DAC®, a Hyaluronan Derivative in the Form of a Gel, Is Effective in Preventing Periprosthetic Joint Infection During Arthroplasty Revision in Patients with Comorbidities: A Retrospective, Observational, 1:1-Matched Case–Control Clinical Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Enrollment
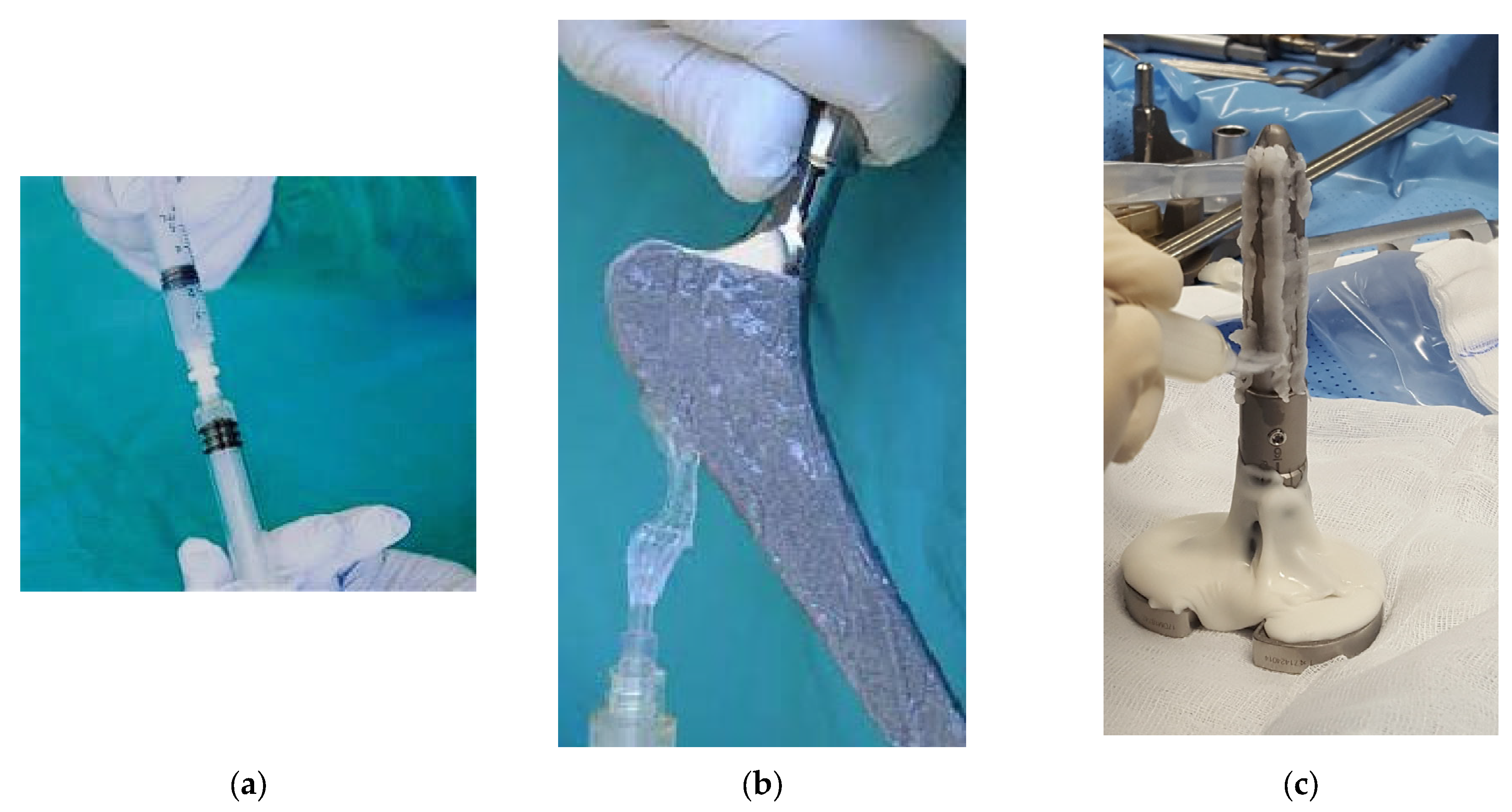
2.2. DAC® Gel Coating and Standard Treatment
2.3. Assessment of PJI
2.4. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics
3.2. PJIs and Adverse Event Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CODP | Chronic Obstructive Pulmonary Disease |
| CDC | Centers for Disease Control |
| DAC® | Defensive Antibacterial Coating |
| DAIR | Debridement, Antibiotics, and Implant Retention |
| F | Female |
| HY | Hyaluronan |
| IAFF | Infection After Fracture Fixation |
| M | Male |
| NS | Not Significant |
| PDLLA | Poly-D, L-Lactic Acid |
| PJIs | Periprosthetic Joint Infections |
| PLA | Poly-Lactic Acid |
| PMMA | Polymethylmethacrylate |
| SD | Standard Deviation |
| SSI | Surgical Site Infection |
| THA | Total Hip Arthroplasty |
| TKA | Total Knee Arthroplasty |
References
- Lustig, S.; Parratte, S.; Magnussen, R.A.; Argenson, J.N.; Neyret, P. Lateral unicompartmental knee arthroplasty relieves pain and improves function in posttraumatic osteoarthritis. Clin. Orthop. Relat. Res. 2012, 470, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Lau, E.C.; Son, M.-S.; Chang, E.T.; Zimmerli, W.; Parvizi, J. Are we winning or losing the battle with periprosthetic joint infection: Trends in periprosthetic joint infection and mortality risk for the Medicare population? J. Arthroplast. 2018, 33, 3238–3245. [Google Scholar] [CrossRef]
- Tsikopoulos, K.; Meroni, G. Periprosthetic Joint Infection Diagnosis: A Narrative Review. Antibiotics 2023, 12, 1485. [Google Scholar] [CrossRef]
- Nelson, S.B.; Pinkney, J.A.; Chen, A.F.; Tande, A.J. Executive Summary: Periprosthetic Joint Infection-Current Clinical Challenges. Clin. Infect. Dis. 2023, 77, 939–940. [Google Scholar] [CrossRef]
- Zeng, Z.J.; Yao, F.M.; He, W.; Wei, Q.S.; He, M.C. Incidence of periprosthetic joint infection after primary total hip arthroplasty is underestimated: A synthesis of meta-analysis and bibliometric analysis. J. Orthop. Surg. Res. 2023, 18, 610. [Google Scholar] [CrossRef]
- European Center for Disease Prevention and Control. Healthcare-associated infections: Surgical site infection. In Annual Epidemiological Report for 2021–2022; ECDC: Stockholm, Sweden, 2025. [Google Scholar]
- Vrancianu, C.O.; Serban, B.; Gheorghe-Barbu, I.; Czobor Barbu, I.; Cristian, R.E.; Chifiriuc, M.C.; Cirstoiu, C. The Challenge of Periprosthetic Joint Infection Diagnosis: From Current Methods to Emerging Biomarkers. Int. J. Mol. Sci. 2023, 24, 4320. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C.; Delgado-Martinez, A.D. Risk Factors for Periprosthetic Joint Infection after Primary Total Knee Arthroplasty. J. Clin. Med. 2022, 11, 6128. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, H.; Chan, P.K.; Fung, W.C.; Fu, H.; Chiu, K.Y. Risk factors associated with surgical site infections following joint replacement surgery: A narrative review. Arthroplasty 2022, 4, 11. [Google Scholar] [CrossRef]
