Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biomaterials
2.2. Ethics Statement
2.3. Clinical Evaluation
2.4. Anesthesia
2.5. Study Design
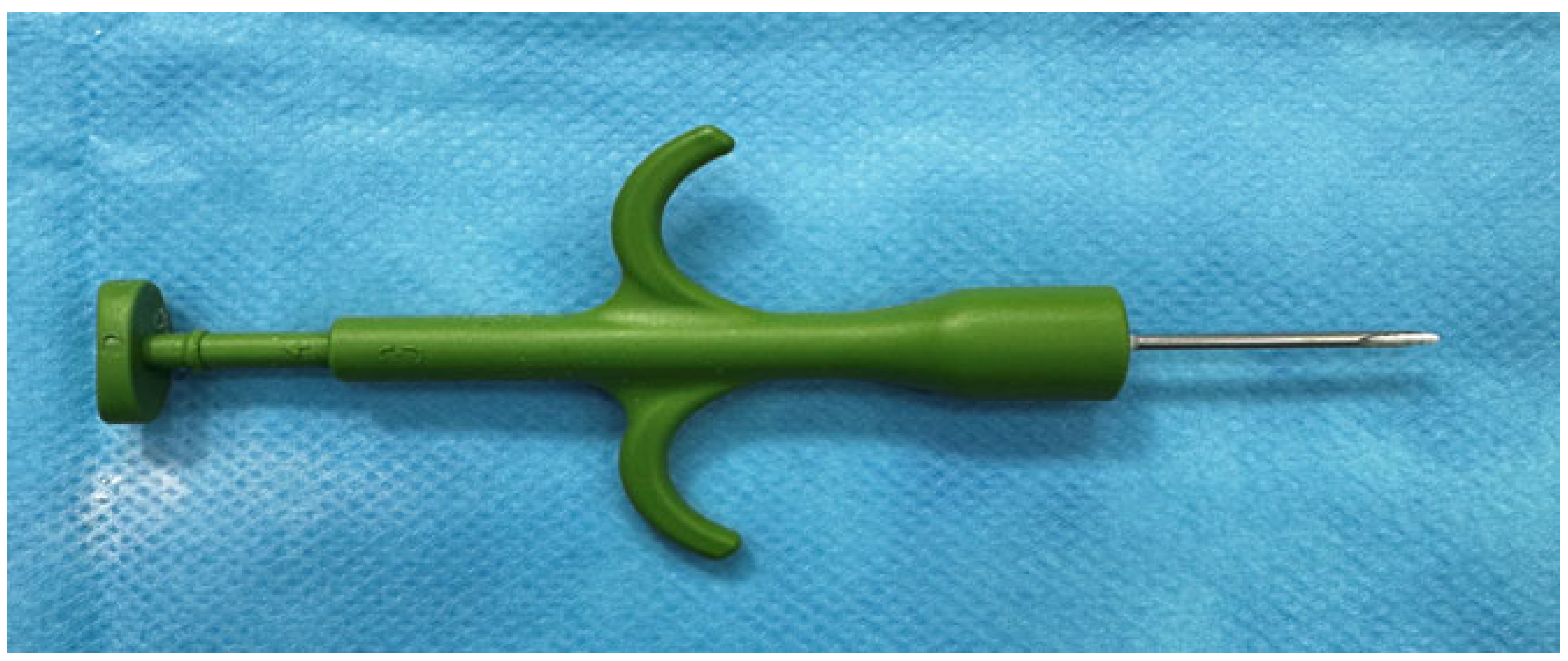
2.6. Biomaterial Implantation
2.6.1. Subcutaneous
2.6.2. Intramuscular/Perimuscular
2.7. Computed Tomography Scans
2.8. Histopathological Examination
3. Results
3.1. Clinical Evaluation
3.2. Postoperative Care
3.2.1. Subcutaneous
3.2.2. Intramuscular/Perimuscular
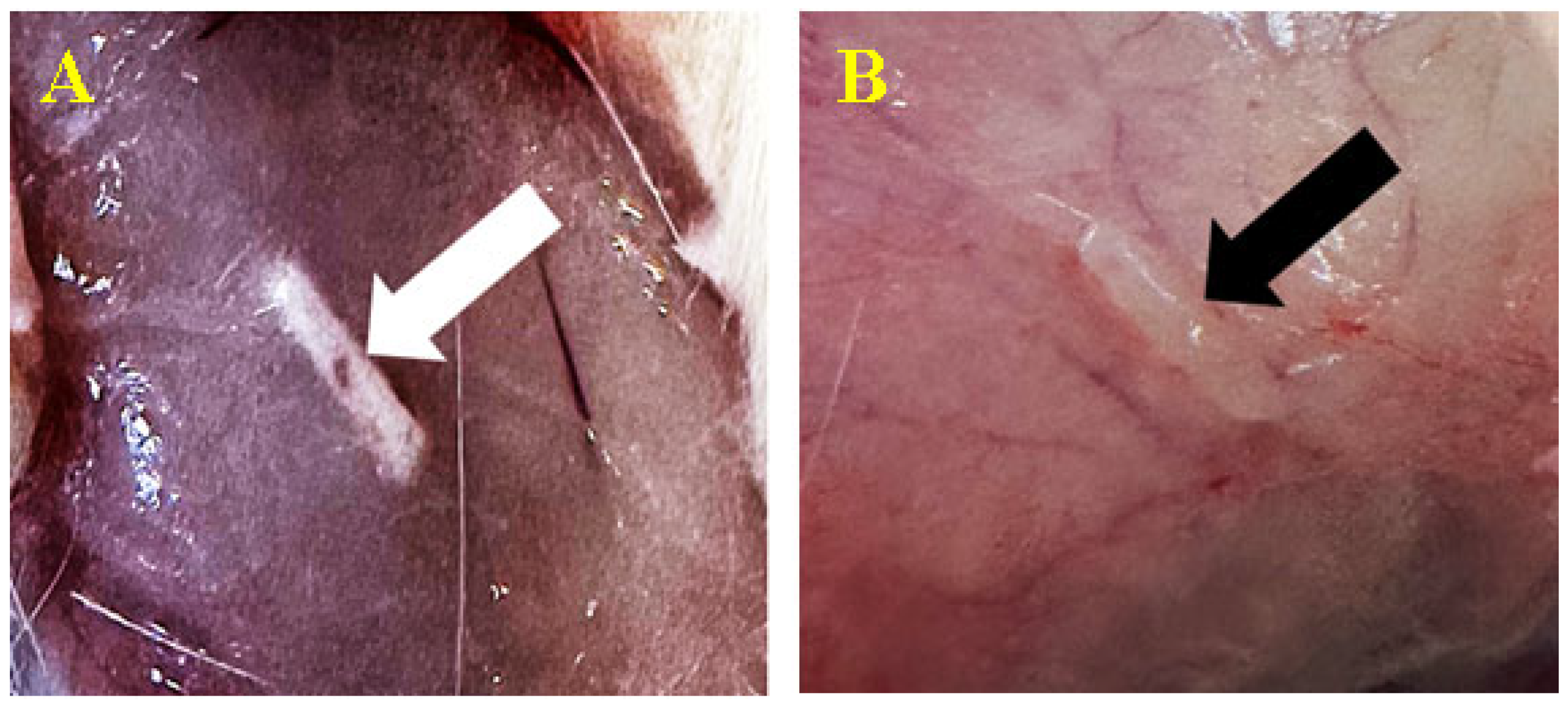
