Layer-Specific Strain Analysis in Patients with Dilated Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Echocardiography
2.2.1. Two-Dimensional Echocardiography
2.2.2. Two-Dimensional Speckle-Tracking Echocardiography
3. Results
3.1. The General Characteristics of the Study Population Are Summarized in Table 1
3.2. 2D Speckle-Tracking Echocardiography Results
3.3. Reproducibility of the Measurements
| Bootstrap for Coefficients | |||||||
|---|---|---|---|---|---|---|---|
| Model | B | Bootstrap | |||||
| Bias | Std. Error | Sig. (2-Tailed) | 95% Confidence Interval | ||||
| Lower | Upper | ||||||
| 1 | (Constant) | 0.046 | 0.000 | 0.112 | 0.683 | −0.163 | 0.275 |
| CSPMend | 0.025 | 0.000 | 0.002 | 0.001 | 0.020 | 0.029 | |
| LAVi | 0.011 | −7.062 × 10−6 | 0.002 | 0.001 | 0.007 | 0.014 | |
| hypertension | −0.215 | −0.002 | 0.033 | 0.001 | −0.282 | −0.151 | |
4. Discussion
5. Conclusions
6. Study Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Donal, E.; Delgado, V.; Bucciarelli-Ducci, C.; Galli, E.; Haugaa, K.; Charron, P.; Voigt, J.-U.; Cardim, N.; Masci, P.G.; Galderisi, M.; et al. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: An expert consensus document from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1075–1093. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Jeferies, J.L.; Towbin, J.A. Dilated cardiomyopathy. Lancet 2010, 375, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An American Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Vendelin, M.; Bovendeerd, P.H.; Engelbrecht, J.; Arts, T. Optimizing ventricular fibers: Uniform strain or stress, but not ATP consumption, leads to high efficiency. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1072–H1081. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Cvijic, M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef]
- Leitman, M.; Lysiansky, M.; Lysyansky, P.; Friedman, Z.; Tyomkin, V.; Fuchs, T.; Adam, D.; Krakover, R.; Vered, Z. Circumferential and Longitudinal Strain in 3 Myocardial Layers in Normal Subjects and in Patients with Regional Left Ventricular Dysfunction. J. Am. Soc. Echocardiogr. 2010, 23, 64–70. [Google Scholar] [CrossRef]
- Collier, P.; Phelan, D.; Klein, A. A Test in context: Myocardial strain measured by speckle-tracking echocardiography. J. Am. Coll. Cardiol. 2017, 69, 1043–1056. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, S.; Tang, X.; Ren, H.; Li, S.; Zou, Q.; Xiong, F.; Zheng, T.; Gong, L. Evaluation of left ventricular strain in patients with dilated cardiomyopathy. J. Int. Med. Res. 2017, 45, 2092–2100. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Du Bois, D. Clinical calorimetry: Tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch. Intern. Med. 1916, 17, 863–871. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Baysan, O.; Ocakli, E.P.; Saglam, Y.; Altuner, T.K. Advances in echocardiography: Global longitudinal strain, intra-cardiac multidirectional flow imaging and automated 3d volume analysis. Heart Vessel. Transplant. 2018, 2, 113–122. [Google Scholar] [CrossRef][Green Version]
- Negishi, K.; Negishi, T.; Kurosawa, K.; Hristova, K.; Popescu, B.A.; Vinereanu, D.; Yuda, S.; Marwick, T.H. Practical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovas Imaging 2015, 8, 489–492. [Google Scholar] [CrossRef]
