The Role of miRNAs in Chemotherapy-Induced Cardiotoxicity
Abstract
1. Introduction
2. Methods
3. Mechanisms of CTRCD and miRNAs’ Correlation
3.1. Oxidative Stress
3.2. Disruption of Mitochondrial Function
3.3. Inhibition of Topoisomerase 2 Beta (Top2β)
3.4. Abnormal Iron (Fe) Metabolism
3.5. Apoptosis
3.6. Fibrosis
4. Association of miRs with CTRCD
4.1. Let-7 Family
4.2. miR-1 Clusters
4.3. miR-29 Family
4.4. miR-30 Family
4.5. miR-34a
4.6. miR-126
4.7. miR-130a
4.8. miR-140
4.9. miR-320a
4.10. miR-499
5. Discrepancies in miR Expression Patterns
6. Cardiotoxicity and Correlated miRs with Other Anticancer Regimes
7. Clinical Implication
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | Fluorouracil |
| ABCB8 | ATP-binding cassette sub-family B member 8 |
| AIFM3 | Apoptosis-inducing factor, mitochondria-associated 3 |
| AKT | Protein kinase B |
| ASK1 | Apoptosis Signal-regulating Kinase 1 |
| Bak | BCL2 Antagonist/Killer 1 |
| Bax | BCL2 Associated X |
| Bcl-2 | B-cell lymphoma-2 |
| BNP | B-type natriuretic peptide |
| CTRCD | Chemotherapy-related cardiac dysfunction |
| cAMP | Cyclic adenosine monophosphate |
| DNA | Deoxyribonucleic acid |
| miRs | MicroRNAs |
| miR | MicroRNA |
| FUNDC1 | FUN14 Domain Containing 1 |
| HER2 | Human epidermal growth factor receptor 2 |
| HGF | Hepatocyte growth factor |
| HO-1 | Hyperoxide ion |
| IRP | Iron regulatory protein |
| LVEF | Left ventricular ejection fraction |
| MFN | Mitofusin |
| MFRN | Mitoferrin |
| MMPs | Matrix metalloproteases |
| mTOR | mechanistic target of rapamycin |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSCLC | Non-small cell lung cancer |
| PI3K | phosphatidylinositol 3-kinase |
| PKA | Protein kinase A |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PTEN | Phosphatase and tensin homolog |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SIRT | Sirtuin |
| SMAD3 | Mothers against decapentaplegic homolog 3 |
| SOD2 | Superoxide dismutase 2 |
| TGF | Transforming growth factor |
| TNF | Tumor necrosis factor |
| Top2β | Topoisomerase 2 beta |
| TRAIL | TNF-related apoptosis inducing ligand |
| VEGF | Vascular Endothelial Growth Factor |
References
- Kourek, C.; Touloupaki, M.; Rempakos, A.; Loritis, K.; Tsougkos, E.; Paraskevaidis, I.; Briasoulis, A. Cardioprotective Strategies from Cardiotoxicity in Cancer Patients: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2022, 9, 259. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Haj-Yehia, E.; Michel, L.; Mincu, R.I.; Rassaf, T.; Totzeck, M. Prevention of cancer-therapy related cardiac dysfunction. Curr. Heart Fail. Rep. 2025, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Gerodias, F.R.; Tan, M.K.; De Guzman, A.; Bernan, A.; Locnen, S.A.; Apostol-Alday, A.; Ybanez, E.J.; Magno, J.D.; Lim, A.; Junia, A.; et al. Anthracycline-Induced Cardiotoxicity in Breast Cancer Patients: A Five-Year Retrospective Study in 10 Centers. Cardiol. Res. 2022, 13, 380–392. [Google Scholar] [CrossRef]
- Mata Caballero, R.; Serrano Antolín, J.M.; Jiménez Hernández, R.M.; Talavera Calle, P.; Curcio Ruigómez, A.; Del Castillo Arrojo, S.; Graupner Abad, C.; Cristóbal Varela, C.; Alonso Martín, J.J. Incidence of long-term cardiotoxicity and evolution of the systolic function in patients with breast cancer treated with anthracyclines. Cardiol. J. 2022, 29, 228–234. [Google Scholar] [CrossRef]
- Kremer, L.C.; van der Pal, H.J.; Offringa, M.; van Dalen, E.C.; Voûte, P.A. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: A systematic review. Ann. Oncol. 2002, 13, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Persson, H.L.; Richardson, D.R. Molecular pharmacology of the interaction of anthracyclines with iron. Mol. Pharmacol. 2005, 68, 261–271. [Google Scholar] [CrossRef]
- Scotti, L.; Franchi, M.; Marchesoni, A.; Corrao, G. Prevalence and incidence of psoriatic arthritis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 48, 28–34. [Google Scholar] [CrossRef]
- Kong, C.Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.P.; Teng, T.; Yan, L.; Tang, Q.Z. Underlying the Mechanisms of Doxorubicin-Induced Acute Cardiotoxicity: Oxidative Stress and Cell Death. Int. J. Biol. Sci. 2022, 18, 760–770. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, X.; Song, P.; Yuan, Y.P.; Kong, C.Y.; Wu, H.M.; Xu, S.C.; Ma, Z.G.; Tang, Q.Z. Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol. 2020, 37, 101747. [Google Scholar] [CrossRef]
