Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale as a Novel Tool to Measure Disease Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Statistical Analyses
3. Results
3.1. Subsection
3.1.1. Basic Information
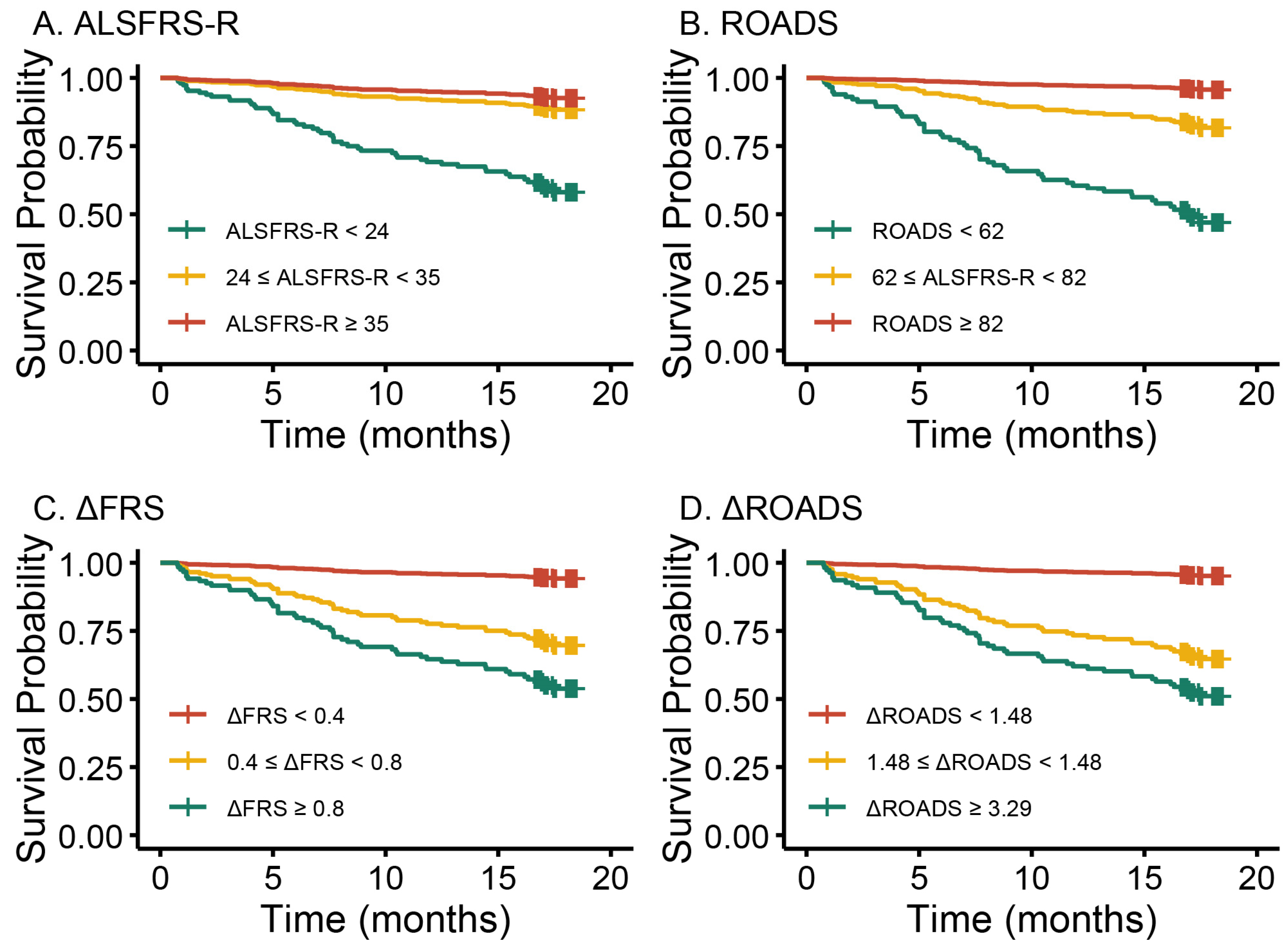
3.1.2. Prognostic Utility of Baseline ROADS and ΔROADS for ALS
3.1.3. Longitudinal Analyses of ROADS to Measure Disease Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A.; Bdnf Als Study Group and 1A complete listing of the BDNF Study Group. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Writing, G.; Edaravone, A.L.S.S.G. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017, 16, 505–512. [Google Scholar]
- Paganoni, S.; Macklin, E.A.; Hendrix, S.; Berry, J.D.; Elliott, M.A.; Maiser, S.; Karam, C.; Caress, J.B.; Owegi, M.A.; Quick, A.; et al. Trial of Sodium Phenylbutyrate-Taurursodiol for Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2020, 383, 919–930. [Google Scholar] [CrossRef]
- Mora, J.S.; Genge, A.; Chio, A.; Estol, C.J.; Chaverri, D.; Hernández, M.; Marín, S.; Mascias, J.; Rodriguez, G.E.; Povedano, M.; et al. Masitinib as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: A randomized clinical trial. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 5–14. [Google Scholar] [CrossRef]
- Statland, J.M.; Moore, D.; Wang, Y.; Walsh, M.; Mozaffar, T.; Elman, L.; Nations, S.P.; Mitsumoto, H.; Fernandes, J.A.; Saperstein, D.; et al. Rasagiline for amyotrophic lateral sclerosis: A randomized, controlled trial. Muscle Nerve 2019, 59, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Macklin, E.A.; Jackson, K.E.; Simpson, E.; Mahoney, K.; Yu, H.; Walker, J.; Simmons, Z.; David, W.S.; Barkhaus, P.E.; et al. Selection design phase II trial of high dosages of tamoxifen and creatine in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 15–23. [Google Scholar] [CrossRef]
- Kaufmann, P.; Levy, G.; Thompson, J.L.P.; Delbene, M.L.; Battista, V.; Gordon, P.H.; Rowland, L.P.; Levin, B.; Mitsumoto, H. The ALSFRSr predicts survival time in an ALS clinic population. Neurology 2005, 64, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Labra, J.; Menon, P.; Byth, K.; Morrison, S.; Vucic, S. Rate of disease progression: A prognostic biomarker in ALS. J. Neurol. Neurosurg. Psychiatry 2016, 87, 628–632. [Google Scholar] [CrossRef]
