miR-155 and miR-21 as Diagnostic and Therapeutic Biomarkers for Ulcerative Colitis: There Is Still a Long Way to Go
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Ethics Statement
2.3. Serum Sample Collection
2.4. RNA Extraction and Reverse Transcription
2.5. miRNA Expression Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mihai, C.; Cijevschi-Prelipcean, C.; Diculescu, M. Rectocolita ulcero-hemoragică (Colita ulcerativă). In Gastroenterologie si Hepatologie Clinica, 2nd ed.; Ed Medicală: Bucharest, Romania, 2023; Volume 1, pp. 132–353. [Google Scholar]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am. J. Gastroenterol. 2021, 116, 1124–1147, Erratum in: Am. J. Gastroenterol. 2022, 117, 358. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.M.; Marks, I.H.; Crowson, R.; Ball, D.; Rampton, D.S. Incidence and outcome of clostridium difficile infection in hospitalized patients with inflammatory bowel disease in the UK. J. Crohn’s Colitis 2017, 11, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.M.; Rea, M.C.; Shanahan, F.; Quigley, E.M.M.; Kiely, B.; Hill, C.; Ross, R.P. The vexed relationship between clostridium difficile and inflammatory bowel disease: An assessment of carriage in an outpatient setting among patients in remission. Am. J. Gastroenterol. 2009, 104, 1162–1169. [Google Scholar] [CrossRef]
- Bishop, E.J.; Tiruvoipati, R. Management of Clostridioides difficile infection in adults and challenges in clinical practice: Review and comparison of current IDSA/SHEA, ESCMID and ASID guidelines. J. Antimicrob. Chemother. 2022, 78, 21–30. [Google Scholar] [CrossRef] [PubMed]
- INSP. Available online: https://insp.gov.ro/download/ghid-diagnostic-tratament-si-prevenire-clostridium-difficile-pdf/ (accessed on 3 May 2024).
- Szałwińska, P.; Włodarczyk, J.; Spinelli, A.; Fichna, J.; Włodarczyk, M. IBS-Symptoms in IBD Patients—Manifestation of Concomitant or Different Entities. J. Clin. Med. 2021, 10, 31. [Google Scholar] [CrossRef]
- Quigley, E.M. Overlapping irritable bowel syndrome and inflammatory bowel disease: Less to this than meets the eye? Therap. Adv. Gastroenterol. 2016, 9, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef]
- Cioffi, M.; Rosa, A.D.; Serao, R.; Picone, I.; Vietri, M.T. Laboratory markers in ulcerative colitis: Current insights and future advances. World J. Gastrointest. Pathophysiol. 2015, 6, 13–22. [Google Scholar] [CrossRef]
- Wen, B.J.; Te, L.G.; Liu, X.X.; Zhao, J.H. The value of fecal calprotectin in Clostridioides difficile infection: A systematic review. Front. Physiol. 2022, 13, 881816. [Google Scholar] [CrossRef] [PubMed]
- Onişor, D.; Boeriu, A.; Pascarenco, O.; Brusnic, O.; Dobru, D. Role of fecal calprotectin as a biomarker of intestinal inflammation in ulcerative colitis: A prospective study. Rev. Romana Med. Lab. 2018, 26, 335–343. [Google Scholar] [CrossRef]
- James, J.P.; Riis, L.B.; Malham, M.; Høgdall, E.; Langholz, E.; Nielsen, B.S. MicroRNA Biomarkers in IBD—Differential Diagnosis and Prediction of Colitis-Associated Cancer. Int. J. Mol. Sci. 2020, 21, 7893. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Birsan, S.; Ichim, C.; Boeras, I.; Roman-Filip, I.; Blanca, G.; Bacila, C.; Fleaca, R.S.; Dura, H.; Roman-Filip, C. Has-miR-129-5p’s Involvement in Different Disorders, from Digestive Cancer to Neurodegenerative Diseases. Biomedicines 2023, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.G.; Pekow, J. The emerging role of miRNAs in inflammatory bowel disease: A review. Therap. Adv. Gastroenterol. 