The Multifaceted Nature of Macrophages in Cardiovascular Disease
Abstract
1. Introduction
2. Regulation of Macrophage Diversity and Function in Healthy and Disease States
3. Macrophages in Cardiovascular Disease
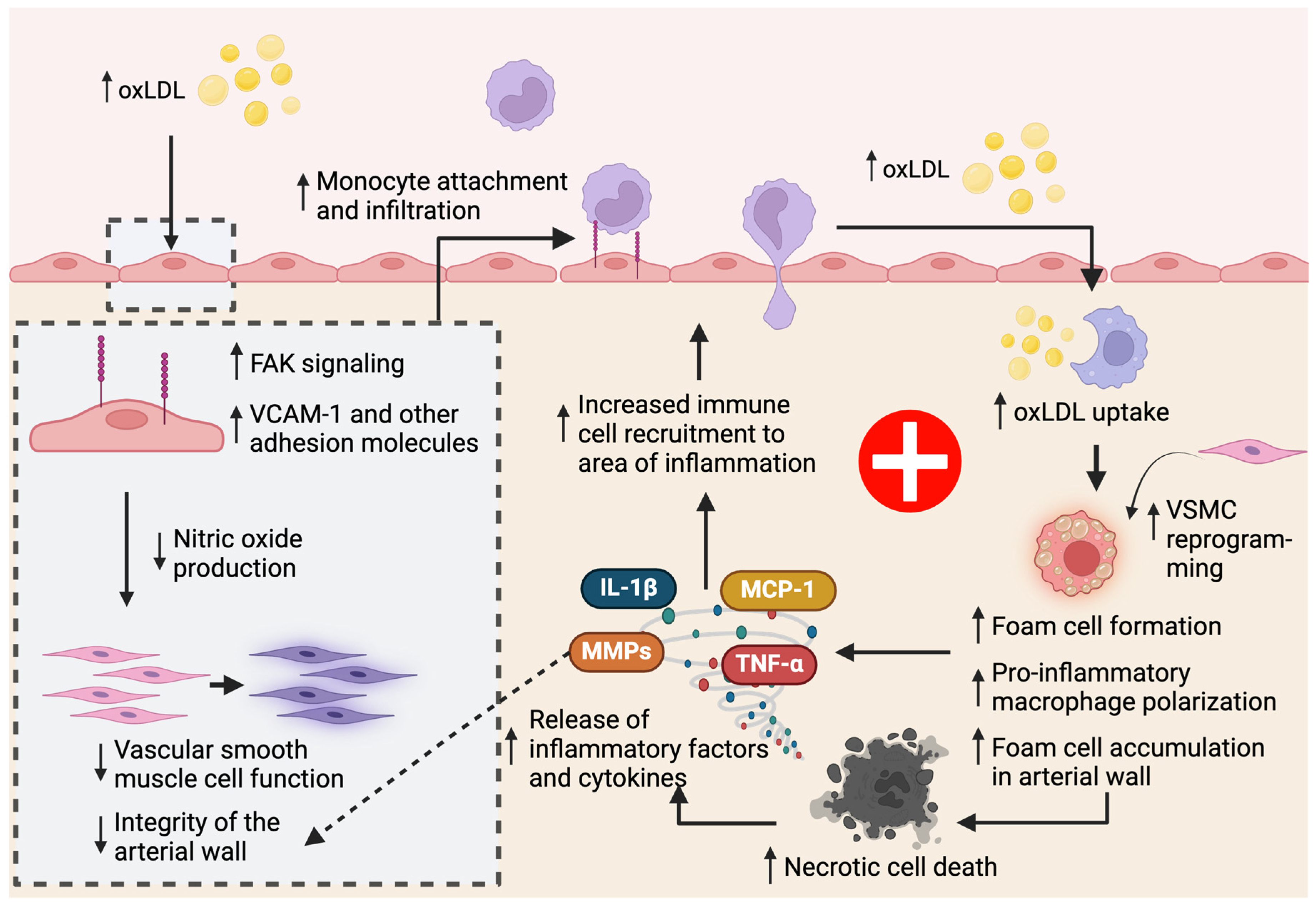
3.1. Macrophages in Atherosclerosis
3.1.1. Increased Macrophage Accumulation in the Plaque Increases Atherosclerosis
3.1.2. Dysfunctional Macrophage Lipoprotein Metabolism Contributes to Atherosclerosis
3.1.3. Macrophages Promote Inflammatory Cell Death Pathways in Atherosclerosis
3.1.4. Efferocytotic Macrophages Are Atheroprotective
3.1.5. Ion Channels Modulate Macrophage Function in Atherosclerosis
3.1.6. Heterogeneity of Macrophages in the Atherosclerotic Plaque
3.1.7. Understanding Macrophages as Potential Targets for Atherosclerosis
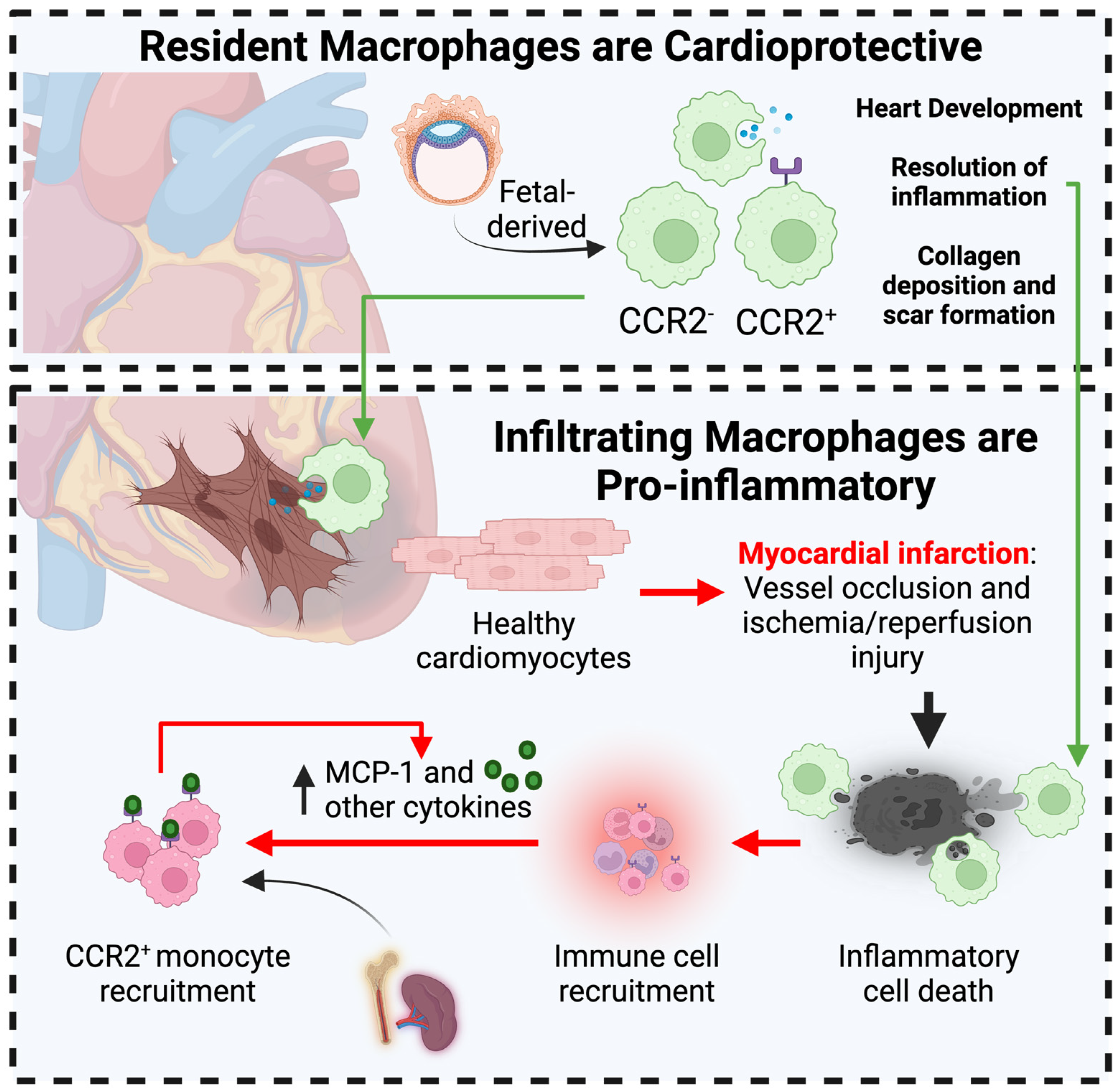
3.2. Macrophages in Myocardial Infarction
3.2.1. Cardioprotective Role of Resident Cardiac Macrophages in MI
3.2.2. Macrophages Contribute to Acute MI-associated Inflammation
3.2.3. Macrophages in MI-Associated Scar Tissue Formation
3.3. Macrophages in Stroke
3.3.1. Infiltrated Macrophages Are Present in the Post-Stroke Brain
3.3.2. Local Microglial and Macrophage Populations Undergo Reprogramming in Post-Stroke Cerebral Tissue
3.4. Macrophages in Other Cardiovascular Diseases
3.4.1. Cardiac Arrythmias
3.4.2. Hypertension
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Fuster, V.; Roth, G.A. A Heart-Healthy and Stroke-Free World: Using Data to Inform Global Action. J. Am. Coll. Cardiol. 2023, 82, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, I. Immune system and cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.J.; Allen, J.E. The expanding world of tissue-resident macrophages. Eur. J. Immunol. 2021, 51, 1882–1896. [Google Scholar] [CrossRef] [PubMed]
- Leid, J.; Carrelha, J.; Boukarabila, H.; Epelman, S.; Jacobsen, S.E.; Lavine, K.J. Primitive embryonic macrophages are required for coronary development and maturation. Circ. Res. 2016, 118, 1498–1511. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 2011, 51, 267–288. [Google Scholar] [CrossRef]
- Lantz, C.; Radmanesh, B.; Liu, E.; Thorp, E.B.; Lin, J. Single-cell RNA sequencing uncovers heterogenous transcriptional signatures in macrophages during efferocytosis. Sci. Rep. 2020, 10, 14333. [Google Scholar] [CrossRef]
- Coulis, G.; Jaime, D.; Guerrero-Juarez, C.; Kastenschmidt, J.M.; Farahat, P.K.; Nguyen, Q.; Pervolarakis, N.; McLinden, K.; Thurlow, L.; Movahedi, S.; et al. Single-cell and spatial transcriptomics identify a macrophage population associated with skeletal muscle fibrosis. Sci. Adv. 2023, 9, eadd9984. [Google Scholar] [CrossRef]
- Garrido-Trigo, A.; Corraliza, A.M.; Veny, M.; Dotti, I.; Melón-Ardanaz, E.; Rill, A.; Crowell, H.L.; Corbí, Á.; Gudiño, V.; Esteller, M.; et al. Macrophage and neutrophil heterogeneity at single-cell spatial resolution in human inflammatory bowel disease. Nat. Commun. 2023, 14, 4506. [Google Scholar] [CrossRef]
