Impact of First- and Second-Generation Tyrosine Kinase Inhibitors on the Development of Graft-Versus-Host Disease in Individuals with Chronic Myeloid Leukemia: A Retrospective Analysis on Behalf of the Polish Adult Leukemia Group
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definitions
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient-, Disease-, and Transplantation-Related Characteristics
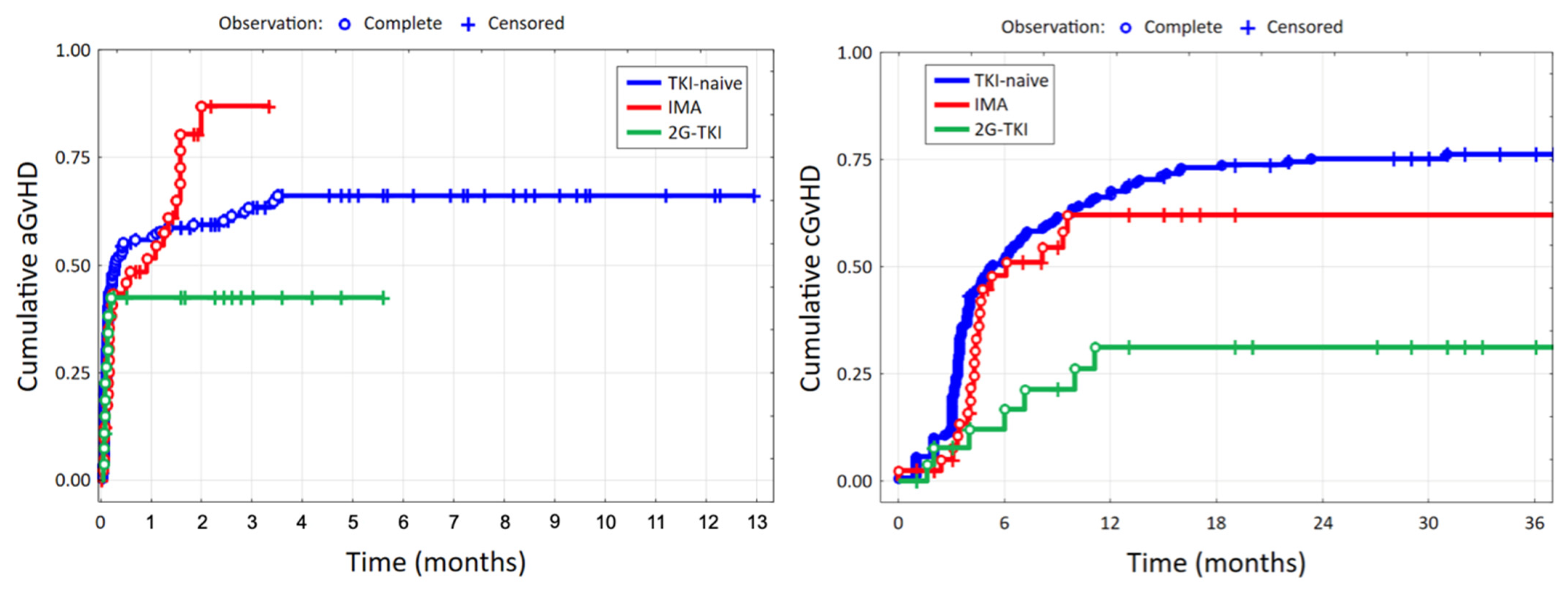
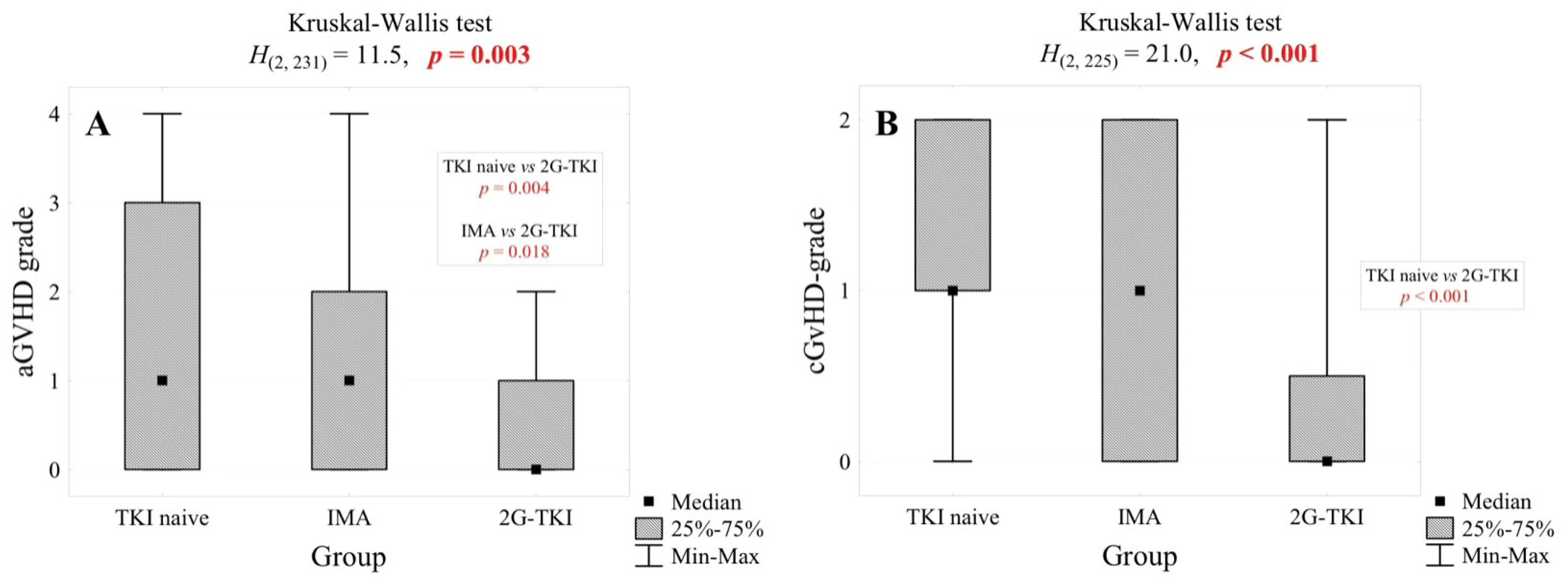
3.2. Acute and Chronic Graft-Versus-Host Disease
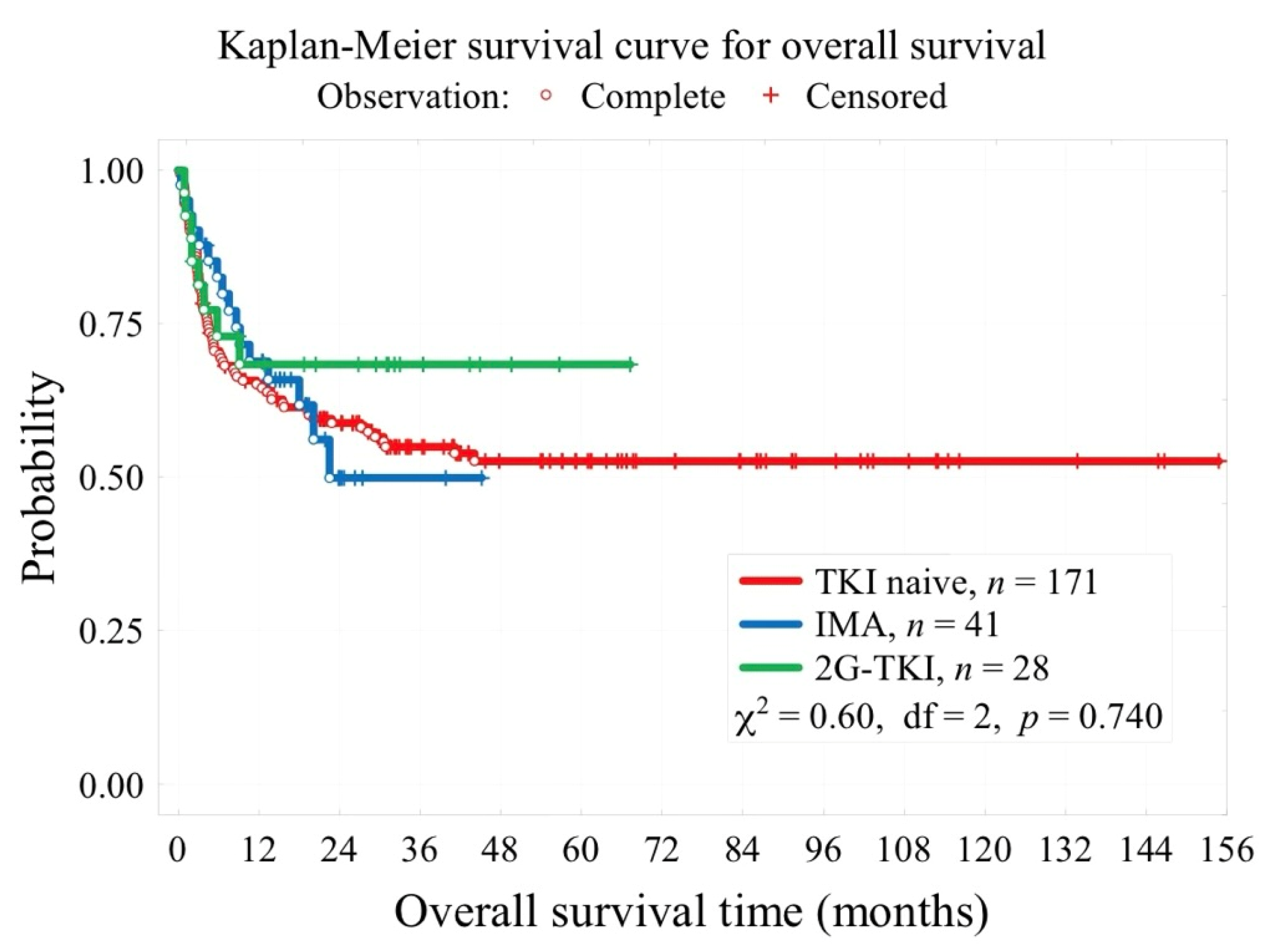
3.3. Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.G.; Guilhot, F.; Larson, R.A.; Gathmann, I.; Baccarani, M.; Cervantes, F.; Cornelissen, J.J.; Fischer, T.; Hochhaus, A.; Hughes, T.; et al. Imatinib Compared with Interferon and Low-Dose Cytarabine for Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. N. Engl. J. Med. 2003, 348, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P.; Guilhot, F.; Cortes, J.E.; Schiffer, C.A.; le Coutre, P.; Brümmendorf, T.H.; Kantarjian, H.M.; Hochhaus, A.; Rousselot, P.; Mohamed, H.; et al. Long-Term Outcome with Dasatinib after Imatinib Failure in Chronic-Phase Chronic Myeloid Leukemia: Follow-up of a Phase 3 Study. Blood 2014, 123, 2317–2324. [Google Scholar] [CrossRef]
- Giles, F.J.; le Coutre, P.D.; Pinilla-Ibarz, J.; Larson, R.A.; Gattermann, N.; Ottmann, O.G.; Hochhaus, A.; Radich, J.P.; Saglio, G.; Hughes, T.P.; et al. Nilotinib in Imatinib-Resistant or Imatinib-Intolerant Patients with Chronic Myeloid Leukemia in Chronic Phase: 48-Month Follow-up Results of a Phase II Study. Leukemia 2013, 27, 107–112. [Google Scholar] [CrossRef]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.-W.; Issaragrisil, S.; le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-Term Benefits and Risks of Frontline Nilotinib vs. Imatinib for Chronic Myeloid Leukemia in Chronic Phase: 5-Year Update of the Randomized ENESTnd Trial. Leukemia 2016, 30, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Saglio, G.; Kantarjian, H.M.; Baccarani, M.; Mayer, J.; Boqué, C.; Shah, N.P.; Chuah, C.; Casanova, L.; Bradley-Garelik, B.; et al. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. J. Clin. Oncol. 2016, 34, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Ito, S. The Role of Stem Cell Transplantation for Chronic Myelogenous Leukemia in the 21st Century. Blood 2015, 125, 3230–3235. [Google Scholar] [CrossRef] [PubMed]
