Amyotrophic Lateral Sclerosis Patients Show Higher Urinary Levels of Lead and Copper: A Pilot Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
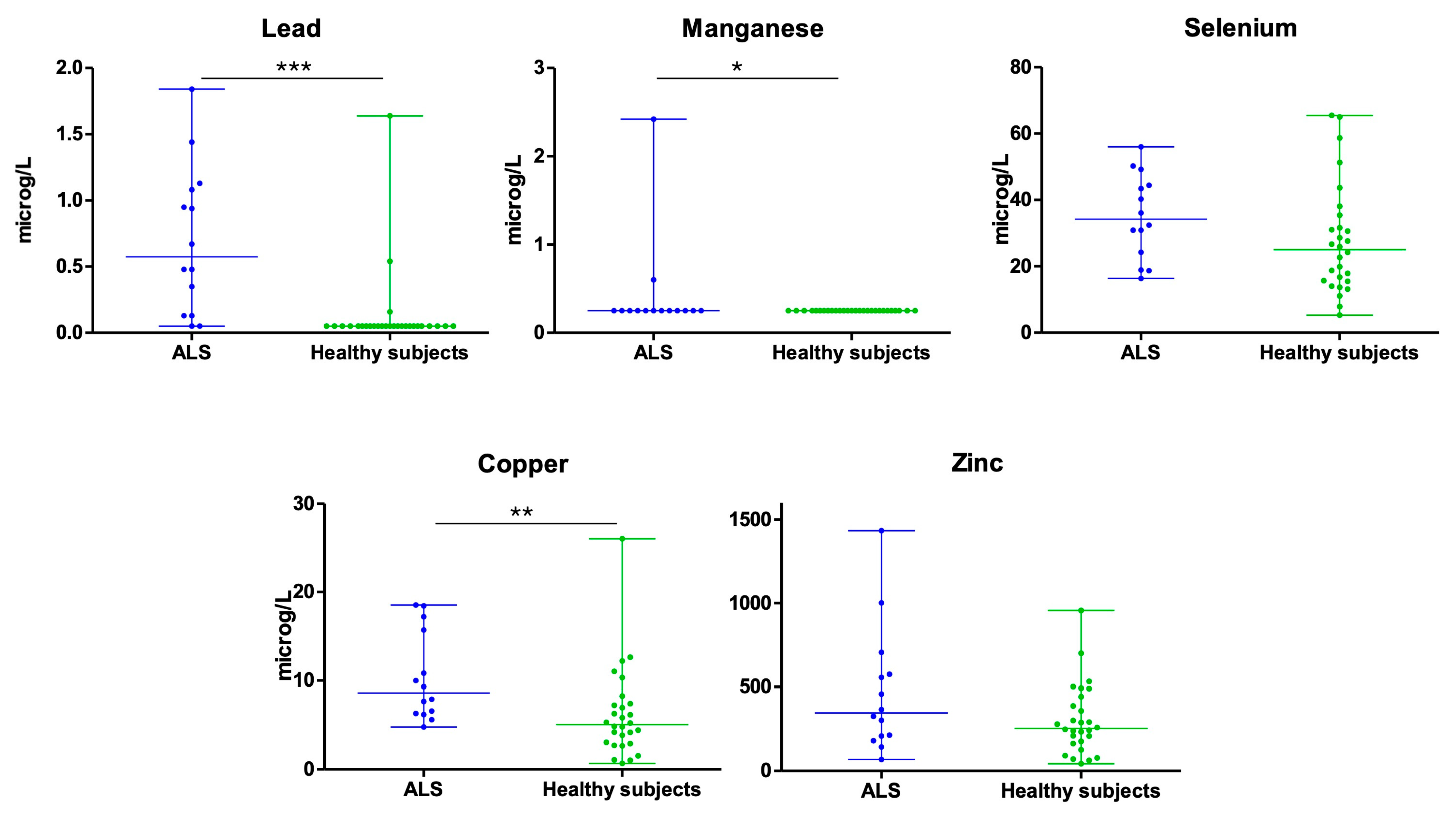
3.1. Lead (Pb)
3.2. Manganese (Mn)
3.3. Selenium (Se)
3.4. Copper (Cu)
3.5. Zinc (Zn)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic lateral sclerosis |
| ALSFRS-R | Revised ALS functional rating scale |
| Al | Aluminium |
| Cu | Copper |
| fALS | Familial Amyotrophic Lateral Sclerosis |
| HCs | Healthy controls |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| Pb | Lead |
| Mg | Magnesium |
| Mn | Manganese |
| P25–P75 | Percentile 25–Percentile 75 |
| sALS | Sporadic Amyotrophic Lateral Sclerosis |
| SD | Standard deviation |
| Se | Selenium |
| Zn | Zinc |
References
- Zufiría, M.; Gil-Bea, F.J.; Fernández-Torrón, R.; Poza, J.J.; Muñoz-Blanco, J.L.; Rojas-García, R.; Riancho, J.; de Munain, A.L. ALS: A bucket of genes, environment, metabolism and unknown ingredients. Prog. Neurobiol. 2016, 142, 104–129. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, E.; Rajasekaran, R. A Systematic and Comprehensive Review on Disease-Causing Genes in Amyotrophic Lateral Sclerosis. J. Mol. Neurosci. 2020, 70, 1742–1770. [Google Scholar] [CrossRef]
- Riancho, J.; Bosque-Varela, P.; Perez-Pereda, S.; Povedano, M.; de Munaín, A.L.; Santurtun, A. The increasing importance of environmental conditions in amyotrophic lateral sclerosis. Int. J. Biometeorol. 2018, 62, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Imam, I.; Ball, S.; Wright, D.; Hanemann, C.O.; Zajicek, J. The epidemiology of motor neurone disease in two counties in the southwest of England. J. Neurol. 2010, 257, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Logroscino, G.; Traynor, B.J.; Hardiman, O.; Chió, A.; Mitchell, D.; Swingler, R.J.; Millul, A.; Benn, E.; Beghi, E. Incidence of amyotrophic lateral sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry 2010, 81, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, A.; Fang, F.; Hanby, M.F.; Leigh, P.N.; Shaw, C.E.; Ye, W.; Rijsdijk, F. An estimate of amyotrophic lateral sclerosis heritability using twin data. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1324–1326. [Google Scholar] [CrossRef]
- Arthur, K.C.; Calvo, A.; Price, T.R.; Geiger, J.T.; Chiò, A.; Traynor, B.J. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat. Commun. 2016, 7, 12408. [Google Scholar] [CrossRef]
- Riancho, J.; Lozano-Cuesta, P.; Santurtún, A.; Sánchez-Juan, P.; López-Vega, J.M.; Berciano, J.; Polo, J.M. Amyotrophic lateral sclerosis in northern Spain 40 Years later: What has changed? Neurodegener. Dis. 2016, 16, 337–341. [Google Scholar] [CrossRef]
