Electrospun Polycaprolactone Membranes Loaded with Gentamicin and Nano-Hidroxyapatite for Guided Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
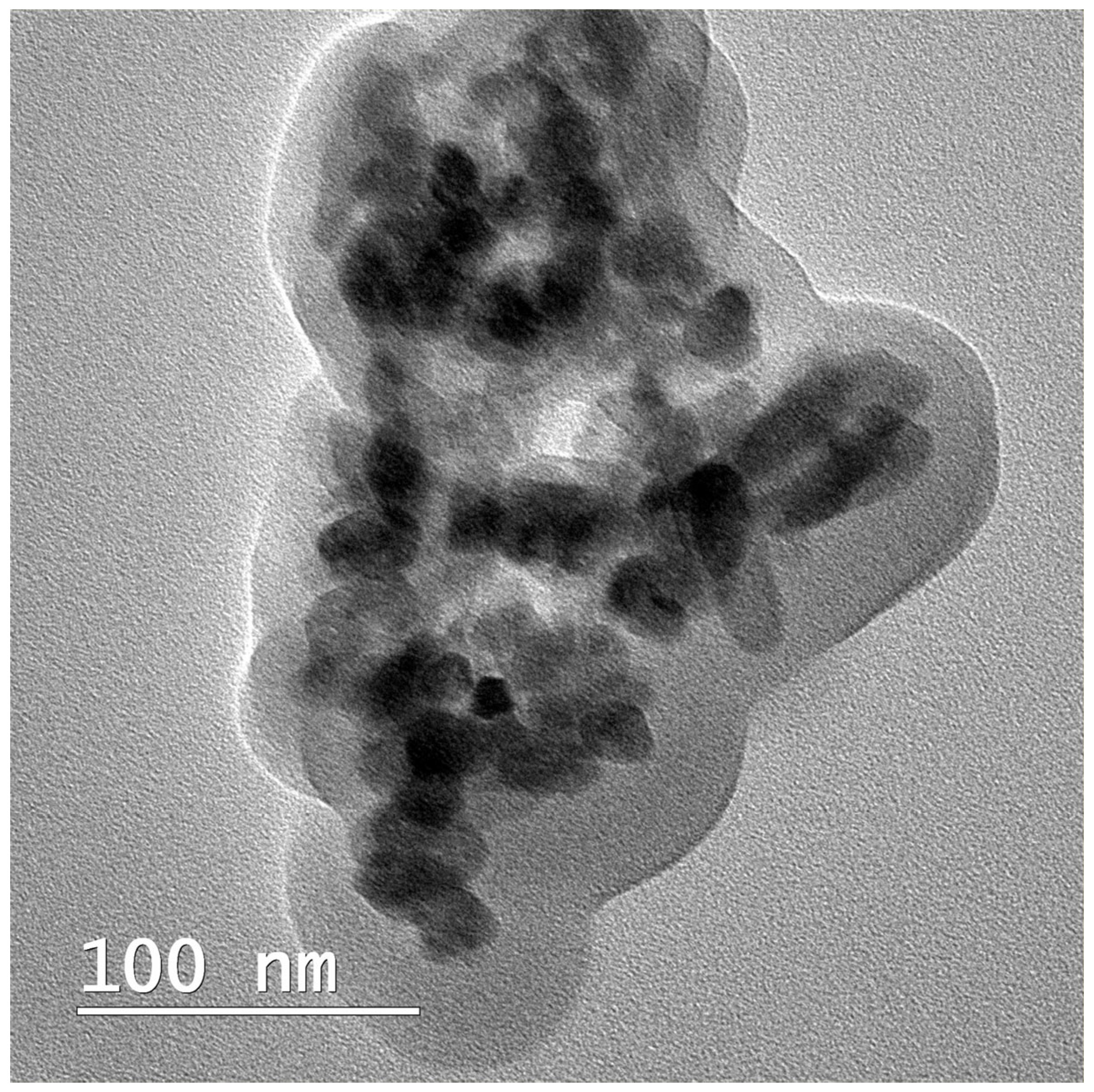
2.2.1. Synthesis of nHAP
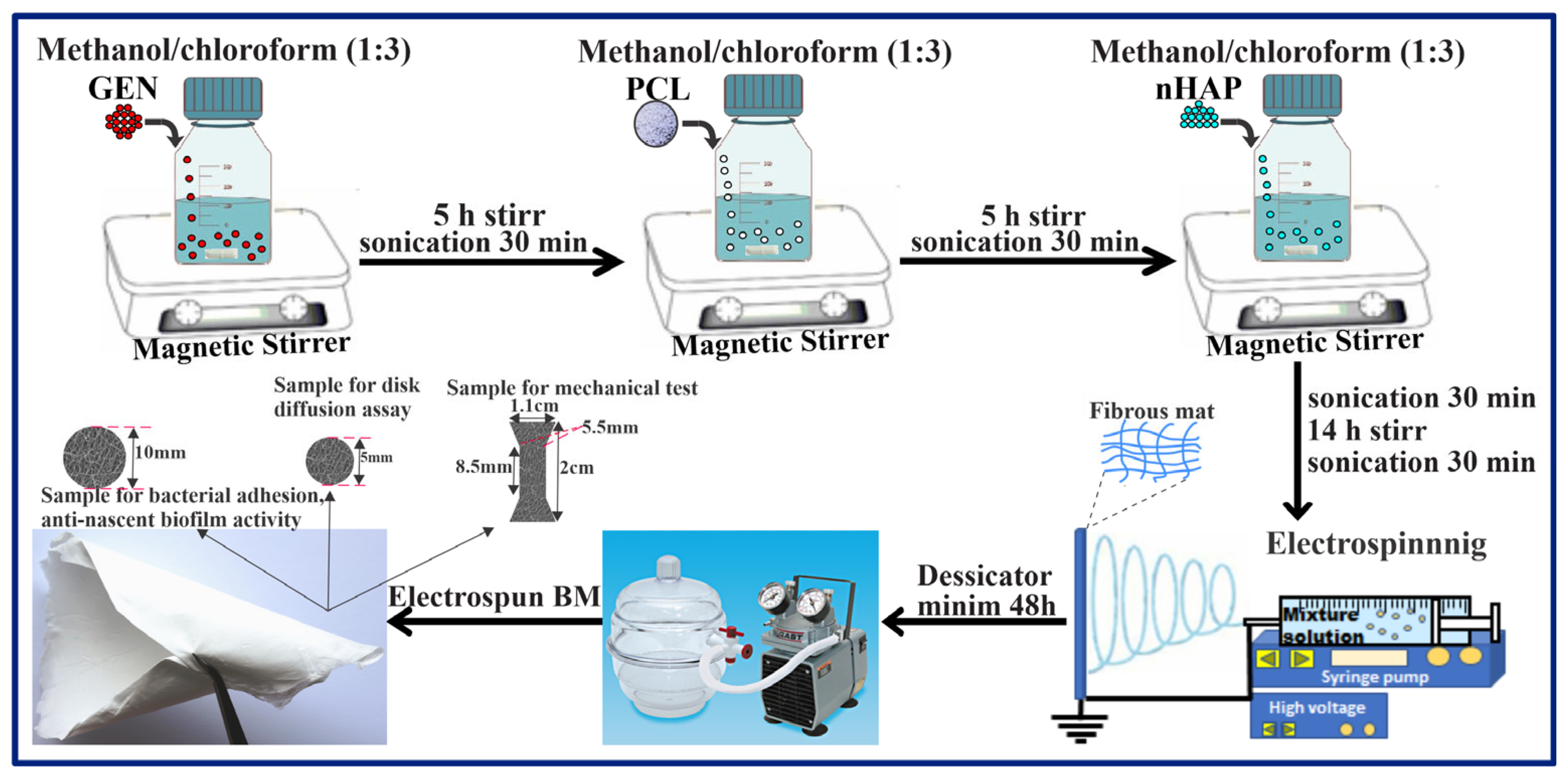
2.2.2. Preparation of the Electrospun BMs
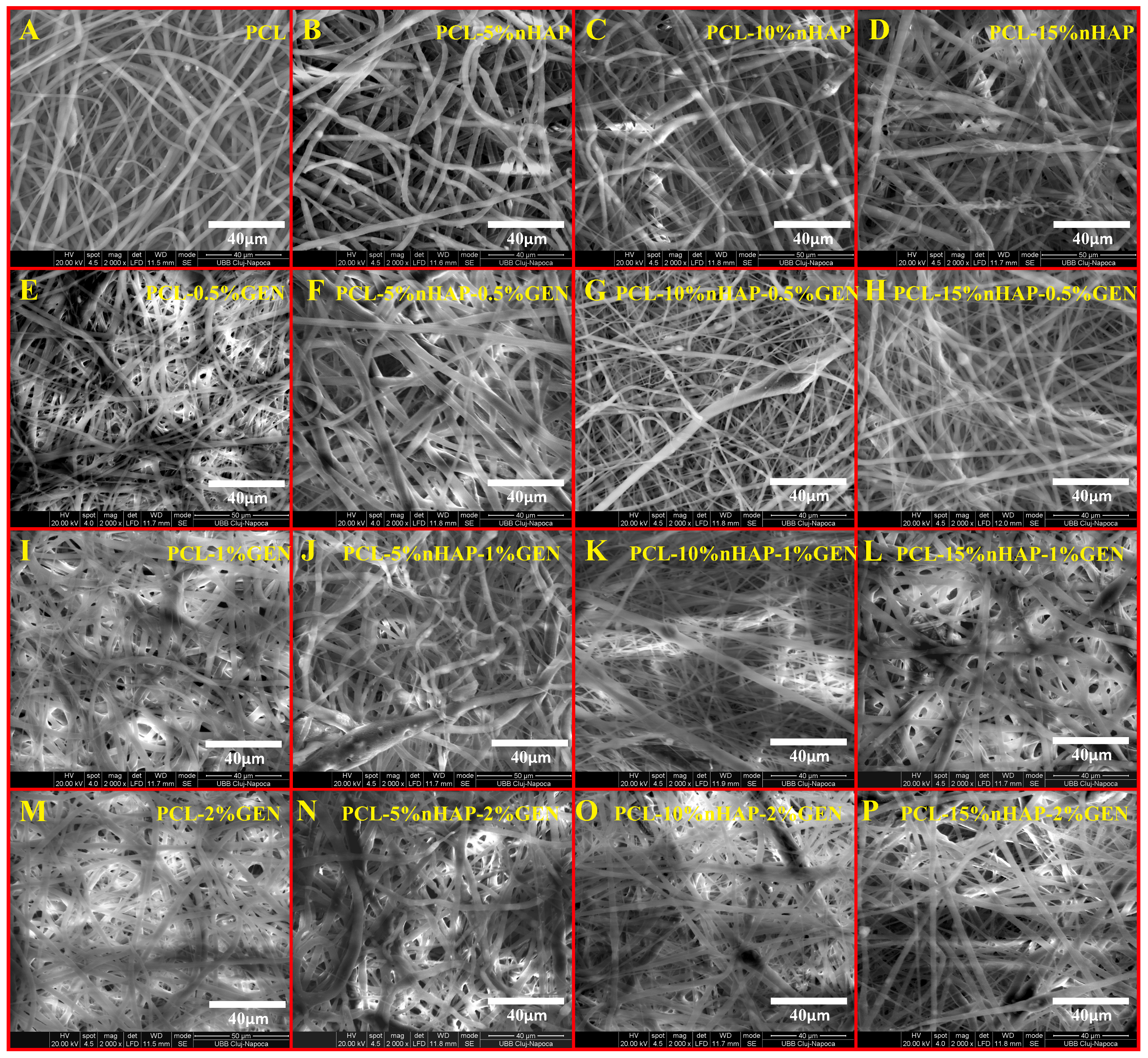
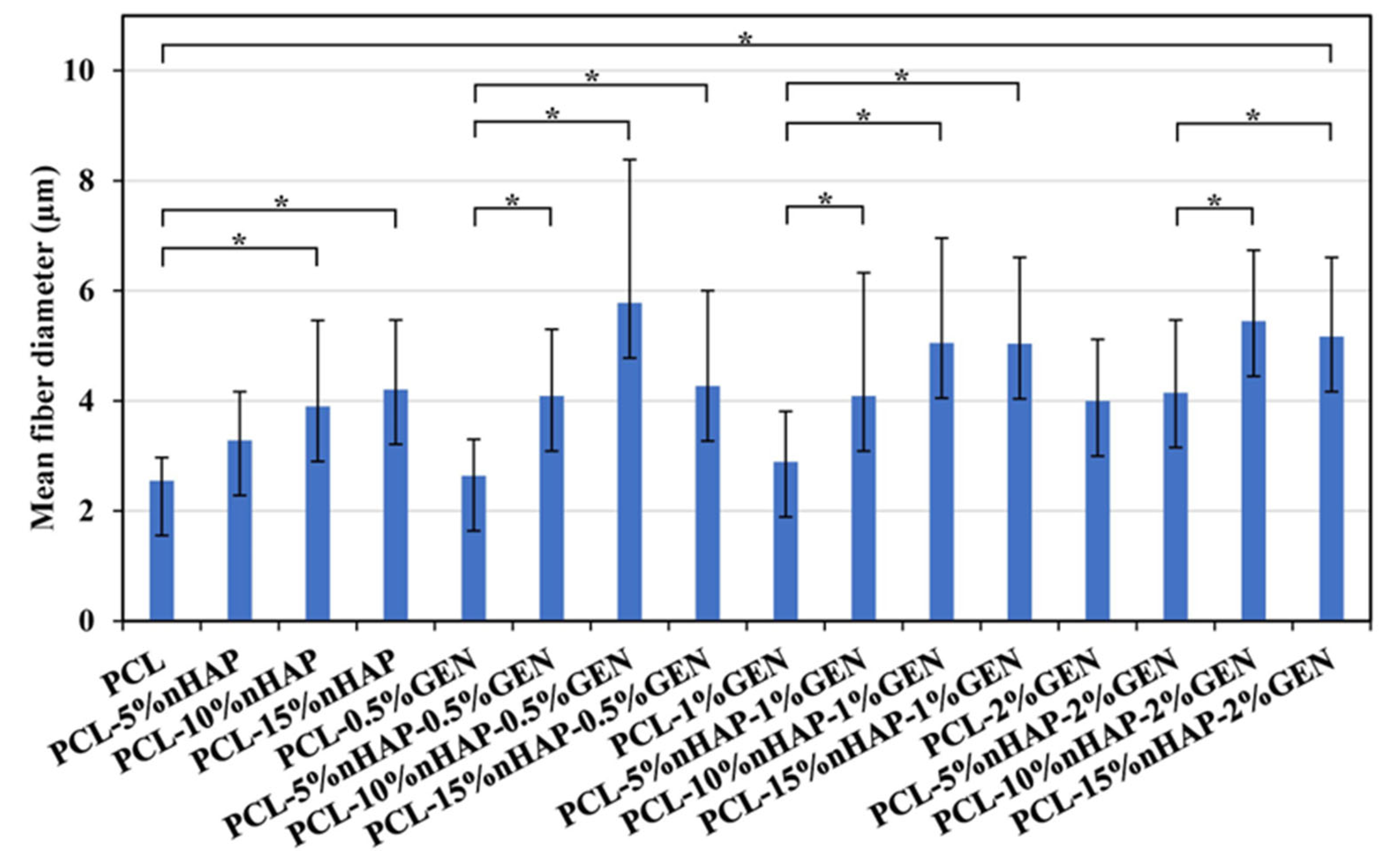
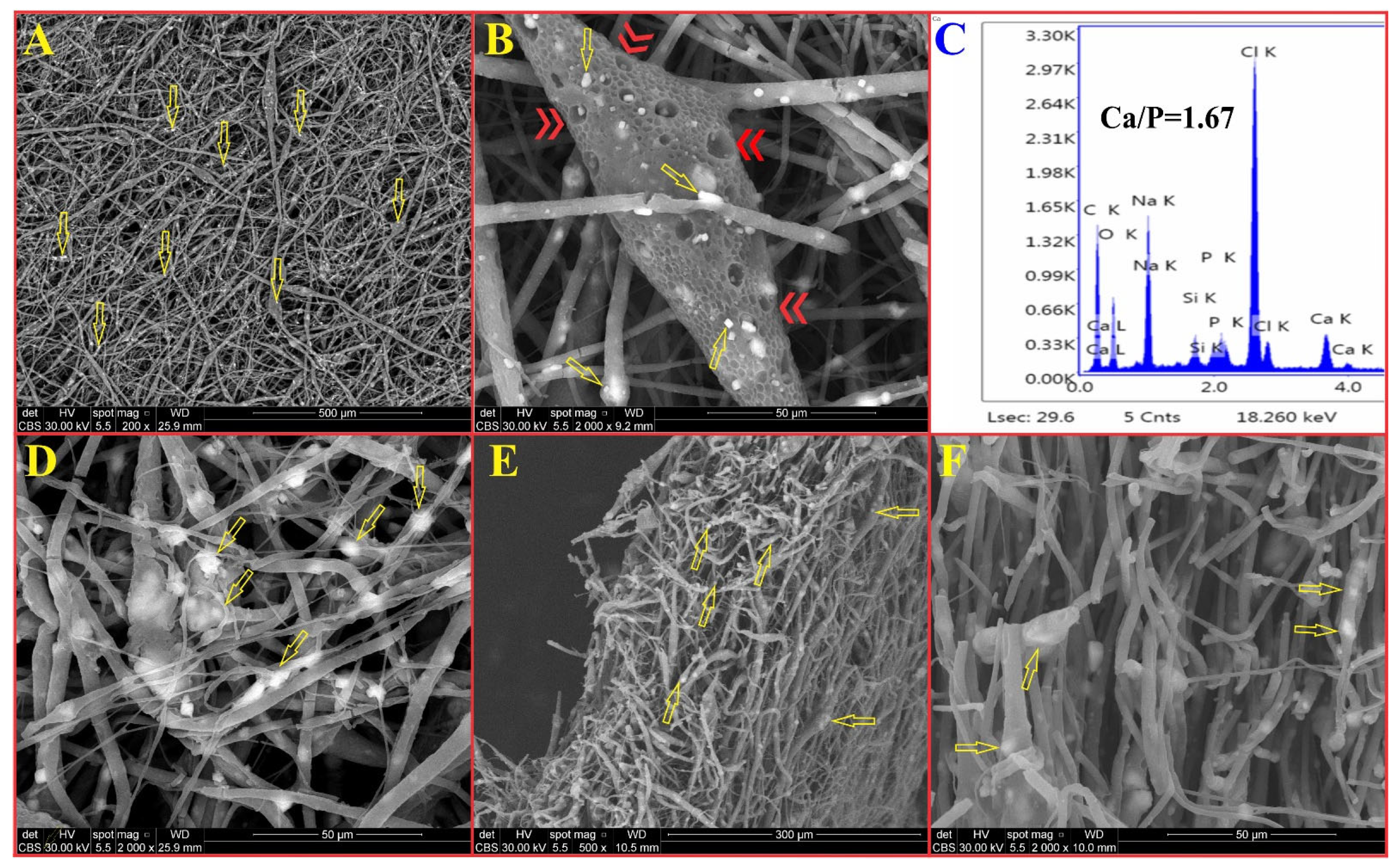
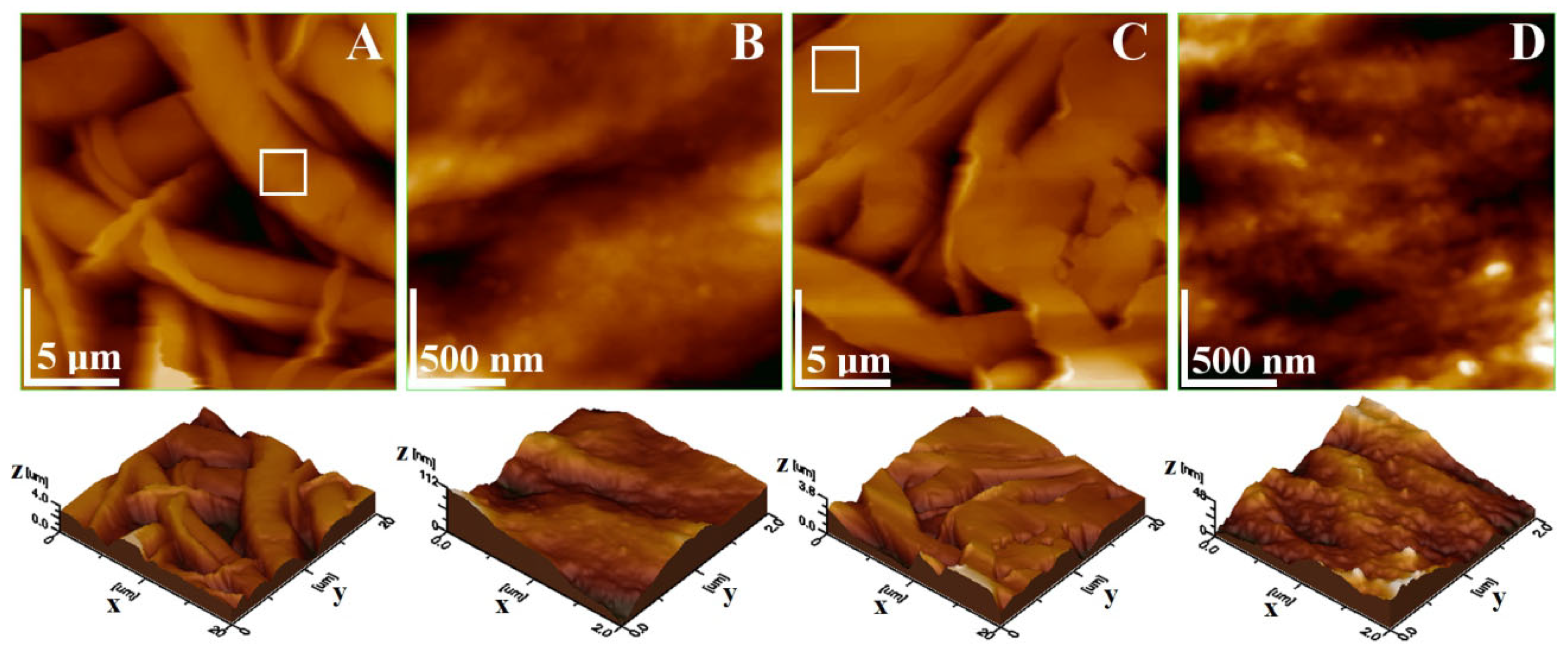
2.2.3. FTIR Spectroscopy, X-Ray Diffraction, TEM, and SEM Analyses
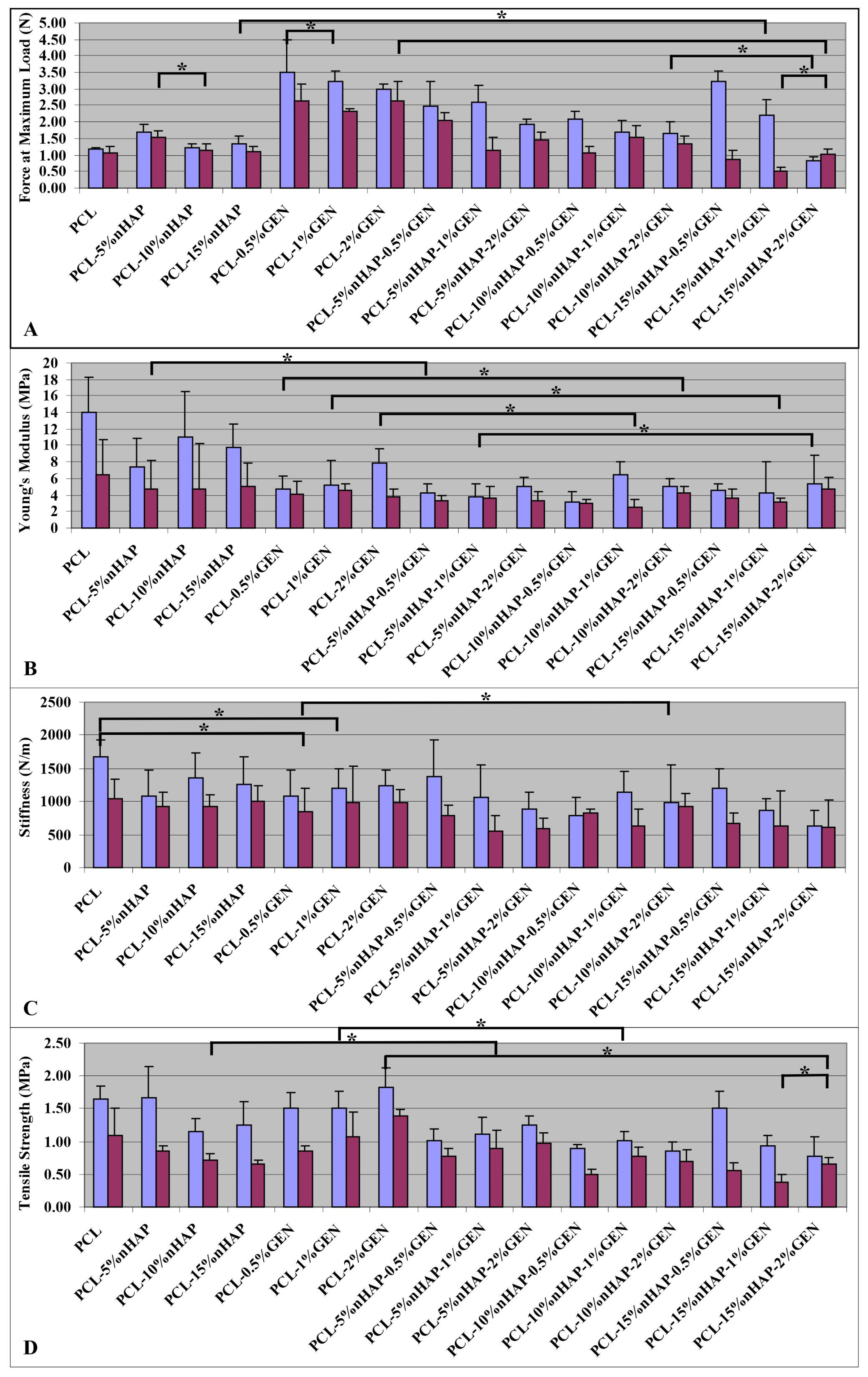
2.2.4. Mechanical Properties
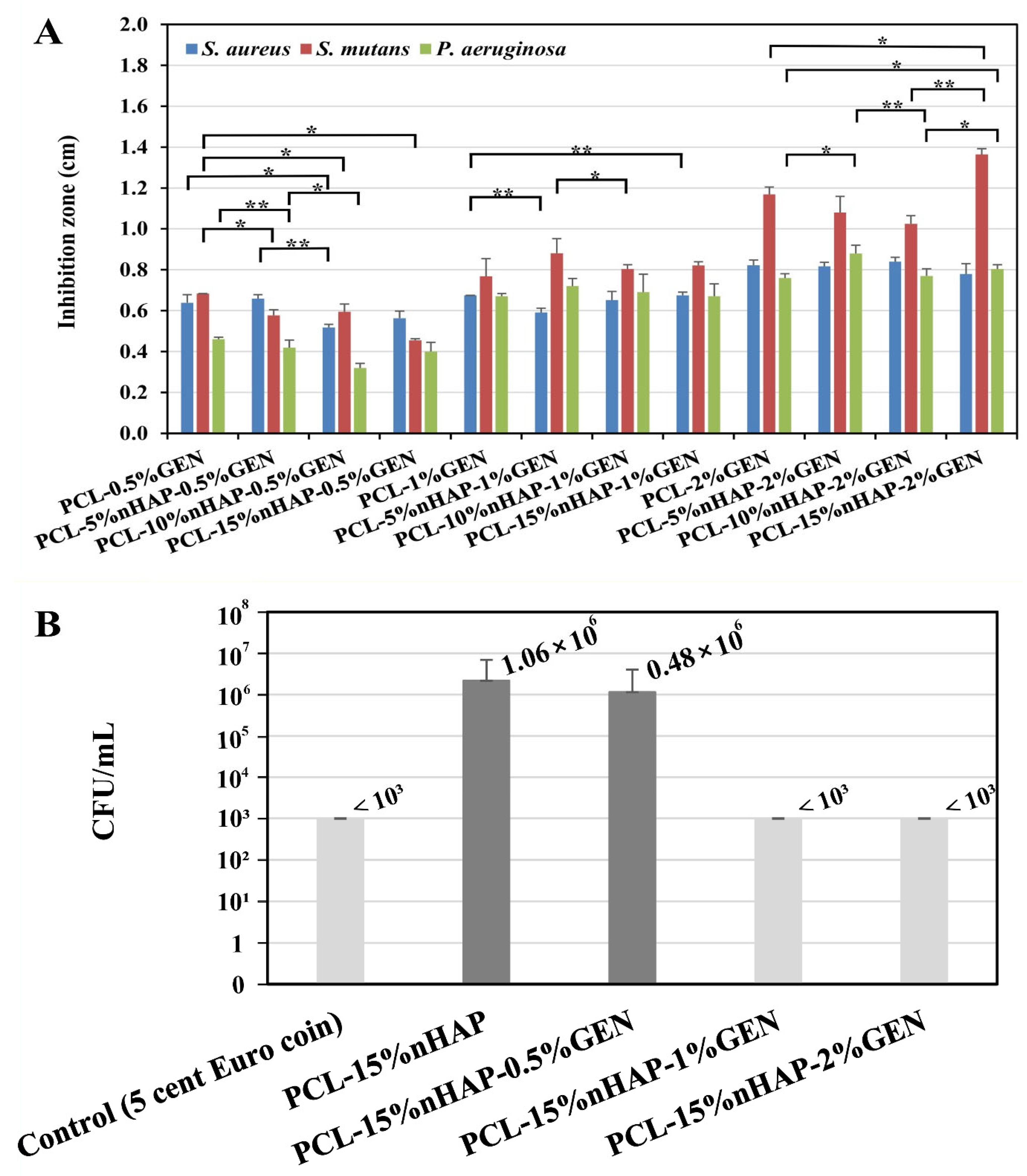
2.2.5. Microbiological Evaluation of Antibacterial Activities of the BMs
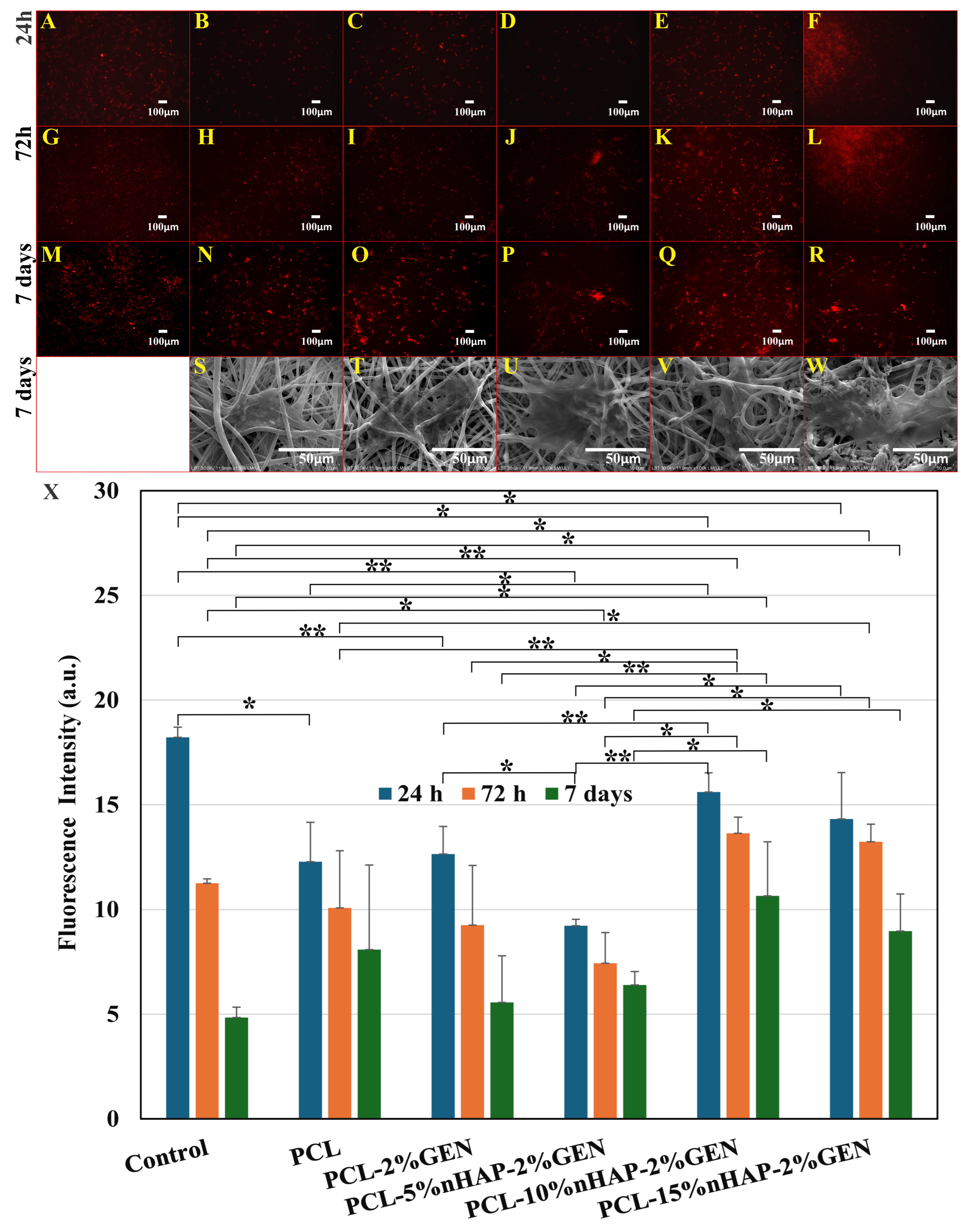
2.2.6. Cytotoxicity, Cell Proliferation, and Cell Adhesion Assay
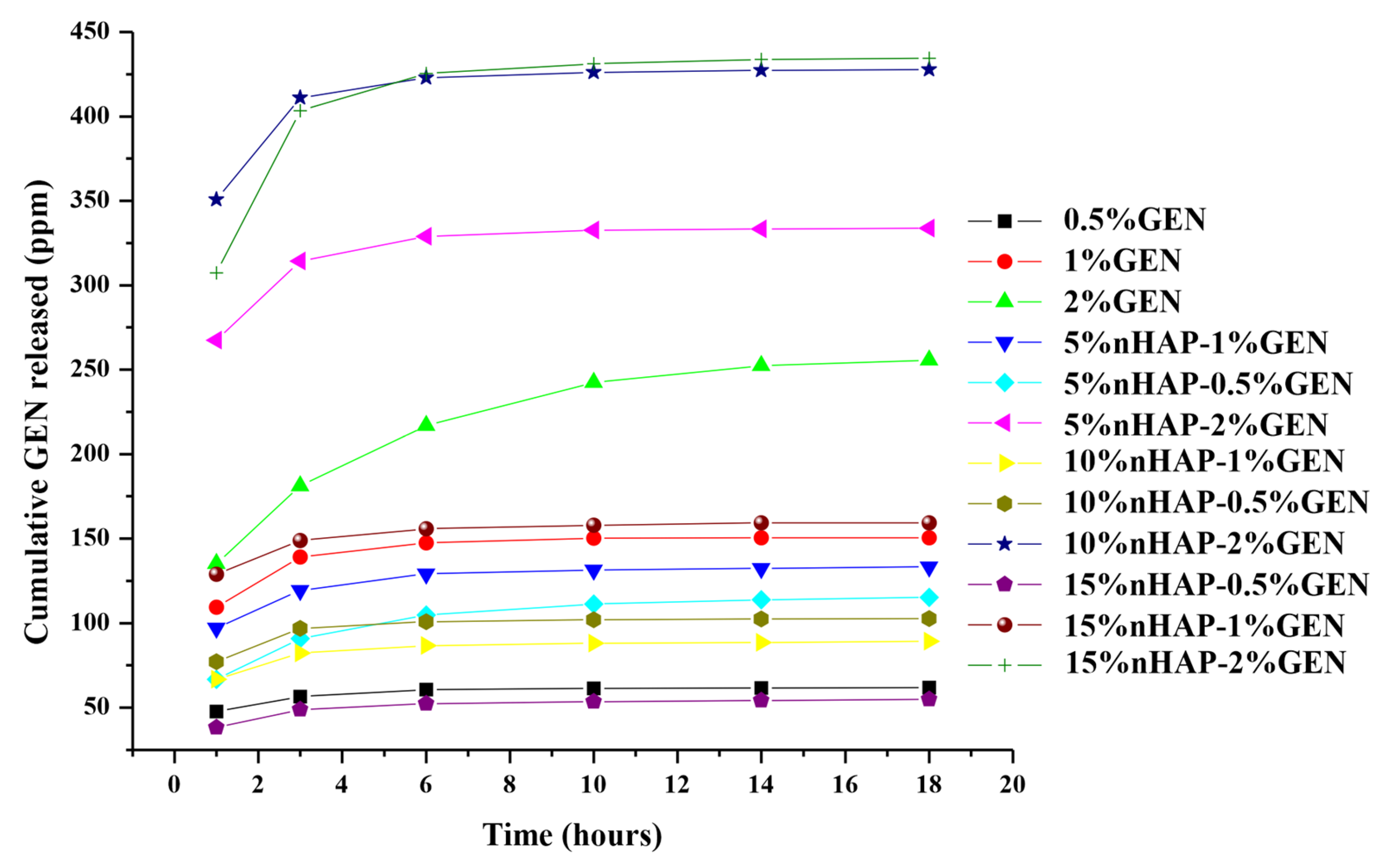
2.2.7. Drug Release
2.2.8. In Vitro Bioactivity
2.2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Bassir, S.; Alhareky, M.; Wangsrimongkol, B.; Jia, Y.; Karimbux, N. Systematic Review and Meta-Analysis of Hard Tissue Outcomes of Alveolar Ridge Preservation. Int. J. Oral Maxillofac. Implant. 2018, 33, 979–994. [Google Scholar] [CrossRef]
- Sun, F.; Kang, H.G.; Ryu, S.-C.; Kim, J.E.; Park, E.Y.; Hwang, D.Y.; Lee, J. Guided Bone Regeneration Using a Flexible Hydroxyapatite Patch. J. Biomed. Nanotechnol. 2013, 9, 1914–1920. [Google Scholar] [CrossRef]
- Wang, H.L.; MacNeil, R.L. Guided Tissue Regeneration. Absorbable Barriers. Dent. Clin. North Am. 1998, 42, 505–522. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Korkusuz, F.; Korkusuz, P.; Eksioglu, F.; Gursel, I.; Hasirci, V. In Vivo Response to Biodegradable Controlled Antibiotic Release Systems. J. Biomed. Mater. Res. 2001, 55, 217–228. [Google Scholar] [CrossRef]