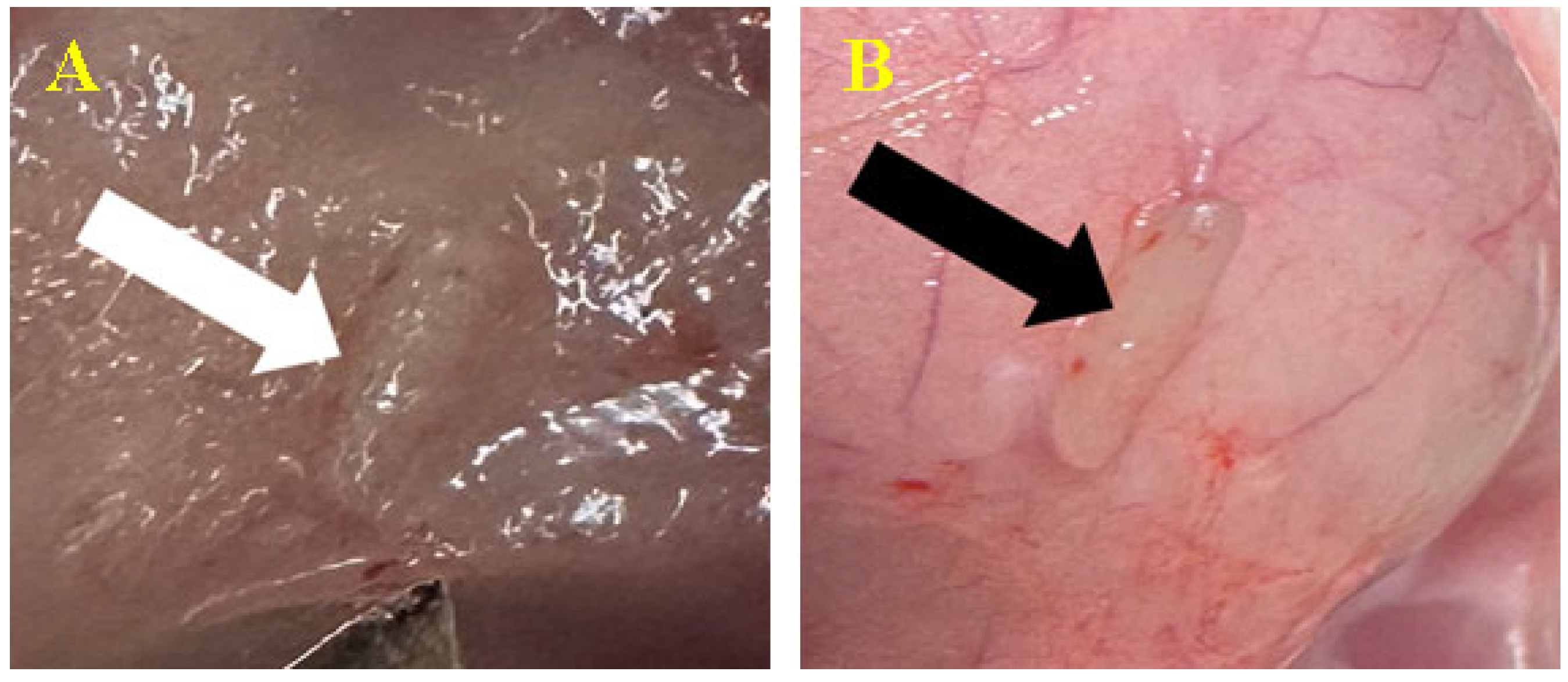
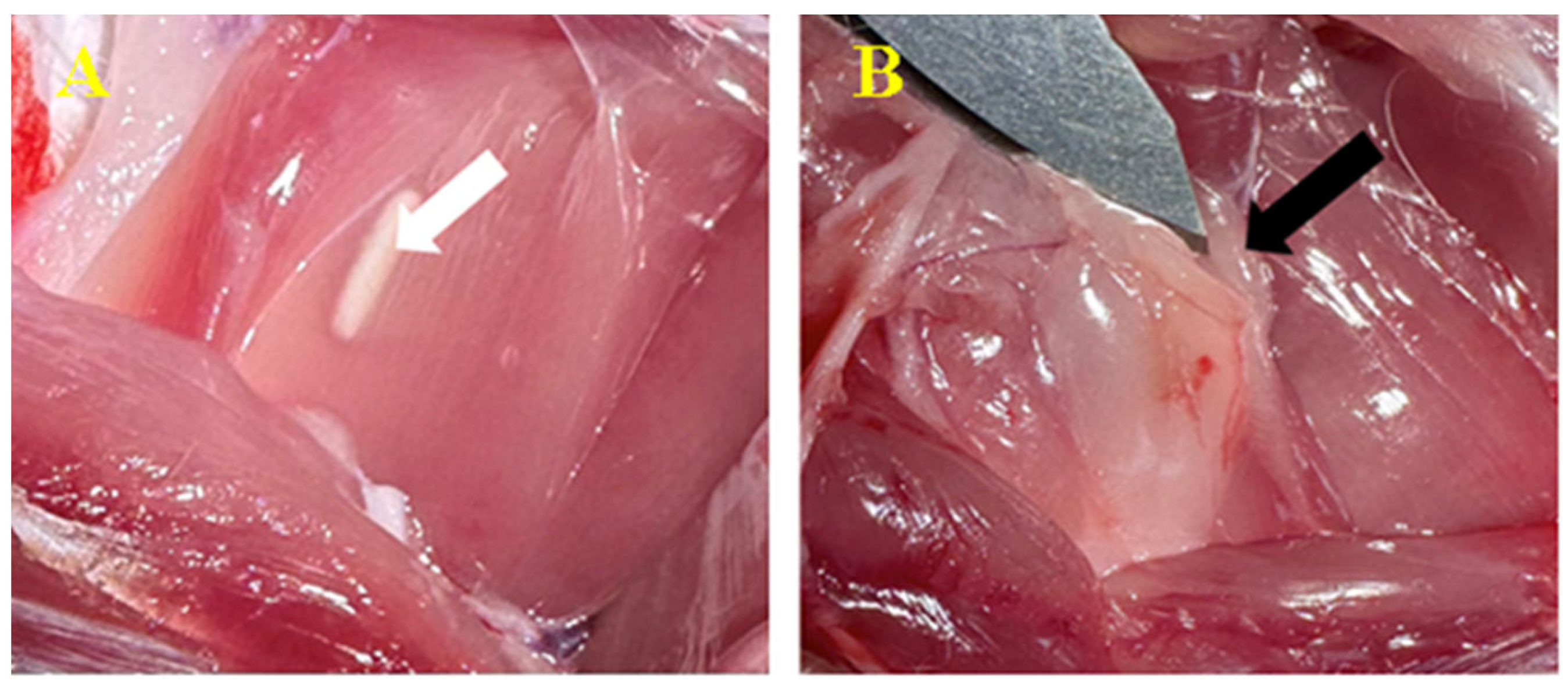
3.3. Biomaterial Implantation
3.3.1. Subcutaneous
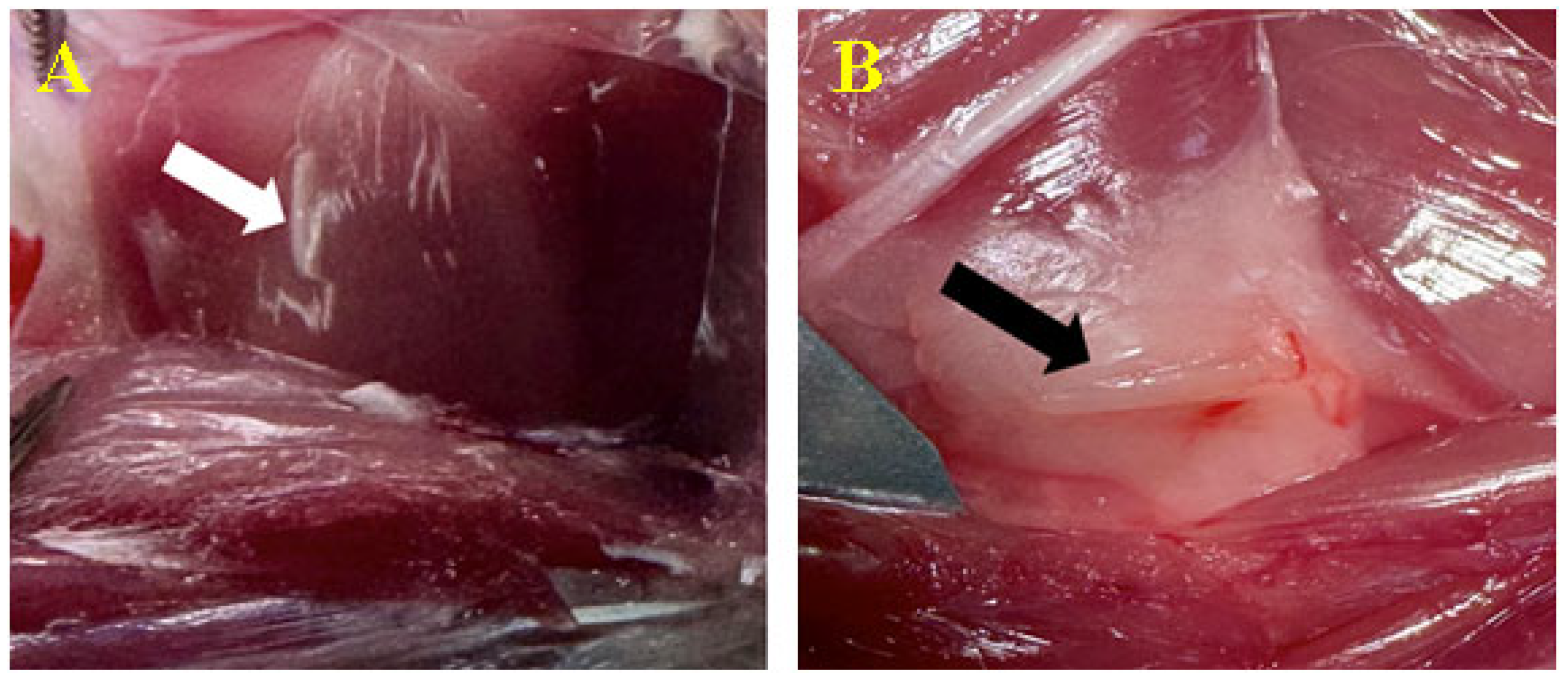
3.3.2. Peri/Intramuscular
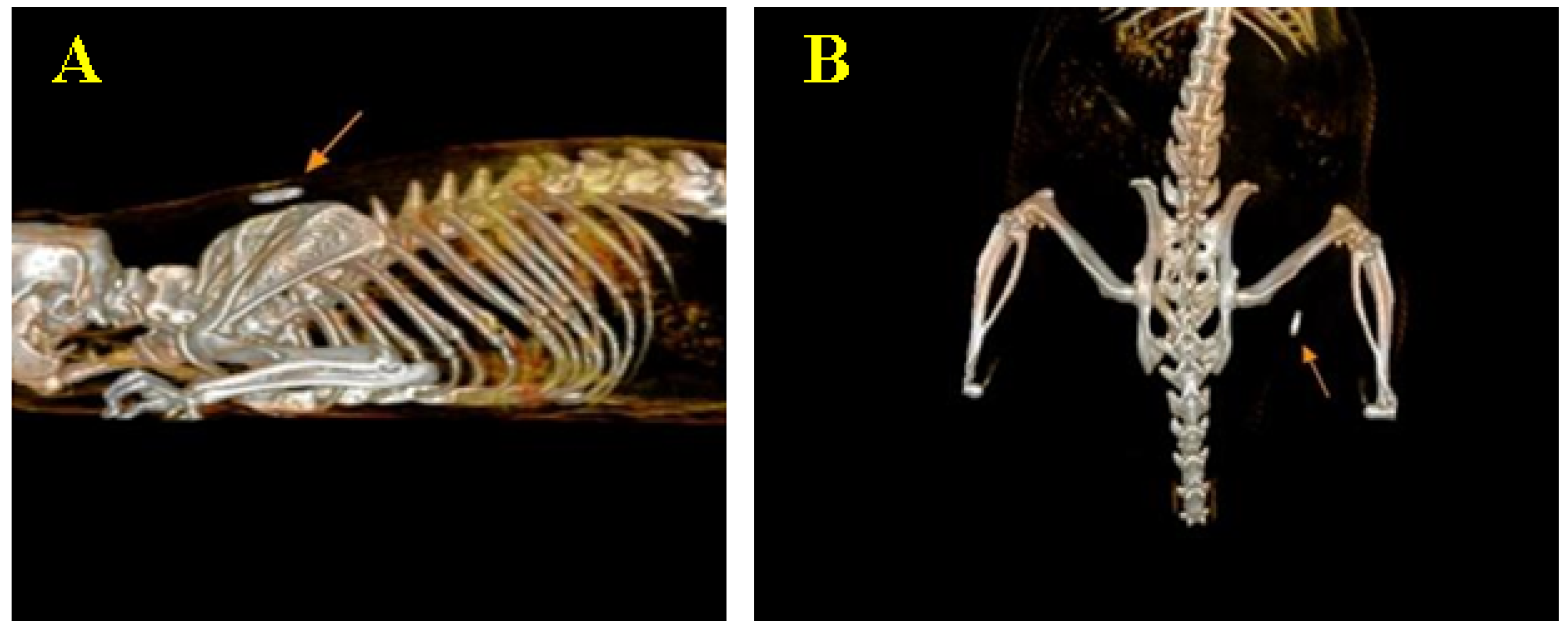
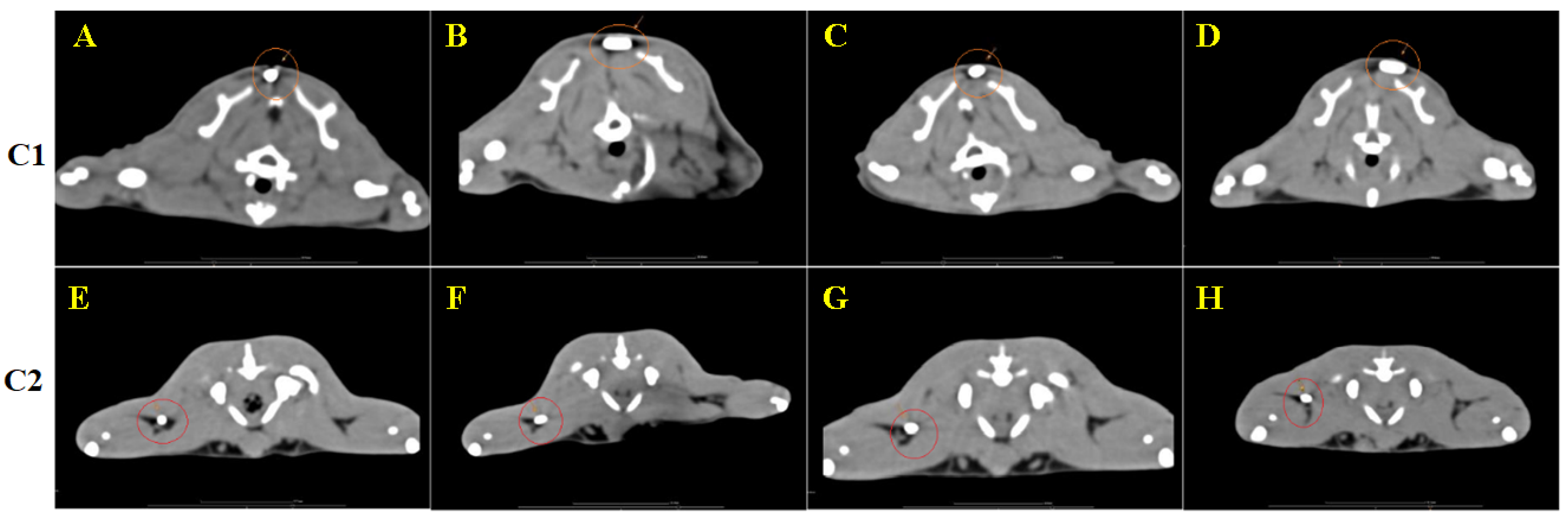
3.4. CT Image Findings
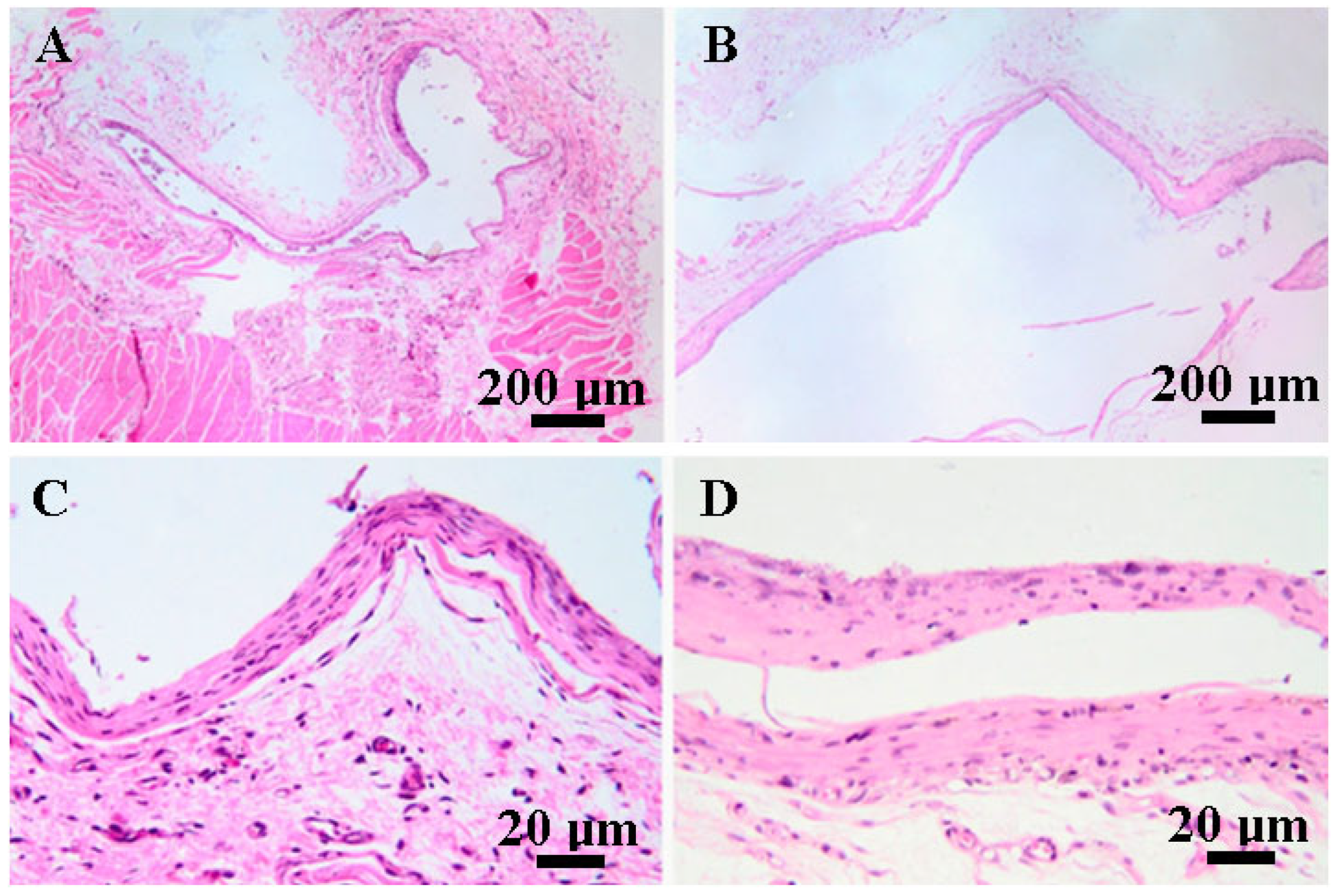
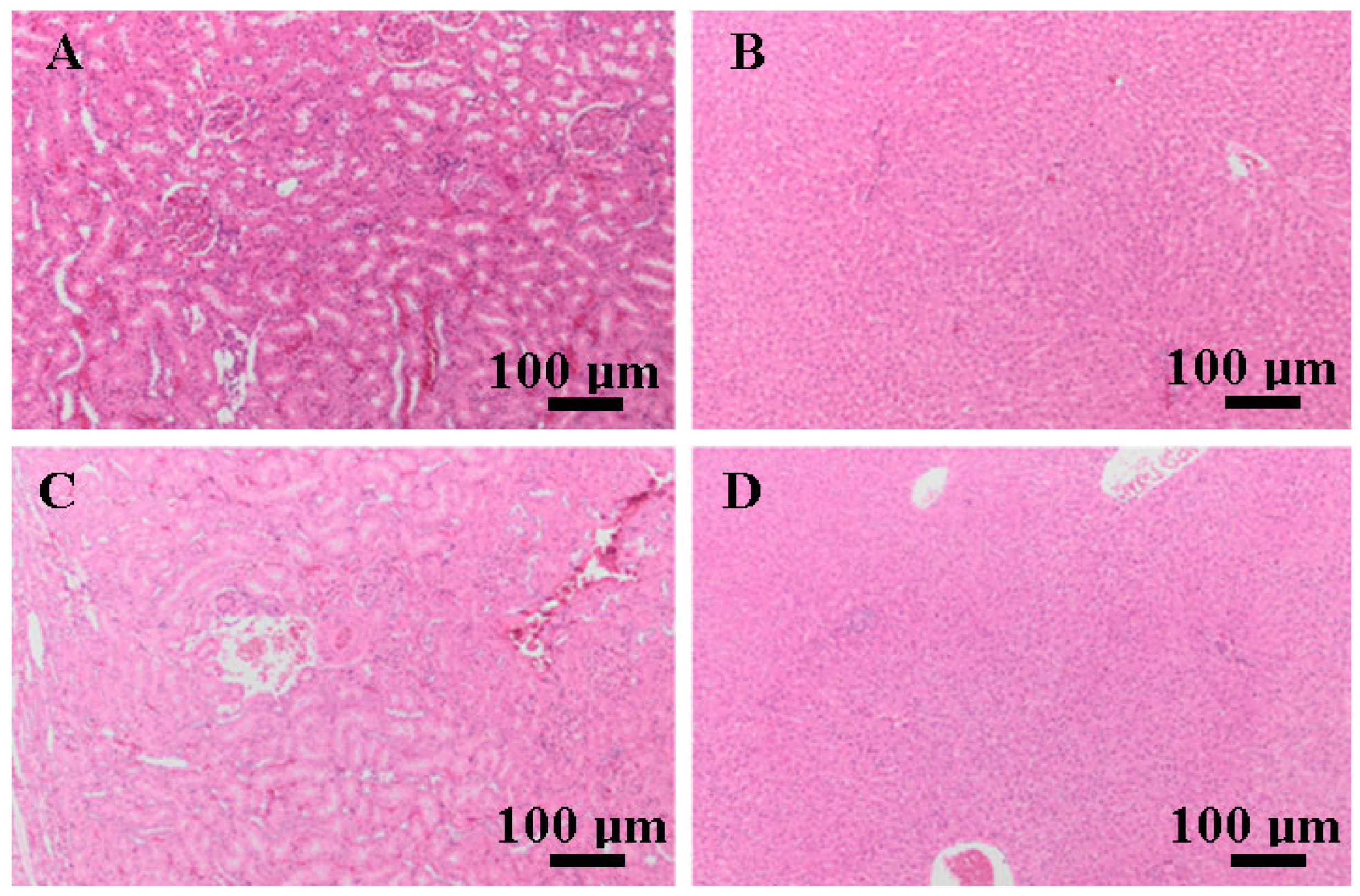
3.5. Histopathological Findings
