Pharmacotherapy from Pre-COVID to Post-COVID: Longitudinal Trends and Predictive Indicators for Long COVID Symptoms
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Classification of Medication Data
- Pre-COVID;
- Acute COVID-19, during the acute infection phase;
- Post-COVID, referring to the immediate phase following recovery from acute infection;
- Long COVID, at 3–6 months post-infection.
- Alimentary tract (A);
- Blood and blood-forming organs (B);
- Cardiovascular system (C);
- Dermatologicals (D);
- Genito-urinary system and sex hormones (G);
- Systemic hormonal preparations, excluding sex hormones and insulins (H);
- Antiinfective for systemic use (J);
- Antineoplastic and immunomodulating agents (L);
- Musculo-skeletal system (M);
- Nervous system (N);
- Antiparasitic products, insecticides, and repellents (P);
- Respiratory system (R);
- Sensory organs (S);
- Various (V).
2.3. Statistical Analysis
3. Results
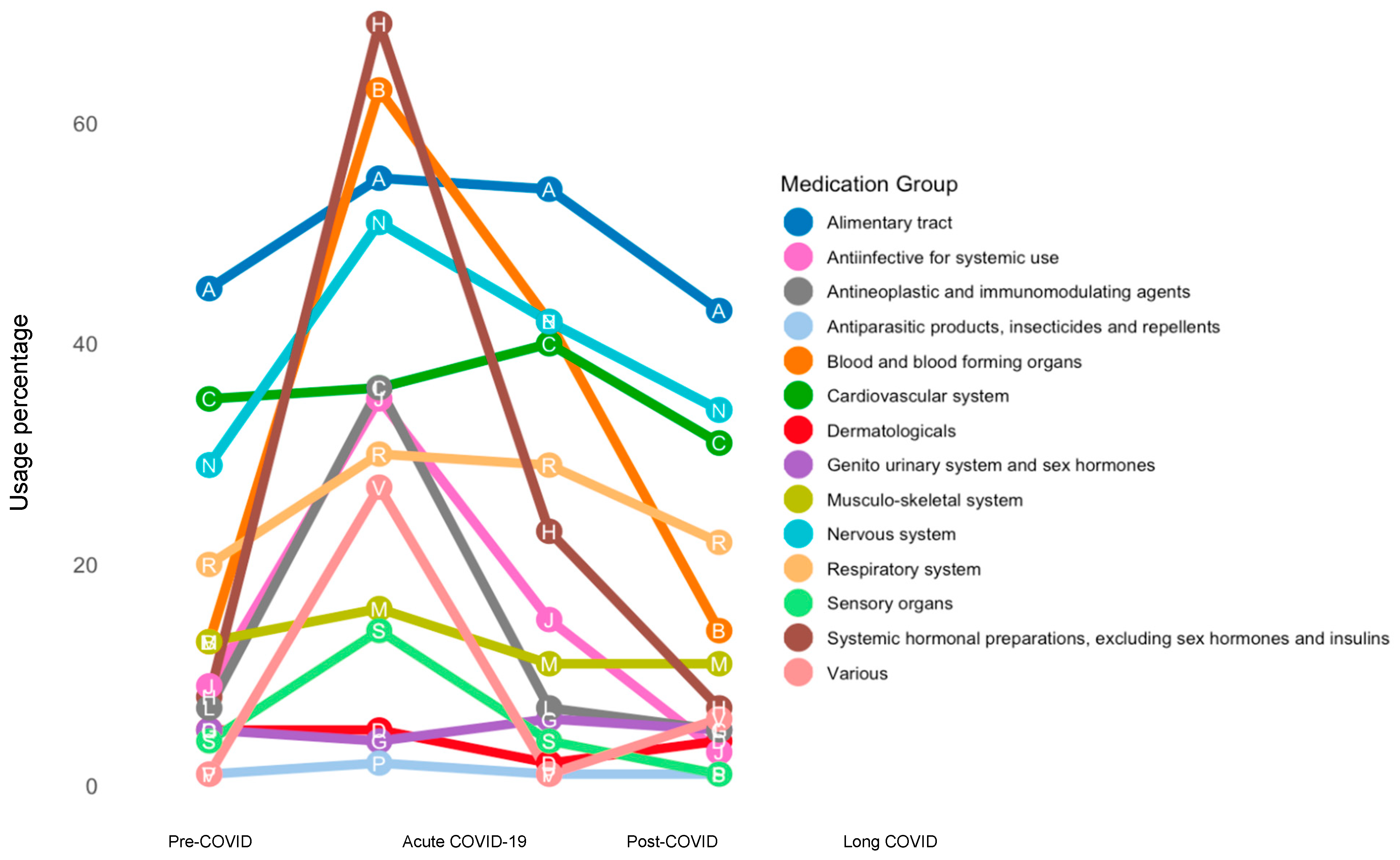
3.1. Study Participant Characteristics and Pharmacotherapy Changes over Time
3.1.1. Alimentary Tract and Metabolism
3.1.2. Blood and Blood-Forming Organs
3.1.3. Cardiovascular System
3.1.4. Dermatologicals and Genito-Urinary System
3.1.5. Systemic Hormonal Preparation and Antiinfectives for Systemic Use
3.1.6. Antineoplastic and Immunomodulating Agents and Musculo-Skeletal System
3.1.7. Nervous System and Respiratory System
3.1.8. Sensory Organ and Various Medications
3.2. Regression Analyses
3.2.1. Fatigue Symptoms
3.2.2. Respiratory Symptoms
3.2.3. Neurological Symptoms
3.2.4. The Number of Persisting Symptom Categories
3.2.5. Pulmonary Radiological Abnormalities
4. Discussion
4.1. Key Pharmacotherapy Trends
4.2. Associations with LC Symptoms
4.3. Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Mills, N.L.; Winter, A.J.; Black, C.; Scott, J.T.; O’donnell, C.A.; Blane, D.N.; Browne, S.; et al. True prevalence of long-COVID in a nationwide, population cohort study. Nat. Commun. 2023, 14, 7892. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Post COVID-19 Condition (Long COVID) [Internet]. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition#:~:text=It%20is%20defined%20as%20the,months%20with%20no%20other%20explanation (accessed on 28 March 2023).