- Moraru, L.; Mirea, O.; Toader, D.; Berceanu, M.; Soldea, S.; Munteanu, A.; Donoiu, I.; Raicea, V. Lower Limit of Normality of Segmental Multilayer Longitudinal Strain in Healthy Adult Subjects. J. Cardiovasc. Dev. Dis. 2024, 11, 102. [Google Scholar] [CrossRef]
- Konstam, M.A.; Abboud, F.M. Ejection fraction: Misunderstood and overrated (changing the paradigm in categorizing heart failure). Circulation 2017, 135, 717–719. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Tanacli, R.; Hashemi, D.; Lapinskas, T.; Edelmann, F.; Gebker, R.; Pedizzetti, G.; Schuster, A.; Nagel, E.; Pieske, B.; Dungen, H.-D.; et al. Range variability in CMR feature tracking multilayer strain across diferent stages of heart failure. Sci. Rep. 2019, 9, 16478. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Lapinskas, T.; Tonti, G.; Stoiber, L.; Zaliunas, R.; Gebker, R.; Pieske, B.; Kelle, S. The Relationship between EF and strain permits a more accurate assessment of LV systolic function. JACC Cardiovasc. Imaging 2019, 12, 1893–1895. [Google Scholar] [CrossRef]
- Marciniak, A.; Claus, P.; Sutherland, G.R.; Marciniak, M.; Karu, T.; Baltabaeva, A.; Merli, E.; Bijnens, B.; Jahangiri, M. Changes in systolic left ventricular function in isolated mitral regurgitation. A strain rate imaging study. Eur. Heart J. 2007, 28, 2627–2636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hofrichter, P.; Hagendorff, A.; Laufs, U.; Fikenzer, S.; Hepp, P.; Marshall, R.P.; Tayal, B.; Stöbe, S. Analysis of left ventricular rotational deformation by 2D speckle tracking echocardiography: A feasibility study in athletes. Int. J. Cardiovasc. Imaging 2021, 37, 2369–2386. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Pan, C.; Kong, D.; Cheng, L.; Shu, X. Left ventricular longitudinal and circumferential layer-specific myocardial strains and their determinants in healthy subjects. Echocardiography 2016, 33, 510–518. [Google Scholar] [CrossRef]
- Altiok, E.; Neizel, M.; Tiemann, S.; Krass, V.; Kuhr, K.; Becker, M.; Zwicker, C.; Koos, R.; Lehmacher, W.; Kelm, M.; et al. Quantitative analysis of endocardial and epicardial left ventricular myocardial deformation-comparison of strain-encoded cardiac magnetic resonance imaging with two-dimensional speckle-tracking echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 117988. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Chien-Chia Wu, V.; Otsuji, Y.; Takeuchi, M. Normal range of myocardial layer-specific strain using two-dimensional speckle tracking echocardiography. PLoS ONE 2017, 12, e0180584. [Google Scholar] [CrossRef]
- Buchi, M.; Hess, O.M.; Murakami, T.; Krayenbuehl, H.P. Left ventricular wall stress distribution in chronic pressure and volume overload: Effect of normal and depressed contractility on regional stress-velocity relations. Basic. ResCardiol. 1990, 85, 367–383. [Google Scholar] [CrossRef]
- Yukihiro, K.; Katsu, T. Transmural Heterogeneity of the Left Ventricular Wall: Subendocardial Layer and Subepicadial Layer. J. Cardiol. 2000, 35, 205–218. [Google Scholar]
- Fredholm, M.; Jörgensen, K.; Houltz, E.; Ricksten, S.-E. Load-dependence of myocardial deformation variables—A clinical strain-echocardiographic study. Acta Anaesthesiol. Scand. 2017, 61, 1155–1165. [Google Scholar] [CrossRef]
