Neuroinflammation at the Dorsolateral Inferior Medulla: A Possible Central Nervous System Localization for POTS and Long COVID
Abstract
1. Introduction
2. POTS, Long COVID and the CNS
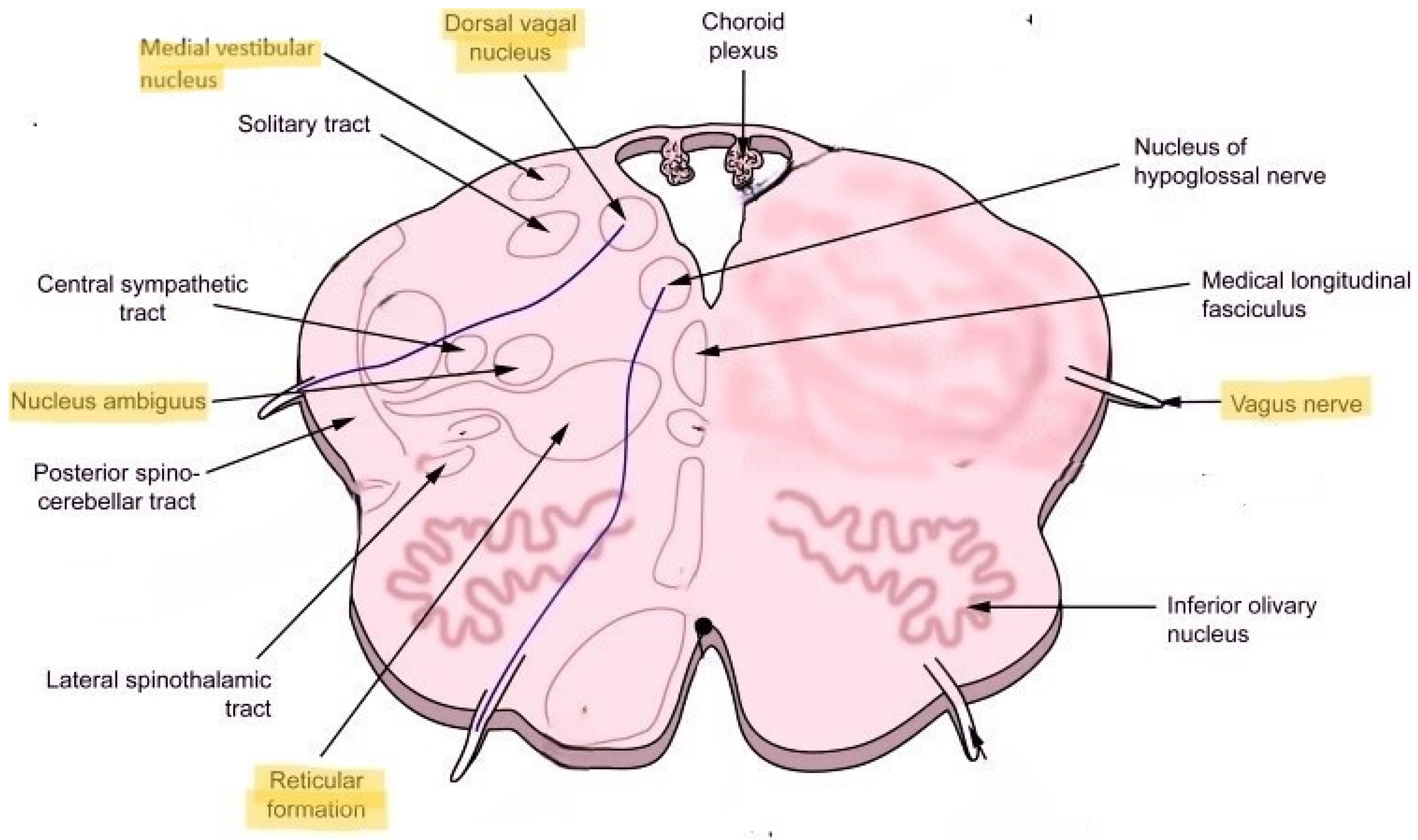
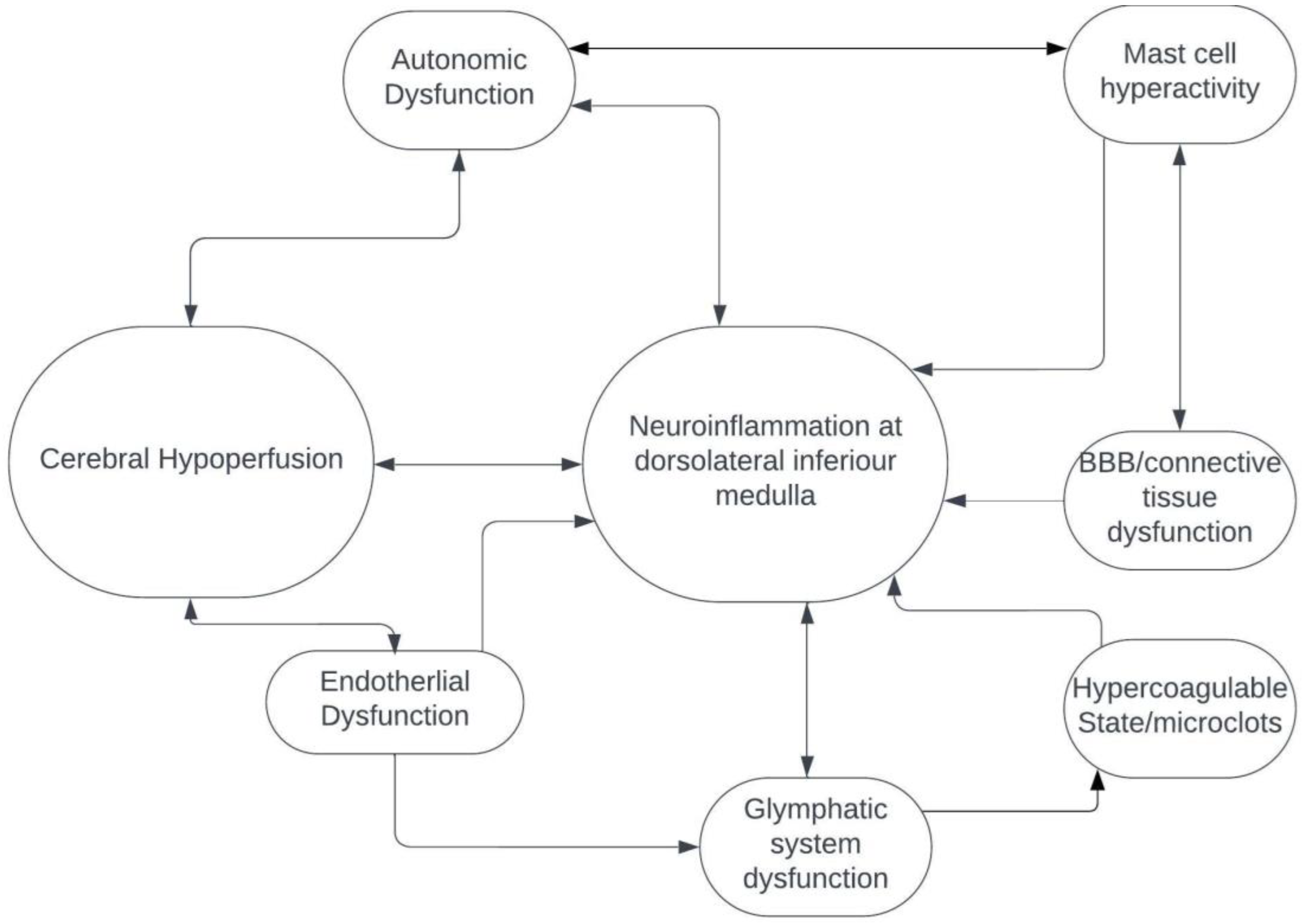
3. Dorsolateral Medulla: Localization for POTS and Long COVID
4. Future Direction
- Why would neuroinflammation affect only specific areas of the brainstem and not the rest of the brainstem or the subcortical and cortical regions in POTS and Long COVID?
- How do hypovolemia, small fiber neuropathy and autoimmunity factor in the development of neuroinflammation at the dorsolateral medulla?
- Is neuroinflammation at the dorsolateral medulla the cause or consequence of systemic immune dysregulation?
- Might there be a mechanical brainstem compression at the level of the dorsolateral inferior medulla with resultant secondary neuroinflammation?
- Could there be structural changes in the ligaments, bones and glymphatic system at the cranio-cervical junction, which may be caused by connective tissue alterations that are triggered by acute infection, possibly in a host with a genetic predisposition toward hypermobility spectrum disorders and other aberrant connective tissue phenotypes?
- How do hyperactive mast cells triggered by infection cause or contribute to neuroinflammation, and do antihistamines and mast cell stabilizing agents reduce neuroinflammation?
- How can we develop clinically available and affordable diagnostic biomarkers to objectively confirm brainstem neuroinflammation when 7T MRI is not available for clinical testing at this time and 3T MRI fails to detect the radiographic signs of neuroinflammation?
- Ultimately, the most important question is the following: What therapeutic targets should be explored in patients with POTS and Long COVID?
