Arrhythmogenic Potential of Myocardial Edema: The Interstitial Osmolality Induces Spiral Waves and Multiple Excitation Wavelets
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects of Animal Experimentation
2.2. Neonatal Rat Ventricular Cardiomyocytes Isolation Protocol
2.3. Preparation of Solutions with Different Osmolality
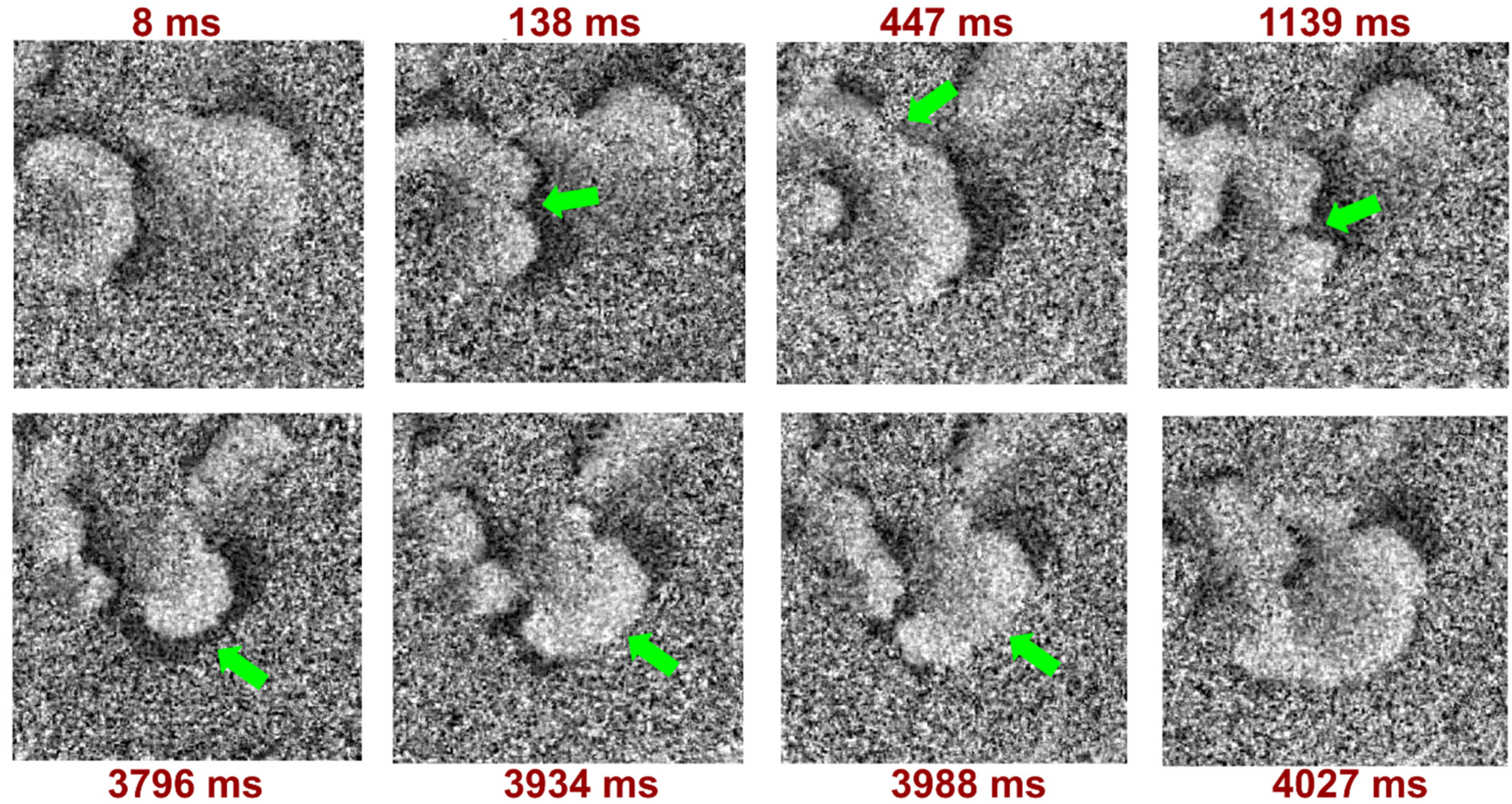
2.4. Registration of Cell Area Changes
2.5. Optical Mapping
2.6. Data Analysis
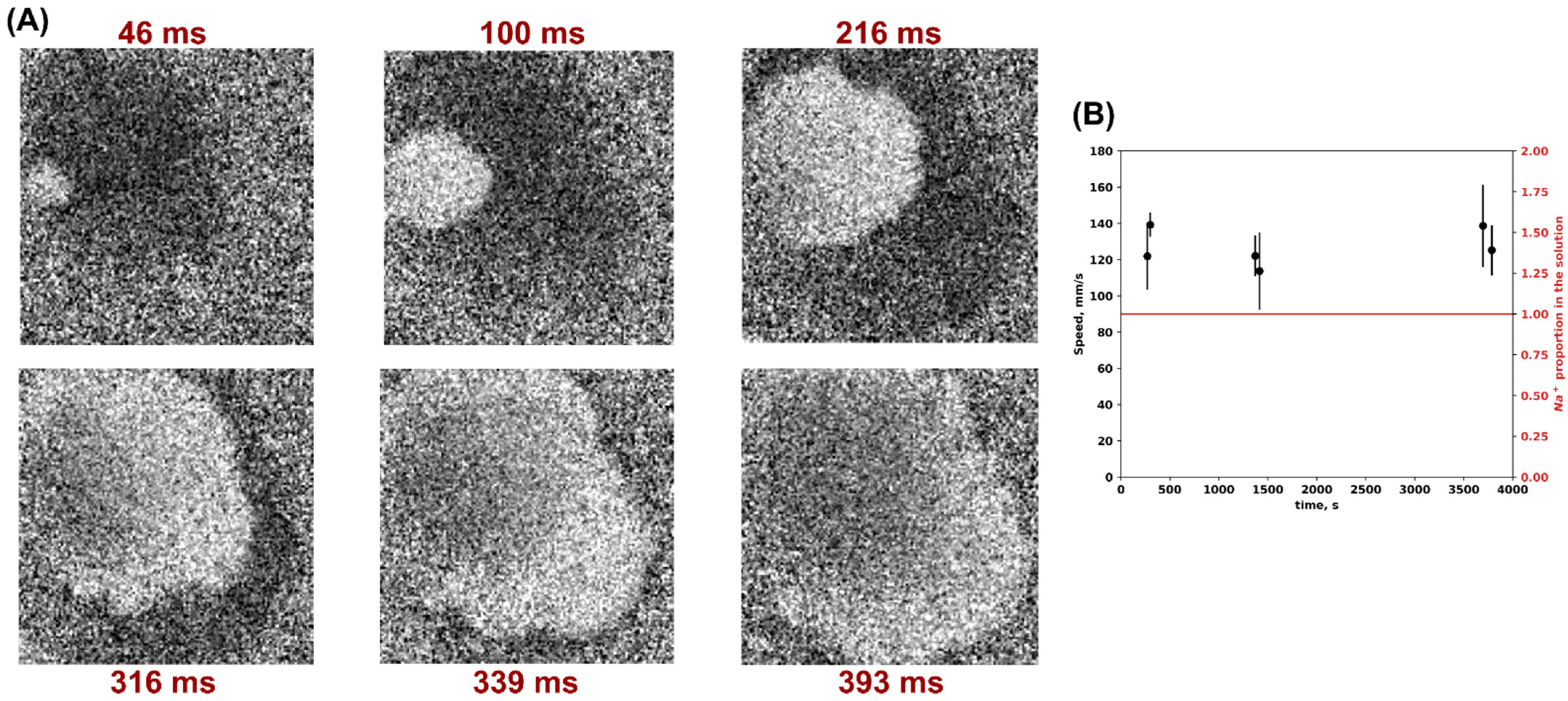