- Sadhwani, S.; Kamson, A.; Frear, A.J.; Sadaka, N.; Urish, K.L. Current Concepts on the Clinical and Economic Impact of Periprosthetic Joint Infections. Orthop. Clin. N. Am. 2024, 55, 151–159. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef] [PubMed]
- Mohler, S.A.; Stambough, J.B.; Mears, S.C.; Kathiresan, A.R.; Barnes, C.L.; Stronach, B.M. Treatment of Hip and Knee Periprosthetic Joint Infection Requires Extensive Administrative Work. J. Arthroplast. 2024, 39, 2898–2903. [Google Scholar] [CrossRef]
- Vasarhelyi, E.M.; Somerville, L.; Barton, K.I.; Howard, J.L.; Lanting, B.A.; Naudie, D.D.R.; McCalden, R.W.; MacDonald, S.J. Survivorship and Outcomes of 2-Stage Revision for Infected Total Hip Arthroplasty at a Mean of 7-Year Follow-Up. J. Arthroplast. 2024, 39, S243–S247. [Google Scholar] [CrossRef]
- Cavagnaro, L.; Chiarlone, F.; Mosconi, L.; Zanirato, A.; Formica, M.; Burastero, G. Two-stage revision for periprosthetic joint infection in unicompartmental knee arthroplasty: Clinical and radiological results. Arch. Orthop. Trauma Surg. 2022, 142, 2031–2038. [Google Scholar] [CrossRef]
- Alrayes, M.M.; Sukeik, M. Two-stage revision in periprosthetic knee joint infections. World J. Orthop. 2023, 14, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hipfl, C.; Leopold, V.; Becker, L.; Pumberger, M.; Perka, C.; Hardt, S. Two-stage revision for periprosthetic joint infection in cemented total hip arthroplasty: An increased risk for failure? Arch. Orthop. Trauma Surg. 2023, 143, 4481–4490. [Google Scholar] [CrossRef]
- Boclé, H.; Lavigne, J.P.; Cellier, N.; Crouzet, J.; Kouyoumdjian, P.; Sotto, A.; Loubet, P. Effectiveness of early switching from intravenous to oral antibiotic therapy in Staphilococcus aureus prosthetic bone and joint or orthopedic metal ware-associated infections. BMC Musculoskelet. Disord. 2021, 22, 315. [Google Scholar] [CrossRef]
- Myers, T.G.; Lipof, J.S.; Chen, A.F.; Ricciardi, B.F. Antibiotic Stewardship for Total Joint Arthroplasty in 2020. J. Am. Acad. Orthop. Surg. 2020, 28, e793–e802. [Google Scholar] [CrossRef]
- Gao, J.; Shu, L.; Jiang, K.; Muheremu, A. Prophylactic use of vancomycin powder on postoperative infection after total joint arthroplasty. BMC Musculoskelet. Disord. 2024, 25, 68. [Google Scholar] [CrossRef] [PubMed]
- Bourget-Murray, J.; Azad, M.; Gofton, W.; Abdelbary, H.; Garceau, S.; Grammatopoulos, G. Is the routine use of local antibiotics in the management of periprosthetic joint infections justified? Hip Int. 2023, 33, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Gasik, M.; Van Mellaert, L.; Pierron, D.; Braem, A.; Hofmans, D.; De Waelheyns, E.; Anne, J.; Harmand, M.F.; Vleugels, J. Reduction of biofilm infection risks and promotion of osteointegration for optimized surfaces of titanium implants. Adv. Healthc. Mater. 2012, 1, 117–127. [Google Scholar] [CrossRef]
