Monocyte-Derived Macrophages Expressing Dopamine D2-Subtype Receptors Drive Alcohol Effects on Mesolimbic Neurons and Microglia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Subjects
2.2. Flow Cytometry for Impact of In Vivo Ethanol on Lymphocyte and Monocyte Dopamine Subtype-2 Receptor Expression
2.3. In Vitro Incubation of Lymphocytes and Monocytes and Expression of D2Rs
2.4. Immunofluorescence Staining for Microglial Dopamine Subtype-2 Receptor Expression
2.5. Confocal Microscopy for Microglia Volume-to-Surface-Area Ratio
2.6. Isolation of Brain Microglia and PBMCs
2.7. Submandibular Blood Collection and Flow Cytometry in Clodronate Studies
2.8. In Vivo Single-Unit Recordings
2.9. Liposomal Clodronate Injection
2.10. Microdialysis and High-Performance Liquid Chromatography
2.11. Behavioral Procedures
2.12. Drugs and Chemicals
2.13. Data Analysis and Statistics
3. Results
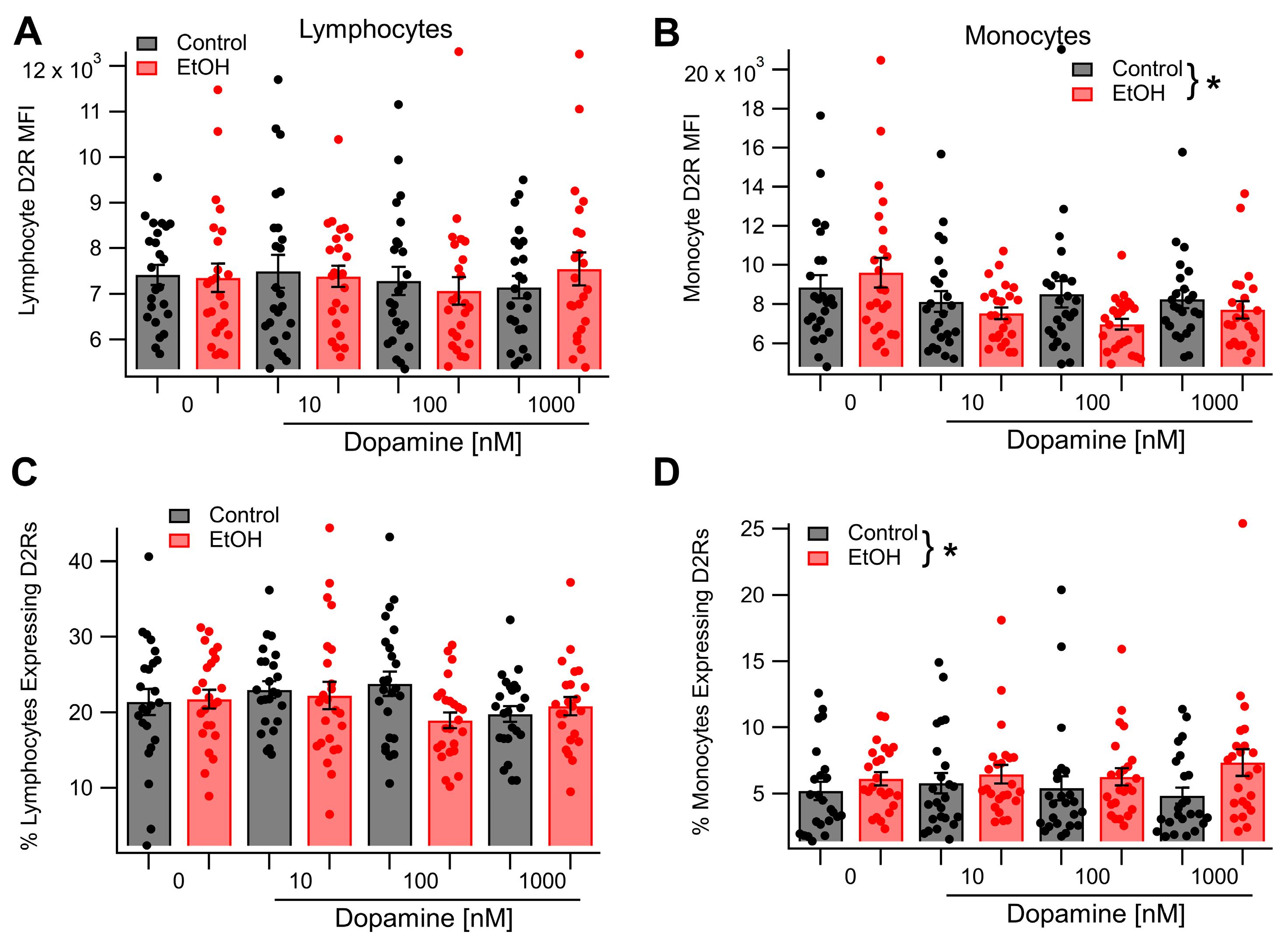
3.1. Acute Ethanol Enhances Lymphocyte and Monocyte D2R Expression In Vivo
3.2. Dopamine Receptor 2 Expression in Monocytes, but Not Lymphocytes, Is Sensitive to Incubation in Dopamine and Ethanol In Vitro
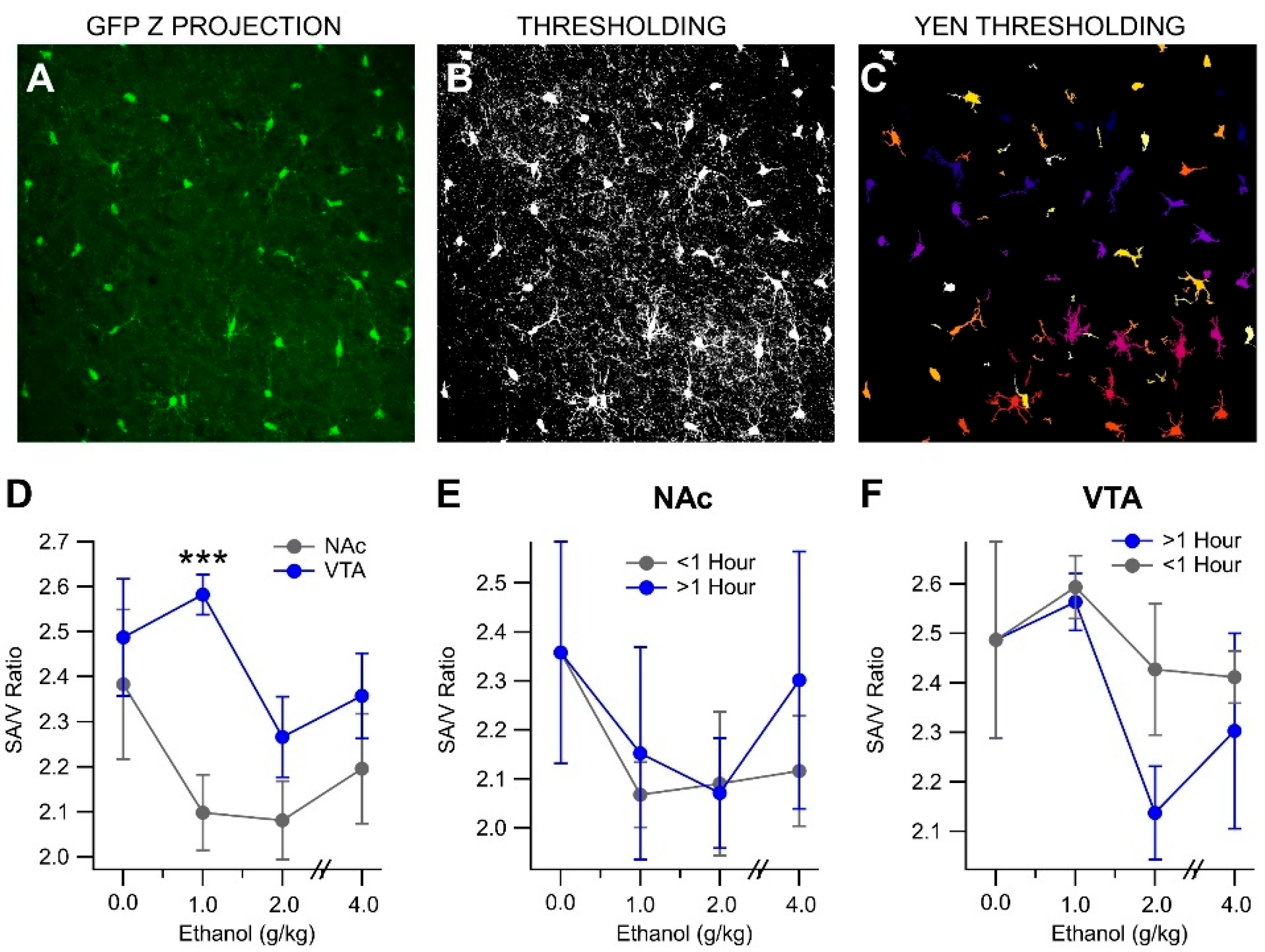
3.3. In Vivo Acute Ethanol Modulates Microglial D2R Expression
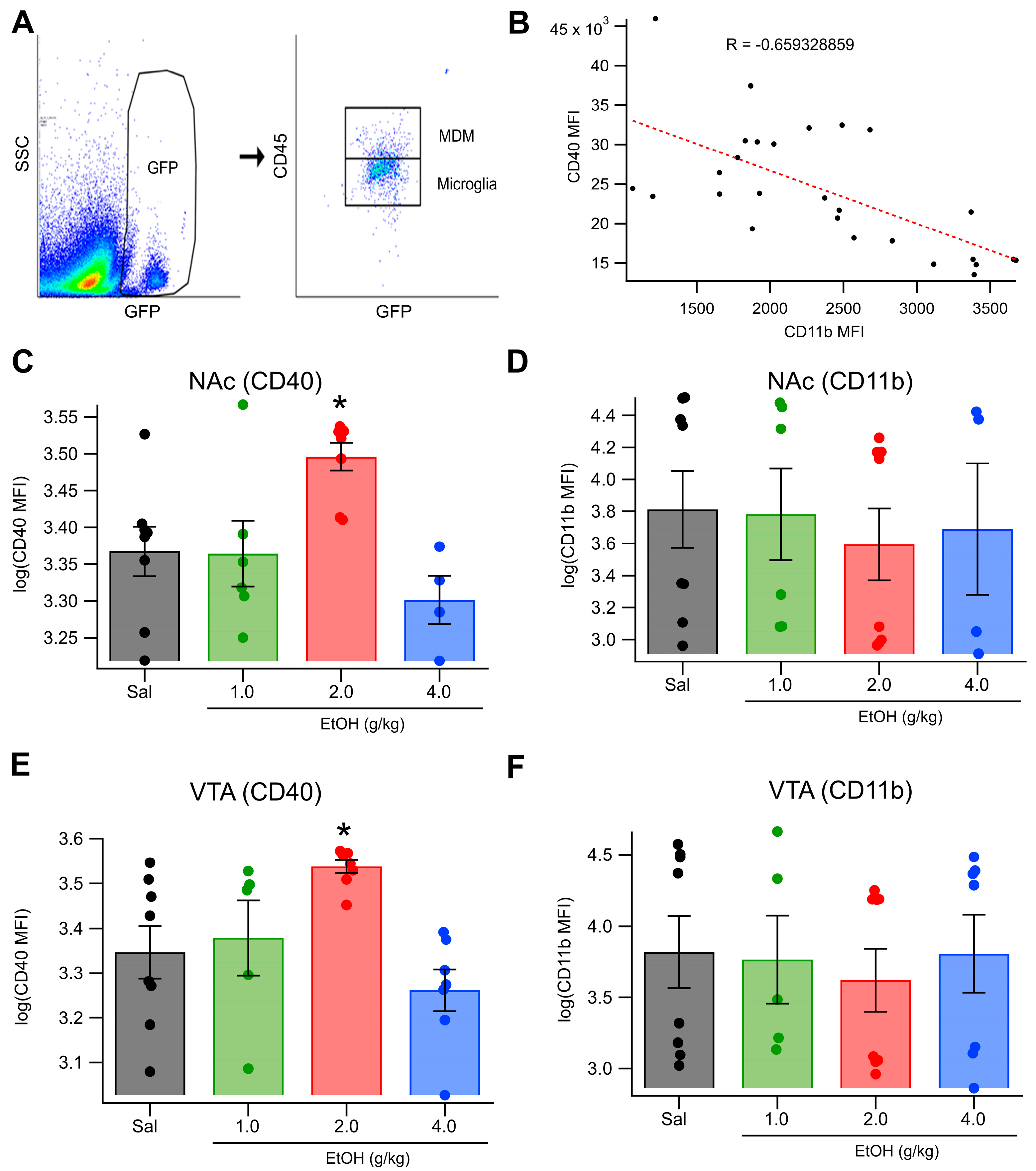
3.4. In Vivo Acute Ethanol Leads to the Activation of Mesolimbic Microglia
3.5. In Vivo Acute Ethanol Alters Microglia Polarization
3.6. Ethanol Enhances D2R-Dependent Activation of VTA GABA Neurons over a Protracted Period
3.7. Clodronate Reduces MDMs and Blocks EtOH Effects on VTA GABA Neurons and Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Alcohol use disorder |
| VTA | Ventral tegmental area |
| DA | Dopamine |
| NAc | Nucleus accumbens |
| EtOH | Ethanol |
| GABA | γ-aminobutyric acid |
| D2Rs | Dopamine 2 receptors |
| NK | Natural killer |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| TLRs | Toll-like receptors |
| MDMs | Monocyte-derived macrophages |
| MaFIA | Macrophage FAS-Induced Apoptosis |
| IP | Intraperitoneal |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| BSA | Bovine serum albumin |
| RLS | Restless legs syndrome |
| MFI | Mean fluorescent intensity |
| IHC | Immunohistochemistry |
| CLOD | Clodronate liposomes |
References
- Stahre, M.; Roeber, J.; Kanny, D.; Brewer, R.D.; Zhang, X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev. Chronic. Dis. 2014, 11, E109. [Google Scholar] [CrossRef]
- Wise, R.A. Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotox. Res. 2008, 14, 169–183. [Google Scholar] [CrossRef]
- Theile, J.W.; Morikawa, H.; Gonzales, R.A.; Morrisett, R.A. GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience 2011, 172, 94–103. [Google Scholar] [CrossRef]