- Riancho, J.; Delgado-Alvarado, M.; Andreu, M.D.; Paz-Fajardo, L.; Arozamena, S.; Gil-Bea, F.J.; López de Munaín, A. Amyotrophic lateral sclerosis (ALS), cancer, autoimmunity and metabolic disorders: An unsolved tantalizing challenge. Br. J. Pharmacol. 2021, 178, 1269–1278. [Google Scholar] [CrossRef]
- Alonso, A.; Logroscino, G.; Hernán, M.A. Smoking and the risk of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1249–1252. [Google Scholar] [CrossRef]
- Miller, Z.A.; Sturm, V.E.; Balci Camsari, G.; Karydas, A.; Yokoyama, J.S.; Grinberg, L.T.; Boxer, A.L.; Rosen, H.J.; Rankin, K.P.; Gorno-Tempini, M.L.; et al. Increased prevalence of au-toimmune disease within C9 and FTD/MND cohorts Completing the picture. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e301. [Google Scholar] [CrossRef] [PubMed]
- Parvanovova, P.; Hnilicova, P.; Kolisek, M.; Tatarkova, Z.; Halasova, E.; Kurca, E.; Holubcikova, S.; Koprusakova, M.T.; Baranovicova, E. Disturbances in Muscle Energy Metabolism in Patients with Amyotrophic Lateral Sclerosis. Metabolites 2024, 14, 356. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Yadav, R.; Vishwakarma, R.K.; Shekhar, S.; Pathak, A.; Singh, C. An Integrative Analysis of Metagenomic and Metabolomic Profiling Reveals Gut Microbiome Dysbiosis and Metabolic Alterations in ALS: Potential Biomarkers and Therapeutic Insights. ACS Chem. Neurosci. 2025, 16, 2691–2706. [Google Scholar] [CrossRef] [PubMed]
- Saucier, D.; Registe, P.P.W.; Bélanger, M.; O’Connell, C. Urbanization, air pollution, and water pollution: Identification of potential environmental risk factors associated with amyotrophic lateral sclerosis using systematic reviews. Front. Neurol. 2023, 14, 1108383. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Tesauro, M.; Fiore, M.; Malagoli, C.; Consonni, M.; Violi, F.; Iacuzio, L.; Arcolin, E.; Oliveri Conti, G.; Cristaldi, A.; et al. Environmental and occupational risk factors of amyotrophic lateral sclerosis: A population-based case-control study. Int. J. Environ. Res. Public Health 2020, 17, 2882. [Google Scholar] [CrossRef]
- Wu, F.; Malek, A.M.; Buchanich, J.M.; Arena, V.C.; Rager, J.R.; Sharma, R.K.; Vena, J.E.; Bear, T.; Talbott, E.O. Exposure to ambient air toxicants and the risk of amyotrophic lateral sclerosis (ALS): A matched case control study. Environ. Res. 2024, 242, 117719. [Google Scholar] [CrossRef]
- Malek, A.M.; Arena, V.C.; Song, R.; Whitsel, E.A.; Rager, J.R.; Stewart, J.; Yanosky, J.D.; Liao, D.; Talbott, E.O. Long-term air pollution and risk of amyotrophic lateral sclerosis mortality in the Women’s Health Initiative cohort. Environ. Res. 2023, 216, 114510. [Google Scholar] [CrossRef]
- Zhang, G.; E, M.; Zhou, X. Environmental and Occupational solvents exposure and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Neurol. Sci. 2023, 44, 2803–2809. [Google Scholar] [CrossRef]
