Vascularization, Innervation, and Inflammation: Pathways Connecting the Heart–Brain Axis and Implications in a Clinical Setting
Abstract
1. Introduction
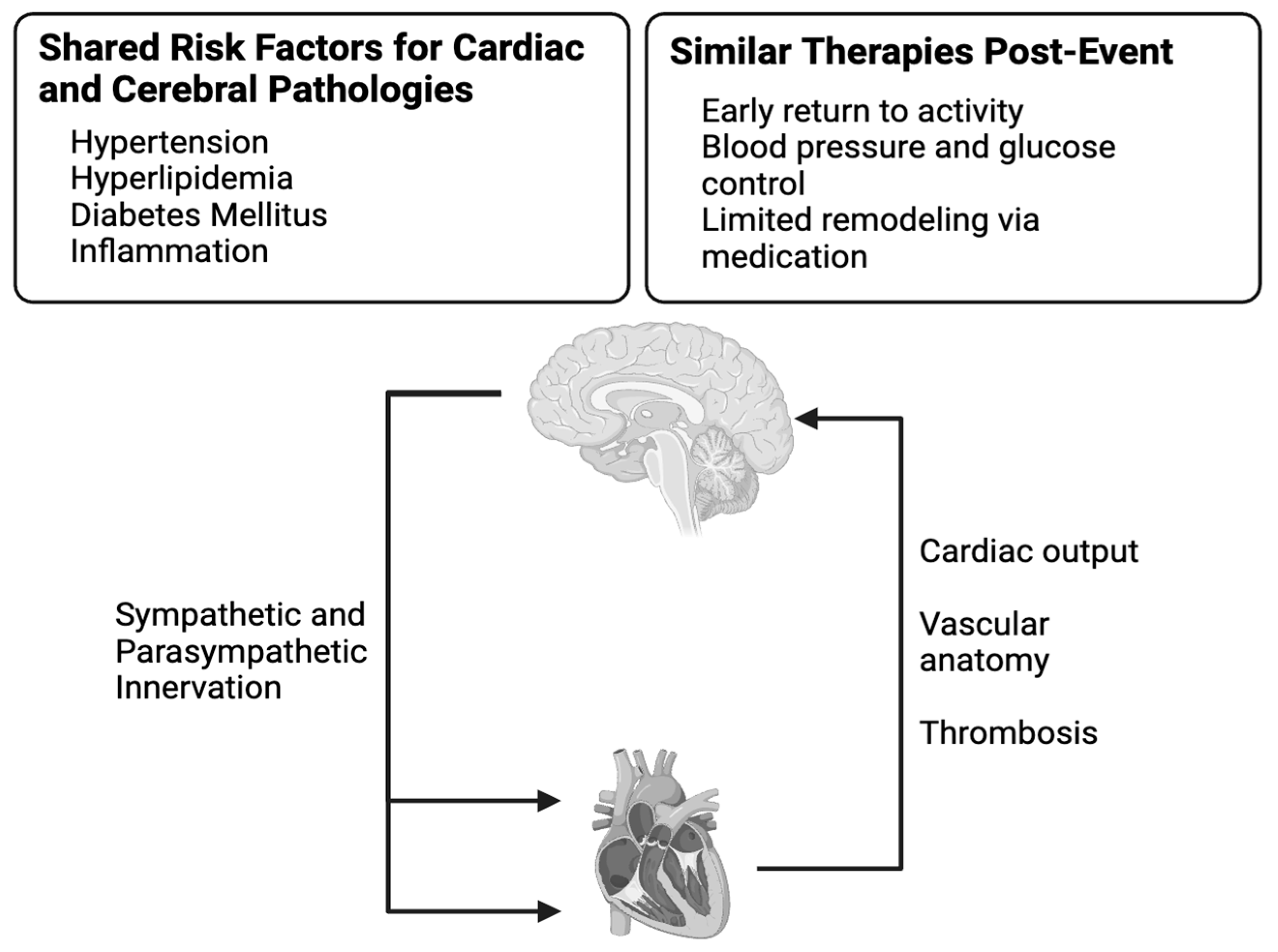
2. Biology of the Heart–Brain Axis
2.1. Innervation in the Heart–Brain Axis
2.2. Vascular Influence of the Brain
2.3. Impacts of Systemic Inflammation
3. Discussion and Therapeutic Opportunities
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; Alkatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Stroke Collaborators. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Zhan, C.; Shi, M.; Wu, R.; He, H.; Liu, X.; Shen, B. MIRKB: A Myocardial Infarction Risk Knowledge Base. Database 2019, 2019, baz125. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S.V. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Rosengren, A.; Young, K.; Jennings, E. Mortality and Morbidity Trends after the First Year in Survivors of Acute Myocardial Infarction: A Systematic Review. BMC Cardiovasc. Disord. 2017, 17, 53. [Google Scholar] [CrossRef]
- van der Wall, E.E.; van Gilst, W.H. Neurocardiology: Close Interaction between Heart and Brain. Neth. Heart J. 2013, 21, 51–52. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G. Update on the Gut Microbiome in Health and Diseases. World J. Methodol. 2024, 14, 89196. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Pan, W.; Xu, Y.; Zhang, J.; Wan, J.; Jiang, H. Microglia-Mediated Neuroinflammation: A Potential Target for the Treatment of Cardiovascular Diseases. J. Inflamm. Res. 2022, 15, 3083–3094. [Google Scholar] [CrossRef]
- Yang, S.; Webb, A.J.S. Associations between Neurovascular Coupling and Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. Eur. Stroke J. 2023, 8, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Andresen, M.C.; Armour, J.A.; Billman, G.E.; Chen, P.-S.; Foreman, R.D.; Herring, N.; O’Leary, D.S.; Sabbah, H.N.; Schultz, H.D.; et al. Translational Neurocardiology: Preclinical Models and Cardioneural Integrative Aspects. J. Physiol. 2016, 594, 3877–3909. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Zhang, Z.-H.; Xue, B.; Weiss, R.M.; Felder, R.B. Inhibition of Brain Proinflammatory Cytokine Synthesis Reduces Hypothalamic Excitation in Rats with Ischemia-Induced Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H227–H236. [Google Scholar] [CrossRef] [PubMed]
- Crestani, C.C.; Alves, F.H.F.; Resstel, L.B.M.; Corrêa, F.M.A. Cardiovascular Effects of Noradrenaline Microinjection in the Bed Nucleus of the Stria Terminalis of the Rat Brain. J. Neurosci. Res. 2007, 85, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Harrison, N.A. Visceral Influences on Brain and Behavior. Neuron 2013, 77, 624–638. [Google Scholar] [CrossRef]
- Nistor, I.R.; Gherasim, L. From Neurocardiology to Stroke-Heart Syndrome. Rom. J. Intern. Med. 2023, 61, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sposato, L.A.; Gurol, M.E. Advances in Neurocardiology: Focus on Atrial Fibrillation. Stroke 2021, 52, 3696–3699. [Google Scholar] [CrossRef]
