Prognostic Protein Biomarker Screening for Thyroid Carcinoma Based on Cancer Proteomics Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Construction of the Protein Prognostic Signature
2.3. Nomogram Construction and Calibration
2.4. DEPs Identification
2.5. Principal Component Analysis (PCA), GO and KEGG Analysis
2.6. Protein–Protein Interaction Network Construction
2.7. Gene Set Enrichment Analysis
2.8. Analysis of Tumor Infiltrating Immune Cells
2.9. Analysis of Tumor Mutation Burden
2.10. Immunotherapy Analysis
2.11. Potential Chemotherapeutic Response
2.12. Statistical Analysis
3. Results
3.1. Establishment of a Proteomic Prognostic Signature in Thyroid Carcinoma
3.2. Construction of Nomogram Based on the Protein Signature and Clinical Data
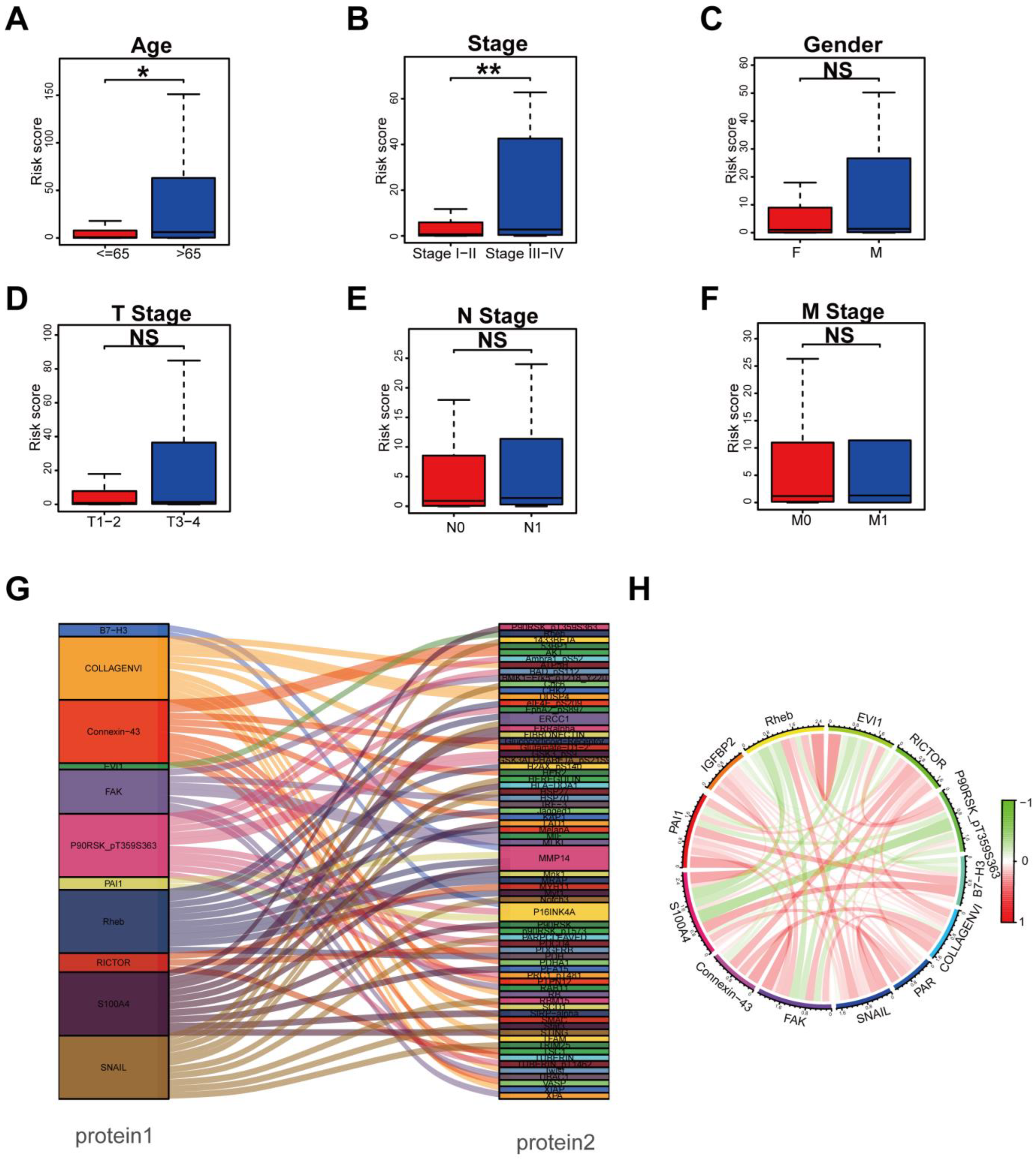
3.3. Clinical Relevance Assessment and Construction of the Protein Coexpression Network
3.4. Functional Enrichment Analysis Based on Risk Model
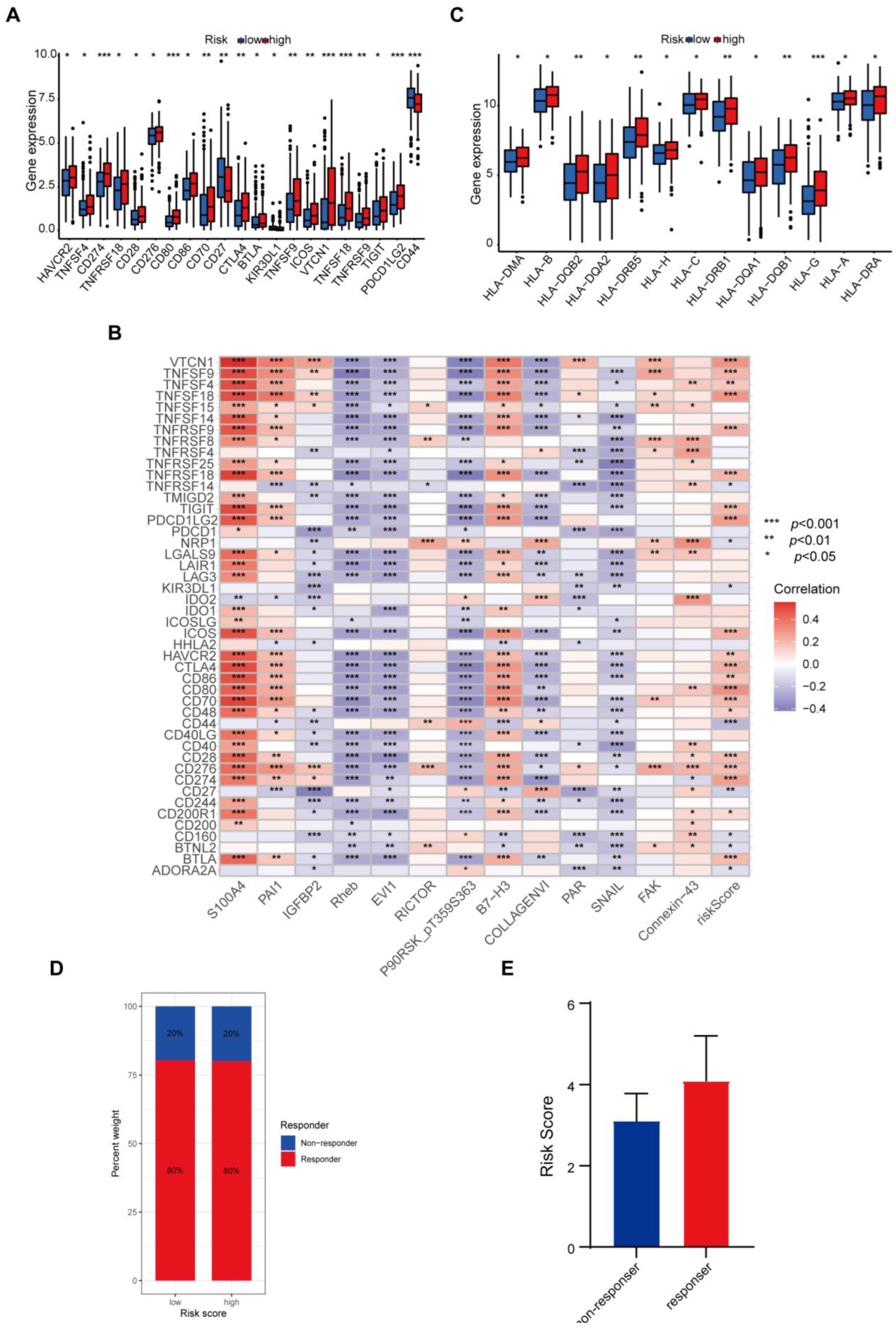
3.5. Difference in Tumor-Infiltrating Immune Cells in Different Risk Groups
3.6. Protein-Based Signature Is Associated with Immunization Checkpoint Block
3.7. Mutational Landscape Based on the Protein-Associated Signature
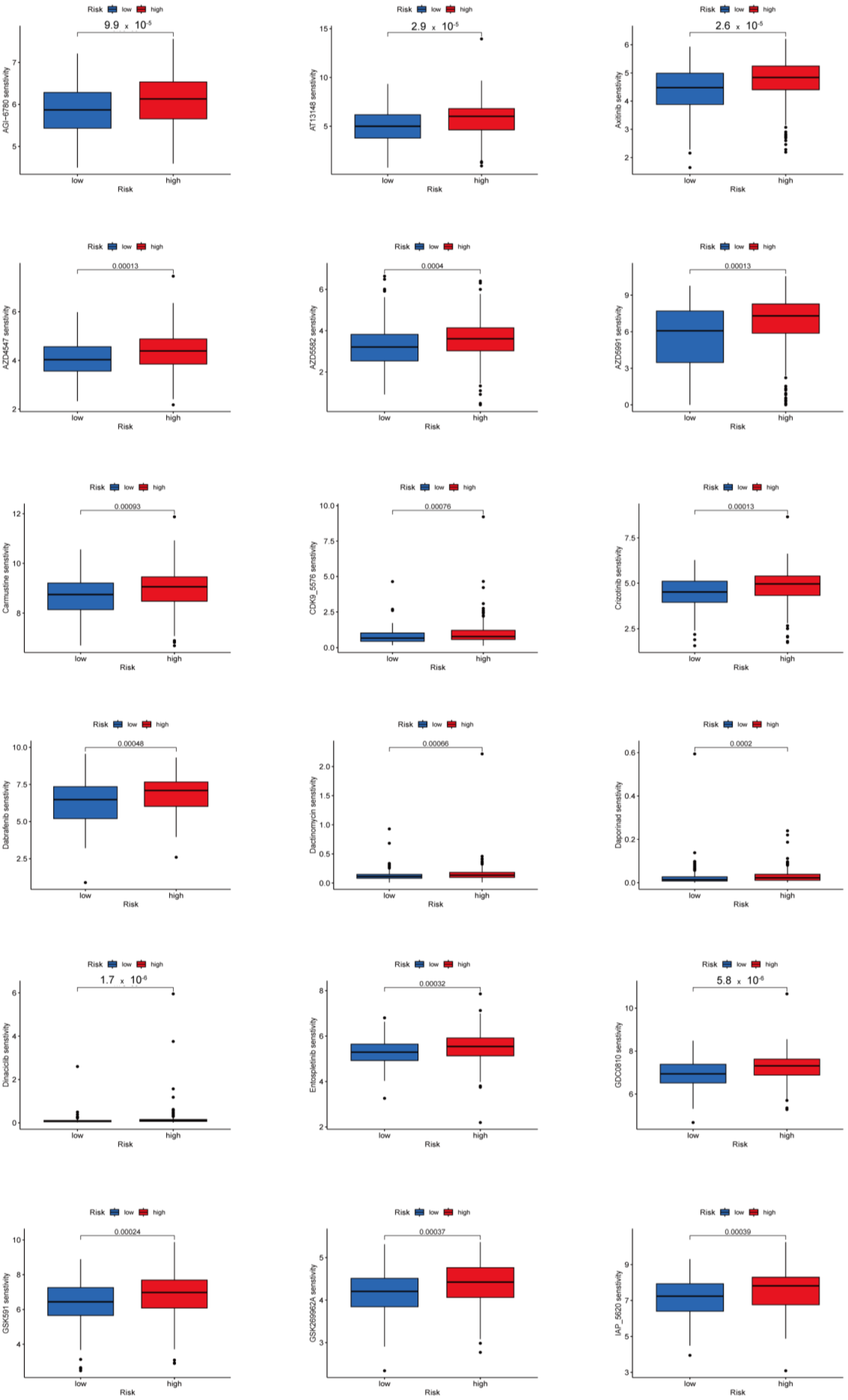
3.8. Potential Predictive Biomarker for Chemotherapy and Targeted Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Gosnell, J.E.; Roman, S.A. Geographic influences in the global rise of thyroid cancer. Nat. Rev. Endocrinol. 2020, 16, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Drozd, V.; Schneider, R.; Platonova, T.; Panasiuk, G.; Leonova, T.; Oculevich, N.; Shimanskaja, I.; Vershenya, I.; Dedovich, T.; Mitjukova, T.; et al. Feasibility Study Shows Multicenter, Observational Case-Control Study Is Practicable to Determine Risk of Secondary Breast Cancer in Females with Differentiated Thyroid Carcinoma Given Radioiodine Therapy in Their Childhood or Adolescence; Findings Also Suggest Possible Fertility Impairment in Such Patients. Front. Endocrinol. 2020, 11, 567385. [Google Scholar]
- Jillard, C.L.; Scheri, R.P.; Sosa, J.A. What Is the Optimal Treatment of Papillary Thyroid Cancer? Adv. Surg. 2015, 49, 79–93. [Google Scholar] [CrossRef]
- Morris, L.G.T.; Tuttle, R.M.; Davies, L. Changing Trends in the Incidence of Thyroid Cancer in the United States. JAMA Otolaryngol. Head. Neck Surg. 2016, 142, 709–711. [Google Scholar] [CrossRef]