- Katbeh, A.; Ondrus, T.; Barbato, E.; Galderesi, M.; Trimarco, B.; Van Camp, G.; Vanderheyden, M.; Penicka, M. Imaging of myocardial fbrosis and its functional correlates in aortic stenosis: A review and clinical potential. Cardiology 2018, 141, 141–149. [Google Scholar] [CrossRef]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Risum, N.; Jons, C.; Olsen, N.T.; Fritz-Hansen, T.; Bruun, N.E.; Hojgaard, M.V.; Valeur, N.; Kronborg, M.B.; Kisslo, J.; Sogaard, P. Simple regional strain pattern analysis to predict response to cardiac resynchronization therapy: Rationale, initial results, and advantages. Am. Heart J. 2012, 163, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Uppu, S.C.; Shah, A.; Weigand, J.; Nielsen, J.C.; Ko, H.H.; Parness, I.A.; Srivastava, S. Two-Dimensional Speckle-Tracking-Derived Segmental Peak Systolic Longitudinal Strain Identifies Regional Myocardial Involvement in Patients with Myocarditis and Normal Global Left Ventricular Systolic Function. Pediatr. Cardiol. 2015, 36, 950–959. [Google Scholar] [CrossRef]
- Sperlongano, S.; D’Amato, A.; Tagliamonte, E.; Russo, V.; Desiderio, A.; Ilardi, F.; Muscogiuri, G.; Esposito, G.; Pontone, G.; Esposito, G.; et al. Acute myocarditis: Prognostic role of speckle tracking echocardiography and comparison with cardiac magnetic resonance features. Heart Vessel. 2022, 37, 121–131. [Google Scholar] [CrossRef]
- Sun, J.P.; Xu, T.Y.; Ni, X.D.; Yang, X.S.; Hu, J.L.; Wang, S.C.; Li, Y.; Bahler, R.C.; Wang, J.G. Echocardiographic strain in hypertrophic cardiomyopathy and hypertensive left ventricular hypertrophy. Echocardiography 2019, 36, 257–265. [Google Scholar] [CrossRef]
- Sakurai, D.; Asanuma, T.; Masuda, K.; Hioki, A.; Nakatani, S. Myocardial layer-specific analysis of ischemic memory using speckle tracking echocardiography. Int. J. Cardiovasc. Imaging 2014, 30, 739–748. [Google Scholar] [CrossRef]
- Hamada, S.; Schroeder, J.; Hoffmann, R.; Altiok, E.; Keszei, A.; Almalla, M.; Napp, A.; Marx, N.; Becker, M. Prediction of outcomes in patients with chronic ischemic cardiomyopathy by layer-specific strain echocardiography: A proof of concept. J. Am. Soc. Echocardiogr. 2016, 29, 412–420. [Google Scholar] [CrossRef]
- Skaarup, K.G.; Iversen, A.; Jorgensen, P.G.; Olsen, F.J.; Grove, G.L.; Jensen, J.S.; Biering-Sorensen, T. Association between layer-specific global longitudinal strain and adverse outcomes following acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1334–1342. [Google Scholar] [CrossRef]
- Hung, C.L.; Verma, A.; Uno, H.; Shin, S.H.; Bourgoun, M.; Hassanein, A.H.; McMurray, J.J.; Velazquez, E.J.; Kober, L.; Pfeffer, M.A.; et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J. Am. Coll. Cardiol. 2010, 56, 1812–1822. [Google Scholar] [CrossRef]
- Abate, E.; Hoogslag, G.E.; Leong, D.P.; Bertini, M.; Antoni, M.L.; Nucifora, G.; Joyce, E.; Holman, E.R.; Siebelink, H.M.; Schalij, M.J.; et al. Association between multilayer left ventricular rotational mechanics and the development of left ventricular remodeling after acute myocardial infarction. J. Am. Soc. Echocardiogr. 2014, 27, 239–248. [Google Scholar] [CrossRef]