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conte, R.; Di Salle, A.; Riccitiello, F.; Petillo, O.; Peluso, G.; Calarco, A. Biodegradable polymers in dental tissue engineering and regeneration. AIMS Mater. Sci. 2018, 5, 1073–1101. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Anju, S.; Prajitha, N.; Sukanya, V.S.; Mohanan, P.V. Complicity of degradable polymers in health-care applications. Mater. Today Chem. 2020, 16, 100236. [Google Scholar] [CrossRef]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What affects the biocompatibility of polymers? Adv. Colloid. Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.-M.; Lesieur, J.; Vacher, C.; Chaussain, C.; Rochefort, G.Y. Strategies Developed to Induce, Direct, and Potentiate Bone Healing. Front. Physiol. 2017, 8, 927. [Google Scholar] [CrossRef]
- Chisnoiu, R.; Moldovan, M.; Păstrav, O.; Delean, A.; Chisnoiu, A. The influence of three endodontic sealers on bone healing: An experimental study. Folia Morphol. 2016, 75, 14–20. [Google Scholar] [CrossRef][Green Version]
- Lee, S.-Y.; Wu, S.-C.; Chen, H.; Tsai, L.-L.; Tzeng, J.-J.; Lin, C.-H.; Lin, Y.-M. Synthesis and Characterization of Polycaprolactone-Based Polyurethanes for the Fabrication of Elastic Guided Bone Regeneration Membrane. BioMed Res. Int. 2018, 2018, 3240571. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2004; pp. 209–222. ISBN 9780080470368. [Google Scholar]
- ISO10993-5; BeomdP. International Organization for Standardization: Geneva, Switzerland, 1999.