- Li, B.; Xia, M.; Zorec, R.; Parpura, V.; Verkhratsky, A. Astrocytes in heavy metal neurotoxicity and neurodegeneration. Brain Res. 2021, 1752, 147234. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Ifenatuoha, C.W.; Aluko, O.M.; Ijomone, O.K.; Aschner, M. The aging brain: Impact of heavy metal neurotoxicity. Crit. Rev. Toxicol. 2020, 50, 801–814. [Google Scholar] [CrossRef]
- Roberts, A.L.; Johnson, N.J.; Cudkowicz, M.E.; Eum, K.D.; Weisskopf, M.G. Job-related formaldehyde exposure and ALS mortality in the USA. J. Neurol. Neurosurg. Psychiatry 2016, 87, 786–788. [Google Scholar] [CrossRef]
- Pupillo, E.; Bianchi, E.; Chiò, A.; Casale, F.; Zecca, C.; Tortelli, R.; Beghi, E.; SLALOM Group; PARALS Group; SLAP Group. Amyotrophic lateral sclerosis and food intake. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 267–274. [Google Scholar] [CrossRef]
- Sánchez-Díaz, G.; Escobar, F.; Badland, H.; Arias-Merino, G.; Posada de la Paz, M.; Alonso-Ferreira, V. Geographic Analysis of Motor Neuron Disease Mortality and Heavy Metals Released to Rivers in Spain. Int. J. Environ. Res. Public Health 2018, 15, 2522. [Google Scholar] [CrossRef]
- Saucier, D.; Bélanger, M.; Liu, Z.; Lavigne, E.; O’Connell, C. Associations between long-term air pollution exposure and the development of amyotrophic lateral sclerosis: A matched case-control study. Environ. Res. 2025, 284, 122232. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhang, X.; Zhao, X.; Chang, H.; Zhang, J.; Gao, Z.; Mi, Y.; Chen, Y.; Zhang, H.; Huang, C.; et al. Global ambient particulate matter pollution and neurodegenerative disorders: A systematic review of literature and meta-analysis. Environ. Sci. Pollut. Res. Int. 2023, 30, 39418–39430. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Govoni, V.; Brancaleoni, L.; Donà, A.; Granieri, E.; Bergamini, M.; Gerdol, R.; Pugliatti, M. Amyotrophic Lateral Sclerosis and Air Pollutants in the Province of Ferrara, Northern Italy: An Ecological Study. Int. J. Environ. Res. Public Health 2023, 20, 5591. [Google Scholar] [CrossRef] [PubMed]
- Santurtún, A.; Villar, A.; Delgado-Alvarado, M.; Riancho, J. Trends in motor neuron disease: Association with latitude and air lead levels in Spain. Neurol. Sci. 2016, 37, 1271–1275. [Google Scholar] [CrossRef]
- Violi, F.; Solovyev, N.; Vinceti, M.; Mandrioli, J.; Lucio, M.; Michalke, B. The study of levels from redox-active elements in cerebrospinal fluid of amyotrophic lateral sclerosis patients carrying disease-related gene mutations shows potential copper dyshomeostasis. Metallomics 2020, 12, 668–681. [Google Scholar] [CrossRef]
- Royce-Nagel, G.; Cudkowicz, M.; Myers, D.; Nicholson, K.; Shui, A.; Schoenfeld, D.; Huang, X.; Brown, R.H., Jr. Vanadium, aluminum, magnesium and manganese are not elevated in hair samples in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010, 11, 492–493. [Google Scholar] [CrossRef]