- García-Alvarez, R.; Izquierdo-Barba, I.; Vallet-Regí, M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2017, 49, 113–126. [Google Scholar] [CrossRef]
- Ashique, S.; Sandhu, N.K.; Chawla, V.; Chawla, P.A. Targeted Drug Delivery: Trends and Perspectives. Curr. Drug Deliv. 2021, 18, 1435–1455. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Mehra, P.; Dhanjal, D.S.; Sharma, P.; Sharma, V.; Singh, R.; Nepovimova, E.; Chopra, C.; Kuča, K. Antibiotics and Antibiotic Resistanc—Flipsides of the Same Coin. Curr. Pharm. Des. 2022, 28, 2312–2329. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Cornejo-Bravo, J.M.; Serrano-Medina, A.; Perez-González, G.L.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Lan, Y.; Li, W.; Jiao, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. Therapeutic efficacy of antibiotic-loaded gelatin microsphere/silk fibroin scaffolds in infected full-thickness burns. Acta Biomater. 2014, 10, 3167–3176. [Google Scholar] [CrossRef]
- Wright, J.A.; Nair, S.P. Interaction of staphylococci with bone. Int. J. Med Microbiol. 2010, 300, 193–204. [Google Scholar] [CrossRef]
- Gomes, D.; Pereira, M.; Bettencourt, A.F. Osteomyelitis: An overview of antimicrobial therapy. Braz. J. Pharm. Sci. 2013, 49, 13–27. [Google Scholar] [CrossRef]
- Woodruff, A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Rajzer, E.; Menaszek, L.; Bacakova, M.; Orzelski, M.; Błażewicz, M. Hyaluronic Acid-coated Carbon Nonwoven Fabrics as Potential Material for Repair of Osteochondral Defects for Medical Applications. Fibres Text. East. Eur. 2013, 3, 102–107. [Google Scholar]