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. North. Am. 2019, 48, 23–35. [Google Scholar] [CrossRef]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef]
- Subbiah, V.; Yang, D.; Velcheti, V.; Drilon, A.; Meric-Bernstam, F. State-of-the-Art Strategies for Targeting RET-Dependent Cancers. J. Clin. Oncol. 2020, 38, 1209–1221. [Google Scholar] [CrossRef]
- Idris, S.F.; Ahmad, S.S.; Scott, M.A.; Vassiliou, G.S.; Hadfield, J. The role of high-throughput technologies in clinical cancer genomics. Expert. Rev. Mol. Diagn. 2013, 13, 167–181. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Edfors, F.; Danielsson, F.; Hallström, B.M.; Käll, L.; Lundberg, E.; Pontén, F.; Forsström, B.; Uhlén, M. Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol. Syst. Biol. 2016, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Nusinow, D.P.; Szpyt, J.; Ghandi, M.; Rose, C.M.; McDonald, E.R.; Kalocsay, M.; Jané-Valbuena, J.; Gelfand, E.; Schweppe, D.K.; Jedrychowski, M.; et al. Quantitative Proteomics of the Cancer Cell Line Encyclopedia. Cell 2020, 180, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Finotello, F.; Rieder, D.; Hackl, H.; Trajanoski, Z. Next-generation computational tools for interrogating cancer immunity. Nat. Rev. Genet. 2019, 20, 724–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lengerich, B.J.; Aragam, B.; Xing, E.P. Precision Lasso: Accounting for correlations and linear dependencies in high-dimensional genomic data. Bioinformatics 2019, 35, 1181–1187. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W.V. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Reisländer, T.; Groelly, F.J.; Tarsounas, M. DNA Damage and Cancer Immunotherapy: A STING in the Tale. Mol. Cell 2020, 80, 21–28. [Google Scholar] [CrossRef]
- George, N.; Agarwal, A.; Kumari, N.; Agarwal, S.; Krisnani, N.; Gupta, S.K. Mutational Profile of Papillary Thyroid Carcinoma in an Endemic Goiter Region of North India. Indian. J. Endocrinol. Metab. 2018, 22, 505–510. [Google Scholar] [PubMed]