- Gallegos, R.A.; Criado, J.R.; Lee, R.S.; Henriksen, S.J.; Steffensen, S.C. Adaptive responses of GABAergic neurons in the ventral tegmental area to chronic ethanol. J. Pharmacol. Exp. Ther. 1999, 291, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Gessa, G.L.; Muntoni, F.; Collu, M.; Vargiu, L.; Mereu, G. Low doses of ethanol activate dopaminergic neurons of the ventral tegmental area. Brain Res. 1985, 348, 201. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.J.; Schallert, T.; Randall, P.K.; Gonzales, R.A. Comparison of local and systemic ethanol effects on extracellular dopamine concentration in rat nucleus accumbens by microdialysis. Alcohol. Clin. Exp. Res. 1998, 22, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.S.; Appel, S.B. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol. Clin. Exp. Res. 1998, 22, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.S.; Appel, S.B. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol. Clin. Exp. Res. 2000, 24, 1120–1124. [Google Scholar] [CrossRef]
- Brodie, M.S.; Dunwiddie, T.V. Cocaine effects in the ventral tegmental area: Evidence for an indirect dopaminergic mechanism of action. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1990, 342, 660–665. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, J.; Krnjevic, K.; Ye, J.H. Effects of ethanol on midbrain neurons: Role of opioid receptors. Alcohol. Clin. Exp. Res. 2007, 31, 1106–1113. [Google Scholar] [CrossRef]
- Steffensen, S.C.; Shin, S.I.; Nelson, A.C.; Pistorius, S.S.; Williams, S.B.; Woodward, T.J.; Park, H.J.; Friend, L.; Gao, M.; Gao, F.; et al. alpha6 subunit-containing nicotinic receptors mediate low-dose ethanol effects on ventral tegmental area neurons and ethanol reward. Addict. Biol. 2017, 23, 1079–1093. [Google Scholar] [CrossRef]
- Steffensen, S.C.; Walton, C.H.; Hansen, D.M.; Yorgason, J.T.; Gallegos, R.A.; Criado, J.R. Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol. Biochem. Behav. 2009, 92, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Stobbs, S.H.; Ohran, A.J.; Lassen, M.B.; Allison, D.W.; Brown, J.E.; Steffensen, S.C. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-Methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 2004, 311, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, K.H.; Bradley, K.D.; Allison, D.W.; Taylor, S.R.; Yorgason, J.T.; Hansen, D.M.; Walton, C.H.; Sudweeks, S.N.; Steffensen, S.C. Acute and chronic ethanol modulate dopamine D2-subtype receptor responses in ventral tegmental area GABA neurons. Alcohol. Clin. Exp. Res. 2009, 33, 804–811. [Google Scholar] [CrossRef]