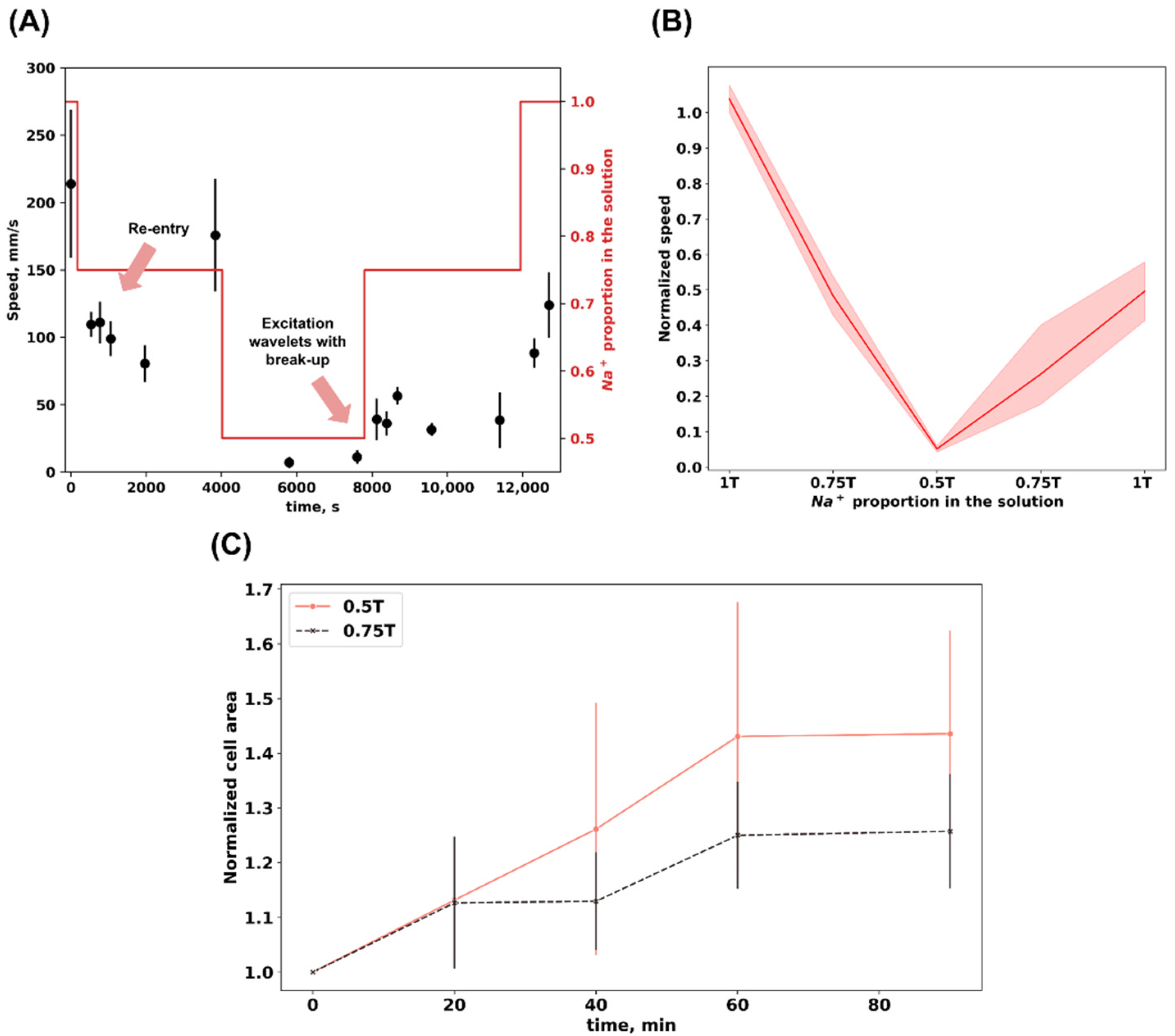
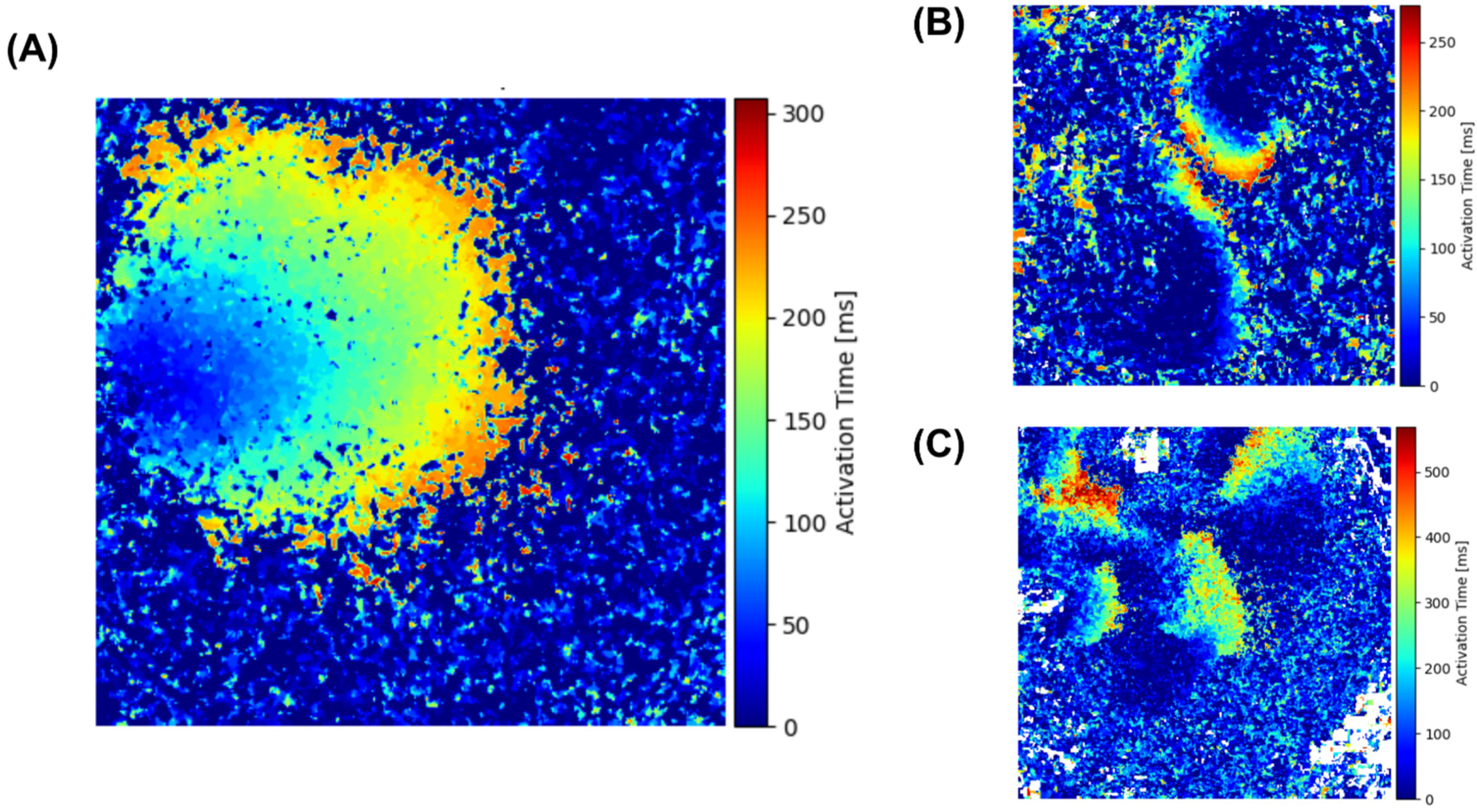
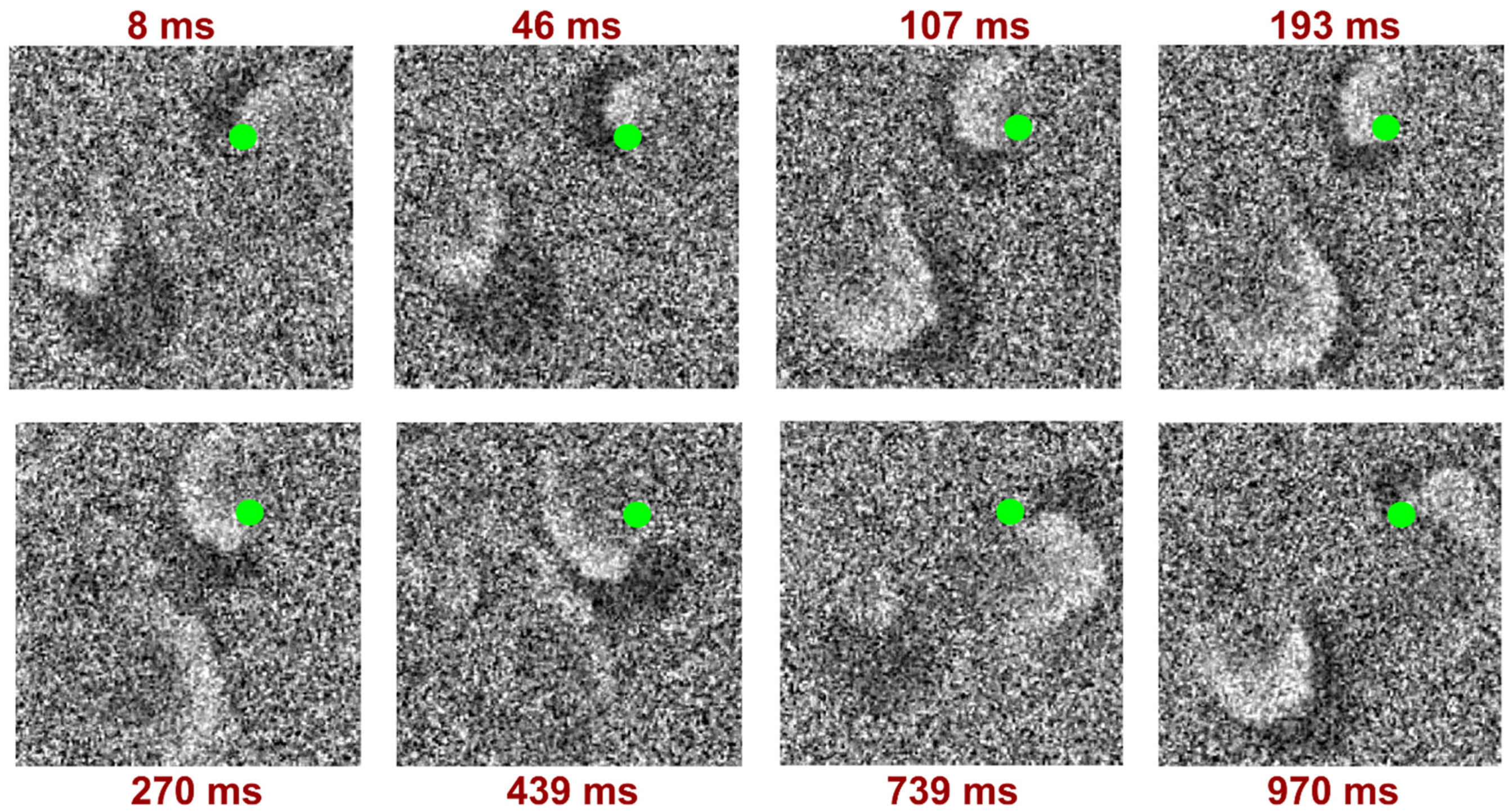
3. Results
4. Discussion
- Changes in the ion channels conductance, in particular, the activation of mechanosensitive channels.
- Changes in the mechanical properties of the sample:
- a heterogeneous increase in cell volume with various swelling rates in different sample areas;
- contractile apparatus disorder;
- rupture of intercellular contacts.