- Higuchi, J.; Klimek, K.; Wojnarowicz, J.; Opalińska, A.; Chodara, A.; Szałaj, U.; Dąbrowska, S.; Fudala, D.; Ginalska, G. Electrospun Membrane Surface Modification by Sonocoating with HA and ZnO:Ag Nanoparticles—Characterization and Evaluation of Osteoblasts and Bacterial Cell Behavior In Vitro. Cells 2022, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Furtos, G.; Rivero, G.; Rapuntean, S.; Abraham, G.A. Amoxicillin-loaded electrospun nanocomposite membranes for dental applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Sultana, N. Characterization, drug loading and antibacterial activity of nanohydroxyapatite/polycaprolactone (nHA/PCL) electrospun membrane. 3 Biotech 2017, 7, 249. [Google Scholar] [CrossRef]
- Ribeiro, N.; Sousa, S.R.; van Blitterswijk, C.A.; Moroni, L.; Monteiro, F.J. A biocomposite of collagen nanofibers and nanohydroxyapatite for bone regeneration. Biofabrication 2014, 6, 035015. [Google Scholar] [CrossRef]
- Mirică, I.-C.; Furtos, G.; Lucaciu, O.; Pascuta, P.; Vlassa, M.; Moldovan, M.; Campian, R.-S. Electrospun Membranes Based on Polycaprolactone, Nano-Hydroxyapatite and Metronidazole. Materials 2021, 14, 931. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Mirica, I.-C.; Furtos, G.; Moldovan, M.; Prodan, D.; Petean, I.; Campian, R.-S.; Pall, E.; Lucaciu, O. Morphology, Cytotoxicity, and Antimicrobial Activity of Electrospun Polycaprolactone Biomembranes with Gentamicin and Nano-Hydroxyapatite. Membranes 2023, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Vanheuverzwijn, J.; Maillard, E.-E.; Mahat, A.; Fowler, L.; Monteyne, D.; Bonnaud, L.; Landercy, N.; Hemberg, A.; Janković, A.; Meyer, F.; et al. Easy, Flexible and Standardizable Anti-Nascent Biofilm Activity Assay to Assess Implant Materials. Microorganisms 2023, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009; pp. 5–9.