- Steffensen, S.C.; Bradley, K.D.; Hansen, D.M.; Wilcox, J.D.; Wilcox, R.S.; Allison, D.W.; Merrill, C.B.; Edwards, J.G. The role of connexin-36 gap junctions in alcohol intoxication and consumption. Synapse 2011, 65, 695–707. [Google Scholar] [CrossRef]
- Nelson, A.C.; Williams, S.B.; Pistorius, S.S.; Park, H.J.; Woodward, T.J.; Payne, A.J.; Obray, J.D.; Shin, S.I.; Mabey, J.K.; Steffensen, S.C. Ventral Tegmental Area GABA Neurons Are Resistant to GABA(A) Receptor-Mediated Inhibition During Ethanol Withdrawal. Front. Neurosci. 2018, 12, 131. [Google Scholar] [CrossRef]
- Williams, S.B.; Yorgason, J.T.; Nelson, A.C.; Lewis, N.; Nufer, T.M.; Edwards, J.G.; Steffensen, S.C. Glutamate Transmission to Ventral Tegmental Area GABA Neurons Is Altered by Acute and Chronic Ethanol. Alcohol. Clin. Exp. Res. 2018, 42, 2186–2195. [Google Scholar] [CrossRef] [PubMed]
- Yorgason, J.T.; Rose, J.H.; McIntosh, J.M.; Ferris, M.J.; Jones, S.R. Greater ethanol inhibition of presynaptic dopamine release in C57BL/6J than DBA/2J mice: Role of nicotinic acetylcholine receptors. Neuroscience 2015, 284, 854–864. [Google Scholar] [CrossRef]
- Yorgason, J.T.; Wadsworth, H.A.; Anderson, E.J.; Williams, B.M.; Brundage, J.N.; Hedges, D.M.; Stockard, A.L.; Jones, S.T.; Arthur, S.B.; Hansen, D.M.; et al. Modulation of dopamine release by ethanol is mediated by atypical GABA(A) receptors on cholinergic interneurons in the nucleus accumbens. Addict. Biol. 2022, 27, e13108. [Google Scholar] [CrossRef]
- Yorgason, J.T.; Ferris, M.J.; Steffensen, S.C.; Jones, S.R. Frequency-dependent effects of ethanol on dopamine release in the nucleus accumbens. Alcohol. Clin. Exp. Res. 2014, 38, 438–447. [Google Scholar] [CrossRef]
- Obray, J.D.; Jang, E.Y.; Klomp, A.M.; Small, C.A.; Richardson, A.P.; LeBaron, J.J.; Lee, J.G.; Yorgason, J.T.; Yang, C.H.; Steffensen, S.C. The peripheral dopamine 2 receptor antagonist domperidone attenuates ethanol enhancement of dopamine levels in the nucleus accumbens. Alcohol. Clin. Exp. Res. 2022, 46, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, U.H.; Obray, J.D.; Hunsaker, E.; Garcia, B.T.; Clarke, T.J.; Hope, S.; Steffensen, S.C. Peripheral Dopamine in Restless Legs Syndrome. Front. Neurol. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Bode, J.C.; Bode, C.; Brenner, D.A.; Choudhry, M.A.; Hamilton, F.; Kang, Y.J.; Keshavarzian, A.; Rao, R.; Sartor, R.B.; et al. Alcohol, Intestinal Bacterial Growth, Intestinal Permeability to Endotoxin, and Medical Consequences: Summary of a Symposium. Alcohol 2008, 42, 349–361. [Google Scholar] [CrossRef]
- Petrasek, J.; Mandrekar, P.; Szabo, G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol. Res. Pract. 2010, 2010, 710381. [Google Scholar] [CrossRef]