5. Conclusions
Funding
Conflicts of Interest
References
- Blitshteyn, S.; Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunol. Res. 2021, 69, 205–211. [Google Scholar] [CrossRef]
- Blitshteyn, S.; Whiteson, J.H.; Abramoff, B.; Azola, A.; Bartels, M.N.; Bhavaraju-Sanka, R.; Chung, T.; Fleming, T.K.; Henning, E.; Miglis, M.G.; et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R 2022, 14, 1270–1291. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences; The National Academies Press: Washington, DC, USA, 2024. [Google Scholar] [CrossRef]
- Sheldon, R.S.; Grubb, B.P., 2nd; Olshansky, B.; Shen, W.K.; Calkins, H.; Brignole, M.; Raj, S.R.; Krahn, A.D.; Morillo, C.A.; Stewart, J.M.; et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015, 12, e41–e63. [Google Scholar] [CrossRef] [PubMed]
- Peltier, A.C.; Garland, A.; Raj, S.R. Distal sudomotor findings in postural tachycardia syndrome. Clin. Auton. Res. 2010, 20, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Blitshteyn, S. Is postural orthostatic tachycardia syndrome (POTS) a central nervous system disorder? J. Neurol. 2022, 269, 725–732. [Google Scholar] [CrossRef]
- Novak, V.; Novak, P.; Opfer-Gehrking, T.L.; O’Brien, P.C.; Low, P.A. Clinical and laboratory indices that enhance the diagnosis of postural tachycardia syndrome. Mayo Clin. Proc. 1998, 73, 1141–1150. [Google Scholar] [CrossRef]
- Benarroch, E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Shaw, B.H.; Stiles, L.E.; Bourne, K.; Green, E.A.; Shibao, C.A.; Okamoto, L.E.; Garland, E.M.; Gamboa, A.; Diedrich, A.; Raj, V.; et al. The face of postural tachycardia syndrome-insights from a large cross-sectional online community-based survey. J. Intern. Med. 2019, 286, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Boris, J.R.; Shadiack EC 3rd McCormick, E.M.; MacMullen, L.; George-Sankoh, I.; Falk, M.J. Long-term POTS outcomes survey: Diagnosis, therapy, and clinical outcomes. J. Am. Heart Assoc. 2024, 3, e033485. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.F.; Grubb, B.P. Postural orthostatic tachycardia syndrome: New concepts in pathophysiology and management. Trends Cardiovasc. Med. 2023, 33, 65–69. [Google Scholar] [CrossRef]
- Wagoner, A.L.; Olson, J.D.; Westwood, B.M.; Fortunato, J.E.; Diz, D.I.; Shaltout, H.A. Children with orthostatic intolerance exhibit elevated markers of inflammation in the dorsal medulla. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H323–H329. [Google Scholar] [CrossRef]
- Rua, C.; Raman, B.; Rodgers, C.T.; Newcombe, V.F.J.; Manktelow, A.; A Chatfield, D.; Sawcer, S.J.; Outtrim, J.G.; Lupson, V.C.; A Stamatakis, E.; et al. Quantitative susceptibility mapping at 7 T in COVID-19: Brainstem effects and outcome associations. Brain 2024, 147, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: Pathophysiological factors and abnormalities of coagulation. Trends Endocrinol. Metab. 2023, 34, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Mangold, S.A.; Das, J.M. Neuroanatomy, Reticular Formation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Nakamura, K.; Matsumura, K.; Kaneko, T.; Kobayashi, S.; Katoh, H.; Negishi, M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J. Neurosci. 2002, 22, 4600–4610. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Z.; Liang, X.; Wang, Y.; Cao, Y.; Li, M.; Zhou, F. Glymphatic system dysfunction in recovered patients with mild COVID-19: A DTI-ALPS study. iScience 2023, 27, 108647. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blitshteyn, S. Neuroinflammation at the Dorsolateral Inferior Medulla: A Possible Central Nervous System Localization for POTS and Long COVID. Biomedicines 2025, 13, 166. https://doi.org/10.3390/biomedicines13010166
Blitshteyn S. Neuroinflammation at the Dorsolateral Inferior Medulla: A Possible Central Nervous System Localization for POTS and Long COVID. Biomedicines. 2025; 13(1):166. https://doi.org/10.3390/biomedicines13010166
Chicago/Turabian StyleBlitshteyn, Svetlana. 2025. "Neuroinflammation at the Dorsolateral Inferior Medulla: A Possible Central Nervous System Localization for POTS and Long COVID" Biomedicines 13, no. 1: 166. https://doi.org/10.3390/biomedicines13010166
APA StyleBlitshteyn, S. (2025). Neuroinflammation at the Dorsolateral Inferior Medulla: A Possible Central Nervous System Localization for POTS and Long COVID. Biomedicines, 13(1), 166. https://doi.org/10.3390/biomedicines13010166