- Lübking, A.; Dreimane, A.; Sandin, F.; Isaksson, C.; Märkevärn, B.; Brune, M.; Ljungman, P.; Lenhoff, S.; Stenke, L.; Höglund, M.; et al. Allogeneic Stem Cell Transplantation for Chronic Myeloid Leukemia in the TKI Era: Population-Based Data from the Swedish CML Registry. Bone Marrow Transplant. 2019, 54, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Mughal, T.I.; Radich, J.P.; Deininger, M.W.; Apperley, J.F.; Hughes, T.P.; Harrison, C.J.; Gambacorti-Passerini, C.; Saglio, G.; Cortes, J.; Daley, G.Q. Chronic Myeloid Leukemia: Reminiscences and Dreams. Haematologica 2016, 101, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Gratwohl, A.; Brand, R.; Apperley, J.; Crawley, C.; Ruutu, T.; Corradini, P.; Carreras, E.; Devergie, A.; Guglielmi, C.; Kolb, H.-J.; et al. Allogeneic Hematopoietic Stem Cell Transplantation for Chronic Myeloid Leukemia in Europe 2006: Transplant Activity, Long-Term Data and Current Results. An Analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 2006, 91, 513–521. [Google Scholar]
- Dazzi, F.; Szydlo, R.M.; Craddock, C.; Cross, N.C.; Kaeda, J.; Chase, A.; Olavarria, E.; van Rhee, F.; Kanfer, E.; Apperley, J.F.; et al. Comparison of Single-Dose and Escalating-Dose Regimens of Donor Lymphocyte Infusion for Relapse after Allografting for Chronic Myeloid Leukemia. Blood 2000, 95, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Pavlu, J.; Szydlo, R.M.; Goldman, J.M.; Apperley, J.F. Three Decades of Transplantation for Chronic Myeloid Leukemia: What Have We Learned? Blood 2011, 117, 755–763. [Google Scholar] [CrossRef]
- Innes, A.J.; Milojkovic, D.; Apperley, J.F. Allogeneic Transplantation for CML in the TKI Era: Striking the Right Balance. Nat. Rev. Clin. Oncol. 2016, 13, 79–91. [Google Scholar] [CrossRef]
- Gratwohl, A.; Heim, D. Current Role of Stem Cell Transplantation in Chronic Myeloid Leukaemia. Best Pract. Res. Clin. Haematol. 2009, 22, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Passweg, J.R.; Baldomero, H.; Gratwohl, A.; Bregni, M.; Cesaro, S.; Dreger, P.; de Witte, T.; Farge-Bancel, D.; Gaspar, B.; Marsh, J.; et al. The EBMT Activity Survey: 1990–2010. Bone Marrow Transplant. 2012, 47, 906–923. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: 2025 Update on Diagnosis, Therapy, and Monitoring. Am. J. Hematol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Cortes, J.; Santos, F.P.S.; Jones, D.; O’Brien, S.; Rondon, G.; Popat, U.; Giralt, S.; Kebriaei, P.; Jones, R.B.; et al. Results of Allogeneic Hematopoietic Stem Cell Transplantation for Chronic Myelogenous Leukemia Patients Who Failed Tyrosine Kinase Inhibitors after Developing BCR-ABL1 Kinase Domain Mutations. Blood 2011, 117, 3641–3647. [Google Scholar] [CrossRef]