- Banks, W.A. Blood-brain barrier transport of cytokines: A mechanism for neuropathology. Curr. Pharm. Des. 2005, 11, 973–984. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef]
- Lawson, L.J.; Perry, V.H.; Gordon, S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 1992, 48, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Schulz, C.; Geissmann, F. Development and homeostasis of “resident” myeloid cells: The case of the microglia. Glia 2013, 61, 112–120. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456. [Google Scholar] [CrossRef]
- Solito, E.; Sastre, M. Microglia Function in Alzheimer’s Disease. Front. Pharmacol. 2012, 3, 14. [Google Scholar] [CrossRef]
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.; Ruffini, F.; Bar-Or, A.; Antel, J.P. Microglia and multiple sclerosis. J. Neurosci. Res. 2005, 81, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.-F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimer’s Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004, 20, 467–473. [Google Scholar] [CrossRef]
- Alfonso-Loeches, S.; Guerri, C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit. Rev. Clin. Lab. Sci. 2011, 48, 19–47. [Google Scholar] [CrossRef]
- Bajo, M.; Montgomery, S.E.; Cates, L.N.; Nadav, T.; Delucchi, A.M.; Cheng, K.; Yin, H.; Crawford, E.F.; Roberts, A.J.; Roberto, M. Evaluation of TLR4 Inhibitor, T5342126, in Modulation of Ethanol-Drinking Behavior in Alcohol-Dependent Mice. Alcohol. Alcohol. 2016, 51, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lizarbe, S.; Pascual, M.; Guerri, C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 2009, 183, 4733–4744. [Google Scholar] [CrossRef]
- Burnett, S.H.; Kershen, E.J.; Zhang, J.; Zeng, L.; Straley, S.C.; Kaplan, A.M.; Cohen, D.A. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J. Leukoc. Biol. 2004, 75, 612–623. [Google Scholar] [CrossRef]
- Wadsworth, H.A.; Warnecke, A.M.P.; Barlow, J.C.; Robinson, J.K.; Steimle, E.; Ronstrom, J.W.; Williams, P.E.; Galbraith, C.J.; Baldridge, J.; Jakowec, M.W.; et al. Ivermectin increases striatal cholinergic activity to facilitate dopamine terminal function. Cell Biosci. 2024, 14, 50. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, A.G. Utilization of Information Measure as a Means of Image Thresholding. CVGIP Graph. Models Image Process. 1994, 56, 414–419. [Google Scholar] [CrossRef]
- Doube, M.; Klosowski, M.M.; Arganda-Carreras, I.; Cordelieres, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Herron, S.; Delpech, J.-C.; Madore, C.; Ikezu, T. Using mechanical homogenization to isolate microglia from mouse brain tissue to preserve transcriptomic integrity. STAR Protoc. 2022, 3, 101670. [Google Scholar] [CrossRef] [PubMed]