- Qin, R.; Li, C.; Wang, X.; Zhong, Z.; Sun, C. Identification and validation of an immune-related prognostic signature and key gene in papillary thyroid carcinoma. Cancer Cell Int. 2021, 21, 378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-A.; Lu, C.-Y.; Cheng, W.-F.; Kuo, K.-T.; Yu, C.-W.; Ho, H.-N.; Chen, H.-F.; Pan, S.-H. An experimental model for ovarian cancer: Propagation of ovarian cancer initiating cells and generation of ovarian cancer organoids. BMC Cancer 2022, 22, 967. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Feng, J.; Wu, X.; Bai, L.; Xu, W.; Zhu, L.; Liu, Y.; Xu, F.; Zhang, X.; Yang, G.; et al. A proteogenomic analysis of clear cell renal cell carcinoma in a Chinese population. Nat. Commun. 2022, 13, 2052. [Google Scholar] [CrossRef]
- Chong, W.; Zhu, X.; Ren, H.; Ye, C.; Xu, K.; Wang, Z.; Jia, S.; Shang, L.; Li, L.; Chen, H. Integrated multi-omics characterization of KRAS mutant colorectal cancer. Theranostics 2022, 12, 5138–5154. [Google Scholar] [CrossRef]
- Saha, S.; Verma, R.; Kumar, C.; Kumar, B.; Dey, A.K.; Surjit, M.; Mylavarapu, S.V.S.; Maiti, T.K. Proteomic analysis reveals USP7 as a novel regulator of palmitic acid-induced hepatocellular carcinoma cell death. Cell Death Dis. 2022, 13, 563. [Google Scholar] [CrossRef]
- Ng, C.K.Y.; Dazert, E.; Boldanova, T.; Coto-Llerena, M.; Nuciforo, S.; Ercan, C.; Suslov, A.; Meier, M.-A.; Bock, T.; Schmidt, A.; et al. Integrative proteogenomic characterization of hepatocellular carcinoma across etiologies and stages. Nat. Commun. 2022, 13, 2436. [Google Scholar] [CrossRef]
- Ambartsumian, N.; Klingelhöfer, J.; Grigorian, M. The Multifaceted S100A4 Protein in Cancer and Inflammation. Methods Mol. Biol. 2019, 1929, 339–365. [Google Scholar]
- Fei, F.; Qu, J.; Zhang, M.; Li, Y.; Zhang, S. S100A4 in cancer progression and metastasis: A systematic review. Oncotarget 2017, 8, 73219–73239. [Google Scholar] [CrossRef]
- Abdelfattah, N.; Kumar, P.; Wang, C.; Leu, J.-S.; Flynn, W.F.; Gao, R.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Wood, S.L.; et al. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat. Commun. 2022, 13, 767. [Google Scholar] [CrossRef]
- Davies, B.R.; O’Donnell, M.; Durkan, G.C.; Rudland, P.S.; Barraclough, R.; Neal, D.E.; Mellon, J.K. Expression of S100A4 protein is associated with metastasis and reduced survival in human bladder cancer. J. Pathol. 2002, 196, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.; Hwang, J.-H.; Shin, E.; Lee, H.S.; Hwang, D.W.; Cho, J.Y.; Yoon, Y.-S.; Han, H.-S.; Cha, B.H. CD24 and S100A4 expression in resectable pancreatic cancers with earlier disease recurrence and poor survival. Pancreas 2014, 43, 380–388. [Google Scholar] [CrossRef]