- Nicolini, F.E.; Basak, G.W.; Kim, D.-W.; Olavarria, E.; Pinilla-Ibarz, J.; Apperley, J.F.; Hughes, T.; Niederwieser, D.; Mauro, M.J.; Chuah, C.; et al. Overall Survival with Ponatinib versus Allogeneic Stem Cell Transplantation in Philadelphia Chromosome-Positive Leukemias with the T315I Mutation. Cancer 2017, 123, 2875–2880. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Shah, N.P.; Hochhaus, A.; Cortes, J.; Shah, S.; Ayala, M.; Moiraghi, B.; Shen, Z.; Mayer, J.; Pasquini, R.; et al. Dasatinib versus Imatinib in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia. N. Engl. J. Med. 2010, 362, 2260–2270. [Google Scholar] [CrossRef]
- Saglio, G.; Kim, D.-W.; Issaragrisil, S.; le Coutre, P.; Etienne, G.; Lobo, C.; Pasquini, R.; Clark, R.E.; Hochhaus, A.; Hughes, T.P.; et al. Nilotinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia. N. Engl. J. Med. 2010, 362, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of Tyrosine Kinase Inhibitor Therapy in Chronic Myeloid Leukaemia (EURO-SKI): A Prespecified Interim Analysis of a Prospective, Multicentre, Non-Randomised, Trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.; Schleuning, M.; Greinix, H.; Sayer, H.G.; Fischer, T.; Martinez, J.; Maziarz, R.; Olavarria, E.; Verdonck, L.; Schaefer, K.; et al. The Effect of Prior Exposure to Imatinib on Transplant-Related Mortality. Haematologica 2006, 91, 452–459. [Google Scholar]
- Oehler, V.G.; Gooley, T.; Snyder, D.S.; Johnston, L.; Lin, A.; Cummings, C.C.; Chu, S.; Bhatia, R.; Forman, S.J.; Negrin, R.S.; et al. The Effects of Imatinib Mesylate Treatment before Allogeneic Transplantation for Chronic Myeloid Leukemia. Blood 2007, 109, 1782–1789. [Google Scholar] [CrossRef]
- Lee, S.J.; Kukreja, M.; Wang, T.; Giralt, S.A.; Szer, J.; Arora, M.; Woolfrey, A.E.; Cervantes, F.; Champlin, R.E.; Gale, R.P.; et al. Impact of Prior Imatinib Mesylate on the Outcome of Hematopoietic Cell Transplantation for Chronic Myeloid Leukemia. Blood 2008, 112, 3500–3507. [Google Scholar] [CrossRef]
- Breccia, M.; Palandri, F.; Iori, A.P.; Colaci, E.; Latagliata, R.; Castagnetti, F.; Torelli, G.F.; Usai, S.; Valle, V.; Martinelli, G.; et al. Second-Generation Tyrosine Kinase Inhibitors before Allogeneic Stem Cell Transplantation in Patients with Chronic Myeloid Leukemia Resistant to Imatinib. Leuk. Res. 2010, 34, 143–147. [Google Scholar] [CrossRef]