- Harris, L.G.; Richardv, R.G. Staphylococci and implant surfaces: A review. Injury 2006, 37 (Suppl. 2), S3–S14. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Urish, K.L.; DeMuth, P.W.; Kwan, B.W.; Craft, D.W.; Ma, D.; Haider, H.; Tuan, R.S.; Wood, T.K.; Davis, C.M., 3rd. Antibiotic-tolerant Staphilococcus aureus Biofilm Persists on Arthroplasty Materials. Clin. Orthop. Relat. Res. 2016, 474, 1649–1656. [Google Scholar] [CrossRef]
- Yassin, M.; Sharma, V.; Butt, F.; Iyer, S.; Tayton, E. Early Peri-Prosthetic Joint Infection after Hemiarthroplasty for Hip Fracture: Outcomes of Debridement, Antibiotics, and Implant Retention. Surg. Infect. 2020, 21, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Steadman, W.; Chapman, P.R.; Schuetz, M.; Schmutz, B.; Trampuz, A.; Tetsworth, K. Local Antibiotic Delivery Options in Prosthetic Joint Infection. Antibiotics 2023, 12, 752. [Google Scholar] [CrossRef]
- Buchholz, H.; Elson, R.; Engelbrecht, E.; Lodenkamper, H.; Rottger, J.; Siegel, A. Management of deep infection of total hip replacement. J. Bone Jt. Surg. Br. 1981, 63, 342–353. [Google Scholar] [CrossRef]
- Lunz, A.; Schonhoff, M.; Omlor, G.W.; Knappe, K.; Bangert, Y.; Lehner, B.; Renkawitz, T.; Jaeger, S. Enhanced antibiotic release from bone cement spacers utilizing dual antibiotic loading with elevated vancomycin concentrations in two-stage revision for periprosthetic joint infection. Int. Orthop. 2023, 47, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Z.; Lin, Y.; Cai, Y.; Zhang, X.; Huang, Z.; Huang, Y.; Li, W.; Fang, X.; Zhang, W. Patient-Reported Outcome on Quality of Life and Pain after Revision Arthroplasty for Periprosthetic Joint Infection: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 7182. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.K.; Gooberman-Hill, R.; Blom, A.W.; Whitehouse, M.R.; Moore, A.J. Post-surgery and recovery experiences following one- and two-stage revision for prosthetic joint infection-A qualitative study of patients’ experiences. PLoS ONE 2020, 15, e0237047. [Google Scholar] [CrossRef]
- Kildow, B.J.; Springer, B.D.; Brown, T.S.; Lyden, E.; Fehring, T.K.; Garvin, K.L. Long Term Results of Two-Stage Revision for Chronic Periprosthetic Hip Infection: A Multicenter Study. J. Clin. Med. 2022, 11, 1657. [Google Scholar] [CrossRef]
- Leta, T.H.; Fenstad, A.M.; Lygre, S.H.L.; Lie, S.A.; Lindberg-Larsen, M.; Pedersen, A.B.; W-Dahl, A.; Rolfson, O.; Bülow, E.; Ashforth, J.A.; et al. The use of antibiotic-loaded bone cement and systemic antibiotic prophylactic use in 2,971,357 primary total knee arthroplasties from 2010 to 2020: An international register-based observational study among countries in Africa; Europe; North America; and Oceania. Acta Orthop. 2023, 94, 416–425. [Google Scholar] [CrossRef]
- Chan, V.W.; Chan, P.K.; Fu, H.; Cheung, M.H.; Cheung, A.; Yan, C.H.; Chiu, K.Y. Preoperative optimization to prevent periprosthetic joint infection in at-risk patients. J. Orthop. Surg. 2020, 28, 2309499020947207. [Google Scholar] [CrossRef]
- de Lachica, J.C.V.; Reyes, S.S.S.; Ureña, J.A.P.; Fragoso, M.A.R. Decrease in acute periprosthetic joint infections incidence with vancomycin-loaded calcium sulphate beads in patients with non-modifiable risk factors. A randomized clinical trial. J. ISAKOS 2022, 7, 201–205. [Google Scholar] [CrossRef]