- Vasconcelos, L.P.B.; da Silva Bastos Vasconcelos, M.C.; Di Flora, F.B.M.E.; de Oliveira, F.A.P.; Lima, P.D.; Silva, L.C.B.E.; Mucelli Spolaor, B.C.; da Silva, J.L.P.; de Magalhães Esteves, W.A.; Nunes, M.C.P.; et al. Neurological and Psychiatric Disorders in Patients with Rheumatic Heart Disease: Unveiling What Is Beyond Cardiac Manifestations. Glob. Heart 2022, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.B.; Essebag, V.; Furlanetto, M.; Yanez, J.P.G.; Farina, M.G.; Garcia, D.; Almeida, E.D.; Stephan, L.; Lima, G.G.; Leiria, T.L.L. Structural Heart Disease as the Cause of Syncope. Braz. J. Med. Biol. Res. 2018, 51, e6989. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Armour, J.A. Neurocardiology: Structure-Based Function. In Comprehensive Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1635–1653. [Google Scholar]
- Filipović, N.; Marinović Guić, M.; Košta, V.; Vukojević, K. Cardiac Innervations in Diabetes Mellitus—Anatomical Evidence of Neuropathy. Anat. Rec. 2023, 306, 2345–2365. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W. Autonomic Cardiac Innervation. Organogenesis 2013, 9, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Fukuda, K. New Aspects for the Treatment of Cardiac Diseases Based on the Diversity of Functional Controls on Cardiac Muscles: The Regulatory Mechanisms of Cardiac Innervation and Their Critical Roles in Cardiac Performance. J. Pharmacol. Sci. 2009, 109, 348–353. [Google Scholar] [CrossRef]
- Jaffe, L.M.; Morin, D.P. Cardiac Resynchronization Therapy: History, Present Status, and Future Directions. Ochsner J. 2014, 14, 596–607. [Google Scholar] [PubMed]
- Parichatikanond, W.; Duangrat, R.; Kurose, H.; Mangmool, S. Regulation of β-Adrenergic Receptors in the Heart: A Review on Emerging Therapeutic Strategies for Heart Failure. Cells 2024, 13, 1674. [Google Scholar] [CrossRef] [PubMed]
- Clyburn, C.; Sepe, J.J.; Habecker, B.A. What Gets on the Nerves of Cardiac Patients? Pathophysiological Changes in Cardiac Innervation. J. Physiol. 2022, 600, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Budohoski, K.P.; Smielewski, P.; Czosnyka, M. Regulation of the Cerebral Circulation: Bedside Assessment and Clinical Implications. Crit. Care 2016, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Sethi, D.; Gofur, E.M.; Munakomi, S. Anatomy, Head and Neck: Carotid Arteries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Reiss, Y.; Bauer, S.; David, B.; Devraj, K.; Fidan, E.; Hattingen, E.; Liebner, S.; Melzer, N.; Meuth, S.G.; Rosenow, F.; et al. The Neurovasculature as a Target in Temporal Lobe Epilepsy. Brain Pathol. 2023, 33, e13147. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.; Petersen, N.H. Physiology, Cerebral Autoregulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Claassen, J.A.H.R.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of Cerebral Blood Flow in Humans: Physiology and Clinical Implications of Autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Czosnyka, M.; Kirkpatrick, P.J.; Smielewski, P.; Steiner, L.A.; Pickard, J.D. Clinical Relevance of Cerebral Autoregulation Following Subarachnoid Haemorrhage. Nat. Rev. Neurol. 2013, 9, 152–163. [Google Scholar] [CrossRef]
- Kaufmann, H.; Norcliffe-Kaufmann, L.; Palma, J.-A. Baroreflex Dysfunction. N. Engl. J. Med. 2020, 382, 163–178. [Google Scholar] [CrossRef]