- Changes in gap junction conductance disconnecting part of the culture from the functional syncytium.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiseleva, D.G.; Kirichenko, T.V.; Markina, Y.V.; Cherednichenko, V.R.; Gugueva, E.A.; Markin, A.M. Mechanisms of Myocardial Edema Development in CVD Pathophysiology. Biomedicines 2024, 12, 465. [Google Scholar] [CrossRef]
- Kadkhodayan, A.; Chareonthaitawee, P.; Raman, S.V.; Cooper, L.T. Imaging of Inflammation in Unexplained Cardiomyopathy. JACC Cardiovasc. Imaging 2016, 9, 603–617. [Google Scholar] [CrossRef]
- Capasso, R.; Imperato, M.C.; Serra, N.; Rodriguez, R.; Rivellini, M.; De Filippo, M.; Pinto, A. Infarct-like versus Non-Infarct-like Clinical Presentation of Acute Myocarditis: Comparison of Cardiac Magnetic Resonance (CMR) Findings. Diagnostics 2023, 13, 2498. [Google Scholar] [CrossRef]
- Filomena, D.; Dresselaers, T.; Bogaert, J. Role of Cardiovascular Magnetic Resonance to Assess Cardiovascular Inflammation. Front. Cardiovasc. Med. 2022, 9, 877364. [Google Scholar] [CrossRef]
- Michael Felker, G. Diuretic Management in Heart Failure. Congest. Heart Fail. 2010, 16, S68–S72. [Google Scholar] [CrossRef]
- Nielsen, N.R.; Rangarajan, K.V.; Mao, L.; Rockman, H.A.; Caron, K.M. A Murine Model of Increased Coronary Sinus Pressure Induces Myocardial Edema with Cardiac Lymphatic Dilation and Fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H895–H907. [Google Scholar] [CrossRef]
- Khanna, S.; Lo, P.; Cho, K.; Subbiah, R. Ventricular Arrhythmias in Cardiac Amyloidosis: A Review of Current Literature. Clin. Med. Insights Cardiol. 2020, 14. [Google Scholar] [CrossRef]
- Amano, Y.; Tachi, M.; Tani, H.; Mizuno, K.; Kobayashi, Y.; Kumita, S. T2-Weighted Cardiac Magnetic Resonance Imaging of Edema in Myocardial Diseases. Sci. World J. 2012, 2012, 194069. [Google Scholar] [CrossRef]
- Kragholm, K.H.; Lindgren, F.L.; Zaremba, T.; Freeman, P.; Andersen, N.H.; Riahi, S.; Pareek, M.; Køber, L.; Torp-Pedersen, C.; Søgaard, P.; et al. Mortality and Ventricular Arrhythmia after Acute Myocarditis: A Nationwide Registry-Based Follow-up Study. Open Heart 2021, 8, e001806. [Google Scholar] [CrossRef]
- Vio, R.; Zorzi, A.; Corrado, D. Arrhythmias in Myocarditis. In Myocarditis; Springer: Cham, Switzerland, 2020; pp. 329–341. [Google Scholar] [CrossRef]
- Dall’Armellina, E.; Karia, N.; Lindsay, A.C.; Karamitsos, T.D.; Ferreira, V.; Robson, M.D.; Kellman, P.; Francis, J.M.; Forfar, C.; Prendergast, B.D.; et al. Dynamic Changes of Edema and Late Gadolinium Enhancement after Acute Myocardial Infarction and Their Relationship to Functional Recovery and Salvage Index. Circ. Cardiovasc. Imaging 2011, 4, 228. [Google Scholar] [CrossRef]
- Sattler, S.M.; Skibsbye, L.; Linz, D.; Lubberding, A.F.; Tfelt-Hansen, J.; Jespersen, T. Ventricular Arrhythmias in First Acute Myocardial Infarction: Epidemiology, Mechanisms, and Interventions in Large Animal Models. Front. Cardiovasc. Med. 2019, 6, 158. [Google Scholar] [CrossRef]
- Lin, J.W.; Garber, L.; Qi, Y.R.; Chang, M.G.; Cysyk, J.; Tung, L. Region of Slowed Conduction Acts as Core for Spiral Wave Reentry in Cardiac Cell Monolayers. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, 58–65. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Laghi-Pasini, F.; Boutjdir, M.; Capecchi, P.L. Cardioimmunology of Arrhythmias: The Role of Autoimmune and Inflammatory Cardiac Channelopathies. Nat. Rev. Immunol. 2018, 19, 63–64. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Ackerman, M.J.; Antzelevitch, C.; Bezzina, C.R.; Borggrefe, M.; Cuneo, B.F.; Wilde, A.A.M. Inherited Cardiac Arrhythmias. Nat. Rev. Dis. Prim. 2020, 6, 58. [Google Scholar] [CrossRef]
- Venetucci, L.; Denegri, M.; Napolitano, C.; Priori, S.G. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nature Reviews. Cardiology 2012, 9, 561–575. [Google Scholar] [CrossRef]
- Manolis, A.A.; Manolis, T.A.; Apostolopoulos, E.J.; Apostolaki, N.E.; Melita, H.; Manolis, A.S. The Role of the Autonomic Nervous System in Cardiac Arrhythmias: The Neuro-Cardiac Axis, More Foe than Friend? Trends Cardiovasc. Med. 2021, 31, 290–302. [Google Scholar] [CrossRef]
- Pravdin, S.F.; Epanchintsev, T.I.; Panfilov, A.V. Overdrive Pacing of Spiral Waves in a Model of Human Ventricular Tissue. Sci. Rep. 2020, 10, 20632. [Google Scholar] [CrossRef]
- Hussaini, S.; Lädke, S.L.; Schröder-Schetelig, J.; Venkatesan, V.; Quiñonez Uribe, R.A.; Richter, C.; Majumder, R.; Luther, S. Dissolution of Spiral Wave’s Core Using Cardiac Optogenetics. PLoS Comput. Biol. 2023, 19, e1011660. [Google Scholar] [CrossRef]
- Valderrábano, M.; Chen, P.S.; Lin, S.F. Spatial Distribution of Phase Singularities in Ventricular Fibrillation. Circulation 2003, 108, 354–359. [Google Scholar] [CrossRef]