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Baalbaki, N.; Blankestijn, J.M.; Abdel-Aziz, M.I.; De Backer, J.; Bazdar, S.; Beekers, I.; Beijers, R.J.H.C.G.; Bergh, J.P.v.D.; Bloemsma, L.D.; Bogaard, H.J.; et al. Precision Medicine for More Oxygen (P4O2)—Study Design and First Results of the Long COVID-19 Extension. J. Pers. Med. 2023, 13, 1060. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 26, n1648. [Google Scholar] [CrossRef]
- Akhoon, N. Precision Medicine: A New Paradigm in Therapeutics. Int. J. Prev. Med. 2021, 12, 12. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post−COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566. [Google Scholar] [CrossRef]
- Giovannucci, E.; Egan, K.M.; Hunter, D.J.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Speizer, F.E. Aspirin and the Risk of Colorectal Cancer in Women. N. Engl. J. Med. 1995, 333, 609–614. [Google Scholar] [CrossRef]
- Li, G.; Higdon, R.; Kukull, W.A.; Peskind, E.; Van Valen Moore, K.; Tsuang, D.; van Belle, G.; McCormick, W.; Bowen, J.D.; Teri, L.; et al. Statin therapy and risk of dementia in the elderly: A community-based prospective cohort study. Neurology 2004, 63, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.L.; Agarwal, S.K.; MacLehose, R.F.; Soliman, E.Z.; Sharrett, A.R.; Huxley, R.R.; Konety, S.; Ballantyne, C.M.; Alonso, A. Blood Lipid Levels, Lipid-Lowering Medications, and the Incidence of Atrial Fibrillation: The Atherosclerosis Risk in Communities Study. Circ. Arrhythmia Electrophysiol. 2012, 5, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tanzadehpanah, H.; Lotfian, E.; Avan, A.; Saki, S.; Nobari, S.; Mahmoodian, R.; Sheykhhasan, M.; Froutagh, M.H.S.; Ghotbani, F.; Jamshidi, R.; et al. Role of SARS-COV-2 and ACE2 in the pathophysiology of peripheral vascular diseases. Biomed. Pharmacother. 2023, 166, 115321. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Tessema, A.G.; Mengiste, Z.M.; Hundie, T.G.; Yosef, H.G.; Huluka, D.K.; Seyoum, A.B.; Abate, H.K.; Howe, R.C. The Effect of Anti-Coagulation Dosage on the Outcome of Hospitalized COVID-19 Patients in Ethiopia: A Multi-Center Retrospective Cohort Study [Internet]. 2022. Available online: https://www.researchsquare.com/article/rs-2094190/v1 (accessed on 29 July 2023).