- Farace, C.; Fiorito, G.; Pisano, A.; Etzi, F.; Sabalic, A.; Fenu, G.; Asara, Y.; Solinas, G.; Madeddu, R. Human tissue lead (Pb) levels and amyotrophic lateral sclerosis: A systematic review and meta-analysis of case-control studies. Neurol. Sci. 2022, 43, 5851–5859. [Google Scholar] [CrossRef]
- Bocca, B.; Forte, G.; Oggiano, R.; Clemente, S.; Asara, Y.; Peruzzu, A.; Farace, C.; Pala, S.; Fois, A.G.; Pirina, P.; et al. Level of neurotoxic metals in amyotrophic lateral scle-rosis: A population-based case-control study. J. Neurol. Sci. 2015, 359, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.G.; Dou, J.F.; Koubek, E.J.; Teener, S.; Zhou, L.; Bakulski, K.M.; Mukherjee, B.; Batterman, S.A.; Feldman, E.L.; Goutman, S.A.; et al. Multiple metal exposures associate with higher amyotrophic lateral sclerosis risk and mortality independent of genetic risk and correlate to self-reported exposures: A case-control study. J. Neurol. Neurosurg. Psychiatry 2024, 96, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Chen, W.Z.; Li, C.; Xu, R.S. Current potential pathogenic mechanisms of copper-zinc superoxide dismutase 1 (SOD1) in amyotrophic lateral sclerosis. Rev. Neurosci. 2024, 35, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, A.; Foroughmand, I.; Koski, L.; Darvish, M.; Saghazadeh, A.; Kamalian, A.; Razavi, S.Z.E.; Abdi, S.; Dehgolan, S.R.; Fotouhi, A.; et al. Metal concentrations in cerebrospinal fluid, blood, serum, plasma, hair, and nails in amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2023, 78, 127165. [Google Scholar] [CrossRef]
- Barbeito, A.G.; Martinez-Palma, L.; Vargas, M.R.; Pehar, M.; Mañay, N.; Beckman, J.S.; Barbeito, L.; Cassina, P. Lead exposure stimulates VEGF ex-pression in the spinal cord and extends survival in a mouse model of ALS. Neurobiol. Dis. 2010, 37, 574–580. [Google Scholar] [CrossRef]
- Giampietro, R.; Spinelli, F.; Contino, M.; Colabufo, N.A. The Pivotal Role of Copper in Neurodegeneration: A New Strategy for the Therapy of Neurodegenerative Disorders. Mol. Pharm. 2018, 15, 808–820. [Google Scholar] [CrossRef]
- Riancho, J.; Gonzalo, I.; Ruiz-Soto, M.; Berciano, J. Why do motor neurons degenerate? Actualization in the pathogenesis of amyotrophic lateral sclerosis. Neurologia 2019, 34, 27–37. [Google Scholar] [CrossRef]
- Kamel, F.; Umbach, D.M.; Hu, H.; Munsat, T.L.; Shefner, J.M.; Taylor, J.A.; Sandler, D.P. Lead exposure as a risk factor for amyotrophic lateral sclerosis. Neurodegener. Dis. 2005, 2, 195–201. [Google Scholar] [CrossRef]
- Baj, J.; Flieger, W.; Barbachowska, A.; Kowalska, B.; Flieger, M.; Forma, A.; Teresiński, G.; Portincasa, P.; Buszewicz, G.; Radzikowska-Büchner, E.; et al. Consequences of Disturbing Manganese Homeostasis. Int. J. Mol. Sci. 2023, 24, 14959. [Google Scholar] [CrossRef]