- de Silva Rudland, S.; Martin, L.; Roshanlall, C.; Winstanley, J.; Leinster, S.; Platt-Higgins, A.; Carroll, J.; West, C.; Barraclough, R.; Rudland, P. Association of S100A4 and osteopontin with specific prognostic factors and survival of patients with minimally invasive breast cancer. Clin. Cancer Res. 2006, 12, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Lademann, U.A.; Rømer, M.U. Regulation of programmed cell death by plasminogen activator inhibitor type 1 (PAI-1). Thromb. Haemost. 2008, 100, 1041–1046. [Google Scholar] [PubMed]
- Valiente, M.; Obenauf, A.C.; Jin, X.; Chen, Q.; Zhang, X.H.F.; Lee, D.J.; Chaft, J.E.; Kris, M.G.; Huse, J.T.; Brogi, E.; et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014, 156, 1002–1016. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Song, Q.; Liu, L.; Forbes, E.; Tian, W.; Zhang, Z.; Kang, Y.A.; Wang, H.; Fleming, J.B.; et al. IGFBP2 promotes tumor progression by inducing alternative polarization of macrophages in pancreatic ductal adenocarcinoma through the STAT3 pathway. Cancer Lett. 2021, 500, 132–146. [Google Scholar] [CrossRef]
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531. [Google Scholar] [CrossRef]
- Fujimoto, D.; Hirono, Y.; Goi, T.; Katayama, K.; Yamaguchi, A. Prognostic value of protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1) in gastric cancer. Anticancer. Res. 2008, 28, 847–854. [Google Scholar]
- Suen, J.Y.; Cotterell, A.; Lohman, R.J.; Lim, J.; Han, A.; Yau, M.K.; Liu, L.; Cooper, M.A.; Vesey, D.A.; Fairlie, D.P. Pathway-selective antagonism of proteinase activated receptor 2. Br. J. Pharmacol. 2014, 171, 4112–4124. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Zhang, P.-F.; Wei, C.-Y.; Peng, R.; Lu, J.-C.; Gao, C.; Cai, J.-B.; Yang, X.; Fan, J.; Ke, A.-W.; et al. Circular RNA circMET drives immunosuppression and anti-PD1 therapy resistance in hepatocellular carcinoma via the miR-30-5p/snail/DPP4 axis. Mol. Cancer 2020, 19, 92. [Google Scholar] [CrossRef]
- Munoz, J.L.; Rodriguez-Cruz, V.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Temozolomide resistance in glioblastoma cells occurs partly through epidermal growth factor receptor-mediated induction of connexin 43. Cell Death Dis. 2014, 5, e1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Chen, A.; Jalali, A.; Liu, S.; Guo, Y.; Na, S.; Nakshatri, H.; Li, B.