2015, 8, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Paraskevi, A.; Theodoropoulos, G.; Papaconstantinou, I.; Mantzaris, G.; Nikiteas, N.; Gazouli, M. Circulating MicroRNA in inflammatory bowel disease. J. Crohn’s Colitis 2012, 6, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Hassan, E.; El-Din Abd El-Rehim, A.S.; Mohammed Kholef, E.F.; Abd-Elgwad Elsewify, W. Potential role of plasma miR-21 and miR-92a in distinguishing between irritable bowel syndrome, ulcerative colitis, and colorectal cancer. Gastroenterol. Hepatol. Bed. Bench. 2020, 13, 147–154. [Google Scholar] [PubMed] [PubMed Central]
- Wu, F.; Guo, N.J.; Tian, H.; Marohn, M.; Gearhart, S.; Bayless, T.M.; Brant, S.R.; Kwon, J.H. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Fasseu, M.; Tréton, X.; Guichard, C.; Pedruzzi, E.; Cazals-Hatem, D.; Richard, C.; Aparicio, T.; Daniel, F.; Soulé, J.C.; Moreau, R.; et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS ONE 2010, 5, e13160. [Google Scholar] [CrossRef]
- Guo, J.G.; Rao, Y.F.; Jiang, J.; Li, X.; Zhu, S.M. MicroRNA-155-5p inhibition alleviates irritable bowel syndrome by increasing claudin-1 and ZO-1 expression. Ann. Transl. Med. 2023, 11, 34. [Google Scholar] [CrossRef]
- Martínez, C.; Rodiño-Janeiro, B.K.; Lobo, B.; Stanifer, M.L.; Klaus, B.; Granzow, M.; González-Castro, A.M.; Salvo-Romero, E.; Alonso-Cotoner, C.; Pigrau, M.; et al. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut 2017, 66, 1537–1538. [Google Scholar] [CrossRef] [PubMed]
- Síbia, C.d.F.d.; Quaglio, A.E.V.; Oliveira, E.C.S.d.; Pereira, J.N.; Ariede, J.R.; Lapa, R.M.L.; Severino, F.E.; Reis, P.P.; Sassaki, L.Y.; Saad-Hossne, R. microRNA–mRNA Networks Linked to Inflammation and Immune System Regulation in Inflammatory Bowel Disease. Biomedicines 2024, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Peng, L.; Yang, Y.; Guo, M.; Wang, W.; Sun, G. MicroRNA-155 is involved in the pathogenesis of ulcerative colitis by targeting FOXO3a. Inflamm. Bowel Dis. 2014, 20, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Grillo, A.R.; Scarpa, M.; Brun, P.; D’Incà, R.; Nai, L.; Banerjee, A.; Cavallo, D.; Barzon, L.; Palù, G.; et al. MiR-155 modulates the inflammatory phenotype of intestinal myofibroblasts by targeting SOCS1 in ulcerative colitis. Exp. Mol. Med. 2015, 47, e164. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, Y.; Shi, C.; Chen, H.; Zhang, H.; Chen, N.; Zhang, P.; Wang, F.; Yang, J.; Yang, J.; et al. Overexpression of miR-21 in patients with ulcerative colitis impairs intestinal epithelial barrier function through targeting the Rho GTPase RhoB. Biochem. Biophys. Res. Commun. 2013, 434, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Song, J.; Bai, Z.; Han, W.; Zhang, J.; Meng, H.; Bi, J.; Ma, X.; Han, S.; Zhang, Z. Identification of suitable reference genes for qPCR analysis of serum microRNA in gastric cancer patients. Dig. Dis. Sci. 2012, 57, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schönauen, K.; Le, N.; von Arnim, U.; Schulz, C.; Malfertheiner, P.; Link, A. Circulating and Fecal microRNAs as Biomarkers for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2018, 24, 1547–1557. [Google Scholar] [CrossRef]
- Wan, J.; Xia, L.; Xu, W.; Lu, N. Expression and Function of miR-155 in Diseases of the Gastrointestinal Tract. Int. J. Mol. Sci. 2016, 17, 709. [Google Scholar] [CrossRef]
- miRBase. Available online: https://www.mirbase.org (accessed on 3 May 2024).