- Kimura, F.C.S.H.D.H.; Fujimura, C.; Ishida, S.; Nakajima, H.; Furutama, D.; Uehara, H.; Shinoda, K.; Sugino, M.; Hanafusa, T. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006, 66, 265–267. [Google Scholar] [CrossRef]
- Franchignoni, F.; Mora, G.; Giordano, A.; Volanti, P.; Chio, A. Evidence of multidimensionality in the ALSFRS-R Scale: A critical appraisal on its measurement properties using Rasch analysis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1340–1345. [Google Scholar] [CrossRef]
- Franchignoni, F.; Mandrioli, J.; Giordano, A.; Ferro, S.; Group, E. A further Rasch study confirms that ALSFRS-R does not conform to fundamental measurement requirements. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.; Burke, T.; Vajda, A.; Heverin, M.; Hardiman, O. What does the ALSFRS-R really measure? A longitudinal and survival analysis of functional dimension subscores in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Bedlack, R.S.; Vaughan, T.; Wicks, P.; Heywood, J.; Sinani, E.; Selsov, R.; Macklin, E.A.; Schoenfeld, D.; Cudkowicz, M.; Sherman, A. How common are ALS plateaus and reversals? Neurology 2016, 86, 808–812. [Google Scholar] [CrossRef]
- Vasta, R.; D’ovidio, F.; Canosa, A.; Manera, U.; Torrieri, M.C.; Grassano, M.; De Marchi, F.; Mazzini, L.; Moglia, C.; Calvo, A.; et al. Plateaus in amyotrophic lateral sclerosis progression: Results from a population-based cohort. Eur. J. Neurol. 2020, 27, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, R.P.; de Jongh, A.D.; Nikolakopoulos, S.; McDermott, C.J.; Eijkemans, M.J.; Roes, K.C.; van den Berg, L.H. An old friend who has overstayed their welcome: The ALSFRS-R total score as primary endpoint for ALS clinical trials. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.N.; Bedlack, R.; Quinn, C.; Russell, J.; Beckwith, D.; Kaminski, K.H.; Tyor, W.; Hertzberg, V.; James, V.; Polak, M.; et al. Development and Validation of the Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS). JAMA Neurol. 2020, 77, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Rasch, G. Probabilistic models for some intelligence and attainment tests. In Copenhagen: Institute of Educational Research; MESA Press: San Diego, CA, USA, 1960. [Google Scholar]