- Choron, R.L.; Iacono, S.; Maharaja, K.; Adams, C.D.; Butts, C.A.; Bargoud, C.; Teichman, A.L.; Krumrei, N.J.; Schroeder, M.E.; Manderski, M.T.B.; et al. Continuation of therapeutic anticoagulation before and during hospitalization is associated with reduced mortality in COVID-19 ICU patients. J. Lung Pulm. Respir. Res. 2021, 8, 120–130. [Google Scholar] [CrossRef]
- Nakamura, A.; Kotaki, T.; Nagai, Y.; Takazawa, S.; Tokunaga, K.; Kameoka, M. Construction and evaluation of a self-replicative RNA vaccine against SARS-CoV-2 using yellow fever virus replicon. PLoS ONE 2022, 17, e0274829. [Google Scholar] [CrossRef]
- Leentjens, J.; Van Haaps, T.F.; Wessels, P.F.; Schutgens, R.E.G.; Middeldorp, S. COVID-19-associated coagulopathy and antithrombotic agents—Lessons after 1 year. Lancet Haematol. 2021, 8, e524–e533. [Google Scholar] [CrossRef]
- Jara-Palomares, L.; Bikdeli, B.; Jiménez, D.; Muriel, A.; Demelo-Rodríguez, P.; Moustafa, F.; Villalobos, A.; López-Miguel, P.; López-Jiménez, L.; Otálora, S.; et al. Risk of recurrence after discontinuing anticoagulation in patients with COVID-19- associated venous thromboembolism: A prospective multicentre cohort study. eClinicalMedicine 2024, 73, 102659. [Google Scholar] [CrossRef]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330. [Google Scholar]
- Yang, J.W.; Yang, L.; Luo, R.G.; Xu, J.F. Corticosteroid administration for viral pneumonia: COVID-19 and beyond. Clin. Microbiol. Infect. 2020, 26, 1171–1177. [Google Scholar] [CrossRef]
- Viana, M.V.; Pellegrini, J.A.S.; Perez, A.V.; Schwarz, P.; Da Silva, D.; Teixeira, C.; Gazzana, M.B.; Rech, T.H. Association between prolonged corticosteroids use in COVID-19 and increased mortality in hospitalized patients: A retrospective study with inverse probability of treatment weighting analysis. Crit. Care 2023, 27, 143. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.I.; Nyberg, F.; Hajiebrahimi, M.; Bertilsson, R.; Nåtman, J.; Santosa, A.; Wettermark, B. Initiation of antihypertensive drugs to patients with confirmed COVID-19—A population-based cohort study in Sweden. Basic Clin. Pharmacol. Toxicol. 2022, 131, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Schreinlechner, M.; Sappler, N.; Dolejsi, T.; Tilg, H.; Aulinger, B.A.; Weiss, G.; Bellmann-Weiler, R.; Adolf, C.; Wolf, D.; et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): A prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir. Med. 2021, 9, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Safizadeh, F.; Nguyen, T.N.M.; Brenner, H.; Schöttker, B. Association of Renin–Angiotensin–Aldosterone System Blocker Use with COVID-19 Hospitalization and All-Cause Mortality in the UK Biobank [Internet]. 2021. Available online: https://www.authorea.com/users/435850/articles/538592-association-of-renin-angiotensin-aldosterone-system-blocker-use-with-covid-19-hospitalization-and-all-cause-mortality-in-the-uk-biobank?commit=9ea4ba8a4b3bdf02cc641c8d233ce6776183f58b (accessed on 30 July 2023).
- Shang, H.; Huang, C.; Chen, Y.; Zhang, S.; Yang, P.; Hong, G.; Zhang, L.; Hou, X. Digestive Involvement in SARS-CoV-2 Infection: A Retrospective Multi-Center Study [Internet]. 2020. Available online: https://www.researchsquare.com/article/rs-21375/v1 (accessed on 29 July 2023).