- Chen, A.X.; Gartrell, R.D.; Zhao, J.; Upadhyayula, P.S.; Zhao, W.; Yuan, J.; Minns, H.E.; Dovas, A.; Bruce, J.N.; Lasorella, A.; et al. Single-cell characterization of macrophages in glioblastoma reveals MARCO as a mesenchymal pro-tumor marker. Genome Med. 2021, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.-Y.; Black, A.; Qian, B.-Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022, 43, 546–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, J.; Chen, R. An M0 macrophage-related prognostic model for hepatocellular carcinoma. BMC Cancer 2022, 22, 791. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Nathan, C.F.; Murray, H.W.; Wiebe, M.E.; Rubin, B.Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983, 158, 670–689. [Google Scholar] [CrossRef]
- Liao, X.; Sharma, N.; Kapadia, F.; Zhou, G.; Lu, Y.; Hong, H.; Paruchuri, K.; Mahabeleshwar, G.H.; Dalmas, E.; Venteclef, N.; et al. Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Investig 2011, 121, 2736–2749. [Google Scholar] [CrossRef]
- Park, C.; Li, S.; Cha, E.; Schindler, C. Immune Response in Stat2 Knockout Mice. Immunity 2000, 13, 795–804. [Google Scholar] [CrossRef]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Mantovani, A.; Sica, A. Chapter Six—Macrophage Activation and Polarization as an Adaptive Component of Innate Immunity. In Advances in Immunology; Murphy, K.M., Merad, M., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 120, pp. 163–184. [Google Scholar]
- Gong, M.; Zhuo, X.; Ma, A. STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis. Med. Sci. Monit. Basic Res. 2017, 23, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional control of macrophage polarization. Arter. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado, J.d.D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-de-Arellano, M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Corrigendum: Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS−) vs. Alternatively Activated Macrophages. Front. Immunol. 2020, 11, 529799. [Google Scholar] [CrossRef] [PubMed]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef]
- Oshi, M.; Tokumaru, Y.; Asaoka, M.; Yan, L.; Satyananda, V.; Matsuyama, R.; Matsuhashi, N.; Futamura, M.; Ishikawa, T.; Yoshida, K.; et al. M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 2020, 10, 16554. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.H.M.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage Polarisation: An Immunohistochemical Approach for Identifying M1 and M2 Macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Jialal, I. Atherosclerosis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507799/ (accessed on 19 April 2024).