- Overstreet, D.; McLaren, A.; Calara, F.; Vernon, B.; McLemore, R. Local gentamicin delivery from resorbable viscous hydrogels is therapeutically effective. Clin. Orthop. Relat. Res. 2015, 473, 337–347. [Google Scholar] [CrossRef] [PubMed]
- De Meo, D.; Martini, P.; Pennarola, M.F.; Guarascio, G.; Rivano Capparuccia, M.; Iaiani, G.; Candela, V.; Gumina, S.; Villani, C. Hydrogel Coating versus Calcium Sulphate Beads as a Local Antibiotic Carrier for Debridement Procedures in Acute Periprosthetic Joint Infection: A Preliminary Study. Gels 2023, 9, 758. [Google Scholar] [CrossRef]
- Romanò, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; Van Der Straeten, C.; Scarponi, S.; Drago, L. Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection After Joint Arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar] [CrossRef]
- Pitarresi, G.; Palumbo, F.S.; Calascibetta, F.; Fiorica, C.; Di Stefano, M.; Giammona, G. Medicated hydrogels of hyaluronic acid derivatives for use in orthopedic field. Int. J. Pharm. 2013, 449, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Boot, W.; Dimas, K.; Malizos, K.; Hänsch, G.M.; Stuyck, J.; Gawlitta, D.; Romanò, C.L. Does Implant Coating With Antibacterial-Loaded Hydrogel Reduce Bacterial Colonization and Biofilm Formation in Vitro? Clin. Orthop. Relat. Res. 2014, 472, 3311–3323. [Google Scholar] [CrossRef] [PubMed]
- Zagra, L.; Gallazzi, E.; Romanò, D.; Scarponi, S.; Romanò, C. Two-stage cementless hip revision for peri-prosthetic infection with an antibacterial hydrogel coating: Results of a comparative series. Int. Orthop. 2019, 43, 111–115. [Google Scholar] [CrossRef]
- Malizos, K.; Blauth, M.; Danita, A.; Capuano, N.; Mezzoprete, R.; Logoluso, N.; Drago, L.; Romanò, C.L. Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: A multicenter randomized controlled trial. J. Orthop. Traumatol. 2017, 18, 159–169. [Google Scholar] [CrossRef]
- Tan, T.L.; Maltenfort, M.G.; Chen, A.F.; Shahi, A.; Higuera, C.A.; Siqueira, M.; Parvizi, J. Development and Evaluation of a Preoperative Risk Calculator for Periprosthetic Joint Infection Following Total Joint Arthroplasty. J. Bone Jt. Surg. Am. 2018, 100, 777–785. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314. [Google Scholar] [CrossRef]
- Berrios-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Szymski, D.; Rupp, M.; Fontalis, A.; Vaznaisiene, D.; Marais, L.C.; Wagner, C.; Walter, N.; Alt, V.; Clauss, M.; et al. The health-economic burden of hip and knee periprosthetic joint infections in Europe: A comprehensive analysis following primary arthroplasty. Bone Jt. Open 2025, 6, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G.; Naylor, P.; Myrvik, Q. Infections from biomaterials and implants: A race for the surface. Med. Prog. Technol. 1988, 14, 205–224. [Google Scholar] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Eka, A.; Chen, A.F. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann. Transl. Med. 2015, 3, 233. [Google Scholar] [CrossRef]