- Mârza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Peștean, C.; Nagy, A.; Gal, A.F.; Tăbăran, F.; Purdoiu, R.C.; Licărete, E. The Impact of Composites with Silicate-Based Glasses and Gold Nanoparticles on Skin Wound Regeneration. Molecules 2021, 26, 620. [Google Scholar] [CrossRef]
- Mirea, R.; Biris, I.M.; Ceatra, L.C.; Ene, R.; Paraschiv, A.; Cucuruz, A.T.; Sbarcea, G.; Popescu, E.; Badea, T. In Vitro Physical-Chemical Behaviour Assessment of 3D-Printed CoCrMo Alloy for Orthopaedic Implants. Metals 2021, 11, 857. [Google Scholar] [CrossRef]
- Wataha, J.C. Principles of biocompatibility for dental practitioners. J. Prosthet. Dent. 2001, 86, 203–209. [Google Scholar] [CrossRef]
- Ardelean, A.I.; Dragomir, M.F.; Moldovan, M.; Sarosi, C.; Paltinean, G.A.; Pall, E.; Tudoran, L.B.; Petean, I.; Oana, L. In Vitro Study of Composite Cements on Mesenchymal Stem Cells of Palatal Origin. Int. J. Mol. Sci. 2023, 24, 10911. [Google Scholar] [CrossRef] [PubMed]
- Mirea, R.; Cucuruz, A.T.; Ceatra, L.C.; Badea, T.; Biris, I.; Popescu, E.; Paraschiv, A.; Ene, R.; Sbarcea, G.; Cretu, M. In-Depth Comparative Assessment of Different Metallic Biomaterials in Simulated Body Fluid. Materials 2021, 14, 2774. [Google Scholar] [CrossRef] [PubMed]
- Pătroi, D.; Gociu, M.; Prejmerean, C.; Colceriu, L.; Silaghi-Dumitrescu, L.; Moldovan, M.; Naicu, V. Assessing the biocompatibility of a dental composite product. Rom. J. Morphol. Embryol. 2013, 54, 321–326. [Google Scholar] [PubMed]
- Páll, E.; Florea, A.; Soriţău, O.; Cenariu, M.; Petruţiu, A.S.; Roman, A. Comparative Assessment of Oral Mesenchymal Stem Cells Isolated from Healthy and Diseased Tissues. Microsc. Microanal. 2015, 21, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhu, W.; Liu, F.; He, J. Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers. Int. J. Mol. Sci. 2016, 17, 2014. [Google Scholar] [CrossRef]
- Ahovuo-Saloranta, A.; Forss, H.; Walsh, T.; Nordblad, A.; Mäkelä, M.; Worthington, H.V. Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database Syst. Rev. 2017, 7, Cd001830. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Marovic, D.; Par, M.; Khai, L.; Thieu, M.; Reseland, J.E.; Johnsen, G.F. Bulk Fill Composites Have Similar Performance to Conventional Dental Composites. Int. J. Mol. Sci. 2020, 21, 5136. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M.; Chrószcz, M.W.; Chladek, G. Novel Urethane-Dimethacrylate Monomers and Compositions for Use as Matrices in Dental Restorative Materials. Int. J. Mol. Sci. 2020, 21, 2644. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Yoshihara, K.; Yoshida, Y. Development of new diacrylate monomers as substitutes for Bis-GMA and UDMA. Dent. Mater. 2021, 37, e391–e398. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Domarecka, M.; Kopacz, K.; Sokolowski, J.; Bociong, K. An Evaluation of the Properties of Urethane Dimethacrylate-Based Dental Resins. Materials 2021, 14, 2727. [Google Scholar] [CrossRef]
- Moszner, N.; Fischer, U.K.; Angermann, J.; Rheinberger, V. A partially aromatic urethane dimethacrylate as a new substitute for Bis-GMA in restorative composites. Dent. Mater. 2008, 24, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Sarosi, C.; Moldovan, M.; Soanca, A.; Roman, A.; Gherman, T.; Trifoi, A.; Chisnoiu, A.M.; Cuc, S.; Filip, M.; Gheorghe, G.F.; et al. Effects of Monomer Composition of Urethane Methacrylate Based Resins on the C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties. Polymers 2021, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Hollander, D.; Krugliak, P.; Katz, K. PEG 400, a hydrophilic molecular probe for measuring intestinal permeability. Gastroenterology 1990, 98, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mohl, S.; Winter, G. Continuous release of rh-interferon α-2a from triglyceride matrices. J. Control. Release 2004, 97, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The future of bioactive ceramics. J. Mater. Sci. Mater. Med. 2015, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Crush, J.; Hussain, A.; Seah, K.T.M.; Khan, W.S. Bioactive Glass: Methods for Assessing Angiogenesis and Osteogenesis. Front. Cell Dev. Biol. 2021, 9, 643781. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Erich Serfözö, N.; Prodan, D.; Diegelmann, J.; Moldovan, M. Synthesis and performance of experimental resin-based dental adhesives reinforced with functionalized graphene and hydroxyapatite fillers. Mater. Des. 2022, 221, 110985. [Google Scholar] [CrossRef]