- Schuh, J.; Fairclough, G.F., Jr.; Haschemeyer, R.H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc. Natl. Acad. Sci. USA 1978, 75, 3173–3177. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, T.; Kume, N.; Aoyama, T.; Moriwaki, H.; Hoshikawa, H.; Aiba, Y.; Tanaka, T.; Miwa, S.; Katsura, Y.; Kita, T. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997, 386, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Ho, Y.; Basu, S.K.; Brown, M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, U.P.; Fisher, M.; Witztum, J.L.; Curtiss, L.K. Immunogenicity of homologous low density lipoprotein after methylation, ethylation, acetylation, or carbamylation: Generation of antibodies specific for derivatized lysine. J. Lipid Res. 1984, 25, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes/Metab. Res. Rev. 2001, 17, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Vergnani, L.; Hatrik, S.; Ricci, F.; Passaro, A.; Manzoli, N.; Zuliani, G.; Brovkovych, V.; Fellin, R.; Malinski, T. Effect of Native and Oxidized Low-Density Lipoprotein on Endothelial Nitric Oxide and Superoxide Production. Circulation 2000, 101, 1261–1266. [Google Scholar] [CrossRef]
- Swirski, F.K.; Libby, P.; Aikawa, E.; Alcaide, P.; Luscinskas, F.W.; Weissleder, R.; Pittet, M.J. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Investig. 2007, 117, 195–205. [Google Scholar] [CrossRef]
- Tacke, F.; Alvarez, D.; Kaplan, T.J.; Jakubzick, C.; Spanbroek, R.; Llodra, J.; Garin, A.; Liu, J.; Mack, M.; van Rooijen, N.; et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 2007, 117, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Pittet, M.J.; Kircher, M.F.; Aikawa, E.; Jaffer, F.A.; Libby, P.; Weissleder, R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl. Acad. Sci. USA 2006, 103, 10340–10345. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.S.; Chudnovskiy, A.; Rauch, P.J.; Figueiredo, J.-L.; Iwamoto, Y.; Gorbatov, R.; Etzrodt, M.; Weber, G.F.; Ueno, T.; Rooijen, N.v.; et al. Extramedullary Hematopoiesis Generates Ly-6Chigh Monocytes That Infiltrate Atherosclerotic Lesions. Circulation 2012, 125, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Yurdagul, A., Jr.; Sulzmaier, F.J.; Chen, X.L.; Pattillo, C.B.; Schlaepfer, D.D.; Orr, A.W. Oxidized LDL induces FAK-dependent RSK signaling to drive NF-κB activation and VCAM-1 expression. J. Cell Sci. 2016, 129, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Potteaux, S.; Gautier, E.L.; Hutchison, S.B.; van Rooijen, N.; Rader, D.J.; Thomas, M.J.; Sorci-Thomas, M.G.; Randolph, G.J. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J. Clin. Investig. 2011, 121, 2025–2036. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Weissleder, R.; Pittet, M.J. Heterogeneous In Vivo Behavior of Monocyte Subsets in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.S.; Hilgendorf, I.; Weber, G.F.; Theurl, I.; Iwamoto, Y.; Figueiredo, J.-L.; Gorbatov, R.; Sukhova, G.K.; Gerhardt, L.M.S.; Smyth, D.; et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013, 19, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Marchini, J.F.; Manica, A.; Crestani, P.; Dutzmann, J.; Folco, E.J.; Weber, H.; Libby, P.; Croce, K. Oxidized Low-Density Lipoprotein Induces Macrophage Production of Prothrombotic Microparticles. J. Am. Heart Assoc. 2020, 9, e015878. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Vengrenyuk, Y.; Ramsey, S.A.; Vila, N.R.; Girgis, N.M.; Liu, J.; Gusarova, V.; Gromada, J.; Weinstock, A.; Moore, K.J.; et al. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J. Clin. Investig. 2017, 127, 2904–2915. [Google Scholar] [CrossRef]
- Newby, A.C. Metalloproteinases and Vulnerable Atherosclerotic Plaques. Trends Cardiovasc. Med. 2007, 17, 253–258. [Google Scholar] [CrossRef]
- Newby, A.C. Metalloproteinase Expression in Monocytes and Macrophages and its Relationship to Atherosclerotic Plaque Instability. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Wu, F.; Li, D.; Yang, L.; Li, Q.; Li, R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol. Cell. Endocrinol. 2018, 461, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Wang, J.; Yang, L.; An, Y.; Zhu, H. AMPK activation enhances the anti-atherogenic effects of high density lipoproteins in apoE−/−mice. J. Lipid Res. 2017, 58, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Feng, K.; Zhang, C.; Zhang, H.; Zhang, J.; Hua, Y.; Dong, Z.; Zhu, Y.; Yang, S.; Ma, C. Metformin attenuates atherosclerosis and plaque vulnerability by upregulating KLF2-mediated autophagy in apoE−/−mice. Biochem. Biophys. Res. Commun. 2021, 557, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, A.; Cave, L.; Hyde, G.; Moestrup, S.K.; Carling, D.; Mason, J.C.; Haskard, D.O.; Boyle, J.J. Metformin directly suppresses atherosclerosis in normoglycaemic mice via haematopoietic adenosine monophosphate-activated protein kinase. Cardiovasc. Res. 2020, 117, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yuan, H.; Chen, M.; Qu, J.; Wang, H.; Yu, B.; Chen, J.; Sun, S.; Tang, X.; Ren, W. Metformin ameliorates the progression of atherosclerosis via suppressing macrophage infiltration and inflammatory responses in rabbits. Life Sci. 2018, 198, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.T.; Firulyova, M.M.; Xu, Y.; Hillman, H.; Bishop, C.; Zhu, A.; Hickok, G.H.; Schrank, P.R.; Ronayne, C.E.; Caillot, Z.; et al. Trem2 promotes foamy macrophage lipid uptake and survival in atherosclerosis. Nat. Cardiovasc. Res. 2023, 2, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, D.; Lei, Q.; Lu, A.; He, X. Roles of Resolvins in Chronic Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 14883. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.E.; De Matteis, R.; Perretti, M.; Dalli, J. Resolvin T4 enhances macrophage cholesterol efflux to reduce vascular disease. Nat. Commun. 2024, 15, 975. [Google Scholar] [CrossRef]
- Xiao, Q.; Hou, R.; Xie, L.; Niu, M.; Pan, X.; Zhu, X. Macrophage metabolic reprogramming and atherosclerotic plaque microenvironment: Fostering each other? Clin. Transl. Med. 2023, 13, e1257. [Google Scholar] [CrossRef]