-Y.; Yokota, H. Effects of a checkpoint kinase inhibitor, AZD7762, on tumor suppression and bone remodeling. Int. J. Oncol. 2018, 53, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Qian, Y.; Li, Z.; Zhang, N.-N.; Xie, Y.-J. Let-7g-5p inhibits epithelial-mesenchymal transition consistent with reduction of glioma stem cell phenotypes by targeting VSIG4 in glioblastoma. Oncol. Rep. 2016, 36, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Wang, Z.; Xu, J.; Zhao, W.; Cao, H.; Zhang, Z. Elevated Rictor expression is associated with tumor progression and poor prognosis in patients with gastric cancer. Biochem. Biophys. Res. Commun. 2015, 464, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Huang, Z.; Zhu, X.; Cai, H.; Huang, Y.; Zhang, X.; Zhang, Z.; Lu, H.; An, C.; Niu, L.; et al. Clinical Significance of the Expression of Co-Stimulatory Molecule B7-H3 in Papillary Thyroid Carcinoma. Front. Cell Dev. Biol. 2022, 10, 819236. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Deng, Q.; Ye, S.; Liu, S.; Fu, Y.; Liu, Y.; Wu, G.; Ouyang, G.; Wu, T. Cancer-associated fibroblast-derived periostin promotes papillary thyroid tumor growth through integrin-FAK-STAT3 signaling. Theranostics 2024, 14, 3014–3028. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, W.; Zhang, Y.; Du, Y.; Zhao, S.; Wang, L.; Sun, Y.; Sha, B.; Yan, J.; Ma, Y.; et al. Glucose deprivation triggers DCAF1-mediated inactivation of Rheb-mTORC1 and promotes cancer cell survival. Cell Death Dis. 2024, 15, 409. [Google Scholar] [CrossRef]
- Nanjundan, M.; Nakayama, Y.; Cheng, K.W.; Lahad, J.; Liu, J.; Lu, K.; Kuo, W.-L.; Smith-McCune, K.; Fishman, D.; Gray, J.W.; et al. Amplification of MDS1/EVI1 and EVI1, located in the 3q26.2 amplicon, is associated with favorable patient prognosis in ovarian cancer. Cancer Res. 2007, 67, 3074–3084. [Google Scholar] [CrossRef]
- Jank, P.; Leichsenring, J.; Kolb, S.; Hoffmann, I.; Bischoff, P.; Kunze, C.A.; Dragomir, M.P.; Gleitsmann, M.; Jesinghaus, M.; Schmitt, W.D.; et al. High EVI1 and PARP1 expression as favourable prognostic markers in high-grade serous ovarian carcinoma. J. Ovarian Res. 2023, 16, 150. [Google Scholar] [CrossRef]