- Al-Husinat, L.; Jouryyeh, B.; Al Sharie, S.; Al Modanat, Z.; Jurieh, A.; Al Hseinat, L.; Varrassi, G. Bone Cement and Its Anesthetic Complications: A Narrative Review. J. Clin. Med. 2023, 12, 2105. [Google Scholar] [CrossRef]
- Lupescu, O.; Nagea, M.; Scurtu, R.; Ciurea, N.M.; Dimitriu, A.L.; Marcov, N.; Popescu, G.I.; Bondari, S. Acute cellulitis as local reaction to orthopedic implant—Case presentation. Rom. J. Morphol. Embryol. 2016, 57, 1137–1143. [Google Scholar]
- Tisler, C.E.; Moldovan, M.; Petean, I.; Buduru, S.D.; Prodan, D.; Sarosi, C.; Leucuţa, D.-C.; Chifor, R.; Badea, M.E.; Ene, R. Human Enamel Fluorination Enhancement by Photodynamic Laser Treatment. Polymers 2022, 14, 2969. [Google Scholar] [CrossRef]
- Mousavinasab, S.M. Biocompatibility of composite resins. Dent. Res. J. 2011, 8 (Suppl. S36), S21–S29. [Google Scholar]
- Schmid-Schwap, M.; Franz, A.; König, F.; Bristela, M.; Lucas, T.; Piehslinger, E.; Watts, D.C.; Schedle, A. Cytotoxicity of four categories of dental cements. Dent. Mater. 2009, 25, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Darmani, H.; Al-Hiyasat, A.S.; Milhem, M.M. Cytotoxicity of dental composites and their leached components. Quintessence Int. 2007, 38, 789–795. [Google Scholar] [PubMed]
- Moldovan, M.; Dudea, D.; Cuc, S.; Sarosi, C.; Prodan, D.; Petean, I.; Furtos, G.; Ionescu, A.; Ilie, N. Chemical and structural assessment of new dental composites with graphene exposed to staining agents. J. Funct. Biomater. 2023, 14, 163. [Google Scholar] [CrossRef]
- Mârza, S.M.; Dăescu, A.M.; Purdoiu, R.C.; Dragomir, M.; Tătaru, M.; Melega, I.; Nagy, A.-L.; Gal, A.; Tăbăran, F.; Bogdan, S.; et al. Healing of Skin Wounds in Rats Using Creams Based on Symphytum Officinale Extract. Int. J. Mol. Sci. 2024, 25, 3099. [Google Scholar] [CrossRef]
- Gociu, M.; Pătroi, D.; Prejmerean, C.; Păstrăv, O.; Boboia, S.; Prodan, D.; Moldovan, M. Biology and cytotoxicity of dental materials: An in vitro study. Rom. J. Morphol. Embryol. 2013, 54, 261–265. [Google Scholar]
- Kirkpatrick, C.; Bittinger, F.; Wagner, M.; Köhler, H.; Van Kooten, T.; Klein, C.; Otto, M. Current trends in biocompatibility testing. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, B.C.; Ivanov, D. Natural and synthetic polymers for designing composite materials. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 233–286. [Google Scholar]
- Whishaw, I.Q.; Kolb, B. Analysis of behavior in laboratory rats. In The Laboratory Rat; Elsevier: Amsterdam, The Netherlands, 2020; pp. 215–242. [Google Scholar]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the Degree of Conversion, Residual Monomers and Mechanical Properties of Some Light-Cured Dental Resin Composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef]
- ISO/DIS 7405; Dentistry-Evaluation of Biocompatibility of Medical Devices Used in Dentistry. International Organization for Standardization: Geneva, Switzerland, 1994.
- ISO 14155; Clinical Investigations of Medical Devices for Human Subjects- Good Practice. International Organization for Standardization: Geneva, Switzerland, 1996.
- ISO. Biological Evaluation of Medical Devices—Part 2: Animal Welfare Requirements neIG-v; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Nagatomo, F.; Fujino, H.; Kondo, H.; Ishihara, A. Oxygen concentration-dependent oxidative stress levels in rats. Oxidative Med. Cell. Longev. 2012, 2012, 381763. [Google Scholar] [CrossRef]
- Didion, J.P.; de Villena, F.P. Deconstructing Mus gemischus: Advances in understanding ancestry, structure, and variation in the genome of the laboratory mouse. Mamm. Genome 2013, 24, 1–20. [Google Scholar] [CrossRef]
- Hutt, S.J.; Hutt, C. Direct Observation and Measurement of Behavior; Springfield: Geneseo, IL, USA, 1970; p. 224. [Google Scholar]
- Greenway, K.S.R.; Vargas Carvajal, D.; Nair, R.; Lunt, J.; Tan, T.; Smith, A.J.; Johnson, M.; Ross, K.A. Hounsfield Unit. Reference Article. Available online: https://radiopaedia.org/ (accessed on 14 April 2024).