- Abou, F.R.; Goedemans, L.; Montero-Cabezas, J.M.; Prihadi, E.A.; el Mahdiui, M.; Schalij, M.J.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Prognostic Value of Multilayer Left Ventricular Global Longitudinal Strain in Patients with ST-segment Elevation Myocardial Infarction with Mildly Reduced Left Ventricular Ejection. Am. J. Cardiol. 2021, 152, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, T.; Skulstad, H.; Aakhus, S.; Urheim, S.; Ihlen, H. Regional myocardial systolic function during acute myocardial ischemia assessed by strain Doppler echocardiography. J. Am. Coll. Cardiol. 2001, 37, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, S.I.; Haugaa, K.H.; Zahid, W.; Bendz, B.; Aakhus, S.; Aaberge, L.; Edvardsen, T. Layer specific quantification of myocardial deformation by strain echocardiography may reveal significant CAD in patients with non-ST-segment elevation acute coronary syndrome. JACC Cardiovasc. Imaging. 2013, 6, 535–544. [Google Scholar] [CrossRef]
- Scharrenbroich, J.; Hamada, S.; Keszei, A.; Schroder, J.; Napp, A.; Almalla, M.; Becker, M.; Altiok, E. Use of two-dimensional speckle tracking echocardiography to predict cardiac events: Comparison of patients with acute myocardial infarction and chronic coronary artery disease. Clin. Cardiol. 2018, 41, 111–118. [Google Scholar] [CrossRef]
- Sun, J.P.; Liang, Y.; Zhang, F.; Xu, L.; Chen, X.; Yuan, W.; Xu, L.; Bahler, R.C.; Yan, J. Assessment of ischemia-reperfusion injury after PCI for ST-elevation myocardial infarction using speckle tracking echocardiography. Echocardiography 2020, 37, 1413–1421. [Google Scholar] [CrossRef]
- Dahlslett, T.; Karlsen, S.; Grenne, B.; Eek, C.; Sjøli, B.; Skulstad, H.; Smiseth, O.A.; Edvardsen, T.; Brunvand, H. Early assessment of strain echocardiography can accurately exclude significant coronary artery stenosis in suspected non-ST-segment elevation acute coronary syndrome. J. Am. Soc. Echocardiogr. 2014, 27, 512–519. [Google Scholar] [CrossRef]
- Bogaert, J.; Maes, A.; Van de Werf, F.; Bosmans, H.; Herregods, M.C.; Nuyts, J.; Desmet, W.; Mortelmans, L.; Marchal, G.; Rademakers, F.E. Functional recovery of subepicardial myocardial tissue in transmural myocardial infarction after successful reperfusion. Circulation 1999, 99, 36–43. [Google Scholar] [CrossRef]
- Reimer, K.A.; Jennings, R.B.; Tatum, A.H. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral fow. Lab. Investig. 1979, 40, 633–644. [Google Scholar]
- Becker, M.; Ocklenburg, C.; Altiok, E.; Futing, A.; Balzer, J.; Krombach, G.; Lysyansky, M.; Kuhl, H.; Krings, R.; Kelm, M.; et al. Impact of infarct transmurality on layer-specific impairment of myocardial function: A myocardial deformation imaging study. Eur. Heart J. 2009, 30, 1467–1476. [Google Scholar] [CrossRef][Green Version]
- Lee, W.H.; Liu, Y.W.; Yang, L.T.; Tsai, W.C. Prognostic value of longitudinal strain of subepicardial myocardium in patients with hypertension. J. Hypertens. 2016, 34, 1195–1200. [Google Scholar] [CrossRef]