- Koep, J.L.; Bond, B.; Barker, A.R.; Ruediger, S.L.; Pizzey, F.K.; Coombes, J.S.; Bailey, T.G. The Relationships between Age, Sex, and Cerebrovascular Reactivity to Hypercapnia Using Traditional and Kinetic-Based Analyses in Healthy Adults. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H782–H796. [Google Scholar] [CrossRef] [PubMed]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral Blood Flow Regulation and Neurovascular Dysfunction in Alzheimer’s Disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef]
- Biose, I.J.; Oremosu, J.; Bhatnagar, S.; Bix, G.J. Promising Cerebral Blood Flow Enhancers in Acute Ischemic Stroke. Transl. Stroke Res. 2023, 14, 863–889. [Google Scholar] [CrossRef]
- Roher, A.E.; Debbins, J.P.; Malek-Ahmadi, M.; Chen, K.; Pipe, J.G.; Maze, S.; Belden, C.; Maarouf, C.L.; Thiyyagura, P.; Mo, H.; et al. Cerebral Blood Flow in Alzheimer’s Disease. Vasc. Health Risk Manag. 2012, 8, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, H.S. Alzheimer’s Disease: A Decreased Cerebral Blood Flow to Critical Intraneuronal Elements Is the Cause. J. Alzheimers Dis. 2022, 85, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Liu, L.; Huang, X.; Zhang, D.; Gao, Y.; Yin, X.; Lin, H.; Dai, Y.; Wu, X. Cerebral Blood Flow Alterations in Migraine Patients with and without Aura: An Arterial Spin Labeling Study. J. Headache Pain. 2022, 23, 131. [Google Scholar] [CrossRef]
- Honda, M.; Ichibayashi, R.; Yokomuro, H.; Yoshihara, K.; Masuda, H.; Haga, D.; Seiki, Y.; Kudoh, C.; Kishi, T. Early Cerebral Circulation Disturbance in Patients Suffering from Severe Traumatic Brain Injury (TBI): A Xenon CT and Perfusion CT Study. Neurol. Med. Chir. 2016, 56, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, E.; Fu, X.; Gong, S.; Han, Y.; Deng, F. Cerebral Blood Flow Self-Regulation in Depression. J. Affect. Disord. 2022, 302, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.N.; Russo, A.F. Vascular Contributions to Migraine: Time to Revisit? Front. Cell. Neurosci. 2018, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.A.; Ramya Krishna, M.; Sadiq, S.; Ramesh Kumar, R.; Venkatarathnam, V.; Saikiran, G. A Study to Evaluate Neurovascular Conflict of Trigeminal Nerve in Trigeminal Neuralgia Patients with the Help of 1.5 T MR Imaging. Egypt. J. Radiol. Nucl. Med. 2022, 53, 66. [Google Scholar] [CrossRef]
- Hannan, C.; Shoakazemi, A.; Quigley, G. Microvascular Decompression for Trigeminal Neuralgia: A Regional Unit’s Experience. Ulst. Med. J. 2018, 87, 30–33. [Google Scholar]
- de Liyis, B.G.; Arini, A.A.I.K.; Karuniamaya, C.P.; Pramana, N.A.K.; Tini, K.; Widyadharma, I.P.E.; Setyopranoto, I. Risk of Intracranial Hemorrhage in Brain Arteriovenous Malformations: A Systematic Review and Meta-Analysis. J. Neurol. 2024, 271, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K.; Davidson, A.S.; Assaad, N.N.A.; Stoodley, M.A. Critical Review of Brain AVM Surgery, Surgical Results and Natural History in 2017. Acta Neurochir. 2017, 159, 1457–1478. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of Inflammation: An Organizing Principle in Biology and Medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, C.K.; Yi, M.; Lui, K.O.; Huang, Y. Targeting Endothelial Dysfunction and Inflammation. J. Mol. Cell. Cardiol. 2022, 168, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Lee, D.H. Inflammation and Resolution Signaling in Cardiac Repair and Heart Failure. EBioMedicine 2022, 79, 103992. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Thadathil, N.; Selvarani, R.; Nicklas, E.H.; Wang, D.; Miller, B.F.; Richardson, A.; Deepa, S.S. Necroptosis Contributes to Chronic Inflammation and Fibrosis in Aging Liver. Aging Cell 2021, 20, e13512. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yu, B.; Armando, I.; Han, F. Mitochondrial DNA-Mediated Inflammation in Acute Kidney Injury and Chronic Kidney Disease. Oxid. Med. Cell Longev. 2021, 2021, 9985603. [Google Scholar] [CrossRef] [PubMed]