- Gray, R.A.; Jalife, J.; Panfilov, A.; Baxter, W.T.; Cabo, C.; Davidenko, J.M.; Pertsov, A.M. Nonstationary vortexlike reentrant activity as a mechanism of polymorphic ventricular tachycardia in the isolated rabbit heart. Circulation 1995, 91, 2454–2469. [Google Scholar] [CrossRef] [PubMed]
- Panfilov, A.V.; Keldermann, R.H.; Nash, M.P. Drift and breakup of spiral waves in reaction–diffusion–mechanics systems. Proc. Natl. Acad. Sci. USA 2007, 104, 7922–7926. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Xie, F.; Garfinkel, A.; Weiss, J.N. Origins of spiral wave meander and breakup in a two-dimensional cardiac tissue model. Ann. Biomed. Eng. 2000, 28, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.A. Termination of spiral wave breakup in a Fitzhugh–Nagumo model via short and long duration stimuli. Chaos 2002, 12, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Agladze, K.; Kay, M.W.; Krinsky, V.; Sarvazyan, N. Interaction between spiral and paced waves in cardiac tissue. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H503–H513. [Google Scholar] [CrossRef]
- Lim, Z.Y.; Maskara, B.; Aguel, F.; Emokpae, R., Jr.; Tung, L. Spiral wave attachment to millimeter-sized obstacles. Circulation 2006, 114, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M. Basic concepts and practical equations on osmolality: Biochemical approach. Clin. Biochem. 2016, 49, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Mandiga, P. Physiology, Plasma Osmolality and Oncotic Pressure; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Ogura, T. Sarcolemmal hydraulic conductivity of guinea-pig and rat ventricular myocytes. Cardiovasc. Res. 2002, 54, 590–600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruiz-Meana, M.; Garcia-Dorado, D.; Gonzalez, M.A.; Barrabes, J.A.; Soler-Soler, J. Effect of osmotic stress on sarcolemmal integrity of isolated cardiomyocytes following transient metabolic inhibition. Cardiovasc. Res. 1995, 30, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.I.; Rees, S.A.; Wright, A.R.; Powell, T. Cell swelling and ion transport pathways in cardiac myocytes. Cardiovasc. Res. 1996, 32, 85–97. [Google Scholar] [CrossRef]
- Nizamieva, A.; Frolova, S.; Slotvitsky, M.; Kovalenko, S.; Tsvelaya, V.; Nikitina, A.; Sergeevichev, D.; Agladze, K. Cellular electrophysiological effects of botulinum toxin A on neonatal rat cardiomyocytes and on cardiomyocytes derived from human-induced pluripotent stem cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 513–524. [Google Scholar] [CrossRef]
- Lebert, J.; Ravi, N.; Kensah, G.; Christoph, J. Real-time optical mapping of contracting cardiac tissues with GPU-accelerated numerical motion tracking. Front. Cardiovasc. Med. 2022, 9, 787627. [Google Scholar] [CrossRef]
- Lebert, J.; Christoph, J. Optimap: An Open-Source Library for the Processing of Fluorescence Video Data. Zenodo. 2023. Available online: https://zenodo.org/records/8336620 (accessed on 2 June 2024).
- Egan, J.R.; Butler, T.L.; Au, C.G.; Tan, Y.M.; North, K.N.; Winlaw, D.S. Myocardial water handling and the role of aquaporins. Biochimica et Biophysica Acta. Biomembranes 2006, 1758, 1043–1052. [Google Scholar] [CrossRef]
- Rame, J.E.; Müller, J. Myocardial edema revisited in a new paradigm of cardiac electrical microcurrent application in heart failure. Bioelectricity 2021, 3, 171–175. [Google Scholar] [CrossRef]
- Moe, G.K. Evidence for reentry as a mechanism of cardiac arrhythmias. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 1975; pp. 55–81. [Google Scholar]
- Ashihara, T.; Namba, T.; Ito, M.; Kinoshita, M.; Nakazawa, K. The dynamics of vortex-like reentry wave filaments in three-dimensional computer models. J. Electrocardiol. 1999, 32, 129–138. [Google Scholar] [CrossRef]
- Tang, L.; Hwang, G.-S.; Hayashi, H.; Song, J.; Ogawa, M.; Kobayashi, K.; Joung, B.; Karagueuzian, H.S.; Chen, P.-S.; Lin, S.-F. Intracellular calcium dynamics at the core of endocardial stationary spiral waves in Langendorff-perfused rabbit hearts. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H297–H304. [Google Scholar] [CrossRef]
- Yamazaki, M.; Honjo, H.; Ashihara, T.; Harada, M.; Sakuma, I.; Nakazawa, K.; Trayanova, N.; Horie, M.; Kalifa, J.; Jalife, J.; et al. Regional cooling facilitates termination of spiral-wave reentry through unpinning of rotors in rabbit hearts. Heart Rhythm Off. J. Heart Rhythm. Soc. 2012, 9, 107–114. [Google Scholar] [CrossRef]
- Podgurskaya, A.D.; Tsvelaya, V.A.; Slotvitsky, M.M.; Dementyeva, E.V.; Valetdinova, K.R.; Agladze, K.I. The use of iPSC-derived cardiomyocytes and optical mapping for erythromycin arrhythmogenicity testing. Cardiovasc. Toxicol. 2019, 19, 518–528. [Google Scholar] [CrossRef]