- Dindelegan, G.C.; Caziuc, A.; Brie, I.; Soritau, O.; Dindelegan, M.G.; Bintintan, V.; Pascalau, V.; Mihu, C.; Popa, C. Multilayered Porous Titanium-Based 3rd Generation Biomaterial Designed for Endosseous Implants. Materials 2021, 14, 1727. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.E. (Lungu); Moldovan, M.; Prejmerean, C.A.; Prodan, D.; Vlassa, M.; Filip, M.; Badea, M.E.; Moldovan, M.A. New Antimicrobial Biomaterials for the Reconstruction of Craniofacial Bone Defects. Coatings 2020, 10, 678. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Ramteke, P.W. Fabrication and Assessment of Gentamicin Loaded Electrospun Nanofibrous Scaffolds as a Quick Wound Healing Dressing Material. Curr. Nanosci. 2015, 11, 222–228. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Arsad, M.S.M.; Lee, P.M.; Hung, L.K. Synthesis and characterization of hydroxyapatite nanoparticles and β-TCP particles. In Proceedings of the 2011 2nd International Conference on Biotechnology and Food Science IPCBEE 7, Bali Island, Indonesia, 1–3 April 2011. [Google Scholar]
- Khodir, W.K.W.A.; Razak, A.H.A.; Ng, M.H.; Guarino, V.; Susanti, D. Encapsulation and Characterization of Gentamicin Sulfate in the Collagen Added Electrospun Nanofibers for Skin Regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Munar-Frau, A.; Delgado, L.; Pérez, R.; Hernández–Alfaro, F. Physicochemical characterization of barrier membranes for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019, 97, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bentley, K.L.d.M.; MacDonald, A.; Schwarz, E.M.; Oh, I. Chronic Osteomyelitis with Staphylococcus aureus Deformation in Submicron Canaliculi of Osteocytes. J. Bone Jt. Surg. 2018, 8, e8. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J.; Little, D.G.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cells Mater. 2013, 26, 120–132. [Google Scholar] [CrossRef]
- Bentley, K.L.d.M.; Trombetta, R.; Nishitani, K.; Bello-Irizarry, S.N.; Ninomiya, M.; Zhang, L.; Chung, H.L.; McGrath, J.L.; Daiss, J.L.; Awad, H.A.; et al. Evidence of Staphylococcus aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. J. Bone Miner. Res. 2016, 32, 985–990. [Google Scholar] [CrossRef]
- Rotman, S.G.; Moriarty, T.F.; Nottelet, B.; Grijpma, D.W.; Eglin, D.; Guillaume, O. Poly(aspartic acid) Functionalized Poly(ϵ-caprolactone) Microspheres with Enhanced Hydroxyapatite Affinity as Bone Targeting Antibiotic Carriers. Pharmaceutics 2020, 12, 885. [Google Scholar] [CrossRef]
- Tupinambá, R.A.; Claro, C.A.d.A.; Pereira, C.A.; Nobrega, C.J.P.; Claro, A.P.R.A. Bacterial adhesion on conventional and self-ligating metallic brackets after surface treatment with plasma-polymerized hexamethyldisiloxane. Dent. Press J. Orthod. 2017, 22, 77–85. [Google Scholar] [CrossRef][Green Version]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, M.; Martins, A.; Fonseca, N.A.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Antibacterial activity of chitosan nanofiber meshes with liposomes immobilized releasing gentamicin. Acta Biomater. 