- Moon, H.-G.; Yi, J.K.; Kim, H.S.; Lee, H.Y.; Lee, K.-M.; Yi, M.; Ahn, S.; Shin, H.-C.; Ju, J.-h.; Shin, I.; et al. Phosphorylation of p90RSK is associated with increased response to neoadjuvant chemotherapy in ER-positive breast cancer. BMC Cancer 2012, 12, 585. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, W.; Zheng, X.; Han, Y.; Liu, R. Research on a Weighted Gene Co-expression Network Analysis method for mining pathogenic genes in thyroid cancer. PLoS ONE 2022, 17, e0272403. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jiang, X.; Huang, Y.; Liu, Q.; Huang, Y.; Zhang, B.; Mei, Z.; Xu, D.; Shi, Y.; Tu, W. Construction of a Tumor Immune Microenvironment-Related Prognostic Model in BRAF-Mutated Papillary Thyroid Cancer. Front. Endocrinol. 2022, 13, 895428. [Google Scholar] [CrossRef] [PubMed]
- Buechel, D.; Sugiyama, N.; Rubinstein, N.; Saxena, M.; Kalathur, R.K.R.; Lüönd, F.; Vafaizadeh, V.; Valenta, T.; Hausmann, G.; Cantù, C.; et al. Parsing β-catenin’s cell adhesion and Wnt signaling functions in malignant mammary tumor progression. Proc. Natl. Acad. Sci. USA 2021, 118, e2020227118. [Google Scholar] [CrossRef] [PubMed]
- Nickless, A.; Zhang, J.; Othoum, G.; Webster, J.; Inkman, M.J.; Coonrod, E.; Fontes, S.; Rozycki, E.B.; Maher, C.A.; White, N.M. Pan-Cancer Analysis Reveals Recurrent BCAR4 Gene Fusions across Solid Tumors. Mol. Cancer Res. 2022, 20, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Donahue, T.R.; Tran, L.M.; Hill, R.; Li, Y.; Kovochich, A.; Calvopina, J.H.; Patel, S.G.; Wu, N.; Hindoyan, A.; Farrell, J.J.; et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clin. Cancer Res. 2012, 18, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.L.; Cantley, L.C. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef] [PubMed]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- De Luca, A.; Carotenuto, A.; Rachiglio, A.; Gallo, M.; Maiello, M.R.; Aldinucci, D.; Pinto, A.; Normanno, N. The role of the EGFR signaling in tumor microenvironment. J. Cell Physiol. 2008, 214, 559–567. [Google Scholar] [CrossRef]
- Sasi, B.; Ethiraj, P.; Myers, J.; Lin, A.-P.; Jiang, S.; Qiu, Z.; Holder, K.N.; Aguiar, R.C.T. Regulation of PD-L1 expression is a novel facet of cyclic-AMP-mediated immunosuppression. Leukemia 2021, 35, 1990–2001. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.-H.; Shin, J.H.; Hahn, S.Y.; et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr. Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef]