- Romanova, S.A.; Berezhnoy, A.K.; Ruppel, L.E.; Aitova, A.A.; Bakumenko, S.S.; Semidetnov, I.S.; Naumov, V.D.; Slotvitsky, M.M.; Tsvelaya, V.A.; Agladze, K.I. Discrete anisotropy model of heterogeneous cardiac tissue predicting the occurrence of symmetry breaking of reentrant activity. JETP Lett. 2024, 119, 722–731. [Google Scholar] [CrossRef]
- Kim, D.T.; Kwan, Y.; Lee, J.J.; Ikeda, T.; Uchida, T.; Kamjoo, K.; Kim, Y.-H.; Ong, J.J.C.; Athill, C.A.; Wu, T.-J.; et al. Patterns of spiral tip motion in cardiac tissues. Chaos 1998, 8, 137–148. [Google Scholar] [CrossRef]
- Woo, S.-J.; Hong, J.H.; Kim, T.Y.; Wook Bae, B.; Lee, K.J. Spiral wave drift and complex-oscillatory spiral waves caused by heterogeneities in two-dimensional in vitro cardiac tissues. New J. Phys. 2008, 10, 015005. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Kim, T.Y.; Lee, K.J. Complex-periodic spiral waves in confluent cardiac cell cultures induced by localized inhomogeneities. Proc. Natl. Acad. Sci. USA 2005, 102, 10363–10368. [Google Scholar] [CrossRef] [PubMed]
- Pravdin, S.F.; Panfilov, A.V. Doppler shift during overdrive pacing of spiral waves. Prediction of the annihilation site. Chaos Solitons Fractals 2006, 155, 111782. [Google Scholar] [CrossRef]
- Bursac, N.; Tung, L. Acceleration of functional reentry by rapid pacing in anisotropic cardiac monolayers: Formation of multi-wave functional reentries. Cardiovasc. Res. 2006, 69, 381–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hayakawa, T.; Kunihiro, T.; Dowaki, S.; Uno, H.; Matsui, E.; Uchida, M.; Kobayashi, S.; Yasuda, A.; Shimizu, T.; Okano, T. Noninvasive evaluation of contractile behavior of cardiomyocyte monolayers based on motion vector analysis. Tissue Eng. Part C Methods 2012, 18, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fast, V.G.; Darrow, B.J.; Saffitz, J.E.; Kléber, A.G. Anisotropic activation spread in heart cell monolayers assessed by high-resolution optical mapping: Role of tissue discontinuities. Circ. Res. 1996, 79, 115–127. [Google Scholar] [CrossRef]
- Fenton, F.; Karma, A. Vortex dynamics in three-dimensional continuous myocardium with fiber rotation: Filament instability and fibrillation. Chaos 1998, 8, 20–47. [Google Scholar] [CrossRef]
- Majumder, R.; Hussaini, S.; Zykov, V.S.; Luther, S.; Bodenschatz, E. Pulsed low-energy stimulation initiates electric turbulence in cardiac tissue. PLoS Comput. Biol. 2021, 17, e1009476. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Garfinkel, A.; Karagueuzian, H.S.; Qu, Z.; Chen, P.-S. Chaos and the transition to ventricular fibrillation: A new approach to antiarrhythmic drug evaluation. Circulation 1999, 99, 2819–2826. [Google Scholar] [CrossRef]
- Xie, F.; Qu, Z.; Garfinkel, A.; Weiss, J.N. Effects of simulated ischemia on spiral wave stability. American Journal of Physiology. Heart Circ. Physiol. 2001, 280, H1667–H1673. [Google Scholar] [CrossRef]
- Panfilov, A.V. Spiral breakup as a model of ventricular fibrillation. Chaos 1998, 8, 57–64. [Google Scholar] [CrossRef]
- Shibata, N.; Inada, S.; Nakazawa, K.; Ashihara, T.; Tomii, N.; Yamazaki, M.; Honjo, H.; Seno, H.; Sakuma, I. Mechanism of ventricular fibrillation: Current status and problems. Adv. Biomed. Eng. 2022, 11, 117–135. [Google Scholar] [CrossRef]
- Rappel, W.-J.; Krummen, D.E.; Baykaner, T.; Zaman, J.; Donsky, A.; Swarup, V.; Miller, J.M.; Narayan, S.M. Stochastic termination of spiral wave dynamics in cardiac tissue. Front. Netw. Physiol. 2022, 2, 809532. [Google Scholar] [CrossRef]
- Weise, L.D.; Panfilov, A.V. Mechanism for mechanical wave break in the heart muscle. Phys. Rev. Lett. 2017, 119, 108101. [Google Scholar] [CrossRef]
- Vandersickel, N.; Defauw, A.; Dawyndt, P.; Panfilov, A.V. Global alternans instability and its effect on non-linear wave propagation: Dynamical Wenckebach block and self terminating spiral waves. Sci. Rep. 2016, 6, 29397. [Google Scholar] [CrossRef]
- Kappadan, V.; Telele, S.; Uzelac, I.; Fenton, F.; Parlitz, U.; Luther, S.; Christoph, J. High-resolution optical measurement of cardiac restitution, contraction, and fibrillation dynamics in beating vs. Blebbistatin-uncoupled isolated rabbit hearts. Front. Physiol. 2020, 11, 464. [Google Scholar] [CrossRef]
- Christoph, J.; Chebbok, M.; Richter, C.; Schröder-Schetelig, J.; Bittihn, P.; Stein, S.; Uzelac, I.; Fenton, F.H.; Hasenfuß, G.; Gilmour, R.F.; et al. Electromechanical vortex filaments during cardiac fibrillation. Nature 2018, 555, 667–672. [Google Scholar] [CrossRef]
- Bär, M. Reaction-diffusion patterns and waves: From chemical reactions to cardiac arrhythmias. In Spirals and Vortices; Springer: Cham, Switzerland, 2019; pp. 239–251. [Google Scholar]