- Vinceti, M.; Guidetti, D.; Pinotti, M.; Rovesti, S.; Merlin, M.; Vescovi, L.; Bergomi, M.; Vivoli, G. Amyotrophic lateral sclerosis after long-term exposure to drinking water with high selenium content. Epidemiology 1996, 7, 529–532. [Google Scholar] [CrossRef]
- Lv, Y.; Li, H. Blood diagnostic and prognostic biomarkers in amyotrophic lateral sclerosis. Neural Regen. Res. 2025, 20, 2556–2570. [Google Scholar] [CrossRef]
- Sata, F.; Araki, S.; Yokoyama, K.; Murata, K. Adjustment of creatinine-adjusted values in urine to urinary flow rate: A study of eleven heavy metals and organic substances. Int. Arch. Occup. Environ. Health 1995, 68, 64–68. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Wang, S.L.; Fadrowski, J.J.; Navas-Acien, A.; Kuo, C.C. Urinary Concentration Correction Methods for Arsenic, Cadmium, and Mercury: A Systematic Review of Practice-Based Evidence. Curr. Environ. Health Rep. 2019, 6, 188–199. [Google Scholar] [CrossRef]
- Anual, Z.F.; Mohammad Sham, N.; Ambak, R.; Othman, F.; Shaharudin, R. Urinary Concentrations of Metals and Metalloids in Malaysian Adults. Expo. Health 2021, 13, 391–401. [Google Scholar] [CrossRef]
- Middleton, D.R.S.; Watts, M.J.; Polya, D.A. A comparative assessment of dilution correction methods for spot urinary analyte concentrations in a UK population exposed to arsenic in drinking water. Environ. Int. 2019, 130, 104721. [Google Scholar] [CrossRef]
- Kuiper, M.M.; Kruithof, W.J.; Broekman-Peters, N.; Schröder-van den Nieuwendijk, D.L.; Visser-Meily, J.M.A.; Beelen, A. Nutritional care practices in ALS: Perspectives of healthcare professionals and people with ALS. Amyotroph. Lateral Scler. Front. Degener. 2025, 26, 452–466. [Google Scholar] [CrossRef] [PubMed]
| Sex | Age | Type of ALS | ALSFRS-R at Sampling | Environment | |
|---|---|---|---|---|---|
| Patients with ALS | |||||
| Patient 1 | Female | 51 | Spinal | 11 | Urban |
| Patient 2 | Male | 62 | Spinal | 45 | Urban |
| Patient 3 | Male | 84 | Spinal | 13 | Rural |
| Patient 4 | Female | 75 | Bulbar | 26 | Urban |
| Patient 5 | Male | 71 | Spinal | 45 | Urban |
| Patient 6 | Male | 67 | Spinal | 33 | Rural |
| Patient 7 | Male | 81 | Spinal | 44 | Rural |
| Patient 8 | Male | 62 | Spinal | 41 | Urban |
| Patient 9 | Female | 68 | Spinal | 43 | Rural |
| Patient 10 | Female | 69 | Spinal | 37 | Urban |
| Patient 11 | Female | 69 | Bulbar | 30 | Urban |
| Patient 12 | Female | 82 | Spinal | 39 | Rural |
| Patient 13 | Male | 69 | Spinal | 33 | Rural |
| Patient 14 | Female | 65 | Spinal | 17 | Urban |
| Healthy controls (HC) | |||||
| HC 1 | Female | 64 | - | - | Urban |
| HC 2 | Male | 67 | - | - | Urban |
| HC 3 | Female | 61 | - | - | Urban |