- Binda, D.; Vanhoutte, E.K.; Cavaletti, G.; Cornblath, D.R.; Postma, T.J.; Frigeni, B.; Alberti, P.; Bruna, J.; Velasco, R.; Argyriou, A.A.; et al. Rasch-built Overall Disability Scale for patients with chemotherapy-induced peripheral neuropathy (CIPN-R-ODS). Eur. J. Cancer 2013, 49, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, E.K.; Hermans, M.C.; Faber, C.G.; Gorson, K.C.; Merkies, I.S.; Thonnard, J.L. Rasch-ionale for neurologists. J. Peripher. Nerv. Syst. 2015, 20, 260–268. [Google Scholar] [CrossRef] [PubMed]
- van Nes, S.I.; Vanhoutte, E.K.; Van Doorn, P.A.; Hermans, M.; Bakkers, M.; Kuitwaard, K.; Faber, C.G.; Merkies, I.S. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology 2011, 76, 337–345. [Google Scholar] [CrossRef]
- Fridman, V.; Sillau, S.; Acsadi, G.; Bacon, C.; Dooley, K.; Burns, J.; Day, J.; Feely, S.; Finkel, R.S.; Grider, T.; et al. A longitudinal study of CMT1A using Rasch analysis based CMT neuropathy and examination scores. Neurology 2020, 94, e884–e896. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Fournier, C.N.; Ye, S.; Zhang, N.; Ma, Y.; Fan, D. Chinese validation of the Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale. Eur. J. Neurol. 2021, 28, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Manera, U.; Solero, L.; Fournier, C.N.; Canosa, A.; Vasta, R.; Bombaci, A.; Grassano, M.; Palumbo, F.; Torrieri, M.C.; Salamone, P.; et al. Validation of the Italian version of the Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS) administered to patients and their caregivers. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Mascías Cadavid, J.; Radakovic, R.; Radakovic, C.; Moran Benito, Y.; Marín Esteban, S.; Rodríguez-Santos, F.; Salas Campos, T. Spanish Adaptation of the Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS). Amyotroph. Lateral Scler. Front. Degener. 2024, 16, 1–4. [Google Scholar] [CrossRef]