- Caspar, T.; Fichot, M.; Ohana, M.; El Ghannudi, S.; Morel, O.; Ohlmann, P. Late detection of left ventricular dysfunction using two-dimensional and three-dimensional speckle-tracking echocardiography in patients with history of nonsevere acute myocarditis. J. Am. Soc. Echocardiogr. 2017, 30, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Yamada, S.; Iwano, H.; Nishino, H.; Nakabachi, M.; Yokoyama, S.; Abe, A.; Ichikawa, A.; Kaga, S.; Nishida, M.; et al. Myocardial shortening in 3 orthogonal directions and its transmural variation in patients with nonobstructive hypertrophic cardiomyopathy. Circ. J. 2015, 79, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- DeVore, A.D.; McNulty, S.; Alenezi, F.; Ersboll, M.; Vader, J.M.; Oh, J.K.; Lin, G.; Redfield, M.M.; Lewis, G.; Semigran, M.J.; et al. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: Insights from the RELAX trial. Eur. J. Heart Fail. 2017, 19, 893–900. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Tops, L.F.; Delgado, V.; Marsan, N.A.; Bax, J.J. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur. J. Heart Fail. 2017, 19, 307–331. [Google Scholar] [CrossRef]
- Foley, J.R.J.; Swoboda, P.P.; Fent, G.J.; Garg, P.; McDiarmid, A.K.; Ripley, D.P.; Erhayiem, B.; AlMusa, T.; Dobson, L.E.; Plein, S.; et al. Quantitative deformation analysis differentiates ischaemic and non-ischaemic cardiomyopathy: Sub-group analysis of the VINDICATE trial. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 816–823. [Google Scholar] [CrossRef]
- Erley, J.; Genovese, D.; Tapaskar, N.; Alvi, N.; Rashedi, N.; Besser, S.A.; Kawaji, K.; Goyal, N.; Kelle, S.; Lang, R.M.; et al. Echo cardiography and cardiovascular magnetic resonance based evaluation of myocardial strain and relationship with late gadolinium enhancement. J. Cardiovasc. Magn. Reson. 2019, 21, 46. [Google Scholar] [CrossRef]
- Xu, L.; Pagano, J.J.; Haykowksy, M.J.; Ezekowitz, J.A.; Oudit, G.Y.; Mikami, Y.; Howarth, A.; White, J.A.; Dyck, J.R.B.; Anderson, T.; et al. Layer-specific strain in patients with heart failure using cardiovascular magnetic resonance: Not all layers are the same. J. Cardiovasc. Magn. Reason. 2020, 22, 81. [Google Scholar] [CrossRef]
- Kuetting, D.L.R.; Feisst, A.; Dabir, D.; Homsi, R.; Sprinkart, A.M.; Luetkens, J. Comparison of magnetic resonance feature tracking with CSPAMM HARP for the assessment of global and regional layer specific strain. Int. J. Cardiol. 2017, 244, 340–346. [Google Scholar] [CrossRef]
- Adamu, U.; Schmitz, F.; Becker, M.; Kelm, M.; Hoffman, R. Advanced speckle tracking echocardiography allowing a three-myocardial layer-specific analysis of deformation parameters. Eur. J. Echocardiogr. 2009, 10, 303–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Wu, W.-C.; Ma, H.; Wang, H. Usefulness of layer-specific strain for identifying complex CAD and predicting the severity of coronary lesions in patients with non-ST-segment elevation acute coronary syndrome: Compared with Syntax score. Int. J. Cardiol. 2016, 223, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.-J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assesment of myocardial mechanics using speckle tracking echocardiography: Fundaments and clinical applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Oishi, Y.; Miyoshi, H.; Iuch, A.; Nagase, N.; Oki, T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: A study with two-dimensional strain imaging. J. Am. Soc. Echocardiogr. 2008, 21, 1138–1144. [Google Scholar]
- Taylor, R.J.; Umar, F.; Lin, E.L.S.; Ahmed, A.; Moody, W.E.; Mazur, W.; Stegemann, B.; Townend, J.N.; Steeds, R.P.; Leyva, F. Mechanical effects of left ventricular midwall fibrosis in non-ischemic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2015, 1, 18. [Google Scholar] [CrossRef]