- Ciesielska, S.; Slezak-Prochazka, I.; Bil, P.; Rzeszowska-Wolny, J. Micro RNAs in Regulation of Cellular Redox Homeostasis. Int. J. Mol. Sci. 2021, 22, 6022. [Google Scholar] [CrossRef]
- Luo, M.; Tan, X.; Mu, L.; Luo, Y.; Li, R.; Deng, X.; Chen, N.; Ren, M.; Li, Y.; Wang, L.; et al. MiRNA-21 mediates the antiangiogenic activity of metformin through targeting PTEN and SMAD7 expression and PI3K/AKT pathway. Sci. Rep. 2017, 7, 43427. [Google Scholar] [CrossRef]
- Yang, L.; He, S.; Ling, L.; Wang, F.; Xu, L.; Fang, L.; Wu, F.; Zhou, S.; Yang, F.; Wei, H.; et al. Crosstalk between miR-144/451 and Nrf2 during Recovery from Acute Hemolytic Anemia. Genes 2023, 14, 1011. [Google Scholar] [CrossRef]
- Zhao, L.; Qi, Y.; Xu, L.; Tao, X.; Han, X.; Yin, L.; Peng, J. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018, 15, 284–296. [Google Scholar] [CrossRef]
- Horie, T.; Ono, K.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Kuwabara, Y.; Nakashima, Y.; Takanabe-Mori, R.; Nishi, E.; et al. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc. Res. 2010, 87, 656–664. [Google Scholar] [CrossRef]
- Zhu, J.N.; Fu, Y.H.; Hu, Z.Q.; Li, W.Y.; Tang, C.M.; Fei, H.W.; Yang, H.; Lin, Q.X.; Gou, D.M.; Wu, S.L.; et al. Activation of miR-34a-5p/Sirt1/p66shc pathway contributes to doxorubicin-induced cardiotoxicity. Sci. Rep. 2017, 7, 11879. [Google Scholar] [CrossRef]
- Nammian, P.; Razban, V.; Tabei, S.M.B.; Asadi-Yousefabad, S.L. MicroRNA-126: Dual Role in Angiogenesis Dependent Diseases. Curr. Pharm. Des. 2020, 26, 4883–4893. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Peng, B.; Chen, H.; Yang, M.; Chen, P.; Ye, L.; Wang, H.; Ren, L.; Xie, J.; Zhu, J.; et al. miR-34a induces neutrophil apoptosis by regulating Cdc42-WASP-Arp2/3 pathway-mediated F-actin remodeling and ROS production. Redox Rep. 2022, 27, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yerrapragada, S.M.; Sawant, H.; Chen, S.; Bihl, T.; Wang, J.; Bihl, J.C. The protective effects of miR-210 modified endothelial progenitor cells released exosomes in hypoxia/reoxygenation injured neurons. Exp. Neurol. 2022, 358, 114211. [Google Scholar] [CrossRef]
- Li, T.; Song, X.; Zhang, J.; Zhao, L.; Shi, Y.; Li, Z.; Liu, J.; Liu, N.; Yan, Y.; Xiao, Y.; et al. Protection of Human Umbilical Vein Endothelial Cells against Oxidative Stress by MicroRNA-210. Oxid. Med. Cell Longev. 2017, 2017, 3565613. [Google Scholar] [CrossRef]
- Pereira, G.C.; Pereira, S.P.; Tavares, L.C.; Carvalho, F.S.; Magalhães-Novais, S.; Barbosa, I.A.; Santos, M.S.; Bjork, J.; Moreno, A.J.; Wallace, K.B.; et al. Cardiac cytochrome c and cardiolipin depletion during anthracycline-induced chronic depression of mitochondrial function. Mitochondrion 2016, 30, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Lebrecht, D.; Kokkori, A.; Ketelsen, U.P.; Setzer, B.; Walker, U.A. Tissue-specific mtDNA lesions and radical-associated mitochondrial dysfunction in human hearts exposed to doxorubicin. J. Pathol. 2005, 207, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kohr, M.; Dunkerly-Eyring, B.; Lee, D.I.; Bedja, D.; Kent, O.A.; Leung, A.K.; Henao-Mejia, J.; Flavell, R.A.; Steenbergen, C. Divergent Effects of miR-181 Family Members on Myocardial Function Through Protective Cytosolic and Detrimental Mitochondrial microRNA Targets. J. Am. Heart Assoc. 2017, 6, e004694. [Google Scholar] [CrossRef]
- Li, Y.; Duan, J.Z.; He, Q.; Wang, C.Q. miR-155 modulates high glucose-induced cardiac fibrosis via the Nrf2/HO-1 signaling pathway. Mol. Med. Rep. 2020, 22, 4003–4016. [Google Scholar] [CrossRef]
- Li, J.; Donath, S.; Li, Y.; Qin, D.; Prabhakar, B.S.; Li, P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010, 6, e1000795. [Google Scholar] [CrossRef]
- Pakravan, G.; Foroughmand, A.M.; Peymani, M.; Ghaedi, K.; Hashemi, M.S.; Hajjari, M.; Nasr-Esfahani, M.H. Downregulation of miR-130a, antagonized doxorubicin-induced cardiotoxicity via increasing the PPARγ expression in mESCs-derived cardiac cells. Cell Death Dis. 2018, 9, 758. [Google Scholar] [CrossRef]
- Yan, Y.; Tian, L.Y.; Jia, Q.; Han, Y.; Tian, Y.; Chen, H.N.; Cui, S.J.; Xi, J.; Yao, Y.M.; Zhao, X.J. MiR-130a-3p regulates FUNDC1-mediated mitophagy by targeting GJA1 in myocardial ischemia/reperfusion injury. Cell Death Discov. 2023, 9, 77. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Li, Z.; Yan, X.; Liu, S. microRNA-130a-5p suppresses myocardial ischemia reperfusion injury by downregulating the HMGB2/NF-κB axis. BMC Cardiovasc. Disord. 2021, 21, 121. [Google Scholar] [CrossRef]