2015, 18, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Asselin, J.; Tazi, N.; Messaddeq, Y.; Levinson, D.; Zhang, Z. Production of Biocompatible and Antimicrobial Bacterial Cellulose Polymers Functionalized by RGDC Grafting Groups and Gentamicin. ACS Appl. Mater. Interfaces 2014, 6, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-J.; Gao, L.; Liu, J.-C.; Wang, J.; Cheng, Q.; Li, J.-P.; Li, S.-Q.; Zhi, K.-Q.; Zeng, R.-C.; Wang, Z.-L. Corrosion resistance and antibacterial properties of hydroxyapatite coating induced by gentamicin-loaded polymeric multilayers on magnesium alloys. Colloids Surf. B Biointerfaces 2019, 179, 429–436. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Ahadian, S.; Khademhosseini, A. Smart scaffolds in tissue regeneration. Regen. Biomater. 2018, 5, 125–128. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Yang, M.; Zhan, X.; Lan, F.; He, J.; Gu, Z.; Wu, Y. Protein Corona of Magnetic Hydroxyapatite Scaffold Improves Cell Proliferation via Activation of Mitogen-Activated Protein Kinase Signaling Pathway. ACS Nano 2017, 11, 3690–3704. [Google Scholar] [CrossRef]
- Quiros, Y.; Vicente-Vicente, L.; Morales, A.I.; López-Novoa, J.M.; López-Hernández, F.J. An Integrative Overview on the Mechanisms Underlying the Renal Tubular Cytotoxicity of Gentamicin. Toxicol. Sci. 2011, 119, 245–256. [Google Scholar] [CrossRef]
- Flores-Arriaga, J.C.; de Jesús Pozos-Guillén, A.; Escobar-García, D.M.; Grandfils, C.; Cerda-Cristerna, B.I. Cell viability and hemocompatibility evaluation of a starch-based hydrogel loaded with hydroxyapatite or calcium carbonate for maxillofacial bone regeneration. Odontology 2017, 105, 398–407. [Google Scholar] [CrossRef]