- Piekarska, A.; Gil, L.; Prejzner, W.; Wiśniewski, P.; Leszczyńska, A.; Gniot, M.; Komarnicki, M.; Hellmann, A. Pretransplantation Use of the Second-Generation Tyrosine Kinase Inhibitors Has No Negative Impact on the HCT Outcome. Ann. Hematol. 2015, 94, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Cortes, J.; Kantarjian, H.; Giralt, S.; Andersson, B.S.; Giles, F.; Shpall, E.; Kebriaei, P.; Champlin, R.; de Lima, M. Novel Tyrosine Kinase Inhibitor Therapy before Allogeneic Stem Cell Transplantation in Patients with Chronic Myeloid Leukemia: No Evidence for Increased Transplant-Related Toxicity. Cancer 2007, 110, 340–344. [Google Scholar] [CrossRef]
- Saidu, N.E.B.; Bonini, C.; Dickinson, A.; Grce, M.; Inngjerdingen, M.; Koehl, U.; Toubert, A.; Zeiser, R.; Galimberti, S. New Approaches for the Treatment of Chronic Graft-Versus-Host Disease: Current Status and Future Directions. Front. Immunol. 2020, 11, 578314. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.J.; Deininger, M. Tyrosine Kinase Inhibitor-Associated Cardiovascular Toxicity in Chronic Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 4210–4218. [Google Scholar] [CrossRef]
- de Lavallade, H.; Khoder, A.; Hart, M.; Sarvaria, A.; Sekine, T.; Alsuliman, A.; Mielke, S.; Bazeos, A.; Stringaris, K.; Ali, S.; et al. Tyrosine Kinase Inhibitors Impair B-Cell Immune Responses in CML through off-Target Inhibition of Kinases Important for Cell Signaling. Blood 2013, 122, 227–238. [Google Scholar] [CrossRef]
- Baccarani, M.; Saglio, G.; Goldman, J.; Hochhaus, A.; Simonsson, B.; Appelbaum, F.; Apperley, J.; Cervantes, F.; Cortes, J.; Deininger, M.; et al. Evolving Concepts in the Management of Chronic Myeloid Leukemia: Recommendations from an Expert Panel on Behalf of the European LeukemiaNet. Blood 2006, 108, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Cortes, J.; Pane, F.; Niederwieser, D.; Saglio, G.; Apperley, J.; Cervantes, F.; Deininger, M.; Gratwohl, A.; Guilhot, F.; et al. Chronic Myeloid Leukemia: An Update of Concepts and Management Recommendations of European LeukemiaNet. J. Clin. Oncol. 2009, 27, 6041–6051. [Google Scholar] [CrossRef]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995, 15, 825–828. [Google Scholar]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Shulman, H.M.; Sullivan, K.M.; Weiden, P.L.; McDonald, G.B.; Striker, G.E.; Sale, G.E.; Hackman, R.; Tsoi, M.S.; Storb, R.; Thomas, E.D. Chronic Graft-versus-Host Syndrome in Man. A Long-Term Clinicopathologic Study of 20 Seattle Patients. Am. J. Med. 1980, 69, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, V.S.; Baccarani, M.; Hasford, J.; Castagnetti, F.; Di Raimondo, F.; Casado, L.F.; Turkina, A.; Zackova, D.; Ossenkoppele, G.; Zaritskey, A.; et al. Treatment and Outcome of 2904 CML Patients from the EUTOS Population-Based Registry. Leukemia 2017, 31, 593–601. [Google Scholar] [CrossRef]