- Wan, Q.; Xu, T.; Ding, W.; Zhang, X.; Ji, X.; Yu, T.; Yu, W.; Lin, Z.; Wang, J. miR-499-5p Attenuates Mitochondrial Fission and Cell Apoptosis via p21 in Doxorubicin Cardiotoxicity. Front. Genet. 2018, 9, 734. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, X. MiR-423-5p inhibition alleviates cardiomyocyte apoptosis and mitochondrial dysfunction caused by hypoxia/reoxygenation through activation of the wnt/β-catenin signaling pathway via targeting MYBL2. J. Cell Physiol. 2019, 234, 22034–22043. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, M.; Huang, Y. Anthracycline-induced cardiotoxicity: An overview from cellular structural perspective. Biomed. Pharmacother. 2024, 179, 117312. [Google Scholar] [CrossRef] [PubMed]
- Tewey, K.M.; Rowe, T.C.; Yang, L.; Halligan, B.D.; Liu, L.F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 1984, 226, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Carvajal-Moreno, J.; Hernandez, V.A.; Wang, X.; Li, J.; Yalowich, J.C.; Elton, T.S. Effects of hsa-miR-9-3p and hsa-miR-9-5p on Topoisomerase II. J. Pharmacol. Exp. Ther. 2023, 384, 265–276. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Zumbrennen, K.B.; Paw, B.H.; Ward, D.M.; Kaplan, J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell Biol. 2009, 29, 1007–1016. [Google Scholar] [CrossRef]
- Minotti, G.; Ronchi, R.; Salvatorelli, E.; Menna, P.; Cairo, G. Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: Evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Res. 2001, 61, 8422–8428. [Google Scholar]
- Menon, A.V.; Kim, J. Iron Promotes Cardiac Doxorubicin Retention and Toxicity Through Downregulation of the Mitochondrial Exporter ABCB8. Front. Pharmacol. 2022, 13, 817951. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kosaka, N.; Ochiya, T.; Kato, T. Micromanaging Iron Homeostasis: Hypoxia-inducible micro-RNA-210 suppresses iron homeostasis-related proteins. J. Biol. Chem. 2012, 287, 34110–34119. [Google Scholar] [CrossRef]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef]
- Wang, S.; Konorev, E.A.; Kotamraju, S.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H2O2- and p53-dependent pathways. J. Biol. Chem. 2004, 279, 25535–25543. [Google Scholar] [CrossRef]
- Meredith, A.M.; Dass, C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J. Pharm. Pharmacol. 2016, 68, 729–741. [Google Scholar] [CrossRef]
- Shen, S.; Shao, Y.; Li, C. Different types of cell death and their shift in shaping disease. Cell Death Discov. 2023, 9, 284. [Google Scholar] [CrossRef]
- Crow, M.T.; Mani, K.; Nam, Y.J.; Kitsis, R.N. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ. Res. 2004, 95, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, Y.; Tuersong, T.; Chen, L.; Zhang, M.L.; Li, T.; Feng, S.M.; Guo, Q. Let-7 family regulates HaCaT cell proliferation and apoptosis via the ΔNp63/PI3K/AKT pathway. Open Med. 2024, 19, 20240925. [Google Scholar] [CrossRef]
- Jing, X.; Yang, J.; Jiang, L.; Chen, J.; Wang, H. MicroRNA-29b Regulates the Mitochondria-Dependent Apoptotic Pathway by Targeting Bax in Doxorubicin Cardiotoxicity. Cell Physiol. Biochem. 2018, 48, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Peng, C.; Wang, W.; Jin, H.; Tang, Q.; Wei, X. Let-7 g is involved in doxorubicin induced myocardial injury. Environ. Toxicol. Pharmacol. 2012, 33, 312–317. [Google Scholar] [CrossRef]
- Alexandri, C.; Stamatopoulos, B.; Rothé, F.; Bareche, Y.; Devos, M.; Demeestere, I. MicroRNA profiling and identification of let-7a as a target to prevent chemotherapy-induced primordial follicles apoptosis in mouse ovaries. Sci. Rep. 2019, 9, 9636. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Telesca, M.; Donniacuo, M.; Bellocchio, G.; Riemma, M.A.; Mele, E.; Dell’Aversana, C.; Sgueglia, G.; Cianflone, E.; Cappetta, D.; Torella, D.; et al. Initial Phase of Anthracycline Cardiotoxicity Involves Cardiac Fibroblasts Activation and Metabolic Switch. Cancers 2023, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, P.; Huang, Y. Anthracycline-induced cardiotoxicity: Mechanisms, monitoring, and prevention. Front. Cardiovasc. Med. 2023, 10, 1242596. [Google Scholar] [CrossRef]
- Xie, S.; Sun, Y.; Zhao, X.; Xiao, Y.; Zhou, F.; Lin, L.; Wang, W.; Lin, B.; Wang, Z.; Fang, Z.; et al. An update of the molecular mechanisms underlying anthracycline induced cardiotoxicity. Front. Pharmacol. 2024, 15, 1406247. [Google Scholar] [CrossRef]
- Chan, B.Y.H.; Roczkowsky, A.; Cho, W.J.; Poirier, M.; Sergi, C.; Keschrumrus, V.; Churko, J.M.; Granzier, H.; Schulz, R. MMP inhibitors attenuate doxorubicin cardiotoxicity by preventing intracellular and extracellular matrix remodelling. Cardiovasc. Res. 2021, 117, 188–200. [Google Scholar] [CrossRef]