| HC 4 | Female | 69 | - | - | Urban |
| HC 5 | Male | 70 | - | - | Urban |
| HC 6 | Male | 72 | - | - | Urban |
| HC 7 | Female | 71 | - | - | Urban |
| HC 8 | Male | 64 | - | - | Rural |
| HC 9 | Female | 84 | - | - | Rural |
| HC 10 | Female | 69 | - | - | Rural |
| HC 11 | Female | 77 | - | - | Rural |
| HC 12 | Female | 62 | - | - | Rural |
| HC 13 | Male | 63 | - | - | Rural |
| HC 14 | Male | 69 | - | - | Rural |
| HC 15 | Female | 62 | - | - | Rural |
| HC 16 | Male | 68 | - | - | Rural |
| HC 17 | Male | 80 | - | - | Rural |
| HC 18 | Female | 74 | - | - | Urban |
| HC 19 | Female | 75 | - | - | Urban |
| HC 20 | Male | 66 | - | - | Urban |
| HC 21 | Male | 65 | - | - | Urban |
| HC 22 | Female | 68 | - | - | Urban |
| HC 23 | Female | 62 | - | - | Urban |
| HC 24 | Male | 63 | - | - | Urban |
| HC 25 | Male | 67 | - | - | Urban |
| HC 26 | Male | 92 | - | - | Urban |
| HC 27 | Female | 84 | - | - | Rural |
| HC 28 | Male | 66 | - | - | Rural |
| Pb | Mn | Se | Cu | Zn | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ALS | HC | ALS | HC | ALS | HC | ALS | HC | ALS | HC | |
| Average (μg/L) | 0.69 | 0.13 | 0.43 | 0.25 | 35.14 | 27.73 | 10.36 | 6.18 | 467.28 | 302.73 |
| Median (μg/L) | 0.58 | 0.05 | 0.25 | 0.25 | 34.25 | 25.05 | 8.62 | 5.06 | 346.36 | 253.59 |
| P25 (percentile 25) | 0.13 | 0.05 | 0.25 | 0.25 | 22.88 | 15.55 | 6.28 | 2.94 | 201.53 | 166.89 |
| P75 (percentile 25) | 1.09 | 0.05 | 0.25 | 0.25 | 45.6 | 34.45 | 16.09 | 7.37 | 607.53 | 428.46 |
| Number of samples below detection limit | 2 | 25 | 12 | 28 | 0 | 0 | 0 | 0 | 0 | 0 |
| Test Statistic | 49.00 | 168.00 | 1.48 | 94.50 | 162 | |||||
| p-value | <0.001 | 0.043 | 0.146 | 0.007 | 0.835 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santurtún, A.; Pérez-Soberón, L.; Sedano, M.J.; Riancho, J. Amyotrophic Lateral Sclerosis Patients Show Higher Urinary Levels of Lead and Copper: A Pilot Case-Control Study. Biomedicines 2025, 13, 2385. https://doi.org/10.3390/biomedicines13102385
Santurtún A, Pérez-Soberón L, Sedano MJ, Riancho J. Amyotrophic Lateral Sclerosis Patients Show Higher Urinary Levels of Lead and Copper: A Pilot Case-Control Study. Biomedicines. 2025; 13(10):2385. https://doi.org/10.3390/biomedicines13102385
Chicago/Turabian StyleSanturtún, Ana, Lucía Pérez-Soberón, María José Sedano, and Javier Riancho. 2025. "Amyotrophic Lateral Sclerosis Patients Show Higher Urinary Levels of Lead and Copper: A Pilot Case-Control Study" Biomedicines 13, no. 10: 2385. https://doi.org/10.3390/biomedicines13102385
APA StyleSanturtún, A., Pérez-Soberón, L., Sedano, M. J., & Riancho, J. (2025). Amyotrophic Lateral Sclerosis Patients Show Higher Urinary Levels of Lead and Copper: A Pilot Case-Control Study. Biomedicines, 13(10), 2385. https://doi.org/10.3390/biomedicines13102385