- You, Y.; Bao, W.-L.; Zhang, S.-L.; Li, H.-D.; Li, H.; Dang, W.-Z.; Zou, S.-L.; Cao, X.-Y.; Wang, X.; Liu, L.-X.; et al. Sorting Nexin 10 Mediates Metabolic Reprogramming of Macrophages in Atherosclerosis Through the Lyn-Dependent TFEB Signaling Pathway. Circ. Res. 2020, 127, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Shimai, R.; Hanafusa, K.; Nakayama, H.; Oshima, E.; Kato, M.; Kano, K.; Matsuo, I.; Miyazaki, T.; Tokano, T.; Hirabayashi, Y.; et al. Lysophosphatidylglucoside/GPR55 signaling promotes foam cell formation in human M2c macrophages. Sci. Rep. 2023, 13, 12740. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qu, L.; Matz, A.J.; Murphy, P.A.; Liu, Y.; Manichaikul, A.W.; Aguiar, D.; Rich, S.S.; Herrington, D.M.; Vu, D.; et al. AtheroSpectrum Reveals Novel Macrophage Foam Cell Gene Signatures Associated with Atherosclerotic Cardiovascular Disease Risk. Circulation 2022, 145, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, D.; Geoffrion, M.; Wei, L.; Gan, W.; Richards, L.; Shangari, P.; DeKemp, E.M.; Beanlands, R.A.; Perisic, L.; Maegdefessel, L.; et al. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci. Adv. 2016, 2, e1600224. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lan, B.; Zheng, T.; Yang, L.; Zhang, X.; Cheng, L.; Tuerhongjiang, G.; Yuan, Z.; Wu, Y. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat. Commun. 2023, 14, 929. [Google Scholar] [CrossRef] [PubMed]
- Seimon, T.A.; Nadolski, M.J.; Liao, X.; Magallon, J.; Nguyen, M.; Feric, N.T.; Koschinsky, M.L.; Harkewicz, R.; Witztum, J.L.; Tsimikas, S.; et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010, 12, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Schroder, K.; Pelegrin, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Abbate, A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat. Rev. Cardiol. 2023, 21. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Kobiyama, K.; Winkels, H.; Ghosheh, Y.; McArdle, S.; Mikulski, Z.; Kiosses, W.B.; Fan, Z.; Wen, L.; Jung, Y.; et al. Olfactory receptor 2 in vascular macrophages drives atherosclerosis by NLRP3-dependent IL-1 production. Science 2022, 375, 214–221. [Google Scholar] [CrossRef]
- Yuan, X.M.; Li, W.; Olsson, A.G.; Brunk, U.T. The toxicity to macrophages of oxidized low-density lipoprotein is mediated through lysosomal damage. Atherosclerosis 1997, 133, 153–161. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Du, Y.; Zhang, X.; Xiang, P.; Chen, G.; Ling, W.; Wang, D. Protocatechuic acid boosts continual efferocytosis in macrophages by derepressing KLF4 to transcriptionally activate MerTK. Sci. Signal. 2023, 16, eabn1372. [Google Scholar] [CrossRef] [PubMed]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schoneberg, T.; Schaefer, M.; Krugel, U.; et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef] [PubMed]
- Jager, E.; Murthy, S.; Schmidt, C.; Hahn, M.; Strobel, S.; Peters, A.; Staubert, C.; Sungur, P.; Venus, T.; Geisler, M.; et al. Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis. Nat. Commun. 2020, 11, 4243. [Google Scholar] [CrossRef]
- House, M.G.; Kohlmeier, L.; Chattopadhyay, N.; Kifor, O.; Yamaguchi, T.; Leboff, M.S.; Glowacki, J.; Brown, E.M. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J. Bone Min. Res. 1997, 12, 1959–1970. [Google Scholar] [CrossRef]
- Murakami, T.; Ockinger, J.; Yu, J.; Byles, V.; McColl, A.; Hofer, A.M.; Horng, T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA 2012, 109, 11282–11287. [Google Scholar] [CrossRef]
- Selezneva, A.; Gibb, A.J.; Willis, D. The contribution of ion channels to shaping macrophage behaviour. Front. Pharmacol. 2022, 13, 970234. [Google Scholar] [CrossRef]
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zheng, Z.; Pan, W.; Zhang, J.; Yin, Z.; Wei, C.; Xu, Y.; Wan, J.; Wang, M. TRPA1 deficiency aggravates dilated cardiomyopathy by promoting S100A8 expression to induce M1 macrophage polarization in rats. FASEB J. 2023, 37, e22982. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, K.; Zhang, F.; Peng, K.; Wang, Z.; Yang, D.; Yang, Y. TRPA1 regulates macrophages phenotype plasticity and atherosclerosis progression. Atherosclerosis 2020, 301, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xu, X.; Sun, Z.; Yang, Y.X.; Guo, H.; Li, J.; Sun, K.; Wu, R.; Xu, J.; Jiang, Q.; et al. TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca2+/CaMKII/Nrf2 signaling pathway. Cell Death Dis. 2021, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Scheraga, R.G.; Abraham, S.; Grove, L.M.; Southern, B.D.; Crish, J.F.; Perelas, A.; McDonald, C.; Asosingh, K.; Hasday, J.D.; Olman, M.A. TRPV4 Protects the Lung from Bacterial Pneumonia via MAPK Molecular Pathway Switching. J. Immunol. 2020, 204, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Scheraga, R.G.; Abraham, S.; Niese, K.A.; Southern, B.D.; Grove, L.M.; Hite, R.D.; McDonald, C.; Hamilton, T.A.; Olman, M.A. TRPV4 Mechanosensitive Ion Channel Regulates Lipopolysaccharide-Stimulated Macrophage Phagocytosis. J. Immunol. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Goswami, R.; Rahaman, S.O. TRPV4 Plays a Role in Matrix Stiffness-Induced Macrophage Polarization. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Sun, Y.; Sukumaran, P.; Quenum Zangbede, F.O.; Jondle, C.N.; Sharma, A.; Evans, D.L.; Chauhan, P.; Szlabick, R.E.; Aaland, M.O.; et al. M1 Macrophage Polarization Is Dependent on TRPC1-Mediated Calcium Entry. iScience 2018, 8, 85–102. [Google Scholar] [CrossRef]
- Solanki, S.; Dube, P.R.; Birnbaumer, L.; Vazquez, G. Reduced Necrosis and Content of Apoptotic M1 Macrophages in Advanced Atherosclerotic Plaques of Mice With Macrophage-Specific Loss of Trpc3. Sci. Rep. 2017, 7, 42526. [Google Scholar] [CrossRef]
- Beceiro, S.; Radin, J.N.; Chatuvedi, R.; Piazuelo, M.B.; Horvarth, D.J.; Cortado, H.; Gu, Y.; Dixon, B.; Gu, C.; Lange, I.; et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017, 10, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T. TRPM2 channel-STAT3 complex regulates the polarity of tumor-associated macrophage and tumor angiogenesis. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, S.; Lu, Y.; Wei, Y.; Tang, J.; Yang, Y.; Li, F.; Chen, Q.; Zheng, J.; Liu, X. Quercetin confers protection of murine sepsis by inducing macrophage M2 polarization via the TRPM2 dependent calcium influx and AMPK/ATF3 activation. J. Funct. Foods 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Li, L.; Wei, C.; Cai, S.; Fang, L. TRPM7 modulates macrophage polarization by STAT1/STAT6 pathways in RAW264.7 cells. Biochem. Biophys. Res. Commun. 2020, 533, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Miralles, F.; Eder, C. TRPM7 regulates proliferation and polarisation of macrophages. J. Cell Sci. 2014, 127, 4561–4566. [Google Scholar] [CrossRef]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Shyue, S.K.; Kou, Y.R.; Lu, T.M.; Lee, T.S. Transient Receptor Potential Ankyrin 1 Channel Involved in Atherosclerosis and Macrophage-Foam Cell Formation. Int. J. Biol. Sci. 2016, 12, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, B.; Yin, M.; Koroleva, M.; Mastrangelo, M.; Ture, S.; Morrell, C.N.; Zhang, D.X.; Fisher, E.A.; Jin, Z.G. A novel TRPV4-specific agonist inhibits monocyte adhesion and atherosclerosis. Oncotarget 2016, 7, 37622–37635. [Google Scholar] [CrossRef] [PubMed]