- Sattary, M.; Rafienia, M.; Khorasani, M.T.; Salehi, H. The effect of collector type on the physical, chemical, and biological properties of polycaprolactone/gelatin/nano-hydroxyapatite electrospun scaffold. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 933–950. [Google Scholar] [CrossRef]
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 668428. [Google Scholar] [CrossRef] [PubMed]
- Keivani, F.; Shokrollahi, P.; Zandi, M.; Irani, S.; Hokrolahi, F.; Khorasani, S. Engineered electrospun poly(caprolactone)/polycaprolactone-g-hydroxyapatite nano-fibrous scaffold promotes human fibroblasts adhesion and proliferation. Mater. Sci. Eng. C 2016, 68, 78–88. [Google Scholar] [CrossRef]
- Rotman, S.; Grijpma, D.; Richards, R.; Moriarty, T.; Eglin, D.; Guillaume, O. Drug delivery systems functionalized with bone mineral seeking agents for bone targeted therapeutics. J. Control. Release 2018, 269, 88–99. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110347. [Google Scholar] [CrossRef]
- Lucaciu, P.O.; Repciuc, C.C.; Matei, I.A.; Fiț, N.I.; Andrei, S.; Marica, R.; Petrescu, B.N.; Crișan, B.; Aghiorghiesei, O.; Mirică, I.C.; et al. In Vivo Validation of a Nanostructured Electrospun Polycaprolactone Membrane Loaded with Gentamicin and Nano-Hydroxyapatite for the Treatment of Periodontitis. Membranes 2024, 14, 60. [Google Scholar] [CrossRef]




| No. | Code | Composition of the BM | ||
|---|---|---|---|---|
| PCL (wt.%) | nHAP (wt.%) | GEN (wt.%) | ||
| 1 | PCL | 100 | 0 | 0 |
| 2 | PCL-5%nHAP | 95 | 5 | 0 |
| 3 | PCL-10%nHAP | 90 | 10 | 0 |
| 4 | PCL-15%nHAP | 85 | 15 | 0 |
| 5 | PCL-0.5%GEN | 99.5 | 0 | 0.5 |
| 6 | PCL-1%GEN | 99 | 0 | 1 |
| 7 | PCL-2%GEN | 98 | 0 | 2 |
| 8 | PCL-5%nHAP-0.5%GEN | 94.5 | 5 | 0.5 |