- Oertli, M.; Engler, D.B.; Kohler, E.; Koch, M.; Meyer, T.F.; Muller, A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pyloriinfection and for the induction of chronic Gastritis and Colitis. J. Immunol. 2011, 187, 3578–3586. [Google Scholar] [CrossRef]
- Oana, S.M.; Claudia, B.; Lelia, R.A.; Simona, M.; Claudia, C.; Daniela, D.E. Differential Expression of Tissular miRNA-155 in Pediatric Gastritis. J. Clin. Med. 2022, 11, 3351. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.F.; Li, L.; Wang, L.M. miR-155 and miR-146b negatively regulates IL6 in Helicobacter pylori (cagA+) infected gastroduodenal ulcer. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 607–613. [Google Scholar] [PubMed]
- Li, R.; Hu, Y.; Hou, S. An Exploration of Oral-Gut Pathogens Mediating Immune Escape of Pancreatic Cancer via miR-21/PTEN Axis. Front. Microbiol. 2022, 13, 928846. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.L.; Wang, H.F.; Sun, Z.Q.; Tang, Y.; Han, X.N.; Yu, X.B.; Liu, K. Up-regulated miR-155–5p promotes cell proliferation, invasion and metastasis in colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 6988–6994. [Google Scholar] [PubMed] [PubMed Central]
- Kennedy, K.F. The Effects of Surface Layer Proteins Isolated from Clostridium Difficile on TLR4 Signalling. Ph.D. Thesis, Dublin City University, Dublin, Ireland, March 2016. [Google Scholar]