- Fortuna, A.; Sabbatini, D.; Frigo, A.; Bello, L.; Calvi, F.; Blasi, L.; Gianferrari, G.; Martinelli, I.; Minicuci, G.; Pegoraro, E.; et al. Italian version of the Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS): Validation and longitudinal performance. J. Neurol. 2023, 270, 1452–1456. [Google Scholar] [CrossRef]
- Fournier, C.N.; James, V.; Glass, J.D. Clinically meaningful change: Evaluation of the Rasch-built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS) and the ALSFRS-R. Amyotroph. Lateral. Scler. Front. Degener. 2023, 24, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Burke, K.M.; Scheier, Z.A.; Keegan, M.A.; Clark, A.P.; Chan, J.; Fournier, C.N.; Berry, J.D. Longitudinal comparison of the self-entry Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-RSE) and Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale (ROADS) as outcome measures in people with amyotrophic lateral sclerosis. Muscle Nerve 2022, 66, 495–502. [Google Scholar] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L.; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Goyal, N.A.; Berry, J.D.; Windebank, A.; Staff, N.P.; Maragakis, N.J.; van den Berg, L.H.; Genge, A.; Miller, R.; Baloh, R.H.; Kern, R.; et al. Addressing heterogeneity in amyotrophic lateral sclerosis CLINICAL TRIALS. Muscle Nerve 2020, 62, 156–166. [Google Scholar] [CrossRef]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Ravits, J.; Appel, S.; Baloh, R.H.; Barohn, R.; Rix Brooks, B.; Elman, L.; Floeter, M.K.; Henderson, C.; Lomen-Hoerth, C.; Macklis, J.D.; et al. Deciphering amyotrophic lateral sclerosis: What phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotroph. Lateral Scler. Front. Degener. 2013, 14 (Suppl. S1), 5–18. [Google Scholar] [CrossRef] [PubMed]
- Hartmaier, S.L.; Rhodes, T.; Cook, S.F.; Schlusser, C.; Chen, C.; Han, S.; Zach, N.; Murthy, V.; Davé, S. Qualitative measures that assess functional disability quality of life in ALS. Health Qual. Life Outcomes 2022, 20, 12. [Google Scholar] [CrossRef]
- Atassi, N.; Berry, J.; Shui, A.; Zach, N.; Sherman, A.; Sinani, E.; Walker, J.; Katsovskiy, I.; Schoenfeld, D.; Cudkowicz, M.; et al. The PRO-ACT database: Design, initial analyses, and predictive features. Neurology 2014, 83, 1719–1725. [Google Scholar] [CrossRef]