- DeMaio, A.; Mehrotra, S.; Sambamurti, K.; Husain, S. The Role of the Adaptive Immune System and T Cell Dysfunction in Neurodegenerative Diseases. J. Neuroinflamm. 2022, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Cells, Tissues, and Disease: Principles of General Pathology; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- García-Escobar, A.; Vera-Vera, S.; Tébar-Márquez, D.; Rivero-Santana, B.; Jurado-Román, A.; Jiménez-Valero, S.; Galeote, G.; Cabrera, J.-Á.; Moreno, R. Neutrophil-to-Lymphocyte Ratio an Inflammatory Biomarker, and Prognostic Marker in Heart Failure, Cardiovascular Disease and Chronic Inflammatory Diseases: New Insights for a Potential Predictor of Anti-Cytokine Therapy Responsiveness. Microvasc. Res. 2023, 150, 104598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Kirabo, A.; Fontana, V.; de Faria, A.P.; Loperena, R.; Galindo, C.L.; Wu, J.; Bikineyeva, A.T.; Dikalov, S.; Xiao, L.; Chen, W.; et al. DC Isoketal-Modified Proteins Activate T Cells and Promote Hypertension. J. Clin. Investig. 2014, 124, 4642–4656. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. C-Reactive Protein Concentration and Risk of Coronary Heart Disease, Stroke, and Mortality: An Individual Participant Meta-Analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Hupe, H.C.; Wang, Y.; Bankstahl, J.P.; Berding, G.; Ross, T.L.; Bauersachs, J.; Wollert, K.C.; Bengel, F.M. Myocardial Inflammation Predicts Remodeling and Neuroinflammation After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Roe, K. An Inflammation Classification System Using Cytokine Parameters. Scand. J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef]
- Scheitz, J.F.; Sposato, L.A.; Schulz-Menger, J.; Nolte, C.H.; Backs, J.; Endres, M. Stroke–Heart Syndrome: Recent Advances and Challenges. J. Am. Heart Assoc. 2022, 11, e026528. [Google Scholar] [CrossRef]
- Hurskainen, M.; Tynkkynen, J.; Eskola, M.; Hernesniemi, J. Incidence of Stroke and Mortality Due to Stroke after Acute Coronary Syndrome. J. Stroke Cerebrovasc. Dis. 2022, 31, 106842. [Google Scholar] [CrossRef]
- George, M.S.; Aston-Jones, G. Noninvasive Techniques for Probing Neurocircuitry and Treating Illness: Vagus Nerve Stimulation (VNS), Transcranial Magnetic Stimulation (TMS) and Transcranial Direct Current Stimulation (tDCS). Neuropsychopharmacology 2010, 35, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Wilson, C.G. A Review of Vagus Nerve Stimulation as a Therapeutic Intervention. J. Inflamm. Res. 2018, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Popović, Z.B.; Bibevski, S.; Fakhry, I.; Sica, D.A.; Van Wagoner, D.R.; Mazgalev, T.N. Chronic Vagus Nerve Stimulation Improves Autonomic Control and Attenuates Systemic Inflammation and Heart Failure Progression in a Canine High-Rate Pacing