- Schultze-Mosgau, S.; Keilholz, L.; Rödel, F.; Labahn, D.; Neukam, F.W. Experimental model for transplantation of a modified free myocutaneous gracilis flap to an irradiated neck region in rats. Int. J. Oral Maxillofac. Surg. 2001, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.N.; Saveleva, M.S.; Kozadaev, M.N.; Matveeva, O.V.; Sal’kovskiy, Y.E.; Lyubun, G.P.; Gorin, D.A.; Norkin, I.A. New Approaches to Scaffold Biocompatibility Assessment. BioNanoScience 2019, 9, 395–405. [Google Scholar] [CrossRef]
- Abou ElReash, A.; Hamama, H.; Abdo, W.; Wu, Q.; Zaen El-Din, A.; Xiaoli, X. Biocompatibility of new bioactive resin composite versus calcium silicate cements: An animal study. BMC Oral Health 2019, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.; Cota, J.; Arras, M.; Dobbins, T.; Geller, E.; Ilgen, J.; O’Neill, R.; Smith, J.; Williams, S.; Yates, J. An evaluation of the effect of post-surgical analgesia on weight gain and food and water consumption in laboratory rats. Lab. Anim. 2005, 34, 1554. [Google Scholar]
- Frazão, L.P.; Vieira de Castro, J.; Neves, N.M. In Vivo Evaluation of the Biocompatibility of Biomaterial Device. Adv. Exp. Med. Biol. 2020, 1250, 109–124. [Google Scholar] [PubMed]
- Biddeci, G.; Spinelli, G.; Colomba, P.; Di Blasi, F. Halloysite Nanotubes and Sepiolite for Health Applications. Int. J. Mol. Sci. 2023, 24, 4801. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Hern, H.G. Wound management principles. In Rosen’s Emergency Medicine, 8th ed.; Marx, J., Hockbergr, R., Walls, R., Eds.; Saunders: Philadelphia, PA, USA, 2014; pp. 751–766. [Google Scholar]
- Kyriakides, T.R.; Kim, H.J.; Zheng, C.; Harkins, L.; Tao, W.; Deschenes, E. Foreign body response to synthetic polymer biomaterials and the role of adaptive immunity. Biomed. Mater. 2022, 17, 022007. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Bercker, S.; Bert, B.; Bittigau, P.; Felderhoff-Müser, U.; Bührer, C.; Ikonomidou, C.; Weise, M.; Kaisers, U.X.; Kerner, T. Neurodegeneration in Newborn Rats Following Propofol and Sevoflurane Anesthesia. Neurotox. Res. 2009, 16, 140–147. [Google Scholar] [CrossRef]
- Xu, D.; Liu, J.; Meng, S.; Sun, M.; Chen, Y.; Hong, Y. Isoflurane-induced neuroinflammation and NKCC1/KCC2 dysregulation result in long-term cognitive disorder in neonatal mice. BMC Anesthesiol. 2024, 24, 200. [Google Scholar] [CrossRef]
- Grzeskowiak, R.M.; Schumacher, J.; Dhar, M.S.; Harper, D.P.; Mulon, P.Y.; Anderson, D.E. Bone and Cartilage Interferences with Orthopedic Implans: A Literature Review. Front. Surg. 2020, 7, 601244. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release?A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Donath, K. The diagnostic value of the new method for the study of undecalcified bones and teeth with attached soft tissue, (Säge-Schliff, (Sawing and Grinding) Technique). Pathol. Res. Pract. 1985, 179, 631–633. [Google Scholar] [CrossRef]
- Schmalz, G.; Arenholt-Bindslev, D. Determination of Biocompatibility. In Biocompatibility of Dental Materials, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 13–43. [Google Scholar]
- Girão, L.B.; de Lima Martins, J.O.; Mota Lemos, J.V.; Pinto, M.R.; Marques Lima Rolim, J.P.; Fernandes Alves e Silva, F.C.; de Paulo Aragão Saboia, V.; Bitu Sousa, F.; de Barros Silva, P.G. Influence of the degree of conversion and Bis-GMA residues of bulk fill resins on tissue toxicity in an subcutaneous model in rats. J. Appl. Biomater. Funct. Mater. 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Khatri, C.A.; Stansbury, J.W.; Schultheisz, C.R.; Antonucci, J.M. Synthesis, characteriza-tion and evaluation of urethane derivatives of Bis-GMA. Dent. Mater. 2003, 19, 584–588. [Google Scholar] [CrossRef]
- de Carvalho, D.K.; de Sousa, D.L.; Barbugli, P.A.; Cerri, P.S.; Salih, V.M.; Vergani, C.E. Development and characterization of a 3D oral mucosa model as a tool for host-pathogen interactions. J. Microbiol. Methods 2018, 152, 52–60. [Google Scholar] [CrossRef]
- Klausner, M.; Handa, Y.; Aizawa, S. In vitro threedimensional organotypic culture models of the oral mucosa. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 148–159. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Threedimensional in vitro oral mucosa models of fungal and bacterial infections. Tissue Eng. Part B Rev. 2020, 26, 443–460. [Google Scholar] [CrossRef]


| Materials | Manufacturer | Matrix Monomers | Total Content Filler |
|---|---|---|---|
| Light-curing hybrid cement composite (C1) | UBB-ICCRR, Cluj-Napoca, Romania | Bis-GMA 1; UDMA; HEMA TEGDMA 3; | 65 weight%,—HA 2 (particle size 0.01–0.06 μm and 5–8 nm); silica, barium glass (BaO) (particle size 0.01–0.035 μm and 2–6 nm); quartz |
| Light-curing hybrid cement composite (C2) | UBB-ICCRR, Cluj-Napoca, Romania | Bis-GMA 1; UDMA; TEGDMA 3; | 65 weight%, HA 2 (particle size 0.01–60 μm and 5–8 nm); silica, glass filler (with BaF2) (particle size 2–6 nm); fluoroaluminosilicate glass (0.04–0.50 μm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardelean, A.I.; Marza, S.M.; Negoescu, A.; Dragomir, M.F.; Sarosi, C.; Moldovan, M.; Ene, R.; Oana, L. Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats. Biomedicines 2024, 12, 1718. https://doi.org/10.3390/biomedicines12081718
Ardelean AI, Marza SM, Negoescu A, Dragomir MF, Sarosi C, Moldovan M, Ene R, Oana L. Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats. Biomedicines. 2024; 12(8):1718. https://doi.org/10.3390/biomedicines12081718
Chicago/Turabian StyleArdelean, Alina Ioana, Sorin Marian Marza, Andrada Negoescu, Madalina Florina Dragomir, Codruta Sarosi, Marioara Moldovan, Razvan Ene, and Liviu Oana. 2024. "Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats" Biomedicines 12, no. 8: 1718. https://doi.org/10.3390/biomedicines12081718
APA StyleArdelean, A. I., Marza, S. M., Negoescu, A., Dragomir, M. F., Sarosi, C., Moldovan, M., Ene, R., & Oana, L. (2024). Assessing Biocompatibility of Composite Cements by Peri/Intramuscular and Subcutaneous Implantation in Rats. Biomedicines, 12(8), 1718. https://doi.org/10.3390/biomedicines12081718