- Alcidi, G.M.; Esposito, R.; Evola, V.; Santoro, C.; Lembo, M.; Sorrentino, R.; Lo Iudice, F.; Borgia, F.; Novo, G.; Trimarco, B.; et al. Normal reference values of multilayer longitudinal strain according to age decades in a healthy population: A single-center experience. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1390–1396. [Google Scholar] [CrossRef]
- Vietheer, J.; Lehmann, L.; Unbehaun, C.; Fischer-Rasokat, U.; Wolter, J.S.; Kriechbaum, S.; Weferling, M.; von Jeinsein, B.; Hain, A.; Liebetrau, C.; et al. CMR-derived myocardial strain analysis diferentiates ischemic and dilated cardiomyopathy—A propensity score-matched study. Int. J. Cardiovasc. Imaging 2022, 38, 863–872. [Google Scholar] [CrossRef]
- Ancedy, Y.; Ederhy, S.; Jean, M.-L.; Nhan, P.; Soulat-Dufour, L.; Adavane-Scheuble, S.; Chauvet-Droit, M.; Boccara, F.; Cohen, A. Does layer-specific strain using speckle tracking echocardiography improve the assessment of left ventricular myocardial deformation? A review. Arch. Cardiovasc. Dis. 2020, 113, 721–735. [Google Scholar] [CrossRef]

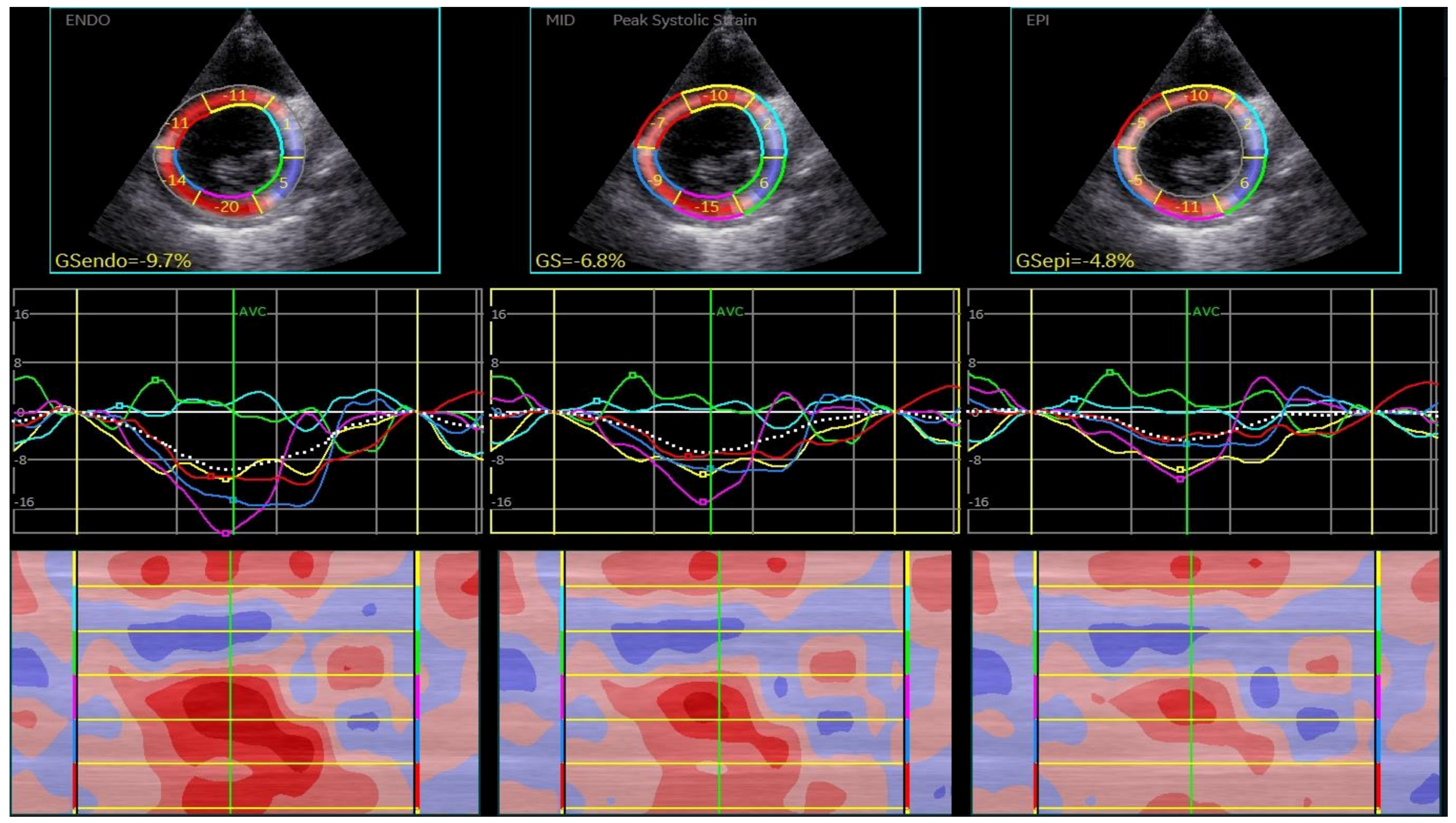

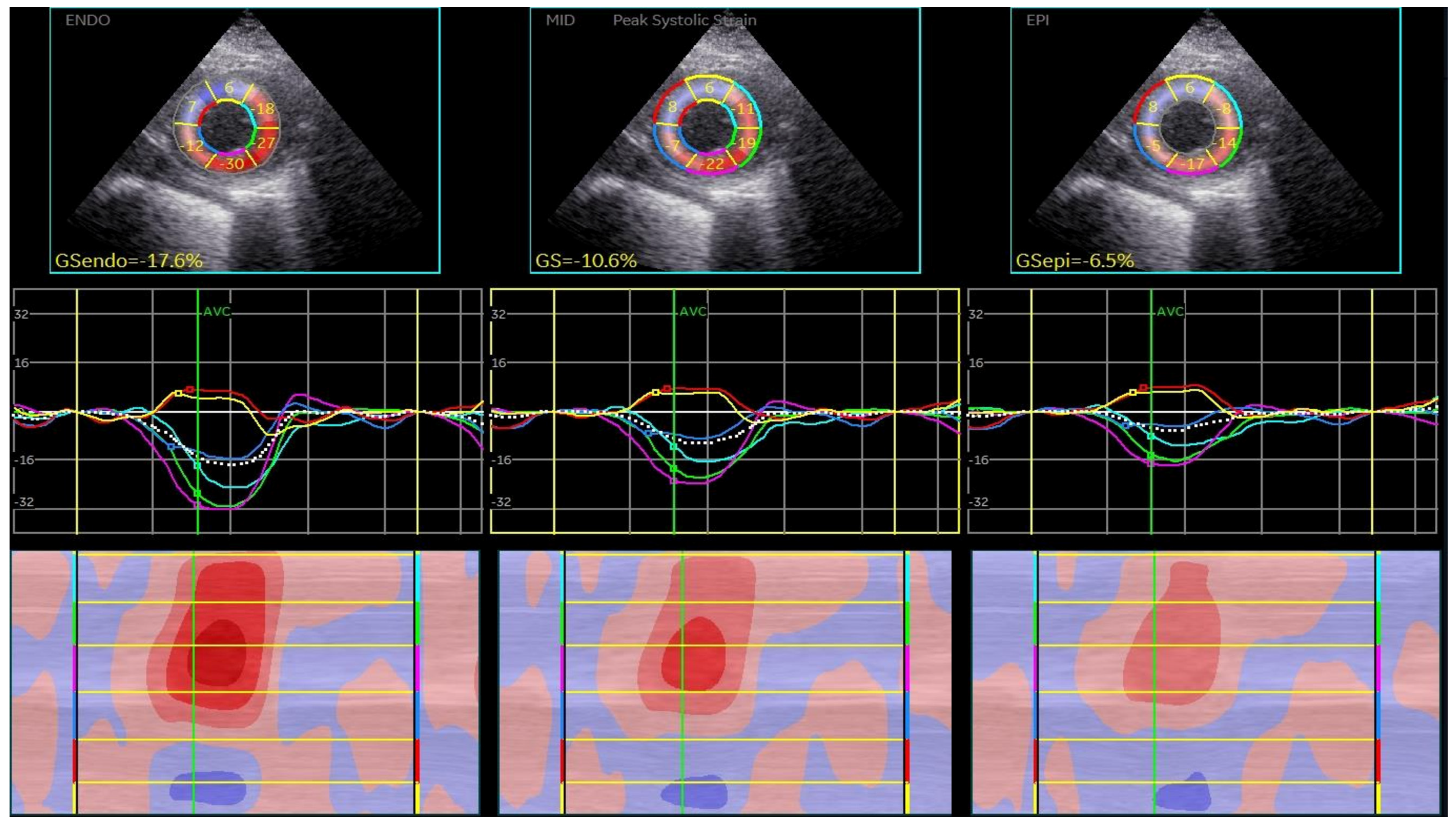


| Parameter | IDCM (51) (52.57%) | NIDCM (46) (47.24%) | p |
|---|---|---|---|
| Age (years) | 62.25 +/− 9.94 | 55.73 +/− 11.68 | 0.0036 |
| Males (%) | 60.78 | 69.56 | 0.37 |
| BSA (m2) | 1.77 (0.16) | 1.78 (0.17) | 0.76 |
| Hypertension (%) | 74.5 | 54.34 | 0.039 |
| DM (%) | 52.94 | 45.65 | 0.47 |
| Dyslipidemia (%) | 64.7 | 63.07 | 0.86 |
| Obesity (%) | 41.17 | 32.6 | 0.38 |
| CKD (%) | 39.21 | 21.73 | 0.063 |
| AF (%) | 13.72 | 16.66 | 0.69 |
| Deaths (%) | 17.64 | 26.08 | 0.32 |
| Parameter | IDCM (51) (52.57%) | NIDCM (46) (47.24%) | p |
|---|---|---|---|
| LVESV (mL) | 192.19 (64.09) | 203.97 (81.26) | 0.43 |
| LVEDV (mL) | 141.11 (55.27) | 152.06 (70.75) | 0.39 |
| LVEF (%) | 27.05 (7.93) | 26.58 (7.48) | 0.35 |
| LAVi (mL/m2) | 55.23 (11.23) | 54.16 (14.69) | 0.68 |
| MR ≥ mild (%) | 33.33 | 41.3 | 0.42 |
| TR > mild (%) | 25.49 | 39.13 | 0.68 |
| TA | 4.29 (0.7) | 4.31 (0.98) | 0.9 |
| Parameter | IDCM MV | SD | NIDCM MV | SD | Diff | SE | 95% CI DF −95 | t | p |
|---|---|---|---|---|---|---|---|---|---|
| GLS | −7.84 | 2.92 | −7.22 | 2.74 | 0.620 | 0.577 | −0.5249 to 1.7649 | 1.075 | 0.2851 |
| GLSend | −9.15 | 3.24 | −8.22 | 3 | 0.930 | 0.636 | −0.3330 to 2.1930 | 1.462 | 0.1471 |
| GLSend-GLSepi | −2.43 | 1.48 | −2.41 | 0.92 | 0.020 | 0.253 | −0.4832 to 0.5232 | 0.079 | 0.9373 |
| CSMV | −7.74 | 2.88 | −7.22 | 2.77 | 0.520 | 0.575 | −0.6218 to 1.6618 | 0.904 | 0.3682 |
| CSMVend | −10.6 | 3.67 | −10.16 | 3.44 | 0.440 | 0.724 | −0.9983 to 1.8783 | 0.607 | 0.5451 |
| CSMVSend-CSMVSepi | −5.8 | 2.76 | −5.59 | 2.89 | 0.210 | 0.574 | −0.9293 to 1.3493 | 0.366 | 0.7152 |
| CSPM | −8.16 | 3.07 | −7.19 | 2.69 | 0.970 | 0.589 | −0.1991 to 2.1391 | 1.647 | 0.1028 |
| CSPMend | −11.57 | 3.96 | −10.5 | 3.88 | 1.070 | 0.799 | −0.5153 to 2.6553 | 1.340 | 0.1834 |
| CSPMend-CSPMepi | −6.75 | 3.02 | −5.78 | 3.4 | 0.970 | 0.653 | −0.3261 to 2.2661 | 1.486 | 0.1406 |
| CSAP | −10.19 | 3.65 | −8.65 | 3.62 | 1.690 | 0.741 | 0.2183 to 3.1617 | 2.280 | 0.0249 |
| CSAPend | −13.77 | 4.67 | −11.67 | 5.1 | 2.100 | 0.992 | 0.1307 to 4.0693 | 2.117 | 0.0369 |