- Shi, R.-L.; Qu, N.; Luo, T.-X.; Xiang, J.; Liao, T.; Sun, G.-H.; Wang, Y.; Wang, Y.-L.; Huang, C.-P.; Ji, Q.-H. Programmed Death-Ligand 1 Expression in Papillary Thyroid Cancer and Its Correlation with Clinicopathologic Factors and Recurrence. Thyroid. 2017, 27, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- St Paul, M.; Ohashi, P.S. The Roles of CD8+ T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.C.A.; Nelson, B.H. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 2018, 24, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Rong, D.; Zhang, B.; Zheng, W.; Wang, X.; Chen, Z.; Tang, W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: Challenges and opportunities. Mol. Cancer 2019, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Bellevicine, C.; Lansione, T.; Ferrara, A.L.; Iannone, R.; di Somma, S.; Borriello, F.; Clery, E.; et al. Potential involvement of neutrophils in human thyroid cancer. PLoS ONE 2018, 13, e0199740. [Google Scholar] [CrossRef]
- Bergdorf, K.; Ferguson, D.C.; Mehrad, M.; Ely, K.; Stricker, T.; Weiss, V.L. Papillary thyroid carcinoma behavior: Clues in the tumor microenvironment. Endocr. Relat. Cancer 2019, 26, 601–614. [Google Scholar] [CrossRef]
- Xie, Z.; Li, X.; He, Y.; Wu, S.; Wang, S.; Sun, J.; He, Y.; Lun, Y.; Zhang, J. Immune Cell Confrontation in the Papillary Thyroid Carcinoma Microenvironment. Front. Endocrinol. 2020, 11, 570604. [Google Scholar] [CrossRef]
- Solinas, C.; Migliori, E.; De Silva, P.; Willard-Gallo, K. LAG3: The Biological Processes That Motivate Targeting This Immune Checkpoint Molecule in Human Cancer. Cancers 2019, 11, 1213. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [PubMed]
- Lozano, E.; Dominguez-Villar, M.; Kuchroo, V.; Hafler, D.A. The TIGIT/CD226 axis regulates human T cell function. J. Immunol. 2012, 188, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; John, T.; Asselin-Labat, M.-L. Regulation of the antigen presentation machinery in cancer and its implication for immune surveillance. Biochem. Soc. Trans. 2022, 50, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, M.L.; Cook, R.S.; Johnson, D.B.; Balko, J.M. Biological Consequences of MHC-II Expression by Tumor Cells in Cancer. Clin. Cancer Res. 2019, 25, 2392–2402. [Google Scholar] [CrossRef]