- Tano, J.Y.; Solanki, S.; Lee, R.H.; Smedlund, K.; Birnbaumer, L.; Vazquez, G. Bone marrow deficiency of TRPC3 channel reduces early lesion burden and necrotic core of advanced plaques in a mouse model of atherosclerosis. Cardiovasc. Res. 2014, 101, 138–144. [Google Scholar] [CrossRef]
- Smedlund, K.B.; Birnbaumer, L.; Vazquez, G. Increased size and cellularity of advanced atherosclerotic lesions in mice with endothelial overexpression of the human TRPC3 channel. Proc. Natl. Acad. Sci. USA 2015, 112, E2201–E2206. [Google Scholar] [CrossRef]
- Feng, M.; Xu, D.; Wang, L. miR-26a inhibits atherosclerosis progression by targeting TRPC3. Cell Biosci. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, Y.; Zhao, Q.; Ru, X. A CA2+-permeable channel TRPM2 promotes atherosclerotic progression in mouse model of atherosclerosis. Atherosclerosis 2023, 379, S28. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhai, Y.; Liang, S.; Mori, Y.; Han, R.; Sutterwala, F.S.; Qiao, L. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat. Commun. 2013, 4, 1611. [Google Scholar] [CrossRef] [PubMed]
- Zong, P.; Feng, J.; Yue, Z.; Yu, A.S.; Vacher, J.; Jellison, E.R.; Miller, B.; Mori, Y.; Yue, L. TRPM2 deficiency in mice protects against atherosclerosis by inhibiting TRPM2–CD36 inflammatory axis in macrophages. Nat. Cardiovasc. Res. 2022, 1, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.; Mieremet, A.; de Vries, C.J.M.; Micha, D.; de Waard, V. Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Kruppel-Like Factor 4). Arter. Thromb. Vasc. Biol. 2021, 41, 2693–2707. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.X.; Shapiro, M.; Trogan, E.; Fisher, E.A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl. Acad. Sci. USA 2003, 100, 13531–13536. [Google Scholar] [CrossRef] [PubMed]
- Decano, J.L.; Maiorino, E.; Matamalas, J.T.; Chelvanambi, S.; Tiemeijer, B.M.; Yanagihara, Y.; Mukai, S.; Jha, P.K.; Pestana, D.V.S.; D’Souza, E.; et al. Cellular Heterogeneity of Activated Primary Human Macrophages and Associated Drug-Gene Networks: From Biology to Precision Therapeutics. Circulation 2023, 148, 1459–1478. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Campanario, M.C.; Cortes, M.; Moreno-Lanceta, A.; Han, L.; Ninfali, C.; Dominguez, V.; Andres-Manzano, M.J.; Farras, M.; Esteve-Codina, A.; Enrich, C.; et al. Atherosclerotic plaque development in mice is enhanced by myeloid ZEB1 downregulation. Nat. Commun. 2023, 14, 8316. [Google Scholar] [CrossRef] [PubMed]
- Gerber, Y.; Weston, S.A.; Berardi, C.; McNallan, S.M.; Jiang, R.; Redfield, M.M.; Roger, V.L. Contemporary Trends in Heart Failure With Reduced and Preserved Ejection Fraction After Myocardial Infarction: A Community Study. Am. J. Epidemiol. 2013, 178, 1272–1280. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; Kaul, P.; Bakal, J.A.; Armstrong, P.W.; Welsh, R.C.; McAlister, F.A. Declining In-Hospital Mortality and Increasing Heart Failure Incidence in Elderly Patients With First Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 53, 13–20. [Google Scholar] [CrossRef]
- Chen, J.; Hsieh, A.F.-C.; Dharmarajan, K.; Masoudi, F.A.; Krumholz, H.M. National Trends in Heart Failure Hospitalization After Acute Myocardial Infarction for Medicare Beneficiaries. Circulation 2013, 128, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Velagaleti, R.S.; Pencina, M.J.; Murabito, J.M.; Wang, T.J.; Parikh, N.I.; D’Agostino, R.B.; Levy, D.; Kannel, W.B.; Vasan, R.S. Long-Term Trends in the Incidence of Heart Failure After Myocardial Infarction. Circulation 2008, 118, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Bagai, A.; Dangas, G.D.; Stone, G.W.; Granger, C.B. Reperfusion Strategies in Acute Coronary Syndromes. Circ. Res. 2014, 114, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Libby, P. Acute Coronary Syndromes. Circulation 2017, 136, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Fedak, P.W.M.; Weisel, R.D.; Butany, J.; Rao, V.; Maitland, A.; Li, R.-K.; Dhillon, B.; Yau, T.M. Fundamentals of Reperfusion Injury for the Clinical Cardiologist. Circulation 2002, 105, 2332–2336. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nojima, T.; Fujisaki, N.; Tsukahara, K.; Yamamoto, H.; Yamada, T.; Aokage, T.; Yumoto, T.; Osako, T.; Nakao, A. Therapeutic strategies for ischemia reperfusion injury in emergency medicine. Acute Med. Surg. 2020, 7, e501. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef] [PubMed]
- Gourdin, M.J.; Bree, B.; De Kock, M. The impact of ischaemia–reperfusion on the blood vessel. Eur. J. Anaesthesiol.|EJA 2009, 26, 537–547. [Google Scholar] [CrossRef]
- Misra, M.K.; Sarwat, M.; Bhakuni, P.; Tuteja, R.; Tuteja, N. Oxidative stress and ischemic myocardial syndromes. Med. Sci. Monit. 2009, 15, 209–219. [Google Scholar]
- De Vries, D.K.; Kortekaas, K.A.; Tsikas, D.; Wijermars, L.G.; van Noorden, C.J.; Suchy, M.T.; Cobbaert, C.M.; Klautz, R.J.; Schaapherder, A.F.; Lindeman, J.H. Oxidative damage in clinical ischemia/reperfusion injury: A reappraisal. Antioxid. Redox Signal 2013, 19, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Visan, I. Myocardial infarct inflammation. Nat. Immunol. 2018, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.-L.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Chakarov, S.; Lim, H.Y.; Tan, L.; Lim, S.Y.; See, P.; Lum, J.; Zhang, X.-M.; Foo, S.; Nakamizo, S.; Duan, K.; et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019, 363, eaau0964. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Paolicelli, R.; Salimova, E.; Gospocic, J.; Slonimsky, E.; Bilbao-Cortes, D.; Godwin, J.W.; Rosenthal, N.A. An Abundant Tissue Macrophage Population in the Adult Murine Heart with a Distinct Alternatively-Activated Macrophage Profile. PLoS ONE 2012, 7, e36814. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.A.; Wong, A.; Hamidzada, H.; Nejat, S.; Nechanitzky, R.; Vohra, S.; Mueller, B.; Zaman, R.; Kantores, C.; Aronoff, L.; et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci. Immunol. 2022, 7, eabf7777. [Google Scholar] [CrossRef]