- Senapati, J.; Sasaki, K.; Issa, G.C.; Lipton, J.H.; Radich, J.P.; Jabbour, E.; Kantarjian, H.M. Management of Chronic Myeloid Leukemia in 2023—Common Ground and Common Sense. Blood Cancer J. 2023, 13, 58. [Google Scholar] [CrossRef]
- Radich, J.P.; Hochhaus, A.; Masszi, T.; Hellmann, A.; Stentoft, J.; Casares, M.T.G.; García-Gutiérrez, J.V.; Conneally, E.; le Coutre, P.D.; Gattermann, N.; et al. Treatment-Free Remission Following Frontline Nilotinib in Patients with Chronic Phase Chronic Myeloid Leukemia: 5-Year Update of the ENESTfreedom Trial. Leukemia 2021, 35, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Gugliotta, G.; Castagnetti, F.; Breccia, M.; Levato, L.; Intermesoli, T.; D’Adda, M.; Salvucci, M.; Stagno, F.; Rege-Cambrin, G.; Tiribelli, M.; et al. Treatment-Free Remission in Chronic Myeloid Leukemia Patients Treated Front-Line with Nilotinib: 10-Year Followup of the GIMEMA CML 0307 Study. Haematologica 2022, 107, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.G.; Sasaki, K.; Issa, G.C.; Garcia-Manero, G.; Ravandi, F.; Kadia, T.; Cortes, J.; Konopleva, M.; Pemmaraju, N.; Alvarado, Y.; et al. Treatment-Free Remission in Patients with Chronic Myeloid Leukemia Following the Discontinuation of Tyrosine Kinase Inhibitors. Am. J. Hematol. 2022, 97, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Khoury, H.J.; Kukreja, M.; Goldman, J.M.; Wang, T.; Halter, J.; Arora, M.; Gupta, V.; Rizzieri, D.A.; George, B.; Keating, A.; et al. Prognostic Factors for Outcomes in Allogeneic Transplantation for CML in the Imatinib Era: A CIBMTR Analysis. Bone Marrow Transplant. 2012, 47, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Liu, Q.; Sun, J.; Jiang, Q.; Ye, Y.; Huang, H.; Meng, F.; Zhou, Y.; Yang, M. Prior Exposure to Imatinib Does Not Impact Outcome of Allogeneic Hematopoietic Transplantation for Chronic Myeloid Leukemia Patients: A Single-Center Experience in China. Int. J. Clin. Exp. Med. 2015, 8, 2495–2505. [Google Scholar] [PubMed]
- Heim, D.; Baldomero, H.; Medinger, M.; Masouridi-Levrat, S.; Schanz, U.; Nair, G.; Güngör, T.; Halter, J.; Passweg, J.R.; Chalandon, Y.; et al. Allogeneic Haematopoietic Cell Transplantation for Chronic Myeloid Leukaemia in Switzerland in the Face of Rapid Development of Effective Drugs. Swiss Med. Wkly 2024, 154, 3754. [Google Scholar]