| 9 | PCL-10%nHAP-0.5%GEN | 89.5 | 10 | 0.5 |
| 10 | PCL-15%nHAP-0.5%GEN | 84.5 | 15 | 0.5 |
| 11 | PCL-5%nHAP-1%GEN | 94 | 5 | 1 |
| 12 | PCL-10%nHAP-1%GEN | 89 | 10 | 1 |
| 13 | PCL-15%nHAP-1%GEN | 84 | 15 | 1 |
| 14 | PCL-5%nHAP-2%GEN | 93 | 5 | 2 |
| 15 | PCL-10%nHAP-2%GEN | 88 | 10 | 2 |
| 16 | PCL-15%nHAP-2%GEN | 83 | 15 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirica, I.-C.; Furtos, G.; Fontaine, V.; Vlassa, M.; Pascuta, P.; Petean, I.; Bâldea, B.; Andercou, O.; Lucaciu, O.P. Electrospun Polycaprolactone Membranes Loaded with Gentamicin and Nano-Hidroxyapatite for Guided Bone Regeneration. Biomedicines 2025, 13, 2349. https://doi.org/10.3390/biomedicines13102349
Mirica I-C, Furtos G, Fontaine V, Vlassa M, Pascuta P, Petean I, Bâldea B, Andercou O, Lucaciu OP. Electrospun Polycaprolactone Membranes Loaded with Gentamicin and Nano-Hidroxyapatite for Guided Bone Regeneration. Biomedicines. 2025; 13(10):2349. https://doi.org/10.3390/biomedicines13102349
Chicago/Turabian StyleMirica, Ioana-Codruta, Gabriel Furtos, Véronique Fontaine, Mihaela Vlassa, Petru Pascuta, Ioan Petean, Bogdan Bâldea, Otilia Andercou, and Ondine Patricia Lucaciu. 2025. "Electrospun Polycaprolactone Membranes Loaded with Gentamicin and Nano-Hidroxyapatite for Guided Bone Regeneration" Biomedicines 13, no. 10: 2349. https://doi.org/10.3390/biomedicines13102349
APA StyleMirica, I.-C., Furtos, G., Fontaine, V., Vlassa, M., Pascuta, P., Petean, I., Bâldea, B., Andercou, O., & Lucaciu, O. P. (2025). Electrospun Polycaprolactone Membranes Loaded with Gentamicin and Nano-Hidroxyapatite for Guided Bone Regeneration. Biomedicines, 13(10), 2349. https://doi.org/10.3390/biomedicines13102349










