Gastrointestinal dysfunction in Parkinson’s disease: Molecular pathology implications of gut microbiome probiotics fecal microbiota transplantation. J. Neurol. 2022, 269, 1154–1163. [Google Scholar] [CrossRef]
- Ponziani, F.R.; De Luca, A.; Picca, A.; Marzetti, E.; Petito, V.; Del Chierico, F.; Reddel, S.; Sterbini, F.P.; Sanguinetti, M.; Putignani, L. Gut dysbiosis and fecal calprotectin predict response to immune checkpoint inhibitors in patients with hepatocellular carcinoma. Hepatol. Commun. 2022, 6, 1492–1501. [Google Scholar] [CrossRef]
- Ho, T.T.; Groer, M.W.; Kane, B.; Yee, A.L.; Torres, B.A.; Gilbert, J.A.; Maheshwari, A. Enteric dysbiosis and fecal calprotectin expression in premature infants. Pediatr. Res. 2019, 85, 361–368. [Google Scholar] [CrossRef]
- Saviano, A.; Candelli, M.; Zanza, C.; Piccioni, A.; Migneco, A.; Ojetti, V. Gastrointestinal Involvement in extra-digestive disease: Which is the role of fecal calprotectin? Medicina 2022, 58, 1384. [Google Scholar] [CrossRef]
- Hor, J.W.; Lim, S.-Y.; Khor, E.S.; Chong, K.K.; Song, S.L.; Ibrahim, N.M.; Teh, C.S.J.; Chong, C.W.; Hilmi, I.N.; Tan, A.H. Fecal calprotectin in Parkinson’s disease multiple system atrophy. J. Mov. Disord. 2022, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 510, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Dajti, E.; Frazzoni, L.; Iascone, V.; Secco, M.; Vestito, A.; Fuccio, L.; Eusebi, L.H.; Fusaroli, P.; Rizzello, F.; Calabrese, C.; et al. Systematic review with meta-analysis: Diagnostic performance of faecal calprotectin in distinguishing inflammatory bowel disease from irritable bowel syndrome in adults. Aliment. Pharmacol. Ther. 2023, 58, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Burri, E.; Beglinger, C. Faecal calprotectin—A useful tool in the management of inflammatory bowel disease. Swiss Med. Wkly. 2012, 142, w13557. [Google Scholar] [CrossRef]
- Sarhan, R.S.R.; Marei, Y.M. Evaluation of the diagnostic performance of estimated fecal calprotectin and serum intelectin-1 and C-reactive protein solo or in combination for differentiation between patients with query ulcerative colitis and irritable bowel syndrome. Egypt. J. Intern. Med. 2023, 35, 79. [Google Scholar] [CrossRef]