- Unviersity Vetrinarian & Animal Resources; Virginia Tech. SOP: Mouse Blood Collecetion from the Submandibular Vein (Cheek Punch); Unviersity Vetrinarian & Animal Resources; Virginia Tech: Blacksburg, VA, USA, 2017. [Google Scholar]
- Allison, D.W.; Ohran, A.J.; Stobbs, S.H.; Mameli, M.; Valenzuela, C.F.; Sudweeks, S.N.; Ray, A.P.; Henriksen, S.H.; Steffensen, S.C. Connexin-36 gap junctions mediate electrical coupling between ventral tegmental area GABA neurons. Synapse 2006, 60, 20–31. [Google Scholar] [CrossRef]
- Steffensen, S.C.; Svingos, A.L.; Pickel, V.M.; Henriksen, S.J. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J. Neurosci. 1998, 18, 8003–8015. [Google Scholar] [CrossRef]
- Steffensen, S.C.; Taylor, S.R.; Horton, M.L.; Barber, E.N.; Lyle, L.T.; Stobbs, S.H.; Allison, D.W. Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. Eur. J. Neurosci. 2008, 28, 2028–2040. [Google Scholar] [CrossRef]
- Van Rooijen, N. Kupffer cell depletion by liposome-delivered drugs: Comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology 1996, 23, 1239–1243. [Google Scholar] [CrossRef]
- Tang, K.; Mahata, S.K. Determination of Catecholamines in a Small Volume (25 μL) of Plasma from Conscious Mouse Tail Vein; Springer: New York, NY, USA, 2023; pp. 331–342. [Google Scholar]
- Cunningham, C.L.; Shields, C.N. Effects of sex on ethanol conditioned place preference, activity and variability in C57BL/6J and DBA/2J mice. Pharmacol. Biochem. Behav. 2018, 173, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Aloyo, V.J.; Weiss, B. Continuous treatment with the D2 dopamine receptor agonist quinpirole decreases D2 dopamine receptors, D2 dopamine receptor messenger RNA and proenkephalin messenger RNA, and increases mu opioid receptors in mouse striatum. Neuroscience 1993, 54, 669–680. [Google Scholar] [CrossRef]
- Feldon, J.; Shofel, A.; Weiner, I. Latent inhibition is unaffected by direct dopamine agonists. Pharmacol. Biochem. Behav. 1991, 38, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Engber, T.M.; Mahan, L.C.; Susel, Z.; Chase, T.N.; Monsma, F.J., Jr.; Sibley, D.R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 1990, 250, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Ivins, K.J.; Luedtke, R.R.; Artymyshyn, R.P.; Molinoff, P.B. Regulation of dopamine D2 receptors in a novel cell line (SUP1). Mol. Pharmacol. 1991, 39, 531–539. [Google Scholar] [CrossRef]
- Filtz, T.M.; Artymyshyn, R.P.; Guan, W.; Molinoff, P.B. Paradoxical regulation of dopamine receptors in transfected 293 cells. Mol. Pharmacol. 1993, 44, 371–379. [Google Scholar] [CrossRef]