- Li, C.; Meng, X.; Wang, L.; Dai, X. Mechanism of action of non-coding RNAs and traditional Chinese medicine in myocardial fibrosis: Focus on the TGF-β/Smad signaling pathway. Front. Pharmacol. 2023, 14, 1092148. [Google Scholar] [CrossRef]
- Leger, K.J.; Leonard, D.; Nielson, D.; de Lemos, J.A.; Mammen, P.P.; Winick, N.J. Circulating microRNAs: Potential Markers of Cardiotoxicity in Children and Young Adults Treated with Anthracycline Chemotherapy. J. Am. Heart Assoc. 2017, 6, e004653. [Google Scholar] [CrossRef]
- Lakhani, H.V.; Pillai, S.S.; Zehra, M.; Dao, B.; Tirona, M.T.; Thompson, E.; Sodhi, K. Detecting early onset of anthracyclines-induced cardiotoxicity using a novel panel of biomarkers in West-Virginian population with breast cancer. Sci. Rep. 2021, 11, 7954. [Google Scholar] [CrossRef]
- Roy, S.; Khanna, S.; Hussain, S.R.; Biswas, S.; Azad, A.; Rink, C.; Gnyawali, S.; Shilo, S.; Nuovo, G.J.; Sen, C.K. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc. Res. 2009, 82, 21–29. [Google Scholar] [CrossRef]
- Piegari, E.; Cozzolino, A.; Ciuffreda, L.P.; Cappetta, D.; De Angelis, A.; Urbanek, K.; Rossi, F.; Berrino, L. Cardioprotective effects of miR-34a silencing in a rat model of doxorubicin toxicity. Sci. Rep. 2020, 10, 12250. [Google Scholar] [CrossRef]
- Pizzamiglio, S.; Ciniselli, C.M.; de Azambuja, E.; Agbor-Tarh, D.; Moreno-Aspitia, A.; Suter, T.M.; Trama, A.; De Santis, M.C.; De Cecco, L.; Iorio, M.V.; et al. Circulating microRNAs and therapy-associated cardiac events in HER2-positive breast cancer patients: An exploratory analysis from NeoALTTO. Breast Cancer Res. Treat. 2024, 206, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Ren, Y.; Hou, A.; Guo, J.; Mao, Z.; Liu, S.; Wang, B.; Bai, Z.; Hou, X. MicroRNA-130a Increases and Predicts Cardiotoxicity during Adjuvant Chemotherapy in Human Epidermal Growth Factor Receptor-2-Positive Breast Cancer. J. Breast Cancer 2021, 24, 153–163. [Google Scholar] [CrossRef]
- Rigaud, V.O.; Ferreira, L.R.; Ayub-Ferreira, S.M.; Ávila, M.S.; Brandão, S.M.; Cruz, F.D.; Santos, M.H.; Cruz, C.B.; Alves, M.S.; Issa, V.S.; et al. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget 2017, 8, 6994–7002. [Google Scholar] [CrossRef]
- Qin, X.; Chang, F.; Wang, Z.; Jiang, W. Correlation of circulating pro-angiogenic miRNAs with cardiotoxicity induced by epirubicin/cyclophosphamide followed by docetaxel in patients with breast cancer. Cancer Biomark. 2018, 23, 473–484. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, X.; Dong, H.; Ke, S.; Zheng, W.H. Let-7f and miRNA-126 correlate with reduced cardiotoxicity risk in triple-negative breast cancer patients who underwent neoadjuvant chemotherapy. Int. J. Clin. Exp. Pathol. 2018, 11, 4987–4995. [Google Scholar] [PubMed]
- Alves, M.T.; da Conceição, I.M.C.A.; de Oliveira, A.N.; Oliveira, H.H.M.; Soares, C.E.; de Paula Sabino, A.; Silva, L.M.; Simões, R.; Luizon, M.R.; Gomes, K.B. microRNA miR-133a as a Biomarker for Doxorubicin-Induced Cardiotoxicity in Women with Breast Cancer: A Signaling Pathway Investigation. Cardiovasc. Toxicol. 2022, 22, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Valderrama, M.R.; Bevilacqua, E.; Echevarría, M.; Salvador-Bofill, F.J.; Ordóñez, A.; López-Haldón, J.E.; Smani, T.; Calderón-Sánchez, E.M. Early Myocardial Strain Reduction and miR-122-5p Elevation Associated with Interstitial Fibrosis in Anthracycline-Induced Cardiotoxicity. Biomedicines 2024, 13, 45. [Google Scholar] [CrossRef]
- Todorova, V.K.; Makhoul, I.; Wei, J.; Klimberg, V.S. Circulating miRNA Profiles of Doxorubicin-induced Cardiotoxicity in Breast Cancer Patients. Ann. Clin. Lab. Sci. 2017, 47, 115–119. [Google Scholar]
- Zhao, Z.; He, J.; Zhang, J.; Liu, M.; Yang, S.; Li, N.; Li, X. Dysregulated miR1254 and miR579 for cardiotoxicity in patients treated with bevacizumab in colorectal cancer. Tumour Biol. 2014, 35, 5227–5235. [Google Scholar] [CrossRef]
- Zhou, F.; Lu, X.; Zhang, X. Serum miR-30c Level Predicted Cardiotoxicity in Non-small Cell Lung Cancer Patients Treated with Bevacizumab. Cardiovasc. Toxicol. 2018, 18, 284–289. [Google Scholar] [CrossRef]