- Molawi, K.; Wolf, Y.; Kandalla, P.K.; Favret, J.; Hagemeyer, N.; Frenzel, K.; Pinto, A.R.; Klapproth, K.; Henri, S.; Malissen, B.; et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 2014, 211, 2151–2158. [Google Scholar] [CrossRef]
- Lavine, K.J.; Epelman, S.; Uchida, K.; Weber, K.J.; Nichols, C.G.; Schilling, J.D.; Ornitz, D.M.; Randolph, G.J.; Mann, D.L. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl. Acad. Sci. USA 2014, 111, 16029–16034. [Google Scholar] [CrossRef]
- Bajpai, G.; Bredemeyer, A.; Li, W.; Zaitsev, K.; Koenig, A.L.; Lokshina, I.; Mohan, J.; Ivey, B.; Hsiao, H.M.; Weinheimer, C.; et al. Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ. Res. 2019, 124, 263–278. [Google Scholar] [CrossRef]
- Chen, B.; Brickshawana, A.; Frangogiannis, N.G. The Functional Heterogeneity of Resident Cardiac Macrophages in Myocardial Injury(CCR2(+) Cells Promote Inflammation, Whereas CCR2(−) Cells Protect). Circ. Res. 2019, 124, 183–185. [Google Scholar] [CrossRef]
- Zaman, R.; Epelman, S. Resident cardiac macrophages: Heterogeneity and function in health and disease. Immunity 2022, 55, 1549–1563. [Google Scholar] [CrossRef]
- Dewald, O.; Zymek, P.; Winkelmann, K.; Koerting, A.; Ren, G.; Abou-Khamis, T.; Michael, L.H.; Rollins, B.J.; Entman, M.L.; Frangogiannis, N.G. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 2005, 96, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Pluijmert, N.J.; Atsma, D.E.; Quax, P.H. Post-ischemic myocardial inflammatory response: A complex and dynamic process susceptible to immunomodulatory therapies. Front. Cardiovasc. Med. 2021, 8, 647785. [Google Scholar] [CrossRef]
- Bujak, M.; Dobaczewski, M.; Chatila, K.; Mendoza, L.H.; Li, N.; Reddy, A.; Frangogiannis, N.G. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am. J. Pathol. 2008, 173, 57–67. [Google Scholar] [CrossRef]
- Saxena, A.; Chen, W.; Su, Y.; Rai, V.; Uche, O.U.; Li, N.; Frangogiannis, N.G. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J. Immunol. 2013, 191, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Masuyama, J.-I.; Ikeda, U.; Kasahara, T.; Kitagawa, S.-I.; Takahashi, Y.-I.; Shimada, K.; Kano, S. Induction of monocyte chemoattractant protein-1 synthesis in human monocytes during transendothelial migration in vitro. Circ. Res. 1995, 76, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Schunk, S.J.; Triem, S.; Schmit, D.; Zewinger, S.; Sarakpi, T.; Becker, E.; Hütter, G.; Wrublewsky, S.; Küting, F.; Hohl, M. Interleukin-1α is a central regulator of leukocyte-endothelial adhesion in myocardial infarction and in chronic kidney disease. Circulation 2021, 144, 893–908. [Google Scholar] [CrossRef]
- Zhang, Y.; McCluskey, K.; Fujii, K.; Wahl, L.M. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-α, granulocyte-macrophage CSF, and IL-1β through prostaglandin-dependent and-independent mechanisms. J. Immunol. 1998, 161, 3071–3076. [Google Scholar] [CrossRef]
- Sager, H.B.; Heidt, T.; Hulsmans, M.; Dutta, P.; Courties, G.; Sebas, M.; Wojtkiewicz, G.R.; Tricot, B.; Iwamoto, Y.; Sun, Y. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation 2015, 132, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- George, M.J.; Jasmin, N.H.; Cummings, V.T.; Richard-Loendt, A.; Launchbury, F.; Woollard, K.; Turner-Stokes, T.; Garcia Diaz, A.I.; Lythgoe, M.; Stuckey, D.J. Selective interleukin-6 trans-signaling blockade is more effective than panantagonism in reperfused myocardial infarction. Basic Transl. Sci. 2021, 6, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Hamid, T.; Gu, Y.; Ortines, R.V.; Bhattacharya, C.; Wang, G.; Xuan, Y.-T.; Prabhu, S.D. Divergent tumor necrosis factor receptor–related remodeling responses in heart failure: Role of nuclear factor-κB and inflammatory activation. Circulation 2009, 119, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Liao, Y.-H.; Cao, Z.; Du, Y.-M.; Xia, J.-D.; Yang, H.; Chen, Z.-J. Effect of tumor necrosis factor-α on neutralization of ventricular fibrillation in rats with acute myocardial infarction. Mediat. Inflamm. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek-Palacz, A.; Sikora, J.; Kondera-Anasz, Z.; Smycz, M. Changes in concentrations of tumor necrosis factor TNF and its soluble receptors type 1 (sTNF-r1) and type 2 (sTNF-R2) in serum of patients with ST-segment elevation myocardial infarction. Wiad. Lek. 2011, 64, 71–74. [Google Scholar] [PubMed]
- Nilsson, L.; Szymanowski, A.; Swahn, E.; Jonasson, L. Soluble TNF receptors are associated with infarct size and ventricular dysfunction in ST-elevation myocardial infarction. PLoS ONE 2013, 8, e55477. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, L.F.; Kwan, H.H.; Kowalski, J.; Prionas, S.; Allison, A. Dual role of tumor necrosis factor-alpha in angiogenesis. Am. J. Pathol. 1992, 140, 539. [Google Scholar] [PubMed]
- Jin, Y.C.; Kim, C.W.; Kim, Y.M.; Nizamutdinova, I.T.; Ha, Y.M.; Kim, H.J.; Seo, H.G.; Son, K.H.; Jeon, S.J.; Kang, S.S. Cryptotanshinone, a lipophilic compound of Salvia miltiorrriza root, inhibits TNF-α-induced expression of adhesion molecules in HUVEC and attenuates rat myocardial ischemia/reperfusion injury in vivo. Eur. J. Pharmacol. 2009, 614, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.K.; Xu, Y.-J.; Zhang, M.; Liu, P.P.; Kirshenbaum, L.A.; Dhalla, N.S. Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp. Clin. Cardiol. 2005, 10, 213. [Google Scholar]
- Dick, S.A.; Macklin, J.A.; Nejat, S.; Momen, A.; Clemente-Casares, X.; Althagafi, M.G.; Chen, J.; Kantores, C.; Hosseinzadeh, S.; Aronoff, L. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019, 20, 29–39. [Google Scholar] [CrossRef]
- Simões, F.C.; Cahill, T.J.; Kenyon, A.; Gavriouchkina, D.; Vieira, J.M.; Sun, X.; Pezzolla, D.; Ravaud, C.; Masmanian, E.; Weinberger, M.; et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 2020, 11, 600. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Zhou, X.; Shan, W.; Chen, C.; Ge, W.; Cui, J.; Yi, W.; Sun, Y. Crosstalk between macrophages and cardiac cells after myocardial infarction. Cell Commun. Signal. 2023, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Boscá, L.; Zandbergen, H.R.; Kovacic, J.C.; Narula, N.; González-Ramos, S.; Fernandez-Velasco, M.; Agrawal, S.; Paz-García, M.; Gupta, S. Transition of macrophages to fibroblast-like cells in healing myocardial infarction. J. Am. Coll. Cardiol. 2019, 74, 3124–3135. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Gibbs, B.B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Types of Stroke and Treatment. Available online: https://www.stroke.org/en/about-stroke/types-of-stroke (accessed on 16 April 2024).