- Friedrichs, B.; Tichelli, A.; Bacigalupo, A.; Russell, N.H.; Ruutu, T.; Shapira, M.Y.; Beksac, M.; Hasenclever, D.; Socié, G.; Schmitz, N. Long-Term Outcome and Late Effects in Patients Transplanted with Mobilised Blood or Bone Marrow: A Randomised Trial. Lancet Oncol. 2010, 11, 331–338. [Google Scholar] [CrossRef]
- Blaise, D.; Kuentz, M.; Fortanier, C.; Bourhis, J.H.; Milpied, N.; Sutton, L.; Jouet, J.P.; Attal, M.; Bordigoni, P.; Cahn, J.Y.; et al. Randomized Trial of Bone Marrow versus Lenograstim-Primed Blood Cell Allogeneic Transplantation in Patients with Early-Stage Leukemia: A Report from the Société Française de Greffe de Moelle. J. Clin. Oncol. 2000, 18, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Vigorito, A.C.; Azevedo, W.M.; Marques, J.F.; Azevedo, A.M.; Eid, K.A.; Aranha, F.J.; Lorand-Metze, I.; Oliveira, G.B.; Correa, M.E.; Reis, A.R.; et al. A Randomised, Prospective Comparison of Allogeneic Bone Marrow and Peripheral Blood Progenitor Cell Transplantation in the Treatment of Haematological Malignancies. Bone Marrow Transplant. 1998, 22, 1145–1151. [Google Scholar] [CrossRef]
- Kim, D.H.; Sohn, S.K.; Baek, J.H.; Kim, J.G.; Lee, J.W.; Min, W.S.; Kim, D.W.; Choi, S.-J.; Lee, J.-H.; Lee, K.-H.; et al. Retrospective Multicenter Study of Allogeneic Peripheral Blood Stem Cell Transplantation Followed by Reduced-Intensity Conditioning or Conventional Myeloablative Regimen. Acta Haematol. 2005, 113, 220–227. [Google Scholar] [CrossRef]
- Aoudjhane, M.; Labopin, M.; Gorin, N.C.; Shimoni, A.; Ruutu, T.; Kolb, H.-J.; Frassoni, F.; Boiron, J.M.; Yin, J.L.; Finke, J.; et al. Comparative Outcome of Reduced Intensity and Myeloablative Conditioning Regimen in HLA Identical Sibling Allogeneic Haematopoietic Stem Cell Transplantation for Patients Older than 50 Years of Age with Acute Myeloblastic Leukaemia: A Retrospective Survey from the Acute Leukemia Working Party (ALWP) of the European Group for Blood and Marrow Transplantation (EBMT). Leukemia 2005, 19, 2304–2312. [Google Scholar]
- Afram, G.; Simón, J.A.P.; Remberger, M.; Caballero-Velázquez, T.; Martino, R.; Piñana, J.L.; Ringden, O.; Esquirol, A.; Lopez-Corral, L.; Garcia, I.; et al. Reduced Intensity Conditioning Increases Risk of Severe cGVHD: Identification of Risk Factors for cGVHD in a Multicenter Setting. Med. Oncol. 2018, 35, 79. [Google Scholar] [CrossRef] [PubMed]
| TKI-Naïve n = 171 | IMA n = 41 | 2G-TKI n = 28 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Patient sex, n (%) | 0.211 | ||||||
| Male | 100 | 58.8% | 26 | 63.4% | 12 | 42.9% | |
| Female | 71 | 41.2% | 15 | 36.6% | 16 | 57.1% | |
| Patient age (years), Me [Q1; Q3] | 37 [28; 44] | 35 [26; 40] | 48 [33; 57] | <0.001 | |||
| Donor sex, n (%) | 0.766 | ||||||