- Pillai, S.S.; Pereira, D.G.; Bonsu, G.; Chaudhry, H.; Puri, N.; Lakhani, H.V.; Tirona, M.T.; Sodhi, K.; Thompson, E. Biomarker panel for early screening of trastuzumab -induced cardiotoxicity among breast cancer patients in west virginia. Front. Pharmacol. 2022, 13, 953178. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Reinal, I.; Peiró-Molina, E.; Buigues, M.; Tejedor, S.; Hernándiz, A.; Selva, M.; Hervás, D.; Cañada, A.J.; Dorronsoro, A.; et al. MicroRNA-4732-3p Is Dysregulated in Breast Cancer Patients with Cardiotoxicity, and Its Therapeutic Delivery Protects the Heart from Doxorubicin-Induced Oxidative Stress in Rats. Antioxidants 2022, 11, 1955. [Google Scholar] [CrossRef]
- Gioffré, S.; Chiesa, M.; Cardinale, D.M.; Ricci, V.; Vavassori, C.; Cipolla, C.M.; Masson, S.; Sandri, M.T.; Salvatici, M.; Ciceri, F.; et al. Circulating MicroRNAs as Potential Predictors of Anthracycline-Induced Troponin Elevation in Breast Cancer Patients: Diverging Effects of Doxorubicin and Epirubicin. J. Clin. Med. 2020, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- Zare, N.; Dana, N.; Mosayebi, A.; Vaseghi, G.; Javanmard, S.H. Evaluation of Expression Level of miR-3135b-5p in Blood Samples of Breast Cancer Patients Experiencing Chemotherapy-Induced Cardiotoxicity. Indian. J. Clin. Biochem. 2023, 38, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Yadi, W.; Shurui, C.; Tong, Z.; Suxian, C.; Qing, T.; Dongning, H. Bioinformatic analysis of peripheral blood miRNA of breast cancer patients in relation with anthracycline cardiotoxicity. BMC Cardiovasc. Disord. 2020, 20, 43. [Google Scholar] [CrossRef]
- Frères, P.; Bouznad, N.; Servais, L.; Josse, C.; Wenric, S.; Poncin, A.; Thiry, J.; Moonen, M.; Oury, C.; Lancellotti, P.; et al. Variations of circulating cardiac biomarkers during and after anthracycline-containing chemotherapy in breast cancer patients. BMC Cancer 2018, 18, 102. [Google Scholar] [CrossRef]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Deng, Z.; Wang, Y.; Zheng, Y.; Jiang, W.; Jiang, L. MicroRNA expression profiling involved in doxorubicin-induced cardiotoxicity using high-throughput deep-sequencing analysis. Oncol. Lett. 2021, 22, 560. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, M.; Zhao, W.; Shu, Y.; Gao, M.; Zhuang, Y.; Yang, T.; Mu, W.; Li, T.; Li, X.; et al. Let-7a regulates expression of β1-adrenoceptors and forms a negative feedback circuit with the β1-adrenoceptor signaling pathway in chronic ischemic heart failure. Oncotarget 2017, 8, 8752–8764. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.H.; Feng, X.; Zhang, Y.W.; Lou, X.Y.; Cheng, Y.; Zhou, H.H. Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. Int. J. Mol. Sci. 2013, 14, 23086–23102. [Google Scholar] [CrossRef]
- Tolonen, A.M.; Magga, J.; Szabó, Z.; Viitala, P.; Gao, E.; Moilanen, A.M.; Ohukainen, P.; Vainio, L.; Koch, W.J.; Kerkelä, R.; et al. Inhibition of Let-7 microRNA attenuates myocardial remodeling and improves cardiac function postinfarction in mice. Pharmacol. Res. Perspect. 2014, 2, e00056. [Google Scholar] [CrossRef]
- Yu, H.; Lu, Y.; Li, Z.; Wang, Q. microRNA-133: Expression, function and therapeutic potential in muscle diseases and cancer. Curr. Drug Targets 2014, 15, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Townley-Tilson, W.H.; Callis, T.E.; Wang, D. MicroRNAs 1, 133, and 206: Critical factors of skeletal and cardiac muscle development, function, and disease. Int. J. Biochem. Cell Biol. 2010, 42, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Totoń-Żurańska, J.; Sulicka-Grodzicka, J.; Seweryn, M.T.; Pitera, E.; Kapusta, P.; Konieczny, P.; Drabik, L.; Kołton-Wróż, M.; Chyrchel, B.; Nowak, E.; et al. MicroRNA composition of plasma extracellular vesicles: A harbinger of late cardiotoxicity of doxorubicin. Mol. Med. 2022, 28, 156. [Google Scholar] [CrossRef]
- Jeyabal, P.; Bhagat, A.; Wang, F.; Roth, M.; Livingston, J.A.; Gilchrist, S.C.; Banchs, J.; Hildebrandt, M.A.T.; Chandra, J.; Deswal, A.; et al. Circulating microRNAs and Cytokines as Prognostic Biomarkers for Doxorubicin-Induced Cardiac Injury and for Evaluating the Effectiveness of an Exercise Intervention. Clin. Cancer Res. 2023, 29, 4430–4440. [Google Scholar] [CrossRef]
- Li, Z.; Ye, Z.; Ma, J.; Gu, Q.; Teng, J.; Gong, X. MicroRNA-133b alleviates doxorubicin-induced cardiomyocyte apoptosis and cardiac fibrosis by targeting PTBP1 and TAGLN2. Int. J. Mol. Med. 2021, 48, 125. [Google Scholar] [CrossRef]
- Roca-Alonso, L.; Castellano, L.; Mills, A.; Dabrowska, A.F.; Sikkel, M.B.; Pellegrino, L.; Jacob, J.; Frampton, A.E.; Krell, J.; Coombes, R.C.; et al. Myocardial MiR-30 downregulation triggered by doxorubicin drives alterations in β-adrenergic signaling and enhances apoptosis. Cell Death Dis. 2015, 6, e1754. [Google Scholar] [CrossRef]