- Jha, A.K.; Chaudhary, M.; Dayal, V.M.; Kumar, A.; Jha, S.K.; Jha, P.; Purkayastha, S.; Ranjan, R. Optimal cut-off value of fecal calprotectin for the evaluation of ulcerative colitis: An unsolved issue? JGH Open. 2018, 2, 207–213. [Google Scholar] [CrossRef]
- Sharbatdaran, M.; Holaku, A.; Kashifard, M.; Bijani, A.; Firozjahi, A.; Hosseini, A.; Siadati, S. Fecal calprotectin Level in patients with IBD and noninflammatory disease of colon: A study in Babol, Northern, Iran. Casp. J. Int. Med. 2018, 9, 60–64. [Google Scholar]
- Moein, S.; Qujeq, D.; Vaghari Tabari, M.; Kashifard, M.; Hajian-Tilaki, K. Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: From laboratory to clinic. Casp. J. Int. Med. 2017, 8, 178–182. [Google Scholar] [CrossRef]
- Diamanti, A.; Panetta, F.; Basso, M.S.; Forgione, A.; Colistro, F.; Bracci, F.; Papadatou, B.; Francalanci, P.; Torroni, F.; Knafelz, D.; et al. Diagnostic work-up of inflammatory bowel disease in children: The role of calprotectin assay. Inflamm. Bowel Dis. 2010, 16, 1926–1930. [Google Scholar] [CrossRef]
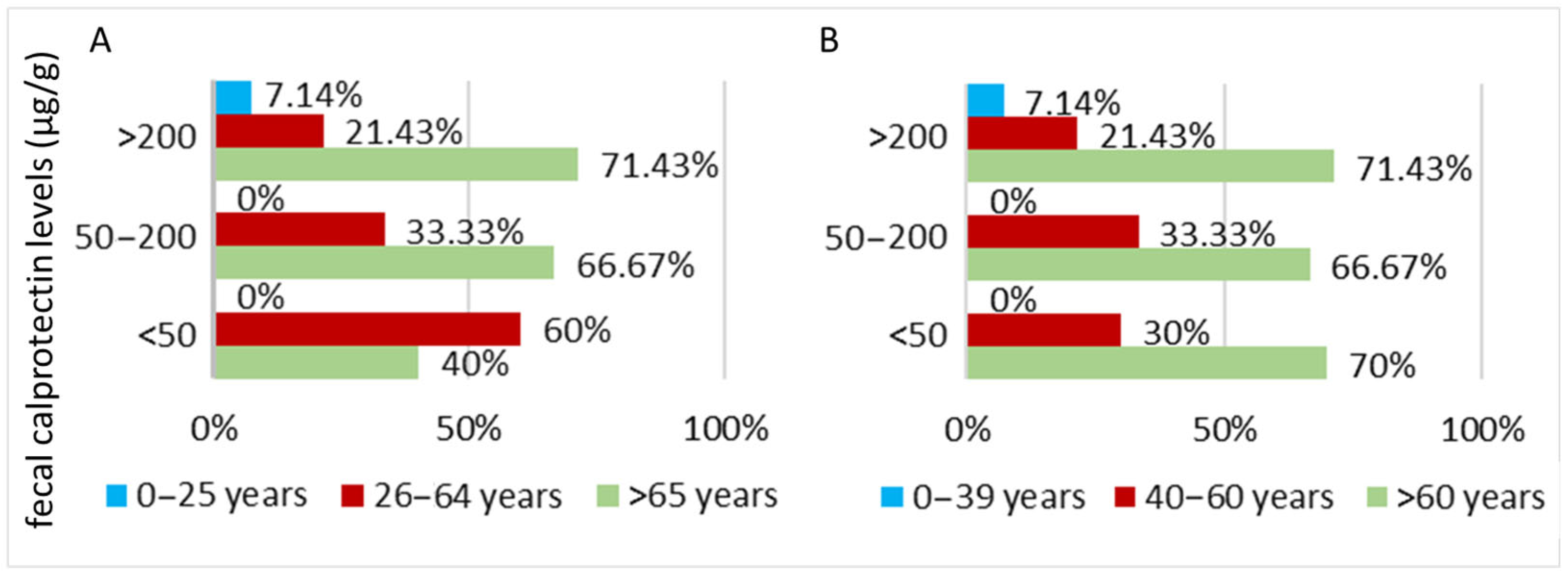

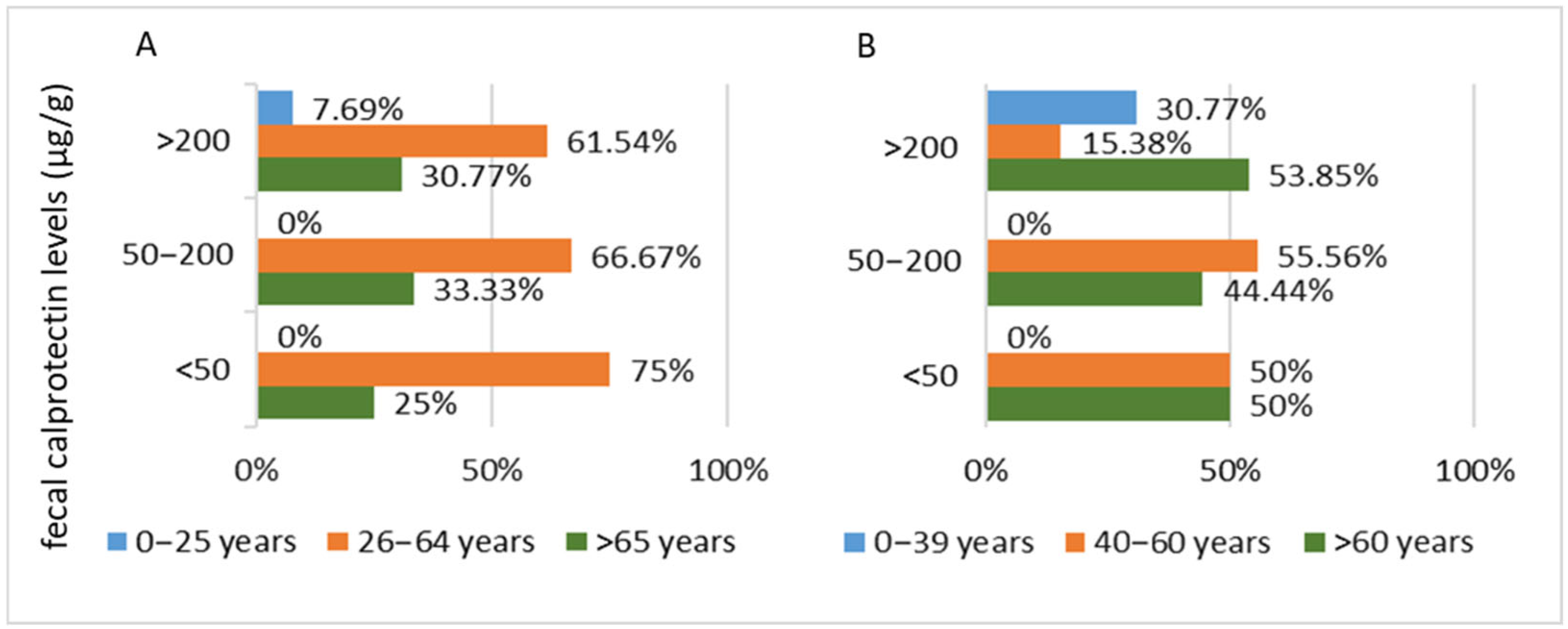
| Parameter | Social Data/ Paraclinical Data | PD Group | IBD Group |
|---|---|---|---|
| N/Value (SD) | |||
| Provenience area | Rural | 7 | 7 |
| Urban | 23 | 23 | |
| Age—mean | 64.6 (16.54) | 57.57 (14.52) | |
| Age | Minimum | 23 | 25 |
| Maximum | 87 | 81 | |
| Gender | Feminine | 18 | 15 |
| Masculine | 12 | 15 | |
| Fecal Calprotectin (µg/g)—mean value | 366.25 (415.14) | 537.70 (795.30) | |
| Fecal Calprotectin (µg/g)—median value | 184.62 | 167.85 | |
| Fecal calprotectin (µg/g)—value | Minimum | 23.33 | 0.1 |
| Maximum | 1345 | 2971.4 | |
| Fecal Calprotectin— number of cases | <50 µg/g | 10 | 8 |
| 50–200 µg/g | 6 | 9 | |
| >200 µg/g | 14 | 13 | |
| Endoscopic diagnostic | Without IBD-specific inflammatory changes | 29 | 0 |
| UC inflammatory aspect | 0 | 12 | |