- Mu, W.; Baochao, J.; Cao, L. Single-stage revision for chronic periprosthetic joint infection after knee and hip arthroplasties: Indications and treatments. Arthroplasty 2023, 5, 11. [Google Scholar] [CrossRef]
- Alamanda, V.K.; Springer, B.D. Perioperative and Modifiable Risk Factors for Periprosthetic Joint Infections (PJI) and Recommended Guidelines. Curr. Rev. Musculoskelet. Med. 2018, 11, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Whitehouse, M.R.; Blom, A.W.; Beswick, A.D.; INFORM Team. Patient-Related Risk Factors for Periprosthetic Joint Infection after Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150866. [Google Scholar] [CrossRef]
- Levack, A.E.; Cyphert, E.L.; Bostrom, M.P.; Hernandez, C.J.; von Recum, H.A.; Carli, A.V. Current Options and Emerging Biomaterials for Periprosthetic Joint Infection. Curr. Rheumatol. Rep. 2018, 30, 33. [Google Scholar] [CrossRef]
- Romanò, C.L.; Tsuchiya, H.; Morelli, I.; Battaglia, A.G.; Drago, L. Antibacterial Coating of Implants: What Surgeons Should Know Antibacterial Coating of Implants. Mo J. Eur. 2022, 8, 14–23. [Google Scholar]
- Trentinaglia, M.T.; Van Der Straeten, C.; Morelli, I.; Logoluso, N.; Drago, L.; Romanò, C.L. Economic Evaluation of Antibacterial Coatings on Healthcare Costs in First Year Following Total Joint Arthroplasty. J. Arthroplast. 2018, 33, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Pacha Olivenza, M.A.; García Blanco, M.B.; Díaz-Fuentes, M.; Vilas-Vilela, J.L. Antibacterial hyaluronic acid/chitosan multilayers onto smooth and micropatterned titanium surfaces. Carbohydr. Polym. 2019, 207, 824–833. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Hook, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphilococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Parbonetti, G.; Puglisi, A.; La Maida, E.; Rizzo, B.; Granata, R. Antibiotic-Loaded Hydrogel Coating for the Prevention of Local Infection after Vertebral Surgery: A Retrospective Cohort Analysis. Surg. Technol. Int. 2021, 39, 441–446. [Google Scholar] [CrossRef]
- Zoccali, C.; Scoccianti, G.; Biagini, R.; Daolio, P.A.; Giardina, F.L.; Campanacci, D.A. Antibacterial hydrogel coating in joint mega-prosthesis: Results of a comparative series. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1647–1655. [Google Scholar] [CrossRef]
| Variables | DAC® Group n = 96 | Control Group n = 96 | p-Value |
|---|---|---|---|
| Basic data | |||
| Age, years, median (IQR) | 63 (12.5) | 63 (9) | 0.7898 ⴕ |
| Gender, n male/female | 82/68 | 82/68 | 1 * |
| Body mass index (kg/m2), median (IQR) | 25.6 (3.3) | 25.7 (3.1) | 0.7307 ⴕ |
| Prevalent comorbidities n (%) | |||
| Smoke | 19 (19.8%) | 8 (8.3%) | 0.0224 * |
| Diabetes | 18 (18.8%) | 13 (13.5%) | 0.3268 * |
| Obesity | 16 (16.7%) | 10 (10.4%) | 0.2057 * |
| Cardiovascular disease | 10 (10.4%) | 10(10.4%) | 1 * |
| Hypertension | 7 (7.3%) | 7 (7.3%) | 1 * |
| CODP | 7 (7.3%) | 9 (9.4%) | 0.6015 * |
| Risk factor, mean (SD) | 3.8 ± 2.3 | 4.5 ± 3.1 | 0.0768 ⴕⴕ |
| Revision n (%) | |||
| Hip | 78 (81.3%) | 78 (81.3%) | 1 * |