- Xing, M.; Westra, W.H.; Tufano, R.P.; Cohen, Y.; Rosenbaum, E.; Rhoden, K.J.; Carson, K.A.; Vasko, V.; Larin, A.; Tallini, G.; et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2005, 90, 6373–6379. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 2007, 28, 742–762. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Fancello, L.; Gandini, S.; Pelicci, P.G.; Mazzarella, L. Tumor mutational burden quantification from targeted gene panels: Major advancements and challenges. J. Immunother. Cancer 2019, 7, 183. [Google Scholar] [CrossRef]
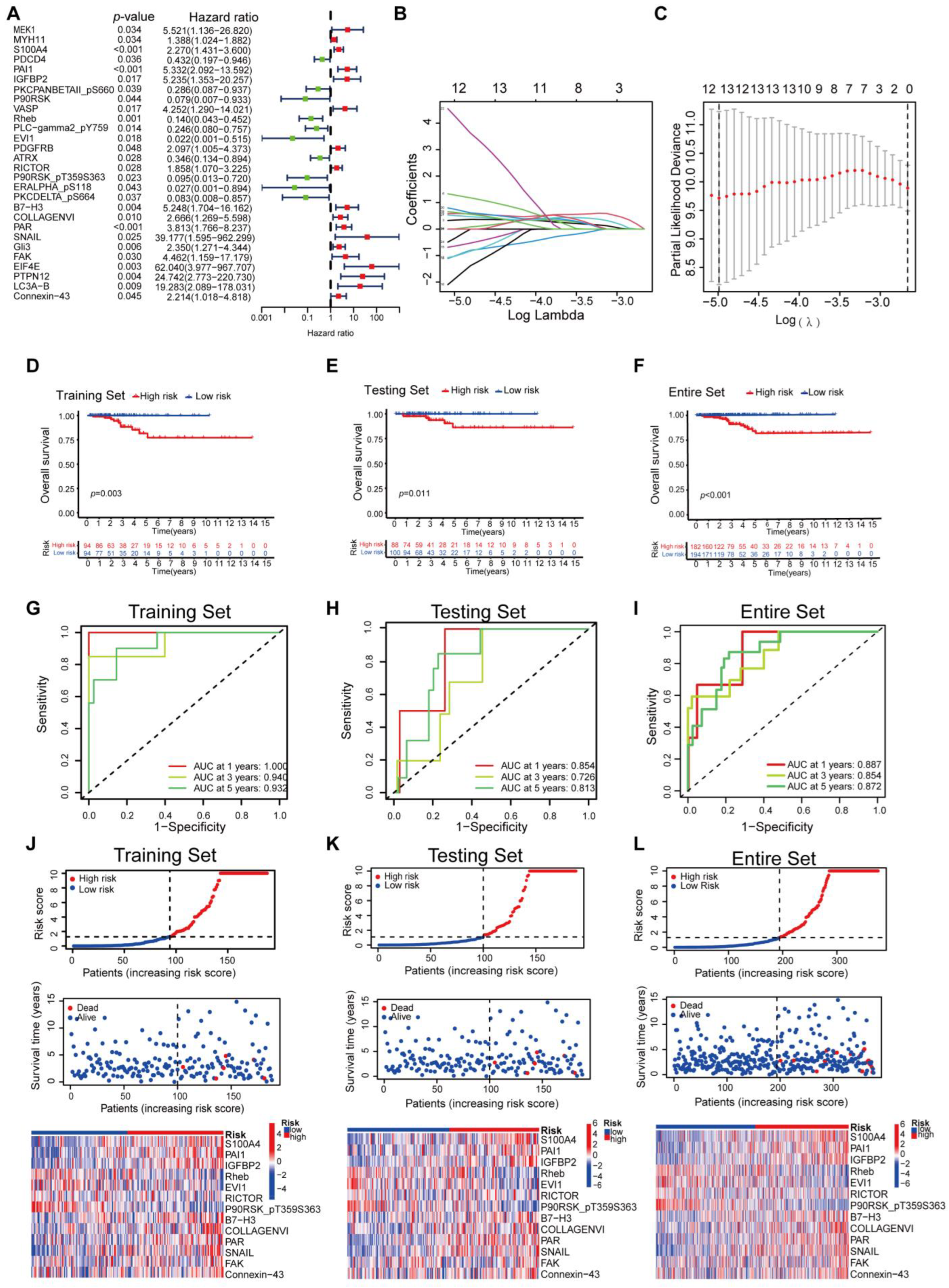



| ID | Coefficient | HR | HR.95L | HR.95H | p Value |
|---|---|---|---|---|---|
| S100A4 | 0.190685583465921 | 2.269585 | 1.430698 | 3.600352 | 0.000499 |
| PAI1 | 0.640128590811076 | 5.331818 | 2.091542 | 13.59202 | 0.000456 |
| IGFBP2 | 2.48571958440727 | 5.234716 | 1.352732 | 20.25697 | 0.016504 |
| Rheb | −3.09616014265545 | 0.13992 | 0.043317 | 0.451963 | 0.001011 |
| EVI1 | −1.96032334732288 | 0.021668 | 0.000912 | 0.514674 | 0.017743 |
| RICTOR | −1.7411650797503 | 1.857881 | 1.070301 | 3.225001 | 0.027707 |
| P90RSK_pT359S363 | −6.6880866540024 | 0.095157 | 0.012577 | 0.719942 | 0.022714 |
| B7-H3 | −0.220113199086768 | 5.247871 | 1.703994 | 16.16211 | 0.003869 |
| COLLAGENVI | 1.84909089061224 | 2.665753 | 1.269488 | 5.597718 | 0.009588 |
| PAR | 1.0667455542712 | 3.813442 | 1.765558 | 8.236686 | 0.000657 |
| SNAIL | 10.6429840082611 | 39.17723 | 1.594989 | 962.2985 | 0.024717 |
| FAK | −3.09948996444977 | 4.461859 | 1.158865 | 17.17904 | 0.029681 |
| Connexin-43 | 0.941781881570662 | 2.214479 | 1.017812 | 4.818098 | 0.045019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P.; Yin, Q.; Wang, S.; Song, D. Prognostic Protein Biomarker Screening for Thyroid Carcinoma Based on Cancer Proteomics Profiles. Biomedicines 2024, 12, 2066. https://doi.org/10.3390/biomedicines12092066
Xie P, Yin Q, Wang S, Song D. Prognostic Protein Biomarker Screening for Thyroid Carcinoma Based on Cancer Proteomics Profiles. Biomedicines. 2024; 12(9):2066. https://doi.org/10.3390/biomedicines12092066
Chicago/Turabian StyleXie, Pu, Qinglei Yin, Shu Wang, and Dalong Song. 2024. "Prognostic Protein Biomarker Screening for Thyroid Carcinoma Based on Cancer Proteomics Profiles" Biomedicines 12, no. 9: 2066. https://doi.org/10.3390/biomedicines12092066
APA StyleXie, P., Yin, Q., Wang, S., & Song, D. (2024). Prognostic Protein Biomarker Screening for Thyroid Carcinoma Based on Cancer Proteomics Profiles. Biomedicines, 12(9), 2066. https://doi.org/10.3390/biomedicines12092066