- Alanis, E.L. Targeting Protein Synthesis in Clostriodioides Difficile to Develop Antimicrobial Candidate. Master’s Thesis, The University of Texas Rio Grande Valley, Edinburg, TX, USA, May 2022. [Google Scholar]
- Jenike, A.E.; Halushka, M.K. miR-21: A non-specific biomarker of all maladies. Biomark. Res. 2021, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Kacimi, S.E.O.; Nguyen, T.L.; Suman, K.H.; Lemus-Martin, R.; Saleem, H.; Do, D.N. MiR-21 in the Cancers of the Digestive System and Its Potential Role as a Diagnostic, Predictive, and Therapeutic Biomarker. Biology 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Spazzafumo Bonafè, M.; Recchioni, R.; Prattichizzo, F.; Marcheselli, F.; Micolucci, L.; Mensà, E.; Giuliani, A.; Santini, G.; Gobbi, M.; et al. MiR-21-5p and miR-126a-3p levels in plasma and circulating angiogenic cells: Relationship with type 2 diabetes complications. Oncotarget 2015, 6, 35372–35382. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Mizushima, K.; Hirata, I.; Yagi, N.; Tomatsuri, N.; Yoshikawa, T. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J. Gastroenterol. Hepatol. 2010, 25, S129–S133. [Google Scholar] [CrossRef]
- Schaefer, J.S.; Attumi, T.; Opekun, A.R.; Abraham, B.; Hou, J.; Shelby, H.; Graham, D.Y.; Streckfus, C.; Klein, J.R. MicroRNA signatures differentiate Crohn’s disease from ulcerative colitis. BMC Immunol. 2015, 16, 5. [Google Scholar] [CrossRef]