- Abdellatif, M. The role of microRNA-133 in cardiac hypertrophy uncovered. Circ. Res. 2010, 106, 16–18. [Google Scholar] [CrossRef]
- Liu, Y.; Taylor, N.E.; Lu, L.; Usa, K.; Cowley, A.W.; Ferreri, N.R.; Yeo, N.C.; Liang, M. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension 2010, 55, 974–982. [Google Scholar] [CrossRef]
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.D.; Esfandyari, D.; Papadopoulou, A.S.; De Strooper, B.; et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat. Commun. 2017, 8, 1614. [Google Scholar] [CrossRef]
- Roncarati, R.; Viviani Anselmi, C.; Losi, M.A.; Papa, L.; Cavarretta, E.; Da Costa Martins, P.; Contaldi, C.; Saccani Jotti, G.; Franzone, A.; Galastri, L.; et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, K.; Ito, R.; Okada, M.; Yoshioka, K.; Uchide, T.; Temma, K.; Mutoh, K.; Uechi, M.; Hara, Y. Enhanced gene expression of myocardial matrix metalloproteinases 2 and 9 after acute treatment with doxorubicin in mice. Pharmacol. Res. 2006, 53, 341–346. [Google Scholar] [CrossRef]
- Lai, L.; Chen, J.; Wang, N.; Zhu, G.; Duan, X.; Ling, F. MiRNA-30e mediated cardioprotection of ACE2 in rats with Doxorubicin-induced heart failure through inhibiting cardiomyocytes autophagy. Life Sci. 2017, 169, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, S.; Zhang, W.; Jia, Q.; Wang, L.; Ding, Y.; Yuan, P.; Zhu, Y.; Liu, L.; Li, Y.; et al. Shenmai injection improves doxorubicin cardiotoxicity via miR-30a/Beclin 1. Biomed. Pharmacother. 2021, 139, 111582. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Gu, J.; Zhuang, T.; Zhang, J.; Fan, C.; Li, Y.; Zhao, M.; Chen, R.; Wang, R.; Kong, Y.; et al. MicroRNA-126: From biology to therapeutics. Biomed. Pharmacother. 2025, 185, 117953. [Google Scholar] [CrossRef]
- Zhu, X.D.; Chi, J.Y.; Liang, H.H.; Huangfu, L.T.; Guo, Z.D.; Zou, H.; Yin, X.H. MicroRNA-377 Mediates Cardiomyocyte Apoptosis Induced by Cyclosporin A. Can. J. Cardiol. 2016, 32, 1249–1259. [Google Scholar] [CrossRef]
- Long, G.; Wang, F.; Duan, Q.; Chen, F.; Yang, S.; Gong, W.; Wang, Y.; Chen, C.; Wang, D.W. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. Int. J. Biol. Sci. 2012, 8, 811–818. [Google Scholar] [CrossRef]
- Fourdinier, O.; Glorieux, G.; Brigant, B.; Diouf, M.; Pletinck, A.; Vanholder, R.; Choukroun, G.; Verbeke, F.; Massy, Z.A.; Metzinger-Le Meuth, V.; et al. Syndecan-1 and Free Indoxyl Sulfate Levels Are Associated with miR-126 in Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 549. [Google Scholar] [CrossRef]
- Bejleri, J.; Jirström, E.; Donovan, P.; Williams, D.J.; Pfeiffer, S. Diagnostic and Prognostic Circulating MicroRNA in Acute Stroke: A Systematic and Bioinformatic Analysis of Current Evidence. J. Stroke 2021, 23, 162–182. [Google Scholar] [CrossRef]
- Liu, H.; Huan, L.; Yin, J.; Qin, M.; Zhang, Z.; Zhang, J.; Wang, S. Role of microRNA-130a in myocardial hypoxia/reoxygenation injury. Exp. Ther. Med. 2017, 13, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Y.; Cao, J.; Gao, Q.; Li, H.; Chen, Y.; Lu, N. MiR-130a inhibition protects rat cardiac myocytes from hypoxia-triggered apoptosis by targeting Smad4. Kardiol. Pol. 2018, 76, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tao, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 2018, 16, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Huang, W.L.; Peng, X.P.; Lv, Y.N.; Li, J.H.; Xiong, J.P. miR-140-5p mediates bevacizumab-induced cytotoxicity to cardiomyocytes by targeting the VEGFA/14-3-3γ signal pathway. Toxicol. Res. 2019, 8, 875–884. [Google Scholar] [CrossRef]
- Yin, Z.; Zhao, Y.; Li, H.; Yan, M.; Zhou, L.; Chen, C.; Wang, D.W. miR-320a mediates doxorubicin-induced cardiotoxicity by targeting VEGF signal pathway. Aging 2016, 8, 192–207. [Google Scholar] [CrossRef]
- Wang, W.; Dong, L.; Lv, H.; An, Y.; Zhang, C.; Zheng, Z.; Guo, Y.; He, L.; Wang, L.; Wang, J.; et al. Downregulating miRNA-199a-5p exacerbates fluorouracil-induced cardiotoxicity by activating the ATF6 signaling pathway. Aging 2024, 16, 5916–5928. [Google Scholar] [CrossRef]
- Nourmohammadi, K.; Bayrami, A.; Naderi, R.; Shirpoor, A. Cyclosporine A induces cardiac remodeling through TGF-β/Smad3/miR-29 signaling pathway and alters gene expression of miR-30b-5p/CaMKIIδ isoforms pathways: Alleviating effects of moderate exercise. Mol. Biol. Rep. 2023, 50, 5859–5870. [Google Scholar] [CrossRef]