- Starr, S.; Kozell, L.B.; Neve, K.A. Drug-induced up-regulation of dopamine D2 receptors on cultured cells. J. Neurochem. 1995, 65, 569–577. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Rasini, E.; Legnaro, M.; Bombelli, R.; Aleksic, I.; Blandini, F.; Comi, C.; Mauri, M.; Minafra, B.; Riboldazzi, G.; et al. Dopaminergic Receptors on CD4+ T Naive and Memory Lymphocytes Correlate with Motor Impairment in Patients with Parkinson’s Disease. Sci. Rep. 2016, 6, 33738. [Google Scholar] [CrossRef] [PubMed]
- Zvara, Á.; Szekeres, G.; Janka, Z.; Kelemen, J.Z.; Cimmer, C.; Sántha, M.; Puskás, L.G. Over-expression of dopamine D_2 receptor and inwardly rectifying potassium channel genes in drug-naive schizophrenic peripheral blood lymphocytes as potential diagnostic markers. Dis. Markers 2005, 21, 61–69. [Google Scholar] [CrossRef]
- Feltmann, K.; Borroto-Escuela, D.O.; Ruegg, J.; Pinton, L.; de Oliveira Sergio, T.; Narvaez, M.; Jimenez-Beristain, A.; Ekstrom, T.J.; Fuxe, K.; Steensland, P. Effects of Long-Term Alcohol Drinking on the Dopamine D2 Receptor: Gene Expression and Heteroreceptor Complexes in the Striatum in Rats. Alcohol. Clin. Exp. Res. 2018, 42, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Logan, J.; Jayne, M.; Ma, Y.; Pradhan, K.; Wong, C. Profound decreases in dopamine release in striatum in detoxified alcoholics: Possible orbitofrontal involvement. J. Neurosci. 2007, 27, 12700–12706. [Google Scholar] [CrossRef]
- He, J.; Crews, F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008, 210, 349–358. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ. Res. 2016, 119, 414–417. [Google Scholar] [CrossRef]
- Lassen, M.B.; Brown, J.E.; Stobbs, S.H.; Gunderson, S.H.; Maes, L.; Valenzuela, C.F.; Ray, A.P.; Henriksen, S.J.; Steffensen, S.C. Brain stimulation reward is integrated by a network of electrically coupled GABA neurons. Brain Res. 2007, 1156, 46–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Basu, S.; Dasgupta, P.S. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol. 2000, 102, 113–124. [Google Scholar] [CrossRef]
- Basu, B.; Sarkar, C.; Chakroborty, D.; Ganguly, S.; Shome, S.; Dasgupta, P.S.; Basu, S. D1 and D2 dopamine receptor-mediated inhibition of activated normal T cell proliferation is lost in jurkat T leukemic cells. J. Biol. Chem. 2010, 285, 27026–27032. [Google Scholar] [CrossRef]
- Ilani, T.; Ben-Shachar, D.; Strous, R.D.; Mazor, M.; Sheinkman, A.; Kotler, M.; Fuchs, S. A peripheral marker for schizophrenia: Increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Fabbrini, G.; Ricci, A.; Bruno, G.; Cerbo, R.; Bronzetti, E.; Amenta, F.; Luigi Lenzi, G. Reduced density of dopamine D2-like receptors on peripheral blood lymphocytes in Alzheimer’s disease. Mech. Ageing Dev. 2000, 120, 65–75. [Google Scholar] [CrossRef]