- Nakata, K.; Sugi, Y.; Narabayashi, H.; Kobayakawa, T.; Nakanishi, Y.; Tsuda, M.; Hosono, A.; Kaminogawa, S.; Hanazawa, S.; Takahashi, K. Commensal microbiota-induced microRNA modulates intestinal epithelial permeability through the small GTPase ARF4. J. Biol. Chem. 2017, 292, 15426–15433. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, X.; Xu, Y. Aberrant expression of miR-21 in patients with inflammatory bowel disease: A protocol for systematic review and meta analysis. Medicine 2020, 99, e19693. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.S.; Ezzat, A.; Nahed, B.; Mohamed, E.l.; Eman, T. Serum expression of microRNA-16 in a cohort of Egyptian patients with ulcerative colitis and its correlation with disease extent and severity. J. Coloproctol. 2020, 40, 253–260. [Google Scholar] [CrossRef]
- Whiteoak, S.R.; Felwick, R.; Sanchez-Elsner, T.; Fraser Cummings, J.R. MicroRNAs in Inflammatory Bowel Diseases: Paradoxes and Possibilities. Inflamm. Bowel Dis. 2015, 21, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Fassan, M.; Mescoli, C.; Pizzi, M.; Balistreri, M.; Albertoni, L.; Pucciarelli, S.; Scarpa, M.; Sturniolo, G.C.; Angriman, I.; et al. PDCD4/miR-21 dysregulation in inflammatory bowel disease-associated carcinogenesis. Virchows Arch. 2013, 462, 57–63. [Google Scholar] [CrossRef]
- Kalla, R.; Adams, A.T.; Ventham, N.T.; A Kennedy, N.; White, R.; Clarke, C.; Ivens, A.; Bergemalm, D.; Vatn, S.; Lopez-Jimena, B.; et al. Whole Blood Profiling of T-Cell Derived miRNA Allows the Development of Prognostic Models in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 1724–1733. [Google Scholar] [CrossRef]