- Jin, R.; Yang, G.; Li, G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Investigational anti-inflammatory agents for the treatment of ischaemic brain injury. Expert Opin. Investig. Drugs 2005, 14, 393–409. [Google Scholar] [CrossRef] [PubMed]
- McColl, B.; Allan, S.; Rothwell, N. Systemic infection, inflammation and acute ischemic stroke. Neuroscience 2009, 158, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, J. Macrophage polarization: A key event in the secondary phase of acute spinal cord injury. J. Cell. Mol. Med. 2017, 21, 941–954. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef]
- Gliem, M.; Schwaninger, M.; Jander, S. Protective features of peripheral monocytes/macrophages in stroke. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 329–338. [Google Scholar] [CrossRef]
- Rajan, W.D.; Wojtas, B.; Gielniewski, B.; Gieryng, A.; Zawadzka, M.; Kaminska, B. Dissecting functional phenotypes of microglia and macrophages in the rat brain after transient cerebral ischemia. Glia 2019, 67, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Y.; Ye, Q.; Hassan, S.H.; Zhao, J.; Li, S.; Hu, X.; Leak, R.K.; Rocha, M.; Wechsler, L.R.; et al. RNA sequencing reveals novel macrophage transcriptome favoring neurovascular plasticity after ischemic stroke. J. Cereb. Blood Flow Metab. 2020, 40, 720–738. [Google Scholar] [CrossRef] [PubMed]
- Beuker, C.; Schafflick, D.; Strecker, J.-K.; Heming, M.; Li, X.; Wolbert, J.; Schmidt-Pogoda, A.; Thomas, C.; Kuhlmann, T.; Aranda-Pardos, I.; et al. Stroke induces disease-specific myeloid cells in the brain parenchyma and pia. Nat. Commun. 2022, 13, 945. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Wildgruber, M.; Ueno, T.; Figueiredo, J.-L.; Panizzi, P.; Iwamoto, Y.; Zhang, E.; Stone, J.R.; Rodriguez, E.; Chen, J.W.; et al. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J. Clin. Investig. 2010, 120, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.L.; Getts, D.R.; Deffrasnes, C.; van Vreden, C.; Campbell, I.L.; King, N.J.C. Inflammatory monocytes and the pathogenesis of viral encephalitis. J. Neuroinflamm. 2012, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- García-Culebras, A.; Durán-Laforet, V.; Peña-Martínez, C.; Ballesteros, I.; Pradillo, J.M.; Díaz-Guzmán, J.; Lizasoain, I.; Moro, M.A. Myeloid cells as therapeutic targets in neuroinflammation after stroke: Specific roles of neutrophils and neutrophil–platelet interactions. J. Cereb. Blood Flow Metab. 2018, 38, 2150–2164. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Ye, W.; Panga, L.; Wu, D.-C.; Yang, G.-Y. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001, 902, 171–177. [Google Scholar] [CrossRef]
- Chu, H.X.; Arumugam, T.V.; Gelderblom, M.; Magnus, T.; Drummond, G.R.; Sobey, C.G. Role of CCR2 in Inflammatory Conditions of the Central Nervous System. J. Cereb. Blood Flow Metab. 2014, 34, 1425–1429. [Google Scholar] [CrossRef]
- Losy, J.; Zaremba, J. Monocyte Chemoattractant Protein-1 Is Increased in the Cerebrospinal Fluid of Patients with Ischemic Stroke. Stroke 2001, 32, 2695–2696. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Chen, L.; Tang, J.; Chen, Y.; Wang, L. The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials. Int. J. Mol. Sci. 2022, 23, 3485. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.Y.; Kim, Y.R.; Huang, M.; Chang, J.Y.; Sim, A.Y.; Jung, H.; Lee, W.T.; Hyun, Y.-M.; Lee, J.E. Reparative System Arising from CCR2(+) Monocyte Conversion Attenuates Neuroinflammation Following Ischemic Stroke. Transl. Stroke Res. 2021, 12, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Granger, D.N. Cell adhesion molecules and ischemic stroke. Neurol. Res. 2008, 30, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Regal-McDonald, K.; Patel, R.P. Selective Recruitment of Monocyte Subsets by Endothelial N-Glycans. Am. J. Pathol. 2020, 190, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef]
- Gliem, M.; Klotz, L.; Rooijen, N.v.; Hartung, H.-P.; Jander, S. Hyperglycemia and PPARγ Antagonistically Influence Macrophage Polarization and Infarct Healing After Ischemic Stroke. Stroke 2015, 46, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, J.; Zhang, Z.; Li, F.; Zhang, C.; Lai, B.; Xiao, L.; Wang, N. Peroxisome proliferator-activated receptor γ (PPARγ) induces the gene expression of integrin αVβ5 to promote macrophage M2 polarization. J. Biol. Chem. 2018, 293, 16572–16582. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, Q.; Wu, C.; Du, G.; Yang, L. Oleoylethanolamide Protects against Acute Ischemic Stroke by Promoting PPARα-Mediated Microglia/Macrophage M2 Polarization. Pharmaceuticals 2023, 16, 621. [Google Scholar] [CrossRef]
- Pan, J.; Jin, J.-l.; Ge, H.-m.; Yin, K.-l.; Chen, X.; Han, L.-j.; Chen, Y.; Qian, L.; Li, X.-x.; Xu, Y. Malibatol A regulates microglia M1/M2 polarization in experimental stroke in a PPARγ-dependent manner. J. Neuroinflamm. 2015, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cao, D.; Guo, C.; Liu, M.; Tao, Y.; Zhou, J.; Wang, F.; Zhao, Y.; Wei, J.; Zhang, Y.; et al. Berberine Facilitates Angiogenesis Against Ischemic Stroke Through Modulating Microglial Polarization via AMPK Signaling. Cell. Mol. Neurobiol. 2019, 39, 751–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Q.; Chen, S.-P.; Sun, J.; Wang, X.-M.; Chen, N.; Zhou, Y.-Q.; Tian, Y.-K.; Ye, D.-W. Berberine protects against ischemia-reperfusion injury: A review of evidence from animal models and clinical studies. Pharmacol. Res. 2019, 148, 104385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, M.; Zhang, X.; Kuai, Y.; Mei, Y.; Tan, Q.; Zhong, K.; Sun, X.; Tan, W. Isosteviol Sodium Protects against Ischemic Stroke by Modulating Microglia/Macrophage Polarization via Disruption of GAS5/miR-146a-5p sponge. Sci. Rep. 2019, 9, 12221. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Dai, X.; Chen, J.; Zhao, J.; Xu, M.; Zhang, L.; Yang, B.; Zhang, W.; Rocha, M.; Nakao, T. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight 2019, 4, e131355. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Fan, W.-H.; Liu, Q.; Shang, K.; Murugan, M.; Wu, L.-J.; Wang, W.; Tian, D.-S. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke 2017, 48, 3336–3346. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Ran, Y.Y.; Qie, S.Y.; Gong, W.J.; Gao, F.H.; Ding, Z.T.; Xi, J.N. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci. Ther. 2019, 25, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Zhong, P.; Fang, L.; Wu, X.; Song, Y.; Yuan, H. miR-183 inhibits microglia activation and expression of inflammatory factors in rats with cerebral ischemia reperfusion via NF-κB signaling pathway. Exp. Ther. Med. 2019, 18, 2540–2546. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α–PU. 1 pathway. Nat. Med. 2011, 17, 64–70. [Google Scholar] [CrossRef]
- Mo, Y.; Xu, W.; Fu, K.; Chen, H.; Wen, J.; Huang, Q.; Guo, F.; Mo, L.; Yan, J. The dual function of microglial polarization and its treatment targets in ischemic stroke. Front. Neurol. 2022, 13, 921705. [Google Scholar] [CrossRef]
- Jin, Q.; Cheng, J.; Liu, Y.; Wu, J.; Wang, X.; Wei, S.; Zhou, X.; Qin, Z.; Jia, J.; Zhen, X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014, 40, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Grissi, M.; Boudot, C.; Assem, M.; Candellier, A.; Lando, M.; Poirot-Leclercq, S.; Boullier, A.; Bennis, Y.; Lenglet, G.; Avondo, C.; et al. Metformin prevents stroke damage in non-diabetic female mice with chronic kidney disease. Sci. Rep. 2021, 11, 7464. [Google Scholar] [CrossRef] [PubMed]