- Bacci, E.D.; Staniewska, D.; Coyne, K.S.; Boyer, S.; White, L.A.; Zach, N.; Cedarbaum, J.M.; Pooled Resource Open-Access ALS Clinical Trials Consortium. Item response theory analysis of the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised in the Pooled Resource Open-Access ALS Clinical Trials Database. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 157–167. [Google Scholar] [CrossRef]
- McDermott, C.J. Stay at home with the amyotrophic lateral sclerosis functional rating scale. J. Neurol. Neurosurg. Psychiatry 2020, 91, 7. [Google Scholar] [CrossRef] [PubMed]
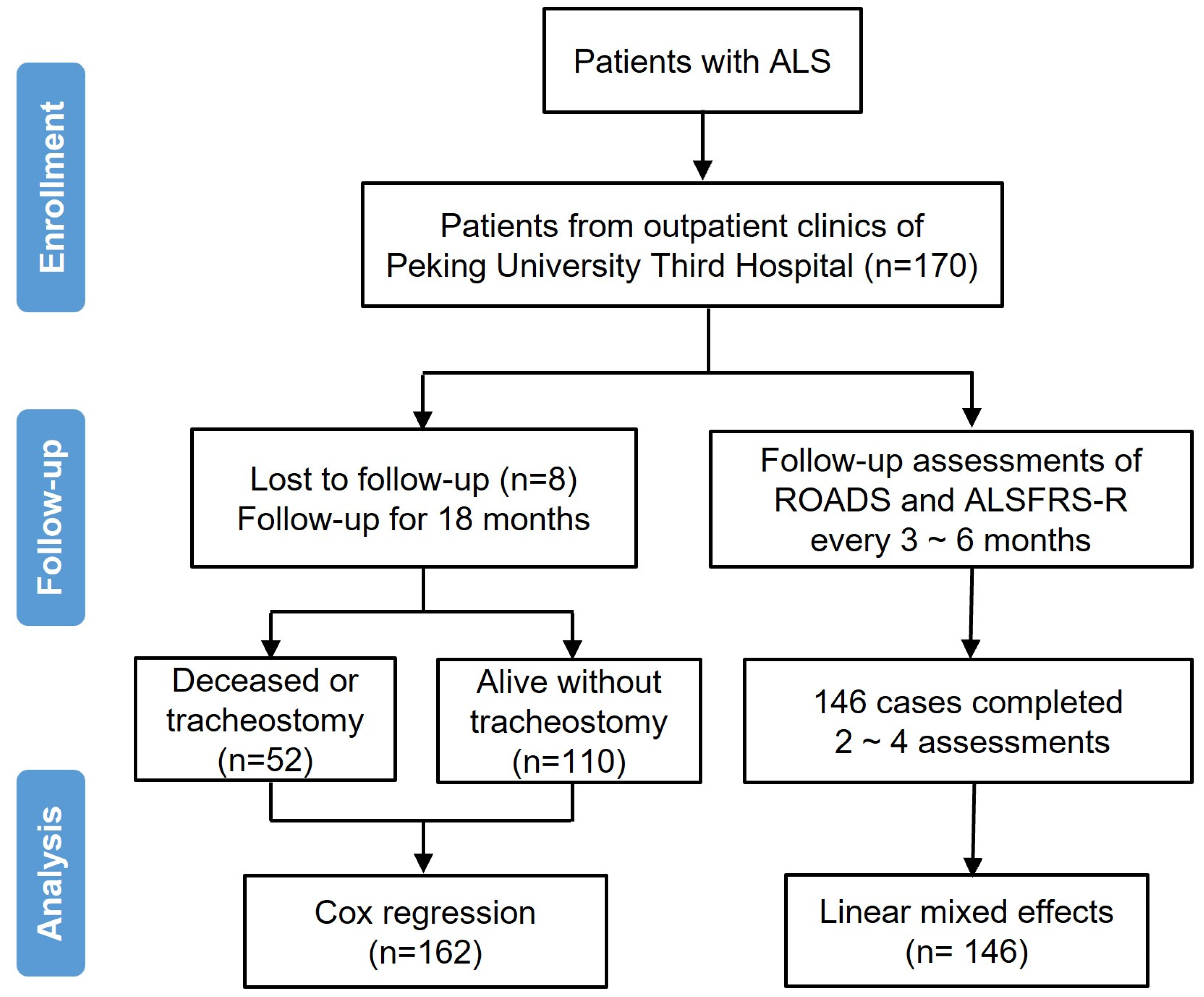

| Variable | Total | Deceased or Tracheostomy | Alive Without Tracheostomy |
|---|---|---|---|
| No. of patients, n (%) | 162 | 52 (32.1) | 110 (67.9) |
| Baseline age *, y, mean (SD) | 55.0 (12.2) | 59.9 (9.9) | 52.7 (12.6) |
| Male, n (%) | 102 (63.0) | 34 (65.4) | 68 (61.8) |
| Site of onset, n (%) | |||
| Spinal cord | 134 (82.7) | 40 (76.9) | 94 (85.5) |
| Bulbar | 28 (17.3) | 12 (23.1) | 16 (14.5) |
| Symptom duration †, m, mean (SD) | 44.5 (40.3) | 31.4 (20.9) | 50.6 (45.6) |
| Baseline normed ROADS †, mean (SD) | 70.9 (20.3) | 57.7 (18.6) | 77.1 (18.0) |
| Baseline ALSFRS-R †, mean (SD) | 27.9 (9.8) | 21.9 (9.6) | 30.8 (8.6) |
| Baseline ΔROADS †, mean (SD) | 2.72 (2.00) | 3.68 (2.24) | 2.26 (1.70) |
| Baseline FRS †, mean (SD) | 0.76 (0.65) | 1.15 (0.82) | 0.57 (0.45) |
| Survival, m, mean (SD) | — | 7.6 (5.0) | 17.6 (0.59) |
| Variable | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| Age, year | ||
| <55 | 1.00 (Reference) | NA |
| ≥55 | 2.55 (1.43–4.56) | 0.002 |
| Gender | ||
| Male | 1.00 (Reference) | NA |
| Female | 0.89 (0.50–1.58) | 0.70 |
| Site of onset | ||
| Spinal cord | 1.00 (Reference) | NA |
| Bulbar | 1.45 (0.76–2.77) | 0.27 |
| Symptom duration | 0.99 (0.98–1.00) | 0.012 |
| ROADS | 0.96 (0.94–0.97) | <0.001 |
| ALSFRS-R | 0.92 (0.89–0.94) | <0.001 |
| ROADS tertiles | ||
| ≥82 | 1.00 (Reference) | NA |
| ≥62, <82 | 4.20 (1.41–12.51) | 0.010 |
| <62 | 11.23 (3.95–31.92) | <0.001 |
| ALSFRS-R tertiles | ||
| ≥35 | 1.00 (Reference) | NA |
| ≥24, <35 | 1.70 (0.67–4.33) | 0.26 |
| <24 | 6.17 (2.72–13.98) | <0.001 |
| ΔROADS | 1.30 (1.15–1.43) | <0.001 |
| ΔFRS | 2.68 (1.94–3.71) | <0.001 |