Model. Circ. Heart Fail. 2009, 2, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus Nerve Stimulation Attenuates the Systemic Inflammatory Response to Endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, G.; Gomez-Pinilla, P.J.; Nemethova, A.; Giovangiulio, M.D.; Cailotto, C.; van Bree, S.H.; Michel, K.; Tracey, K.J.; Schemann, M.; Boesmans, W.; et al. A Distinct Vagal Anti-Inflammatory Pathway Modulates Intestinal Muscularis Resident Macrophages Independent of the Spleen. Gut 2014, 63, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, A.; Urechie, V.; Shiffer, D.; Rigo, S.; Minonzio, M.; Cairo, B.; Smith, E.C.; Okamoto, L.E.; Barbic, F.; Bisoglio, A.; et al. Transdermal Auricular Vagus Stimulation for the Treatment of Postural Tachycardia Syndrome. Auton. Neurosci. 2021, 236, 102886. [Google Scholar] [CrossRef] [PubMed]
- Chapleau, M.W.; Rotella, D.L.; Reho, J.J.; Rahmouni, K.; Stauss, H.M. Chronic Vagal Nerve Stimulation Prevents High-Salt Diet-Induced Endothelial Dysfunction and Aortic Stiffening in Stroke-Prone Spontaneously Hypertensive Rats. Am. J. Physiol.-Heart Circ. Physiol. 2016, 311, H276–H285. [Google Scholar] [CrossRef]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.J.; Hu, Y.; Jackman, W.M.; Nakagawa, H.; Lockwood, D.; Lazzara, R.; Po, S.S. Low-Level Transcutaneous Electrical Vagus Nerve Stimulation Suppresses Atrial Fibrillation. J. Am. Coll. Cardiol. 2015, 65, 867–875. [Google Scholar] [CrossRef]
- Stavrakis, S.; Humphrey, M.B.; Scherlag, B.; Iftikhar, O.; Parwani, P.; Abbas, M.; Filiberti, A.; Fleming, C.; Hu, Y.; Garabelli, P.; et al. Low-Level Vagus Nerve Stimulation Suppresses Post-Operative Atrial Fibrillation and Inflammation. JACC Clin. Electrophysiol. 2017, 3, 929–938. [Google Scholar] [CrossRef]
- Yap, J.Y.Y.; Keatch, C.; Lambert, E.; Woods, W.; Stoddart, P.R.; Kameneva, T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front. Neurosci. 2020, 14, 284. [Google Scholar] [CrossRef] [PubMed]
- Chinda, K.; Tsai, W.-C.; Chan, Y.-H.; Lin, A.Y.-T.; Patel, J.; Zhao, Y.; Tan, A.Y.; Shen, M.J.; Lin, H.; Shen, C.; et al. Intermittent Left Cervical Vagal Nerve Stimulation Damages the Stellate Ganglia and Reduces the Ventricular Rate during Sustained Atrial Fibrillation in Ambulatory Dogs. Heart Rhythm. 2016, 13, 771–780. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation Mechanisms of Neuromodulation Therapies for Anxiety and Depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Blake, M.R.; Gardner, R.T.; Jin, H.; Staffenson, M.A.; Rueb, N.J.; Barrios, A.M.; Dudley, G.B.; Cohen, M.S.; Habecker, B.A. Small Molecules Targeting PTPσ-Trk Interactions Promote Sympathetic Nerve Regeneration. ACS Chem. Neurosci. 2022, 13, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Sepe, J.J.; Gardner, R.T.; Blake, M.R.; Brooks, D.M.; Staffenson, M.A.; Betts, C.B.; Sivagnanam, S.; Larson, W.; Kumar, S.; Bayles, R.G.; et al. Therapeutics That Promote Sympathetic Reinnervation Modulate the Inflammatory Response After Myocardial Infarction. JACC. Basic. Transl. Sci. 2022, 7, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Song, L.; Guo, J.-M.; Fu, D.; Shi, J.; Ma, Y.; Ge, Z.-J.; Li, L.; Zhang, S.-Q. Baicalin Improves Isoproterenol-Induced Cardiac Remodeling by Regulating the Nrf2-Dependent Signaling Pathway. BMC Cardiovasc. Disord. 2024, 24, 733. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Coveney, S.; McCabe, J.J.; Murphy, S.; O’Donnell, M.; Kelly, P.J. Anti-inflammatory Therapy for Preventing Stroke and Other Vascular Events after Ischaemic Stroke or Transient Ischaemic Attack. Cochrane Database Syst. Rev. 2020, 2020, CD012825. [Google Scholar] [CrossRef]