| CSAPend-CSAPepi | −6.87 | 3.71 | −5.17 | 3.06 | 1.700 | 0.695 | 0.3204 to 3.0796 | 2.446 | 0.0163 |
| Parameter | Coefficient | SE | OR | 95% CI | AUC | p |
|---|---|---|---|---|---|---|
| Age | −0.0055909 | 0.022025 | 0.9944 | 0.9524 to 1.0383 | 0.504 | 0.8 |
| BSA | 4.67658 | 1.65489 | 107.4017 | 4.1912 to 2752.2135 | 0.717 | 0.0047 |
| Male | −1.41908 | 0.66586 | 0.2419 | 0.0656 to 0.8923 | 0.633 | 0.0331 |
| Hypertension | 1.18562 | 0.50835 | 3.2727 | 1.2084 to 8.8639 | 0.641 | 0.0197 |
| DM | −0.85137 | 0.51696 | 0.4268 | 0.1550 to 1.1757 | 0.603 | 0.0996 |
| Dyslipidemia | 0.053489 | 0.50840 | 1.0549 | 0.3895 to 2.8575 | 0.506 | 0.9162 |
| CKD | 1.45775 | 0.51695 | 4.2963 | 1.5597 to 11.8341 | 0.667 | 0.0048 |
| AF | 0.095310 | 0.70996 | 1.1000 | 0.2736 to 4.4230 | 0.506 | 0.8932 |
| LVEDV | 0.0073219 | 0.0033724 | 1.0073 | 1.0007 to 1.0140 | 0.638 | 0.0299 |
| LVESV | 0.010617 | 0.0042349 | 1.0107 | 1.0023 to 1.0191 | 0.704 | 0.0122 |
| LVEF | −0.12205 | 0.038815 | 0.8851 | 0.8203 to 0.9551 | 0.740 | 0.0017 |
| LAVi | 0.073302 | 0.021835 | 1.0761 | 1.0310 to 1.1231 | 0.733 | 0.0002 |
| MR | 0.55862 | 0.49937 | 1.7483 | 0.6569 to 4.6524 | 0.567 | 0.2633 |
| TA | 0.53870 | 0.28886 | 1.7138 | 0.9729 to 3.0188 | 0.604 | 0.0622 |
| Parameter | Coefficient | SE | OR | 95%CI | p |
|---|---|---|---|---|---|
| BSA | 4.94503 | 3.48548 | 140.4758 | 0.1516 to 130.168 | 0.1560 |
| Males | 0.45761 | 0.91754 | 1.5803 | 0.2616 to 9.5446 | 0.6180 |
| Hypertension = 0 | 1.77494 | 0.77035 | 5.9000 | 1.3035 to 26.7044 | 0.0212 |
| CKD = 1 | 1.40238 | 0.75463 | 4.0648 | 0.9262 to 17.8402 | 0.0631 |
| LVEDV | 0.041878 | 0.068994 | 1.0428 | 0.9109 to 1.1938 | 0.5439 |
| LVESV | −0.058942 | 0.084732 | 0.9428 | 0.7985 to 1.1131 | 0.4867 |
| LVEF | −0.23021 | 0.17691 | 0.7944 | 0.5616 to 1.1236 | 0.1932 |
| LAVi | 0.066052 | 0.031852 | 1.0683 | 0.5616 to 1.1236 | 0.0381 |
| Parameter | DCM Nonsurvivors (21) MV | SD | DCM Survivors (76) MV | SD | Diff | SE | CI DF 95 | t | P |
|---|---|---|---|---|---|---|---|---|---|
| GLS | −6.02 | 1.52 | −8 | 3.01 | −1.980 | 0.682 | −3.3333 to −0.6267 | −2.973 | 0.0046 |
| GLSend | −6.98 | 1.89 | −9.24 | 3.33 | −2.260 | 0.760 | −3.7690 to −0.7510 | −2.973 | 0.0037 |
| GLSend-GLSepi | −1.86 | 0.74 | −2.705 | −0.900 | 0.345 | −1.5858 to −0.2142 | −2.605 | 0.0107 | |
| CSMV | −7.07 | 3.26 | −7.56 | 1.53 | −0.290 | 0.700 | −1.6806 to 1.1006 | 0.414 | 0.6798 |
| CSMVend | −9.58 | 4.19 | −10.62 | 3.35 | −1.040 | 0.874 | −2.7742 to 0.6942 | −1.191 | 0.2368 |
| CSMVend-CSMVepi | −4.66 | 2.72 | −5.98 | 2.77 | −1.320 | 0.682 | −2.6745 to 0.0345 | −1.935 | 0.0560 |
| CSPM | −6.64 | 2.62 | −7.99 | 2.96 | −1.350 | 0.713 | −2.7653 to 0.0653 | −1.894 | 0.0613 |
| CSPMend | −8.92 | 3.28 | −11.66 | 3.92 | −2.740 | 0.935 | −4.5970 to −0.8830 | −2.929 | 0.0043 |
| CSPMemd-CSPMepi | −4.73 | 2.62 | −6.72 | 3.26 | −1.990 | 0.773 | −3.5249 to −0.4551 | −2.574 | 0.0116 |
| CSAP | −7.97 | 2.73 | −9.78 | 3.87 | −1.810 | 0.902 | −3.6011 to −0.0189 | −2.006 | 0.0477 |
| CSAPend | −10.48 | 3.47 | −13.41 | 5.14 | −2.930 | 1.192 | −5.2971 to −0.5629 | −2.457 | 0.0158 |
| CSAPend-CSAPepi | −4.71 | 2.61 | −6.44 | 3.64 | −1.730 | 0.851 | −3.4187 to −0.0413 | −2.034 | 0.0448 |
| Parameter | Coefficient | SE | OR | 95% CI | AUC | p |