| Male | 101 | 58.7% | 26 | 63.4% | 0 | 0.0% | |
| Female | 70 | 41.3% | 15 | 36.6% | 28 | 100% | |
| Donor age (years), M ± SD | 37.0 ± 10.4 | 37.6 ± 11.3 | - | 0.769 | |||
| Type of donor, n (%) | <0.001 | ||||||
| Matched-related donor | 129 | 75.5% | 14 | 34.1% | 10 | 35.7% | |
| Matched-unrelated donor | 37 | 21.6% | 27 | 65.9% | 18 | 64.3% | |
| Haploidentical donor | 5 | 2.9% | 0 | 0.0% | 0 | 0.0% | |
| Source of stem cells, n (%) | <0.001 | ||||||
| BM | 100 | 58.5% | 19 | 46.3% | 4 | 14.3% | |
| PBSC | 71 | 41.5% | 22 | 53.7% | 24 | 85.7% | |
| Transplant risk category, Me [Q1; Q3] | 2 [1; 3] | 3 [3; 4] | - | 1.000 | |||
| Median CD34+ count ×106/kg, Me [Q1; Q3] | 4.0 [2.7; 5.7] | 5.5 [2.2; 7.1] | 4.0 [3.6; 4.7] | 0.543 | |||
| Donor-positive CMV status, n (%) | 99 | 77.3% | 21 | 51.2% | NA | 0.006 | |
| CML phase at day of transplant, n (%) | <0.001 | ||||||
| Chronic phase | 147 | 88.0% | 14 | 37.9% | 20 | 71.4% | |
| Accelerated phase | 14 | 8.4% | 8 | 21.6% | 4 | 14.3% | |
| Blast crisis phase | 0 | 0.0% | 3 | 8.1% | 4 | 14.3% | |
| Second/next chronic phase | 6 | 3.6% | 12 | 32.4% | 0 | 0.0% | |
| HU 1 year before transplant | 140 | 98.6% | 14 | 66.7% | NA | <0.001 | |
| IFNα (yes), n (%) | 22 | 14.8% | 16 | 47.1% | 0 | 0.0% | <0.001 |
| RIC (yes), n (%) | 20 | 11.8% | 7 | 17.1% | 13 | 46.4% | <0.001 |
| High dose TBI (yes), n (%) | 6 | 3.5% | 11 | 28.9% | 1 | 3.6% | <0.001 |
| ATG in conditioning regimen | 48 | 28.1% | 27 | 71.1% | NA | <0.001 | |
| TKI-Naïve n = 171 | IMA n = 41 | 2G-TKI n = 28 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| aGvHD day, Me [Q1; Q3] | 36 [24; 54] | 44 [23; 59] | 32 [24; 49] | 0.894 | |||
| cGvHD day, Me [Q1; Q3] | 139 [103; 250] | 131 [120; 151] | 257 [132; 317] | 0.625 | |||
| aGvHD (yes), n (%) | for 163 pt. | for 27 pt. | 0.044 | ||||
| 100 | 61.4% | 29 | 70.7% | 11 | 40.7% | ||
| aGvHD grade, n (%) | for 163 pt. | for 27 pt. | 0.002 | ||||
| 0 | 63 | 38.7% | 12 | 29.3% | 16 | 59.3% | |
| 1 | 25 | 15.3% | 9 | 22.0% | 9 | 33.3% | |
| 2 | 33 | 20.2% | 13 | 31.7% | 2 | 7.4% | |
| 3 | 18 | 11.0% | 6 | 14.6% | 0 | 0.0% | |
| 4 | 24 | 14.7% | 1 | 2.4% | 0 | 0.0% | |
| cGvHD (yes), n (%) | for 157 pt. | <0.001 | |||||
| 120 | 76.4% | 21 | 51.2% | 7 | 25.0% | ||
| cGvHD grade, n (%) | for 157 pt. | <0.001 | |||||
| 0 | 37 | 23.6% | 20 | 48.8% | 21 | 75.0% | |
| 1 | 49 | 31.2% | 7 | 17.1% | 2 | 7.1% | |
| 2 | 71 | 45.2% | 14 | 34.1% | 5 | 17.9% | |
| Karnofsky scale at the day of last contact, Me [Q1; Q3] | 85 [0; 100] | 90 [80; 100] | NA | 1.000 | |||
| Patient status at the day of last contact, n (%) | |||||||