- Lui, A.; Alzayat, O.; Do, T.; Perekopskiy, D.; Gann, M.; Elgokhy, T.S.; Gao, J.; Liu, D. Multi-Targeted Anti-Inflammatory Drugs for the Treatment of Neurological Disorders. Neural Regen. Res. 2022, 18, 805–806. [Google Scholar]
- Ozben, T.; Ozben, S. Neuro-Inflammation and Anti-Inflammatory Treatment Options for Alzheimer’s Disease. Clin. Biochem. Alzheimer’s Dis. Mak. Point 2019, 72, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-J.; Hu, T.; Jiang, C.-L. Cool the Inflamed Brain: A Novel Anti-Inflammatory Strategy for the Treatment of Major Depressive Disorder. Curr. Neuropharmacol. 2024, 22, 810–842. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Deng, Y.; Wu, S.; Ren, J.; Sun, G.; Yang, J.; Jiang, Y.; Xu, X.; Wang, T.-D.; et al. Trial of Intensive Blood-Pressure Control in Older Patients with Hypertension. N. Engl. J. Med. 2021, 385, 1268–1279. [Google Scholar] [CrossRef]
- Carcel, C.; Haghdoost, F.; Shen, J.; Nanda, P.; Bai, Y.; Atkins, E.; Torii-Yoshimura, T.; Clough, A.J.; Davies, L.; Cordato, D.; et al. The Effect of Blood Pressure Lowering Medications on the Prevention of Episodic Migraine: A Systematic Review and Meta-Analysis. Cephalalgia Int. J. Headache 2023, 43, 3331024231183166. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Desai, S.M.; Jovin, T.G. Indications for Mechanical Thrombectomy for Acute Ischemic Stroke: Current Guidelines and Beyond. Neurology 2021, 97 (Suppl. S2), S126–S136. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cheng, H. Surgical Technique Management of Microvascular Decompression for Trigeminal Neuralgia. Ideggyogy. Szle. 2022, 75, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Redd, M.A.; Fang, C.; Mizikovsky, D.; Li, X.; Macdonald, P.S.; King, G.F.; Palpant, N.J. New Drug Targets and Preclinical Modelling Recommendations for Treating Acute Myocardial Infarction. Heart Lung Circ. 2023, 32, 852–869. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauinger, A.R.; Sepe, J.J. Vascularization, Innervation, and Inflammation: Pathways Connecting the Heart–Brain Axis and Implications in a Clinical Setting. Biomedicines 2025, 13, 171. https://doi.org/10.3390/biomedicines13010171
Lauinger AR, Sepe JJ. Vascularization, Innervation, and Inflammation: Pathways Connecting the Heart–Brain Axis and Implications in a Clinical Setting. Biomedicines. 2025; 13(1):171. https://doi.org/10.3390/biomedicines13010171
Chicago/Turabian StyleLauinger, Alexa R., and Joseph J. Sepe. 2025. "Vascularization, Innervation, and Inflammation: Pathways Connecting the Heart–Brain Axis and Implications in a Clinical Setting" Biomedicines 13, no. 1: 171. https://doi.org/10.3390/biomedicines13010171
APA StyleLauinger, A. R., & Sepe, J. J. (2025). Vascularization, Innervation, and Inflammation: Pathways Connecting the Heart–Brain Axis and Implications in a Clinical Setting. Biomedicines, 13(1), 171. https://doi.org/10.3390/biomedicines13010171