- Sorodoc, V.; Sirbu, O.; Lionte, C.; Haliga, R.E.; Stoica, A.; Ceasovschih, A.; Petris, O.R.; Constantin, M.; Costache, I.I.; Petris, A.O.; et al. The Value of Troponin as a Biomarker of Chemotherapy-Induced Cardiotoxicity. Life 2022, 12, 1183. [Google Scholar] [CrossRef]
- Simões, R.; Silva, L.M.; Cruz, A.L.V.M.; Fraga, V.G.; de Paula Sabino, A.; Gomes, K.B. Troponin as a cardiotoxicity marker in breast cancer patients receiving anthracycline-based chemotherapy: A narrative review. Biomed. Pharmacother. 2018, 107, 989–996. [Google Scholar] [CrossRef]
- Hinrichs, L.; Mrotzek, S.M.; Mincu, R.I.; Pohl, J.; Röll, A.; Michel, L.; Mahabadi, A.A.; Al-Rashid, F.; Totzeck, M.; Rassaf, T. Troponins and Natriuretic Peptides in Cardio-Oncology Patients-Data From the ECoR Registry. Front. Pharmacol. 2020, 11, 740. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Chen, C.; Han, C.; Xue, L.; Xing, D.; Huang, O.; Tao, M. BNP as a marker for early prediction of anthracycline-induced cardiotoxicity in patients with breast cancer. Oncol. Lett. 2019, 18, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wu, Q.; Hu, C. Early Predictive Value of NT-proBNP Combined With Echocardiography in Anthracyclines Induced Cardiotoxicity. Front. Surg. 2022, 9, 898172. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef]
- Schneider, S.I.D.R.; Silvello, D.; Martinelli, N.C.; Garbin, A.; Biolo, A.; Clausell, N.; Andrades, M.; Dos Santos, K.G.; Rohde, L.E. Plasma levels of microRNA-21, -126 and -423-5p alter during clinical improvement and are associated with the prognosis of acute heart failure. Mol. Med. Rep. 2018, 17, 4736–4746. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, C.; Liu, W.; Chen, X.; Wang, Y.; Liu, X.; Yue, J.; Yang, J.; Zhou, X. Upregulation of let-7f-2-3p by long noncoding RNA NEAT1 inhibits XPO1-mediated HAX-1 nuclear export in both in vitro and in vivo rodent models of doxorubicin-induced cardiotoxicity. Arch. Toxicol. 2019, 93, 3261–3276. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhao, P.; Zhou, Y.; Xing, C.; Zhao, L.; Li, Z.; Yuan, L. Ultrasound targeted microbubble destruction assisted exosomal delivery of miR-21 protects the heart from chemotherapy associated cardiotoxicity. Biochem. Biophys. Res. Commun. 2020, 532, 60–67. [Google Scholar] [CrossRef]
- Yu, X.; Ruan, Y.; Shen, T.; Qiu, Q.; Yan, M.; Sun, S.; Dou, L.; Huang, X.; Wang, Q.; Zhang, X.; et al. Dexrazoxane Protects Cardiomyocyte from Doxorubicin-Induced Apoptosis by Modulating miR-17-5p. Biomed. Res. Int. 2020, 2020, 5107193. [Google Scholar] [CrossRef]
| miR | Mechanism of CTRCD | Regulation | Associated Pathways |
|---|---|---|---|
| miR-1 | Oxidative stress and endogenous apoptosis | Upregulation | Bcl-2 in cardiomyocytes |
| let-7 (a, b, f, g) | Apoptosis and fibrosis | Down- or upregulation | BCL2 family members expression and caspase-3 activity |
| miR-21 | Oxidative stress and endogenous apoptosis | Upregulation | Targeting redox genes such as SOD2, PTEN, caspase-9 |
| miR-29b | Apoptosis and fibrosis, extracellular matrix remodeling | Upregulation | Pro-apoptotic Bax and collagen protein |
| miR-30 | Oxidative stress, mitochondrial function and apoptosis | Downregulation | GATA-6 and other pro-apoptotic proteins |
| miR-34a | Apoptosis, Fe-mediated oxidative stress and fibrosis | Upregulation | Regulation of SIRT1 |
| miR-126 | Angiogenesis and fibrosis | Downregulation | Regulation of VEGF inhibitor |
| miR-130a | Mitochondrial dysfunction, oxidative stress, exogenous apoptosis and inflammation | Upregulation | Increase in mitochondrial fission and caspase-3 activation, reduce of mitochondrial fusion |
| miR-133a/b | Apoptosis and fibrosis | Down- or Upregulation | Pro-inflammatory signals |
| miR-140-5p | Oxidative stress, VEGFA pathway | Upregulation | Targeting Nrf2 and Sirt2 |
| miR-144 | Oxidative stress | Downregulation | Nrf2 suppression |
| miR-146a | Apoptosis and fibrosis | Up- or downregulation | Regulation of pro-inflammatory proteins |
| miR-200a | Oxidative stress | Downregulation | Nrf2 suppression |
| miR-210 | Oxidative stress, Fe homeostasis, apoptosis and angiogenesis | Downregulation | Targeting caspase-3, caspase-8, AIFM3, VEGF, HGF |
| miR-423 | Apoptosis and oxidative stress | Upregulation | Activation of caspases |
| miR-499 | Mitochondrial dysfunction, apoptosis and fibrosis | Up- or downregulation | P21 pathway |
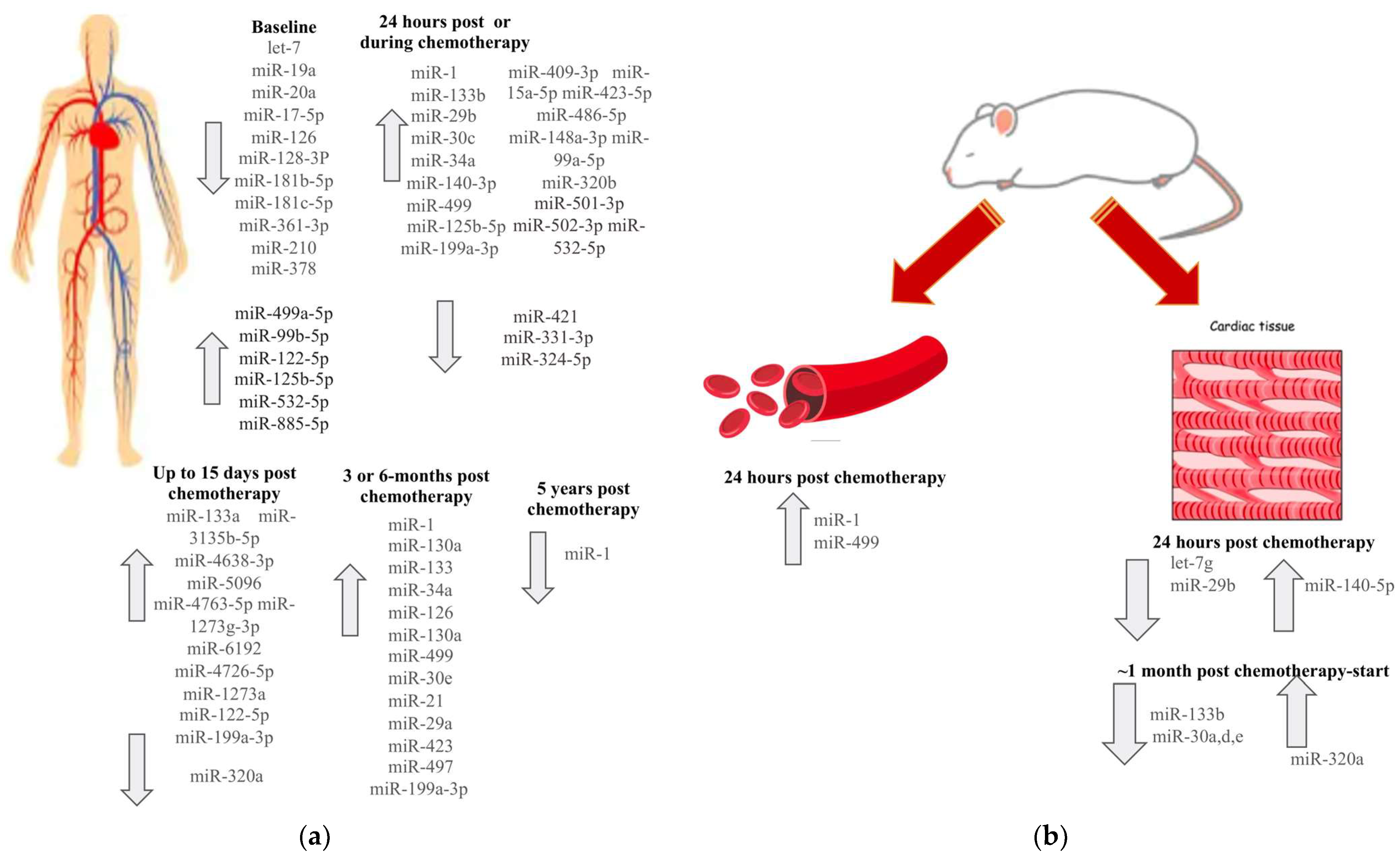
| Time of Blood Samples Collection | Chemotherapy Regimens | N of Patients (N with Cardiotoxicity) | miR | Expression Correlated with Cardiotoxicity | Reference |
|---|---|---|---|---|---|