| CD inflammatory aspect | 0 | 18 | |
| Polyps or diverticulosis | 0 | 0 | |
| Gastritis and ulcers | 1 | 0 | |
| Inflammation level | No inflammation | 21 | 0 |
| Minimal nonspecific inflammation | 9 | 8 | |
| Minimal inflammation IBD-specific | 0 | 8 | |
| Moderate inflammation IBD-specific | 0 | 8 | |
| Profound inflammation IBD-specific | 0 | 6 | |
| Cohort | Variable 1 | Variable 2 | r | p |
|---|---|---|---|---|
| IBD patients | age | disease subtype | 0.370 | 0.044 |
| fecal calprotectin | endoscopic aspects of inflammation | 0.917 | 0.000 | |
| PD patients | age | fecal calprotectin | 0.393 | 0.032 |
| fecal calprotectin | endoscopic inflammatory lesions | 0.282 | 0.132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisoi, A.; Dobrin, N.; Cozaru, G.-C.; Ionescu, A.-C.; Aschie, M.; Kajanto, L.; Vlad, S.E.; Enciu, M.; Popovici, I.A.; Cîmpineanu, B. Inflammatory Insights: Analysis of a Fecal Biomarker in Neurodegenerative and Gastrointestinal Disorders. Biomedicines 2025, 13, 2411. https://doi.org/10.3390/biomedicines13102411
Chisoi A, Dobrin N, Cozaru G-C, Ionescu A-C, Aschie M, Kajanto L, Vlad SE, Enciu M, Popovici IA, Cîmpineanu B. Inflammatory Insights: Analysis of a Fecal Biomarker in Neurodegenerative and Gastrointestinal Disorders. Biomedicines. 2025; 13(10):2411. https://doi.org/10.3390/biomedicines13102411
Chicago/Turabian StyleChisoi, Anca, Nicolae Dobrin, Georgeta-Camelia Cozaru, Anita-Cristina Ionescu, Mariana Aschie, Lidia Kajanto, Sabina Elena Vlad, Manuela Enciu, Ion Alexandru Popovici, and Bogdan Cîmpineanu. 2025. "Inflammatory Insights: Analysis of a Fecal Biomarker in Neurodegenerative and Gastrointestinal Disorders" Biomedicines 13, no. 10: 2411. https://doi.org/10.3390/biomedicines13102411
APA StyleChisoi, A., Dobrin, N., Cozaru, G.-C., Ionescu, A.-C., Aschie, M., Kajanto, L., Vlad, S. E., Enciu, M., Popovici, I. A., & Cîmpineanu, B. (2025). Inflammatory Insights: Analysis of a Fecal Biomarker in Neurodegenerative and Gastrointestinal Disorders. Biomedicines, 13(10), 2411. https://doi.org/10.3390/biomedicines13102411