- Krishnachaitanya, S.S.; Liu, M.; Fujise, K.; Li, Q. MicroRNAs in Inflammatory Bowel Disease and Its Complications. Int. J. Mol. Sci. 2022, 23, 8751. [Google Scholar] [CrossRef]
| Parameter | Group 1 (n = 34) (Mean ± SD) | Group 2 (n = 17) (Mean ± SD) | Control Group (n = 33) (Mean ± SD) | p Value |
|---|---|---|---|---|
| Age (years) | 45.91 ± 15.23 | 65 ± 12.51 | 59.18 ± 13.86 | <0.0001 |
Gender (n)
| 12 22 | 8 9 | 25 8 | <0.0001 |
Background (n)
| 24 10 | 8 9 | 15 18 | 0.08 |
| Parameter | Group 1 (n = 34) (Mean ± SD) | Group 2 (n = 17) (Mean ± SD) | Control Group (n = 33) (Mean ± SD) | p Value |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 11.32 ± 1.87 | 11.82 ± 1.25 | 12.52 ± 0.92 | <0.0001 |
| Leukocytes * (cells/µL) | 10205 ± 2275 | 12182 ± 2776 | 8473 ± 1847 | <0.0001 |
| Serum iron (µg/dL) | 54.24 ± 24.02 | 52.94 ± 13.44 | 63.09 ± 15.14 | 0.14 |
| Albumin (g/L) | 34.47 ± 2.60 | 36.24 ± 3.54 | 37.21 ± 1.83 | <0.01 |
| Fecal calprotectin (µg/mg) | 551.5 ± 424.1 | 339.4 ± 125.7 | 49.82 ± 22.22 | <0.0001 |
| C reactive protein (mg/dL) | 2.29 ± 1.42 | 5.18 ± 1.82 | 0.36 ± 0.16 | <0.0001 |
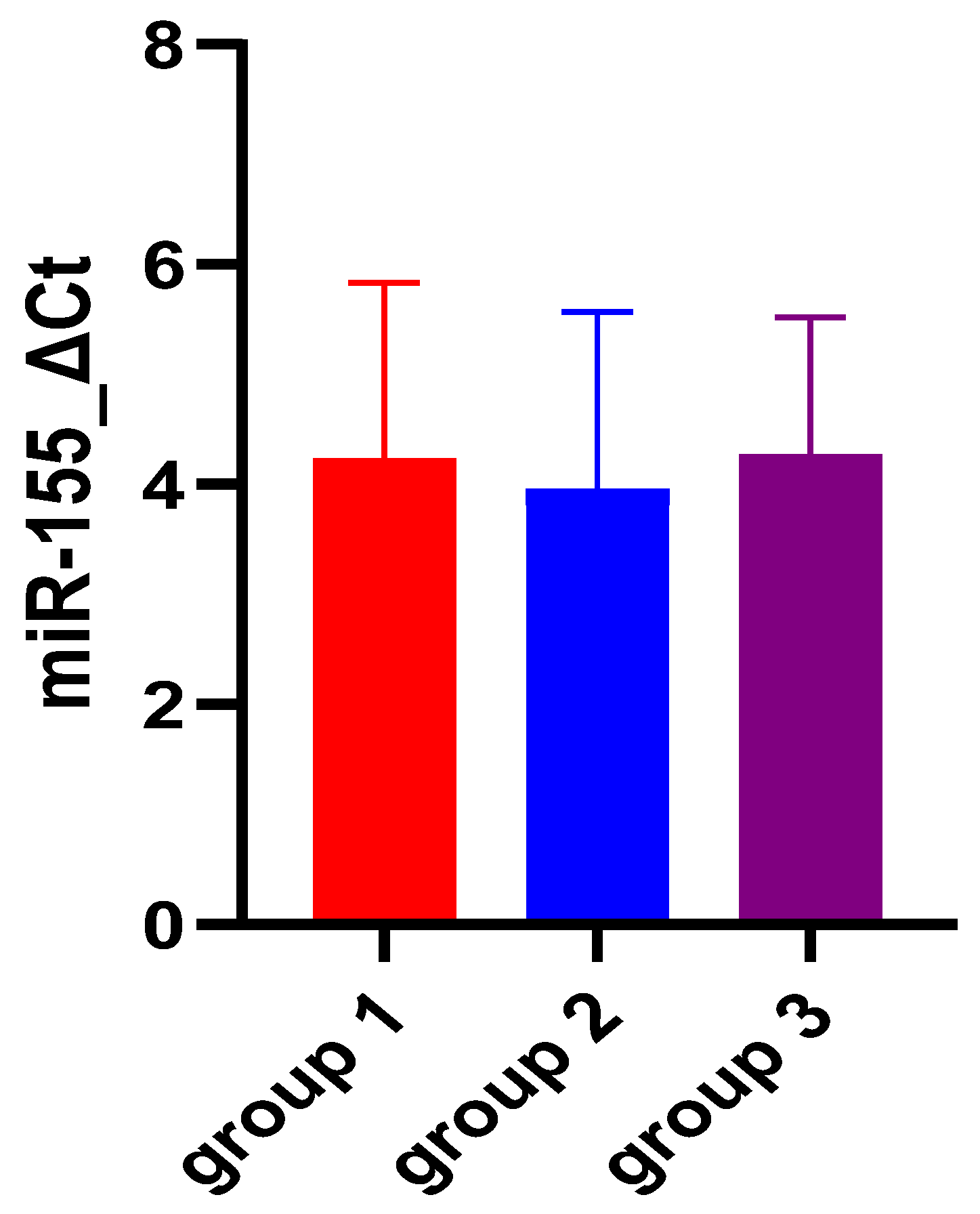
| miR-155 ΔCt | 4.22 ± 1.61 | 3.94 ± 1.62 | 4.26 ± 1.26 | 0.74 |
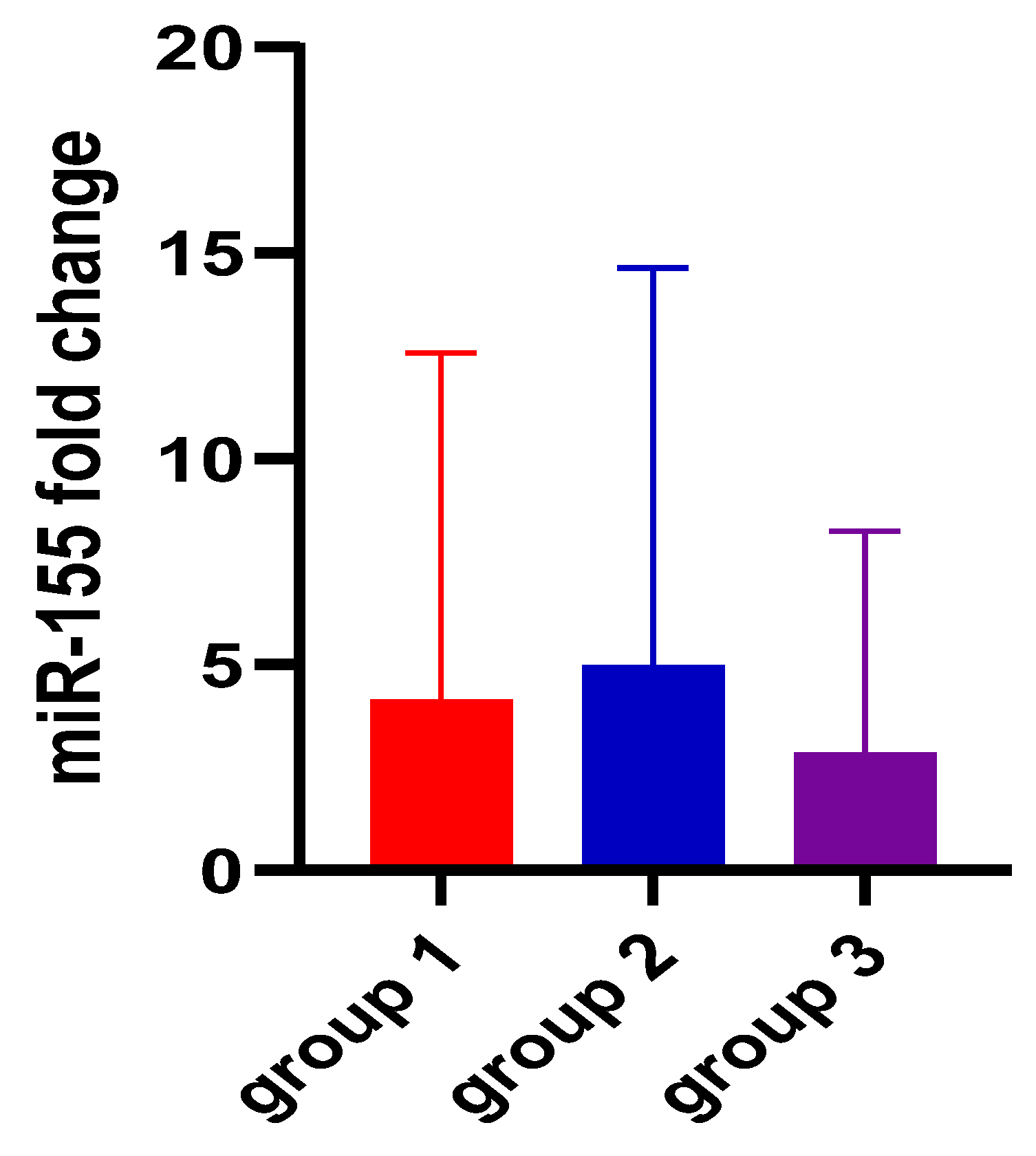