| During chemotherapy | |||||
| At baseline, after 2 weeks of neo-adjuvant anti-HER2 therapy, and immediately before surgery | Trastuzumab + Paclitaxel or Lapatinib + Paclitaxel or Trastuzumab + Lapatinib + Paclitaxel → surgery → FECx3 | 9 (9) | At 2 weeks interval: miR-125b-5p, miR-409-3p, miR-15a-5p, miR-423-5p, miR-148a-3p, miR-99a-5p, and miR-320b | Upregulation | [63] |
| Every 3 months throughout the 15-month of adjuvant treatment for HER-2 positive breast cancer | EC-D + Trastuzumab | 72 (12) | miR-130a | Upregulation | [64] |
| Baseline, 3 weeks after each cycle of doxorubicin | Doxorubicin + cyclophosphamide every 3 weeks for 4 cycles followed by paclitaxel for 12 weeks or docetaxel every 3 weeks | 56 (10) | miR-1 | Upregulation | [65] |
| Baseline | EC-D neoadjuvant chemotherapy | 363 (19) | let-7f, miR-17-5p, miR-20a, miR-126, miR-210 and miR-378 | Downregulation | [66] |
| Before and after a cycle of chemotherapy (6, 12 and 24 h) | 24 patients AC, 9 patients non-anthracycline-chemotherapy | 33 | miR-1, miR-29b, miR-499 | Upregulation | [59] |
| Baseline | EC-D | 179 (9) | let-7f, miR-19a, miR-20a, miR-126, and miR-210 | Downregulation | [67] |
| Baseline and up to 7 days after the completion of chemotherapy | All patients received doxorubicin. 4 patients with HER2+ cancer and 2 patients with TNBC in each group | 12 (6) | miR-133a | Upregulation | [68] |
| Baseline and 4-cycle treatment | 4 cycles of anthracycline (one every 21 days) | 10 (5) | miR-122-5p | Upregulation | [69] |
| Baseline and after the first cycle of chemotherapy | AC | 20 (8) | miR-140-3p, miR-486-5p, miR-501-3p, miR-502-3p, miR-532-5p/miR-421 miR-331-3p, miR-324-5p | Upregulation/Downregulation | [70] |
| Within 24 h since the onset of symptoms of CHF in comparison with control patients | Bevacizumab | 178 (88) | miR-1254, miR-579 | Upregulation | [71] |
| Before, during (at 2, 4 and 8 weeks) and 1 month after chemotherapy | Bevacizumab + chemotherapy | 80 (30) | miR-30c | Upregulation | [72] |
| Long-term follow up | |||||
| At baseline, 3 and 6 months post trastuzumab therapy | Trastuzumab ± pertuzumab + Paclitaxel or Docetaxel ± carboplatin | 17 (3) | miR-34a, miR-21, miR-133, miR-1, miR-30e | Upregulation | [73] |
| At baseline, 3 and 6 months post doxorubicin therapy | Doxorubicin | 17 (4) | miR-423, miR-499, miR-126, miR-29a, miR-34a | Upregulation | [60] |
| Baseline and different intervals after completion of treatment | TAC or AC | 20 (10) in main cohort and 32 (7) in validation cohort | miR-4732-3p | Downregulation | [74] |
| Baseline and at 1, 3, 6 and 12 months after the end of chemotherapy | Doxorubicin or epirubicin | 88 (30) | At baseline: miR-499a-5p, miR-99b-5p, miR-122-5p, miR-125b-5p, miR-532-5p and miR-885-5p/miR-128-3p, miR-181b-5p, miR-181c-5p and miR-361-3p | Upregulation/Downregulation | [75] |
| After adjuvant chemotherapy | Anthracycline-based regimen, followed by paclitaxel or docetaxel | 70 (33) | miR-3135b-5p | Upregulation | [76] |
| Before and after chemotherapy | Anthracyclines | 19 (5) | miR-4638-3p, miR-5096, miR-4763-5p, miR-1273 g-3p, miR-6192, miR-4726-5p, and miR-1273a | Upregulation | [77] |
| Baseline, after 2 cycles, 8 days before surgery and 3 months later | Neoadjuvant 4 cycles EC → Paclitaxel 12 weeks 17 patients with HER2-positive cancer received trastuzumab or lapatinib | 45 (7) | miR-126-3p, miR-199a-3p, miR-423-5p, miR-34a-5p | Upregulation | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiou, M.; Oikonomou, E.; Theofilis, P.; Gazouli, M.; Psyrri, A.; Zagouri, F.; Siasos, G.; Tousoulis, D. The Role of miRNAs in Chemotherapy-Induced Cardiotoxicity. Biomedicines 2025, 13, 2331. https://doi.org/10.3390/biomedicines13102331
Anastasiou M, Oikonomou E, Theofilis P, Gazouli M, Psyrri A, Zagouri F, Siasos G, Tousoulis D. The Role of miRNAs in Chemotherapy-Induced Cardiotoxicity. Biomedicines. 2025; 13(10):2331. https://doi.org/10.3390/biomedicines13102331
Chicago/Turabian StyleAnastasiou, Maria, Evangelos Oikonomou, Panagiotis Theofilis, Maria Gazouli, Amanda Psyrri, Flora Zagouri, Gerasimos Siasos, and Dimitrios Tousoulis. 2025. "The Role of miRNAs in Chemotherapy-Induced Cardiotoxicity" Biomedicines 13, no. 10: 2331. https://doi.org/10.3390/biomedicines13102331
APA StyleAnastasiou, M., Oikonomou, E., Theofilis, P., Gazouli, M., Psyrri, A., Zagouri, F., Siasos, G., & Tousoulis, D. (2025). The Role of miRNAs in Chemotherapy-Induced Cardiotoxicity. Biomedicines, 13(10), 2331. https://doi.org/10.3390/biomedicines13102331