| Dead | 76 | 44.4% | 16 | 39.0% | 8 | 29.6% | 0.268 |
| Relapse | 73 | 42.7% | 14 | 35.9% | 3 | 11.1% | 0.007 |
| Variable | Risk FACTOR for | HR | 95% CI | p Value |
|---|---|---|---|---|
| Donor sex: female | Acute GvHD | 2.17 | 1.15–4.08 | 0.017 |
| GvHD prophylaxis: ATG | 2.80 | 1.15–6.79 | 0.023 | |
| Donor sex: female | Chronic GvHD | 2.43 | 1.26–4.69 | 0.009 |
| RIC conditioning | 0.24 | 0.11–0.51 | <0.001 | |
| CML accelerated phase | 3.13 | 1.05–9.36 | 0.041 |
| TKI-Naïve n = 171 | IMA n = 41 | 2G-TKI n = 28 | p-Value | |
|---|---|---|---|---|
| Median follow-up time, months | 41 [28; 73] for 163 pt. | 19 [14; 24] | 30 [16; 40] | <0.001 |
| 3-year overall survival OS (t = 3 years) | 55% | 49.9% | 69.6% | 0.740 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, U.; Piekarska, A.; Prejzner, W.; Gil, L.; Zaucha, J.M.; Kujawska, J.; Dybko, Z.; Dudek, K.; Giebel, S.; Dybko, J. Impact of First- and Second-Generation Tyrosine Kinase Inhibitors on the Development of Graft-Versus-Host Disease in Individuals with Chronic Myeloid Leukemia: A Retrospective Analysis on Behalf of the Polish Adult Leukemia Group. Biomedicines 2025, 13, 163. https://doi.org/10.3390/biomedicines13010163
Giordano U, Piekarska A, Prejzner W, Gil L, Zaucha JM, Kujawska J, Dybko Z, Dudek K, Giebel S, Dybko J. Impact of First- and Second-Generation Tyrosine Kinase Inhibitors on the Development of Graft-Versus-Host Disease in Individuals with Chronic Myeloid Leukemia: A Retrospective Analysis on Behalf of the Polish Adult Leukemia Group. Biomedicines. 2025; 13(1):163. https://doi.org/10.3390/biomedicines13010163
Chicago/Turabian StyleGiordano, Ugo, Agnieszka Piekarska, Witold Prejzner, Lidia Gil, Jan Maciej Zaucha, Joanna Kujawska, Zuzanna Dybko, Krzysztof Dudek, Sebastian Giebel, and Jarosław Dybko. 2025. "Impact of First- and Second-Generation Tyrosine Kinase Inhibitors on the Development of Graft-Versus-Host Disease in Individuals with Chronic Myeloid Leukemia: A Retrospective Analysis on Behalf of the Polish Adult Leukemia Group" Biomedicines 13, no. 1: 163. https://doi.org/10.3390/biomedicines13010163
APA StyleGiordano, U., Piekarska, A., Prejzner, W., Gil, L., Zaucha, J. M., Kujawska, J., Dybko, Z., Dudek, K., Giebel, S., & Dybko, J. (2025). Impact of First- and Second-Generation Tyrosine Kinase Inhibitors on the Development of Graft-Versus-Host Disease in Individuals with Chronic Myeloid Leukemia: A Retrospective Analysis on Behalf of the Polish Adult Leukemia Group. Biomedicines, 13(1), 163. https://doi.org/10.3390/biomedicines13010163