| miR-155 2−ΔΔCt | 4.11 ± 8.46 | 4.94 ± 9.68 | 2.83 ± 5.41 | 0.73 |
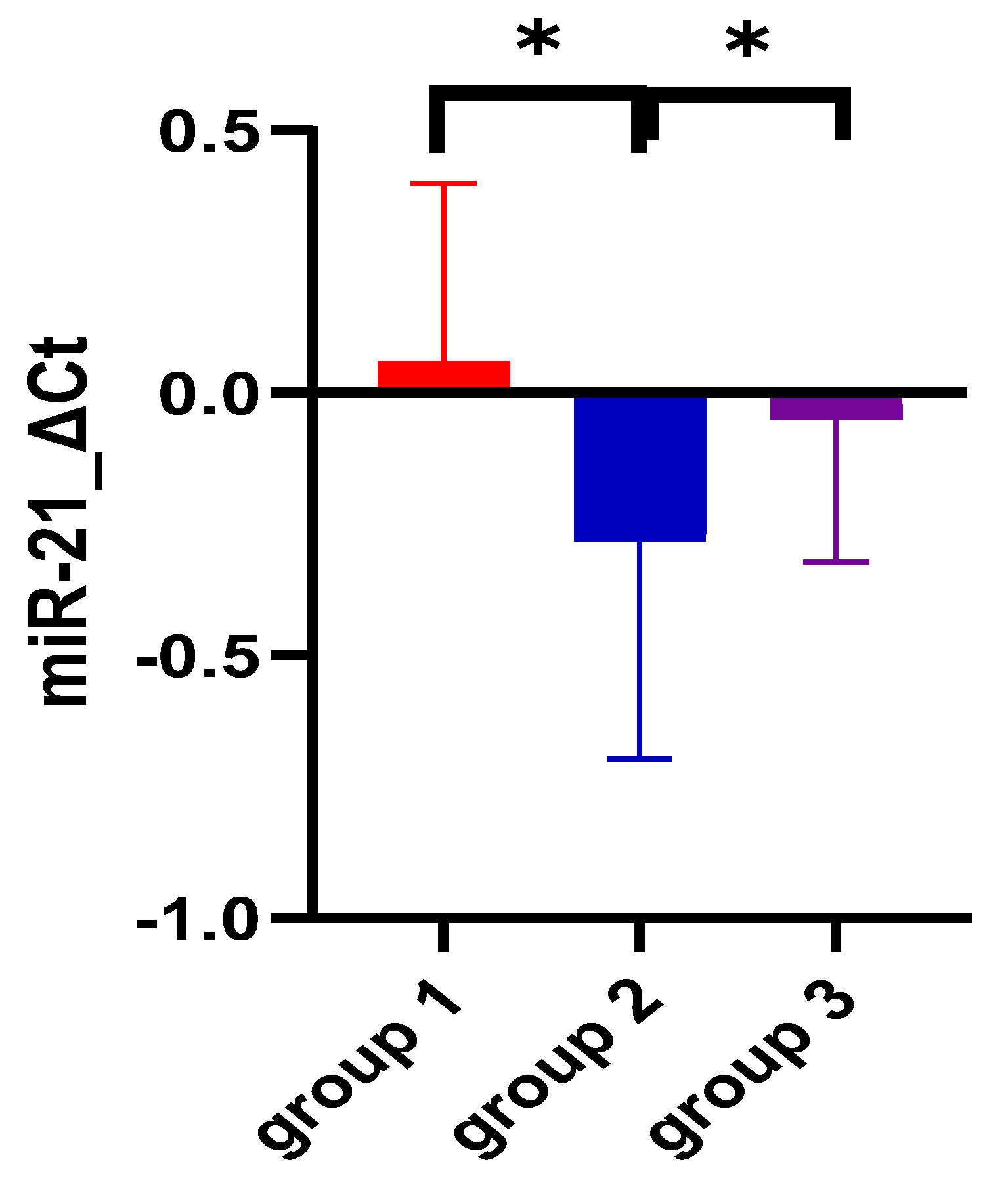
| miR-21 ΔCt | 0.05 ± 0.34 | −0.27 ± 0.41 | −0.04 ± 0.27 | <0.0001 |
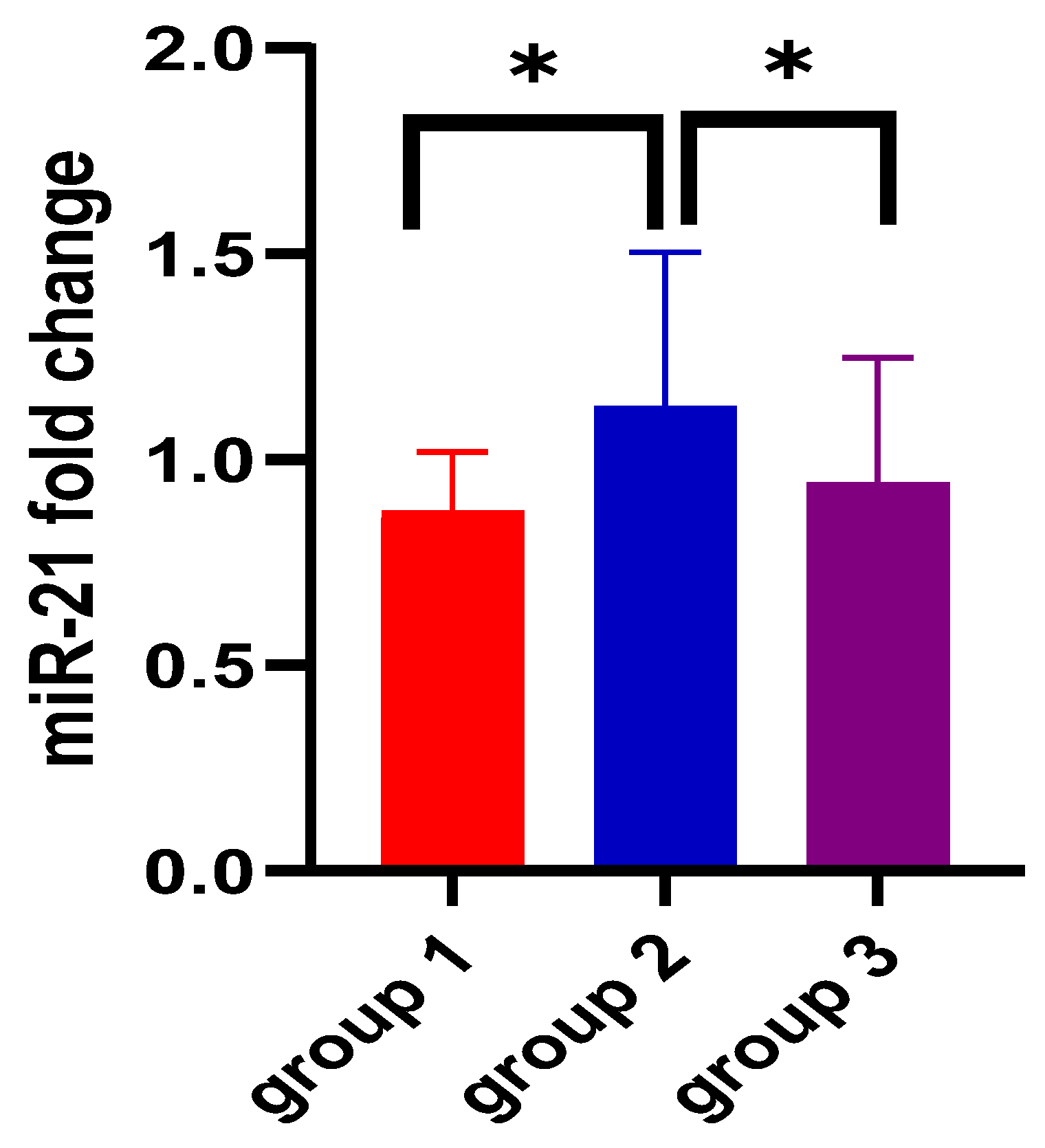
| miR-21 2−ΔΔCt | 0.87 ± 0.14 | 1.12 ± 0.37 | 0.94 ± 0.30 | <0.0001 |
| Parameter | Compared Groups | AUC (95% CI) | Cut-off | Sensitivity (95% CI) | Specificity (95% CI) | p Value |
|---|---|---|---|---|---|---|
| miR-21 ΔCt | group 1 vs. control group | 0.50 (0.36–0.64) | −0.99 | 100% (89.85–100%) | 3.03% (0.15–15.32%) | 0.99 |
| group 2 vs. control group | 0.68 (0.51–0.85) | −0.29 | 35.29% (17.31–58.70%) | 96.97% (84.68–99.84%) | 0.03 | |
| group 1 vs. group 2 | 0.69 (0.52–0.85) | −0.06 | 88.24% (73.38–95.33%) | 41.18% (21.61–63.99%) | 0.02 | |
| miR-21 2−ΔΔCt | group 1 vs. control group | 0.50 (0.36–0.64) | 0.82 | 100% (89.57–100%) | 8.82% (3.04–22.96%) | 0.99 |
| group 2 vs. control group | 0.68 (0.51–0.85) | 0.92 | 96.97% (84.68–99.84%) | 41.18% (21.61–63.99%) | 0.03 | |
| group 1 vs. group 2 | 0.69 (0.52–0.85) | 0.92 | 88.24% (73.38–95.33%) | 41.18% (21.61–63.99%) | 0.02 |
| Independent Variable | Dependent Variable | Spearman’s Rank Correlation Coefficient | p Value |
|---|---|---|---|
| miR-155 ΔCt | UCDAI | −0.005 | 0.97 |
| UC extension | −0.084 | 0.63 | |
| UC severity | −0.716 | 0.68 | |
| fecal calprotectin | 0.070 | 0.52 | |
| miR-21 ΔCt | UCDAI | −0.168 | 0.34 |
| UC extension | 0.026 | 0.88 | |