- Ozoglu Aytac, S.; Kilic, S.P.; Ovayolu, N. Effect of inhaler drug education on fatigue, dyspnea severity, and respiratory function tests in patients with COPD. Patient Educ. Couns. 2020, 103, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.M.J.; Spruit, M.A.; Van ’t Hul, A.J.; Peters, J.B.; Van Herck, M.; Nakken, N.; Djamin, R.S.; Burtin, C.; Thong, M.S.Y.; Coors, A.; et al. Fatigue is highly prevalent in patients with COPD and correlates poorly with the degree of airflow limitation. Ther. Adv. Respir. Dis. 2019, 13, 1753466619878128. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Degli Antoni, M.; Minisci, D.; Amadasi, S.; Castelli, F.; Odone, A.; Quiros-Roldan, E. The impact of early therapies for COVID-19 on death, hospitalization and persisting symptoms: A retrospective study. Infection 2023, 51, 1633–1644. [Google Scholar] [CrossRef]
- Jacobs, E.T.; Catalfamo, C.J.; Colombo, P.M.; Khan, S.M.; Austhof, E.; Cordova-Marks, F.; Ernst, K.C.; Farland, L.V.; Pogreba-Brown, K. Pre-existing conditions associated with post-acute sequelae of COVID-19. J. Autoimmun. 2023, 135, 102991. [Google Scholar] [CrossRef]
- Guragai, N.; Vasudev, R.; Hosein, K.; Habib, H.; Patel, B.; Kaur, P.; Patel, B.; Santana, M.; Elkattawy, S.; Noori, M.A.M.; et al. Does Baseline Diuretics Use Affect Prognosis in Patients with COVID-19? Cureus [Internet]. 2021. Available online: https://www.cureus.com/articles/59052-does-baseline-diuretics-use-affect-prognosis-in-patients-with-covid-19 (accessed on 30 July 2023).
- Sato, K.; Sinclair, J.E.; Sadeghirad, H.; Fraser, J.F.; Short, K.R.; Kulasinghe, A. Cardiovascular disease in SARS-CoV-2 infection. Clin. Trans. Imm. 2021, 10, e1343. [Google Scholar] [CrossRef]
- Rezel-Potts, E.; Douiri, A.; Chowienczyk, P.J.; Gulliford, M.C. Antihypertensive medications and COVID-19 diagnosis and mortality: Population-based case-control analysis in the United Kingdom. Br. J. Clin. Pharmacol. 2021, 87, 4598–4607. [Google Scholar] [CrossRef]
- Chudzik, M.; Lewek, J.; Kapusta, J.; Banach, M.; Jankowski, P.; Bielecka-Dabrowa, A. Predictors of Long COVID in Patients without Comorbidities: Data from the Polish Long-COVID Cardiovascular (PoLoCOV-CVD) Study. J. Clin. Med. 2022, 11, 4980. [Google Scholar] [CrossRef]
- Kataoka, M.; Hazumi, M.; Usuda, K.; Okazaki, E.; Nishi, D. Association of preexisting psychiatric disorders with post-COVID-19 prevalence: A cross-sectional study. Sci. Rep. 2023, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Schönenberg, A.; Prell, T. Factors Influencing Self-Reported Medication Use in the Survey of Health Aging and Retirement in Europe (SHARE) Dataset. Healthcare 2021, 9, 1752. [Google Scholar] [CrossRef]
- Hafferty, J.D.; Campbell, A.I.; Navrady, L.B.; Adams, M.J.; MacIntyre, D.; Lawrie, S.M.; Nicodemus, K.; Porteous, D.J.; McIntosh, A.M. Self-reported medication use validated through record linkage to national prescribing data. J. Clin. Epidemiol. 2018, 94, 132–142. [Google Scholar] [CrossRef]
- Nwabufo, C.K.; Luc, J.; McGeer, A.; Hirota, J.A.; Mubareka, S.; Doxey, A.C.; Moraes, T.J. COVID-19 severity gradient differentially dysregulates clinically relevant drug processing genes in nasopharyngeal swab samples. Br. J. Clin. Pharmacol. 2024, 90, 2137–2158. [Google Scholar] [CrossRef]