| ΔROADS tertiles | ||
| <1.48 | 1.00 (Reference) | NA |
| ≥1.48, <3.29 | 5.07 (1.91–13.46) | 0.001 |
| ≥3.29 | 6.46 (2.48–16.83) | <0.001 |
| ΔFRS tertiles | ||
| <0.40 | 1.00 (Reference) | NA |
| ≥0.40, <0.80 | 4.32 (1.61–11.60) | 0.004 |
| ≥0.80 | 7.33 (2.83–19.01) | <0.001 |
| Variable | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| ROADS | 0.95 (0.94–0.97) | <0.001 |
| ALSFRS-R | 0.90 (0.87–0.93) | <0.001 |
| ROADS tertiles | <0.001 | |
| ≥82 | 1.00 (reference) | NA |
| ≥62, <82 | 4.82 (1.60–14.54) | 0.005 |
| <62 | 17.99 (6.17–52.47) | <0.001 |
| ALSFRS-R tertiles | <0.001 | |
| ≥35 | 1.00 (reference) | NA |
| ≥24, <35 | 1.63 (0.63–4.20) | 0.32 |
| <24 | 7.73 (3.34–17.94) | <0.001 |
| ΔROADS | 1.26 (1.10–1.45) | 0.001 |
| ΔFRS | 2.54 (1.73–3.73) | <0.001 |
| ΔROADS tertiles | ||
| <1.48 | 1.00 (Reference) | NA |
| ≥1.48, <3.29 | 8.71 (2.38–31.10) | 0.001 |
| ≥3.29 | 13.90 (3.36–57.55) | <0.001 |
| ΔFRS tertiles | ||
| <0.40 | 1.00 (Reference) | NA |
| ≥0.40, <0.80 | 5.90 (1.86–18.69) | 0.003 |
| ≥0.80 | 10.90 (3.10–38.73) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Chen, Y.; Xu, L.; Wang, W.; Zhang, N.; Fournier, C.N.; Li, N.; Fan, D. Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale as a Novel Tool to Measure Disease Progression. Biomedicines 2025, 13, 178. https://doi.org/10.3390/biomedicines13010178
Sun C, Chen Y, Xu L, Wang W, Zhang N, Fournier CN, Li N, Fan D. Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale as a Novel Tool to Measure Disease Progression. Biomedicines. 2025; 13(1):178. https://doi.org/10.3390/biomedicines13010178
Chicago/Turabian StyleSun, Can, Yong Chen, Lu Xu, Wenjing Wang, Nan Zhang, Christina N. Fournier, Nan Li, and Dongsheng Fan. 2025. "Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale as a Novel Tool to Measure Disease Progression" Biomedicines 13, no. 1: 178. https://doi.org/10.3390/biomedicines13010178
APA StyleSun, C., Chen, Y., Xu, L., Wang, W., Zhang, N., Fournier, C. N., Li, N., & Fan, D. (2025). Rasch-Built Overall Amyotrophic Lateral Sclerosis Disability Scale as a Novel Tool to Measure Disease Progression. Biomedicines, 13(1), 178. https://doi.org/10.3390/biomedicines13010178