- Boddaert, J.; Bielen, K.; ’s Jongers, B.; Manocha, E.; Yperzeele, L.; Cras, P.; Pirici, D.; Kumar-Singh, S. CD8 signaling in microglia/macrophage M1 polarization in a rat model of cerebral ischemia. PLoS ONE 2018, 13, e0186937. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liao, X.-Y.; Pan, M.-X.; Tang, J.-C.; Chen, S.-F.; Zhang, Y.; Lu, P.-X.; Lu, L.J.; Zou, Y.-Y.; Qin, X.-P. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-κB p65/Hif-1α signaling pathway. J. Immunol. 2019, 202, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, M.-Y.; Zhang, D.-F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.-F.; Gao, H.; Wei, M.-J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Pena-Philippides, J.C.; Caballero-Garrido, E.; Lordkipanidze, T.; Roitbak, T. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J. Neuroinflamm. 2016, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, S.; Kim, J.-S.; Hagendorff, A.; Thönnissen, E.; Krüger, O.; Lamers, W.H.; Willecke, K. Abnormal cardiac conduction and morphogenesis in connexin40 and connexin43 double-deficient mice. Circ. Res. 2000, 87, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522.e520. [Google Scholar] [CrossRef]
- Sugita, J.; Fujiu, K.; Nakayama, Y.; Matsubara, T.; Matsuda, J.; Oshima, T.; Liu, Y.; Maru, Y.; Hasumi, E.; Kojima, T.; et al. Cardiac macrophages prevent sudden death during heart stress. Nat. Commun. 2021, 12, 1910. [Google Scholar] [CrossRef]
- Fei, Y.-D.; Wang, Q.; Hou, J.-W.; Li, W.; Cai, X.-X.; Yang, Y.-L.; Zhang, L.-H.; Wei, Z.-X.; Chen, T.-Z.; Wang, Y.-P. Macrophages facilitate post myocardial infarction arrhythmias: Roles of gap junction and KCa3. 1. Theranostics 2019, 9, 6396. [Google Scholar] [CrossRef]
- Simon-Chica, A.; Fernández, M.C.; Wülfers, E.M.; Lother, A.; Hilgendorf, I.; Seemann, G.; Ravens, U.; Kohl, P.; Schneider-Warme, F. Novel insights into the electrophysiology of murine cardiac macrophages: Relevance of voltage-gated potassium channels. Cardiovasc. Res. 2022, 118, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Grune, J.; Lewis, A.J.M.; Yamazoe, M.; Hulsmans, M.; Rohde, D.; Xiao, L.; Zhang, S.; Ott, C.; Calcagno, D.M.; Zhou, Y.; et al. Neutrophils incite and macrophages avert electrical storm after myocardial infarction. Nat. Cardiovasc. Res. 2022, 1, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Schloss, M.J.; Lee, I.-H.; Bapat, A.; Iwamoto, Y.; Vinegoni, C.; Paccalet, A.; Yamazoe, M.; Grune, J.; Pabel, S.; et al. Recruited macrophages elicit atrial fibrillation. Science 2023, 381, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Time-Updated Systolic Blood Pressure and the Progression of Chronic Kidney Disease. Ann. Intern. Med. 2015, 162, 258–265. [CrossRef] [PubMed]
- Klag, M.J.; Whelton, P.K.; Randall, B.L.; Neaton, J.D.; Brancati, F.L.; Ford, C.E.; Shulman, N.B.; Stamler, J. Blood Pressure and End-Stage Renal Disease in Men. N. Engl. J. Med. 1996, 334, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.; Gu, D.; Muntner, P.; Kusek, J.W.; Chen, J.; Wu, X.; Duan, X.; Chen, C.-S.; Klag, M.J.; Whelton, P.K.; et al. A Population-Based, Prospective Study of Blood Pressure and Risk for End-Stage Renal Disease in China. J. Am. Soc. Nephrol. 2007, 18, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- McMaster, W.G.; Kirabo, A.; Madhur, M.S.; Harrison, D.G. Inflammation, Immunity, and Hypertensive End-Organ Damage. Circ. Res. 2015, 116, 1022–1033. [Google Scholar] [CrossRef]
- Rudemiller, N.P.; Crowley, S.D. Interactions Between the Immune and the Renin–Angiotensin Systems in Hypertension. Hypertension 2016, 68, 289–296. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Mohebati, M.; Disfani, M.F.; Karimian, M.S.; Ebrahimi, M.; Avan, A.; Eslami, S.; Pasdar, A.; Rooki, H.; Esmaeili, H. An imbalance in serum concentrations of inflammatory and anti-inflammatory cytokines in hypertension. J. Am. Soc. Hypertens. 2014, 8, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Capers, Q.; Alexander, R.W.; Lou, P.; Leon, H.D.; Wilcox, J.N.; Ishizaka, N.; Howard, A.B.; Taylor, W.R. Monocyte Chemoattractant Protein-1 Expression in Aortic Tissues of Hypertensive Rats. Hypertension 1997, 30, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Hiasa, K.-i.; Zhao, Q.; Inoue, S.; Ohtani, K.; Kitamoto, S.; Tsuchihashi, M.; Sugaya, T.; Charo, I.F.; Kura, S.; et al. Critical Role of Monocyte Chemoattractant Protein-1 Receptor CCR2 on Monocytes in Hypertension-Induced Vascular Inflammation and Remodeling. Circ. Res. 2004, 94, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, P.; Knorr, M.; Kossmann, S.; Stratmann, J.; Hausding, M.; Schuhmacher, S.; Karbach, S.H.; Schwenk, M.; Yogev, N.; Schulz, E.; et al. Lysozyme M–Positive Monocytes Mediate Angiotensin II–Induced Arterial Hypertension and Vascular Dysfunction. Circulation 2011, 124, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Kossmann, S.; Hu, H.; Steven, S.; Schönfelder, T.; Fraccarollo, D.; Mikhed, Y.; Brähler, M.; Knorr, M.; Brandt, M.; Karbach, S.H.; et al. Inflammatory Monocytes Determine Endothelial Nitric-oxide Synthase Uncoupling and Nitro-oxidative Stress Induced by Angiotensin II*. J. Biol. Chem. 2014, 289, 27540–27550. [Google Scholar] [CrossRef] [PubMed]
- Hughson, M.D.; Gobe, G.C.; Hoy, W.E.; Manning, R.D.; Douglas-Denton, R.; Bertram, J.F. Associations of Glomerular Number and Birth Weight With Clinicopathological Features of African Americans and Whites. Am. J. Kidney Dis. 2008, 52, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kranzhöfer, R.; Browatzki, M.; Schmidt, J.; Kübler, W. Angiotensin II Activates the Proinflammatory Transcription Factor Nuclear Factor-κB in Human Monocytes. Biochem. Biophys. Res. Commun. 1999, 257, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rudemiller, N.P.; Patel, M.B.; Karlovich, N.S.; Wu, M.; McDonough, A.A.; Griffiths, R.; Sparks, M.A.; Jeffs, A.D.; Crowley, S.D.; et al. Interleukin-1 Receptor Activation Potentiates Salt Reabsorption in Angiotensin II-Induced Hypertension via the NKCC2 Co-transporter in the Nephron. Cell Metab. 2016, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.; Vinh, A.; Tuck, K.L.; Sakkal, S.; Krishnan, S.M.; Chan, C.T.; Lieu, M.; Samuel, C.S.; Diep, H.; Kemp-Harper, B.K.; et al. M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H906–H917. [Google Scholar] [CrossRef]
- Liao, K.P. Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc. Med. 2017. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. X 2020, 2, 100050. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.X.; Yue, L. The Multifaceted Nature of Macrophages in Cardiovascular Disease. Biomedicines 2024, 12, 1317. https://doi.org/10.3390/biomedicines12061317
Li CX, Yue L. The Multifaceted Nature of Macrophages in Cardiovascular Disease. Biomedicines. 2024; 12(6):1317. https://doi.org/10.3390/biomedicines12061317
Chicago/Turabian StyleLi, Cindy X., and Lixia Yue. 2024. "The Multifaceted Nature of Macrophages in Cardiovascular Disease" Biomedicines 12, no. 6: 1317. https://doi.org/10.3390/biomedicines12061317
APA StyleLi, C. X., & Yue, L. (2024). The Multifaceted Nature of Macrophages in Cardiovascular Disease. Biomedicines, 12(6), 1317. https://doi.org/10.3390/biomedicines12061317