| Knee | 18 (18.8%) | 18 (18.8%) | 1 * |
| Septic | 5 (5.2%) | 4 (4.2%) | 0.7328 * |
| Aseptic | 91 (94.7%) | 92 (95.8%) | 0.7328 * |
| Length of surgery (min.), median (IQR) | 80 (20) | 90 (15) | 0.0001 ⴕ |
| Variables | DAC® Group n = 96 | Control Group n = 96 | p-Value |
|---|---|---|---|
| 3-month follow-up | |||
| Follow-up (days), median (IQR) | 95 (12) | 105 (4) | 0.0001 ⴕ |
| Infection n (%) | 0 | 5 (5.2%) | 0.0235 * |
| Pathogen | |||
| Methicillin-Susceptible S. aureus (MSSA) | - | 3 | |
| S. Epidermidis | - | 1 | |
| Methicillin-Resistant S. Aureus (MRSA) | - | 1 | |
| Onset of infection (days), mean (SD) | 54 ± 14 | ||
| Surgical wound | |||
| Regular | 92 (95.8%) | 89 (93%) | 0.3515 * |
| Dehiscence | 0 | 3 (3.1%) | 0.0809 * |
| Exudate | 1 (1%) | 0 | 0.3160 * |
| Swelling | 0 | 2 (2.1%) | 0.1551 * |
| Redness | 2 (2.1%) | 1 (1%) | 0.5606 * |
| Delayed wound healing | 0 | 1 (1.3%) | 0.3160 * |
| Requiring advanced wound medication | 1 (1%) | 0 | 0.3160 * |
| Other complications, n (%) | |||
| DAIR | 0 | 2 (2.1%) | 0.1551 * |
| Prolonged antibiotic therapy | 0 | 5 (5.2%) | 0.0235 * |
| 6 m follow-up | |||
| Follow-up (days), median (IQR) | 182.5 (14) | 192 (3.5) | 0.0001 ⴕ |
| Surgical wound | |||
| Regular | 96 (100%) | 96 (100%) | 1 * |
| Other complications, n (%) | |||
| Revision (implant removal) | 0 | 3 (3.1%) | 0.0809 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, G.; Giuliani, G.; Armando, A.; Quitadamo, R.; Demita, R.; Stigliani, C. DAC®, a Hyaluronan Derivative in the Form of a Gel, Is Effective in Preventing Periprosthetic Joint Infection During Arthroplasty Revision in Patients with Comorbidities: A Retrospective, Observational, 1:1-Matched Case–Control Clinical Investigation. Biomedicines 2025, 13, 2408. https://doi.org/10.3390/biomedicines13102408
Ricciardi G, Giuliani G, Armando A, Quitadamo R, Demita R, Stigliani C. DAC®, a Hyaluronan Derivative in the Form of a Gel, Is Effective in Preventing Periprosthetic Joint Infection During Arthroplasty Revision in Patients with Comorbidities: A Retrospective, Observational, 1:1-Matched Case–Control Clinical Investigation. Biomedicines. 2025; 13(10):2408. https://doi.org/10.3390/biomedicines13102408
Chicago/Turabian StyleRicciardi, Giuseppe, Giancarlo Giuliani, Arminio Armando, Raffaele Quitadamo, Rosario Demita, and Costantino Stigliani. 2025. "DAC®, a Hyaluronan Derivative in the Form of a Gel, Is Effective in Preventing Periprosthetic Joint Infection During Arthroplasty Revision in Patients with Comorbidities: A Retrospective, Observational, 1:1-Matched Case–Control Clinical Investigation" Biomedicines 13, no. 10: 2408. https://doi.org/10.3390/biomedicines13102408
APA StyleRicciardi, G., Giuliani, G., Armando, A., Quitadamo, R., Demita, R., & Stigliani, C. (2025). DAC®, a Hyaluronan Derivative in the Form of a Gel, Is Effective in Preventing Periprosthetic Joint Infection During Arthroplasty Revision in Patients with Comorbidities: A Retrospective, Observational, 1:1-Matched Case–Control Clinical Investigation. Biomedicines, 13(10), 2408. https://doi.org/10.3390/biomedicines13102408