- Barbanti, P.; Fabbrini, G.; Ricci, A.; Pascali, M.P.; Bronzetti, E.; Amenta, F.; Lenzi, G.L.; Cerbo, R. Migraine patients show an increased density of dopamine D3 and D4 receptors on lymphocytes. Cephalalgia Int. J. Headache 2000, 20, 15–19. [Google Scholar] [CrossRef]
- Nagai, Y.; Ueno, S.; Saeki, Y.; Soga, F.; Hirano, M.; Yanagihara, T. Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson’s disease. Neurology 1996, 46, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Fabbrini, G.; Ricci, A.; Cerbo, R.; Bronzetti, E.; Caronti, B.; Calderaro, C.; Felici, L.; Stocchi, F.; Meco, G.; et al. Increased expression of dopamine receptors on lymphocytes in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 1999, 14, 764–771. [Google Scholar] [CrossRef]
- Ersche, K.D.; Roiser, J.P.; Lucas, M.; Domenici, E.; Robbins, T.W.; Bullmore, E.T. Peripheral biomarkers of cognitive response to dopamine receptor agonist treatment. Psychopharmacology 2011, 214, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Gladkevich, A.; Kauffman, H.F.; Korf, J. Lymphocytes as a neural probe: Potential for studying psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Xue, X.; Tian, H.; Wang, X.X.; Dong, Y.X.; Wang, F.; Zhao, Y.N.; Yao, X.C.; Cui, W.; Wu, C.F. Role of microglia in ethanol-induced neurodegenerative disease: Pathological and behavioral dysfunction at different developmental stages. Pharmacol. Ther. 2014, 144, 321–337. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Wang, F.; Fan, Y.X.; Ping, G.F.; Yang, J.Y.; Wu, C.F. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav. Brain Res. 2013, 236, 270–282. [Google Scholar] [CrossRef]
- Färber, K.; Pannasch, U.; Kettenmann, H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol. Cell. Neurosci. 2005, 29, 128–138. [Google Scholar] [CrossRef]
- Xia, Q.-P.; Cheng, Z.-Y.; He, L. The modulatory role of dopamine receptors in brain neuroinflammation. Int. Immunopharmacol. 2019, 76, 105908. [Google Scholar] [CrossRef]
- Ortiz, J.; Fitzgerald, L.W.; Charlton, M.; Lane, S.; Trevisan, L.; Guitart, X.; Shoemaker, W.; Duman, R.S.; Nestler, E.J. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse 1995, 21, 289–298. [Google Scholar] [CrossRef]
- Rommelspacher, H.; Raeder, C.; Kaulen, P.; Bruning, G. Adaptive changes of dopamine-D2 receptors in rat brain following ethanol withdrawal: A quantitative autoradiographic investigation. Alcohol 1992, 9, 355–362. [Google Scholar] [CrossRef]
- Yin, J.; Valin, K.L.; Dixon, M.L.; Leavenworth, J.W. The Role of Microglia and Macrophages in CNS Homeostasis, Autoimmunity, and Cancer. J. Immunol. Res. 2017, 2017, 5150678. [Google Scholar] [CrossRef]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia Development and Function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef]