- Handa, B.S.; Li, X.; Baxan, N.; Roney, C.H.; Shchendrygina, A.; Mansfield, C.A.; Jabbour, R.J.; Pitcher, D.S.; Chowdhury, R.A.; Peters, N.S.; et al. Ventricular fibrillation mechanism and global fibrillatory organization are determined by gap junction coupling and fibrosis pattern. Cardiovasc. Res. 2021, 117, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhao, Q.; Malhi, M.; Liu, X.; Zhang, Z.; Wang, L.; Masse, S.; Nanthakumar, K.; Hamilton, R.; Maynes, J.T.; et al. Label-free conduction velocity mapping and gap junction assessment of functional iPSC-Cardiomyocyte monolayers. Biosens. Bioelectron. 2020, 167, 112468. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Dhont, S.; Banerjee, D.; Bayes-Genis, A.; Cannata, A.; Chioncel, O.; Cikes, M.; Ezekowitz, J.; Flammer, A.J.; et al. Dietary sodium and fluid intake in heart failure. A clinical consensus statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2024, 26, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Marti, C.N.; Mentz, R.J.; Greene, S.J.; Ambrosy, A.P.; Subacius, H.P.; Fonarow, G.C.; Chioncel, O.; Bazari, H.; Maggioni, A.P.; et al. Serum osmolality and postdischarge outcomes after hospitalization for heart failure. Am. J. Cardiol. 2016, 117, 1144–1150. [Google Scholar] [CrossRef]
- Mohiuddin, N.; Frinak, S.; Yee, J. Sodium-based osmotherapy for hyponatremia in acute decompensated heart failure. Heart Fail. Rev. 2022, 27, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.F.; Cheng, M.; Jong, B.; Hwang, N.C.; Roscoe, A. Sodium abnormalities in cardiac surgery with cardiopulmonary bypass in adults: A narrative review. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3374–3384. [Google Scholar] [CrossRef] [PubMed]
- Bonfig, N.L.; Soukup, C.R.; Shah, A.A.; Olet, S.; Davidson, S.J.; Schmidt, C.W.; Peterson, R.; Henry, T.D.; Traverse, J.H. Increasing myocardial edema is associated with greater microvascular obstruction in ST-segment elevation myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H818–H824. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Bhar-Amato, J.; Davies, W.; Agarwal, S. Ventricular arrhythmia after acute myocardial infarction: ‘the perfect storm’. Arrhythmia Electrophysiol. Rev. 2017, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Pena Escobar, J.A.; Aung, M.; Amin, S.; Gulraiz, A.; Gandhi, F.R.; Malik, B.H. Pathogenesis of ventricular arrhythmias and its effect on long-term prognosis in patients with takotsubo cardiomyopathy. Cureus 2020, 12, e11171. [Google Scholar] [CrossRef] [PubMed]
- Couch, L.S.; Thomas, K.E.; Marin, F.; Terentes-Printzios, D.; Kotronias, R.A.; Chai, J.; Lukaschuk, E.; Shanmuganathan, M.; Kellman, P.; Langrish, J.P.; et al. The role of coronary blood flow and myocardial edema in the pathophysiology of takotsubo syndrome. JACC Cardiovasc. Imaging 2024, 17, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Redfors, B.; Sáinz-Jaspeado, M.; Shi, S.; Martinsson, P.; Padhan, N.; Scharin Täng, M.; Borén, J.; Levin, M.; Claesson-Welsh, L. Suppressed vascular leakage and myocardial edema improve outcome from myocardial infarction. Front. Physiol. 2020, 11, 763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiseleva, D.G.; Dzhabrailov, V.D.; Aitova, A.A.; Turchaninova, E.A.; Tsvelaya, V.A.; Kazakova, M.A.; Plyusnina, T.Y.; Markin, A.M. Arrhythmogenic Potential of Myocardial Edema: The Interstitial Osmolality Induces Spiral Waves and Multiple Excitation Wavelets. Biomedicines 2024, 12, 1770. https://doi.org/10.3390/biomedicines12081770
Kiseleva DG, Dzhabrailov VD, Aitova AA, Turchaninova EA, Tsvelaya VA, Kazakova MA, Plyusnina TY, Markin AM. Arrhythmogenic Potential of Myocardial Edema: The Interstitial Osmolality Induces Spiral Waves and Multiple Excitation Wavelets. Biomedicines. 2024; 12(8):1770. https://doi.org/10.3390/biomedicines12081770
Chicago/Turabian StyleKiseleva, Diana G., Vitalii D. Dzhabrailov, Aleria A. Aitova, Elena A. Turchaninova, Valeriya A. Tsvelaya, Maria A. Kazakova, Tatiana Yu. Plyusnina, and Alexander M. Markin. 2024. "Arrhythmogenic Potential of Myocardial Edema: The Interstitial Osmolality Induces Spiral Waves and Multiple Excitation Wavelets" Biomedicines 12, no. 8: 1770. https://doi.org/10.3390/biomedicines12081770
APA StyleKiseleva, D. G., Dzhabrailov, V. D., Aitova, A. A., Turchaninova, E. A., Tsvelaya, V. A., Kazakova, M. A., Plyusnina, T. Y., & Markin, A. M. (2024). Arrhythmogenic Potential of Myocardial Edema: The Interstitial Osmolality Induces Spiral Waves and Multiple Excitation Wavelets. Biomedicines, 12(8), 1770. https://doi.org/10.3390/biomedicines12081770