|---|---|---|---|---|---|---|
| GLS | 0.32093 | 0.12197 | 1.3784 | 1.0853 to 1.7507 | 0.694 | 0.0085 |
| GLSend | 0.29082 | 0.10808 | 1.3375 | 1.0822 to 1.6531 | 0.700 | 0.0071 |
| GLSend-GLSepi | 0.55197 | 0.23152 | 1.73671 | 1.1032 to 2.7339 | 0.729 | 0.0067 |
| CSMV | 0.037002 | 0.089136 | 1.0377 | 0.8714 to 1.2358 | 0.526 | 0.6781 |
| CSMVend | 0.085917 | 0.072650 | 1.0897 | 0.9451 to 1.2565 | 0.590 | 0.2370 |
| CSMVend-CSMVepi | 0.183310 | 0.097038 | 1.2012 | 0.9931 to 1.4528 | 0.644 | 0.0589 |
| CSPM | 0.18874 | 0.10263 | 1.2077 | 0.9877 to 1.4768 | 0.650 | 0.0659 |
| CSPMend | 0.21235 | 0.079006 | 1.2366 | 1.0592 to 1.4437 | 0.705 | 0.0072 |
| CSPMend-CSPMepi | 0.22991 | 0.094868 | 1.2585 | 1.0449 to 1.5157 | 0.680 | 0.0154 |
| CSAP | 1.1634 | 0.078330 | 1.1634 | 0.9978 to 1.3564 | 0.629 | 0.0534 |
| CSAPend | 0.14511 | 0.062149 | 1.1562 | 1.0236 to 1.3059 | 0.659 | 0.0196 |
| CSAPend-CSAPepi | 0.18520 | 0.093819 | 1.2035 | 1.0013 to 1.4464 | 0.640 | 0.0484 |
| Parameter | Coefficient | SE | OR | 95%CI | p |
|---|---|---|---|---|---|
| Hypertension = 0 | 1.61529 | 0.61661 | 5.0293 | 1.5019 to 16.8415 | 0.0088 |
| LAVi | 0.080420 | 0.025152 | 1.0837 | 1.0316 to 1.1385 | 0.0014 |
| CSPMend | 0.22247 | 0.089506 | 1.2492 | 1.0482 to 1.4887 | 0.0129 |
| Area Under the ROC Curve (AUC) | 0.846 |
|---|---|
| Standard Error | 0.0456 |
| 95% Confidence interval | 0.759 to 0.912 |
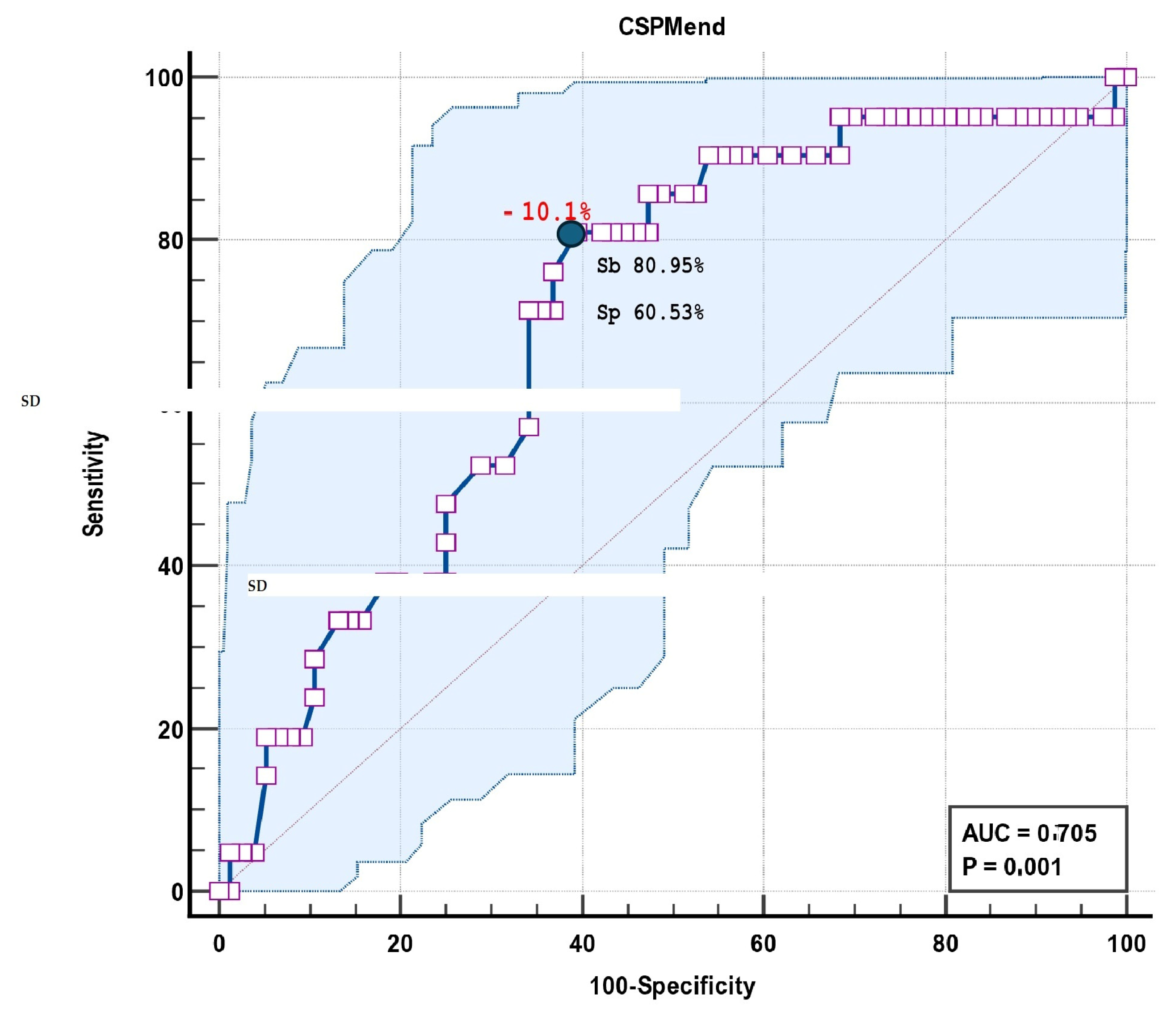
| Area Under the ROC Curve (AUC) | 0.705 |
|---|---|
| Standard Error | 0.0618 |
| 95% Confidence interval | 0.603 to 0.793 |
| z statistic | 3.308 |
| Significance level p (Area = 0.5) | 0.001 |
| Associated criterion | >−10.1 |
| Sensitivity | 80.95 |
| Specificity | 60.53 |
| Parameter | 95% CI | ICC |
|---|---|---|
| GLS | 0.821 to 0.988 | 0.952 |
| GLSend | 0.882 to 0.992 | 0.969 |
| GLSend-GLSepi | 0.794 to 0.986 | 0.945 |
| CSMV | 0.655 to 0.975 | 0.901 |
| CSMVend | 0.64 to 0.973 | 0.897 |
| CSMVend-CSMVepi | 0.566 to 0.966 | 0.871 |
| CSPM | 0.666 to 0.976 | 0.905 |
| CSPMend | 0.771 to 0.984 | 0.938 |
| CSPMend-CSPMepi | 0.636 to 0.973 | 0.895 |
| CSAP | 0.855 to 0.99 | 0.962 |
| CSAPend | 0.891 to 0.993 | 0.972 |
| CDAPend-CSAPepi | 0.858 to 0.991 | 0.963 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toader, D.-M.; Paraschiv, A.; Târtea, G.; Tiucu, G.; Chițu, M.; Stănișor, R.; Mirea, O. Layer-Specific Strain Analysis in Patients with Dilated Cardiomyopathy. Biomedicines 2025, 13, 11. https://doi.org/10.3390/biomedicines13010011
Toader D-M, Paraschiv A, Târtea G, Tiucu G, Chițu M, Stănișor R, Mirea O. Layer-Specific Strain Analysis in Patients with Dilated Cardiomyopathy. Biomedicines. 2025; 13(1):11. https://doi.org/10.3390/biomedicines13010011
Chicago/Turabian StyleToader, Despina-Manuela, Alina Paraschiv, Georgică Târtea, Gabriela Tiucu, Mihai Chițu, Raluca Stănișor, and Oana Mirea. 2025. "Layer-Specific Strain Analysis in Patients with Dilated Cardiomyopathy" Biomedicines 13, no. 1: 11. https://doi.org/10.3390/biomedicines13010011
APA StyleToader, D.-M., Paraschiv, A., Târtea, G., Tiucu, G., Chițu, M., Stănișor, R., & Mirea, O. (2025). Layer-Specific Strain Analysis in Patients with Dilated Cardiomyopathy. Biomedicines, 13(1), 11. https://doi.org/10.3390/biomedicines13010011