- Wickramasinghe, S.N. Role of macrophages in the pathogenesis of alcohol induced tissue damage. Br. Med. J. 1987, 294, 1137–1139. [Google Scholar] [CrossRef]
- Laso, F.J.; Vaquero, J.M.; Almeida, J.; Marcos, M.; Orfao, A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol. Clin. Exp. Res. 2007, 31, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Laso, F.J.; Vaquero, J.M.; Almeida, J.; Marcos, M.; Orfao, A. Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: Relationship with ethanol intake and liver disease. Cytom. Part B Clin. Cytom. 2007, 72, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, B.D.S.; Kahoud, R.J.; McCarthy, C.B.; Howe, C.L. Inflammatory cytokine-induced changes in neural network activity measured by waveform analysis of high-content calcium imaging in murine cortical neurons. Sci. Rep. 2017, 7, 9037. [Google Scholar] [CrossRef] [PubMed]
- Ronstrom, J.W.; Williams, S.B.; Payne, A.; Obray, D.J.; Hafen, C.; Burris, M.; Scott Weber, K.; Steffensen, S.C.; Yorgason, J.T. Interleukin-10 enhances activity of ventral tegmental area dopamine neurons resulting in increased dopamine release. Brain Behav. Immun. 2023, 113, 145–155. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, C.A.; Obray, J.D.; Clarke, T.J.; Brundage, J.N.; Folsom, R.J.; Moreno, C.M.; Williams, P.E.; Ford, L.H.; Hope, S.; Weber, K.S.; et al. Monocyte-Derived Macrophages Expressing Dopamine D2-Subtype Receptors Drive Alcohol Effects on Mesolimbic Neurons and Microglia. Biomedicines 2025, 13, 2327. https://doi.org/10.3390/biomedicines13102327
Nelson CA, Obray JD, Clarke TJ, Brundage JN, Folsom RJ, Moreno CM, Williams PE, Ford LH, Hope S, Weber KS, et al. Monocyte-Derived Macrophages Expressing Dopamine D2-Subtype Receptors Drive Alcohol Effects on Mesolimbic Neurons and Microglia. Biomedicines. 2025; 13(10):2327. https://doi.org/10.3390/biomedicines13102327
Chicago/Turabian StyleNelson, Christina A., J. Daniel Obray, Travis J. Clarke, James N. Brundage, Ryan J. Folsom, Carlos M. Moreno, Pacen E. Williams, Lauren H. Ford, Sandra Hope, K. Scott Weber, and et al. 2025. "Monocyte-Derived Macrophages Expressing Dopamine D2-Subtype Receptors Drive Alcohol Effects on Mesolimbic Neurons and Microglia" Biomedicines 13, no. 10: 2327. https://doi.org/10.3390/biomedicines13102327
APA StyleNelson, C. A., Obray, J. D., Clarke, T. J., Brundage, J. N., Folsom, R. J., Moreno, C. M., Williams, P. E., Ford, L. H., Hope, S., Weber, K. S., Bills, K. B., Yorgason, J. T., & Steffensen, S. C. (2025). Monocyte-Derived Macrophages Expressing Dopamine D2-Subtype Receptors Drive Alcohol Effects on Mesolimbic Neurons and Microglia. Biomedicines, 13(10), 2327. https://doi.org/10.3390/biomedicines13102327