| Fatigue Symptoms | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted * | |||||
| Medication Group | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Cardiovascular system | 0.56 | 0.23–1.36 | 0.20 | 0.35 | 0.12–1.06 | 0.06 |
| Antiinfective for systemic use | 0.20 | 0.05–0.85 | 0.03 | 0.22 | 0.04–1.23 | 0.09 |
| Respiratory system | 5.48 | 1.18–25.41 | 0.03 | 5.74 | 1.16–28.54 | 0.03 |
| Respiratory symptoms | ||||||
| Unadjusted | Adjusted * | |||||
| Medication Group | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Respiratory system | 2.89 | 0.61–13.69 | 0.18 | 2.67 | 0.51–14.00 | 0.25 |
| Neurological symptoms | ||||||
| Unadjusted | Adjusted * | |||||
| Medication Group | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Cardiovascular system | 0.51 | 0.21–1.25 | 0.14 | 0.39 | 0.13–1.14 | 0.09 |
| Antiinfective for systemic use | 0.10 | 0.02–0.52 | 0.01 | 0.11 | 0.02–0.66 | 0.02 |
| Number of symptom categories | ||||||
| Unadjusted | Adjusted * | |||||
| Medication Group | β | 95% CI | p-value | β | 95% CI | p-value |
| Cardiovascular system | −0.50 | −1.18–0.19 | 0.15 | −0.76 | −1.49–−0.03 | 0.04 |
| Antiinfective for systemic use | −1.59 | −2.68–−0.50 | 0.01 | −1.21 | −2.40–−0.03 | 0.046 |
| Pulmonary radiological abnormalities | ||||||
| Unadjusted | Adjusted * | |||||
| Medication Group | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Nervous system | 0.50 | 0.18–1.39 | 0.18 | 0.29 | 0.09–0.97 | 0.045 |
| Respiratory system | 2.79 | 0.58–13.39 | 0.20 | 5.04 | 0.73–34.87 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baalbaki, N.; Verbeek, S.T.; Bogaard, H.J.; Blankestijn, J.M.; van den Brink, V.C.; Cornelissen, M.E.B.; Twisk, J.W.R.; Golebski, K.; Maitland-van der Zee, A.H., on behalf of the P4O2 consortium. Pharmacotherapy from Pre-COVID to Post-COVID: Longitudinal Trends and Predictive Indicators for Long COVID Symptoms. Biomedicines 2024, 12, 2694. https://doi.org/10.3390/biomedicines12122694
Baalbaki N, Verbeek ST, Bogaard HJ, Blankestijn JM, van den Brink VC, Cornelissen MEB, Twisk JWR, Golebski K, Maitland-van der Zee AH on behalf of the P4O2 consortium. Pharmacotherapy from Pre-COVID to Post-COVID: Longitudinal Trends and Predictive Indicators for Long COVID Symptoms. Biomedicines. 2024; 12(12):2694. https://doi.org/10.3390/biomedicines12122694
Chicago/Turabian StyleBaalbaki, Nadia, Sien T. Verbeek, Harm Jan Bogaard, Jelle M. Blankestijn, Vera C. van den Brink, Merel E. B. Cornelissen, Jos W. R. Twisk, Korneliusz Golebski, and Anke H. Maitland-van der Zee on behalf of the P4O2 consortium. 2024. "Pharmacotherapy from Pre-COVID to Post-COVID: Longitudinal Trends and Predictive Indicators for Long COVID Symptoms" Biomedicines 12, no. 12: 2694. https://doi.org/10.3390/biomedicines12122694
APA StyleBaalbaki, N., Verbeek, S. T., Bogaard, H. J., Blankestijn, J. M., van den Brink, V. C., Cornelissen, M. E. B., Twisk, J. W. R., Golebski, K., & Maitland-van der Zee, A. H., on behalf of the P4O2 consortium. (2024). Pharmacotherapy from Pre-COVID to Post-COVID: Longitudinal Trends and Predictive Indicators for Long COVID Symptoms. Biomedicines, 12(12), 2694. https://doi.org/10.3390/biomedicines12122694