| UC severity | −0.169 | 0.33 | |
| fecal calprotectin | 0.060 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onisor, D.; Brusnic, O.; Banescu, C.; Carstea, C.; Sasaran, M.; Stoian, M.; Avram, C.; Boicean, A.; Boeriu, A.; Dobru, D. miR-155 and miR-21 as Diagnostic and Therapeutic Biomarkers for Ulcerative Colitis: There Is Still a Long Way to Go. Biomedicines 2024, 12, 1315. https://doi.org/10.3390/biomedicines12061315
Onisor D, Brusnic O, Banescu C, Carstea C, Sasaran M, Stoian M, Avram C, Boicean A, Boeriu A, Dobru D. miR-155 and miR-21 as Diagnostic and Therapeutic Biomarkers for Ulcerative Colitis: There Is Still a Long Way to Go. Biomedicines. 2024; 12(6):1315. https://doi.org/10.3390/biomedicines12061315
Chicago/Turabian StyleOnisor, Danusia, Olga Brusnic, Claudia Banescu, Claudia Carstea, Maria Sasaran, Mircea Stoian, Calin Avram, Adrian Boicean, Alina Boeriu, and Daniela Dobru. 2024. "miR-155 and miR-21 as Diagnostic and Therapeutic Biomarkers for Ulcerative Colitis: There Is Still a Long Way to Go" Biomedicines 12, no. 6: 1315. https://doi.org/10.3390/biomedicines12061315
APA StyleOnisor, D., Brusnic, O., Banescu, C., Carstea, C., Sasaran, M., Stoian, M., Avram, C., Boicean, A., Boeriu, A., & Dobru, D. (2024). miR-155 and miR-21 as Diagnostic and Therapeutic Biomarkers for Ulcerative Colitis: There Is Still a Long Way to Go. Biomedicines, 12(6), 1315. https://doi.org/10.3390/biomedicines12061315








