Anemia of Chronic Kidney Disease—A Narrative Review of Its Pathophysiology, Diagnosis, and Management
Abstract
1. Introduction
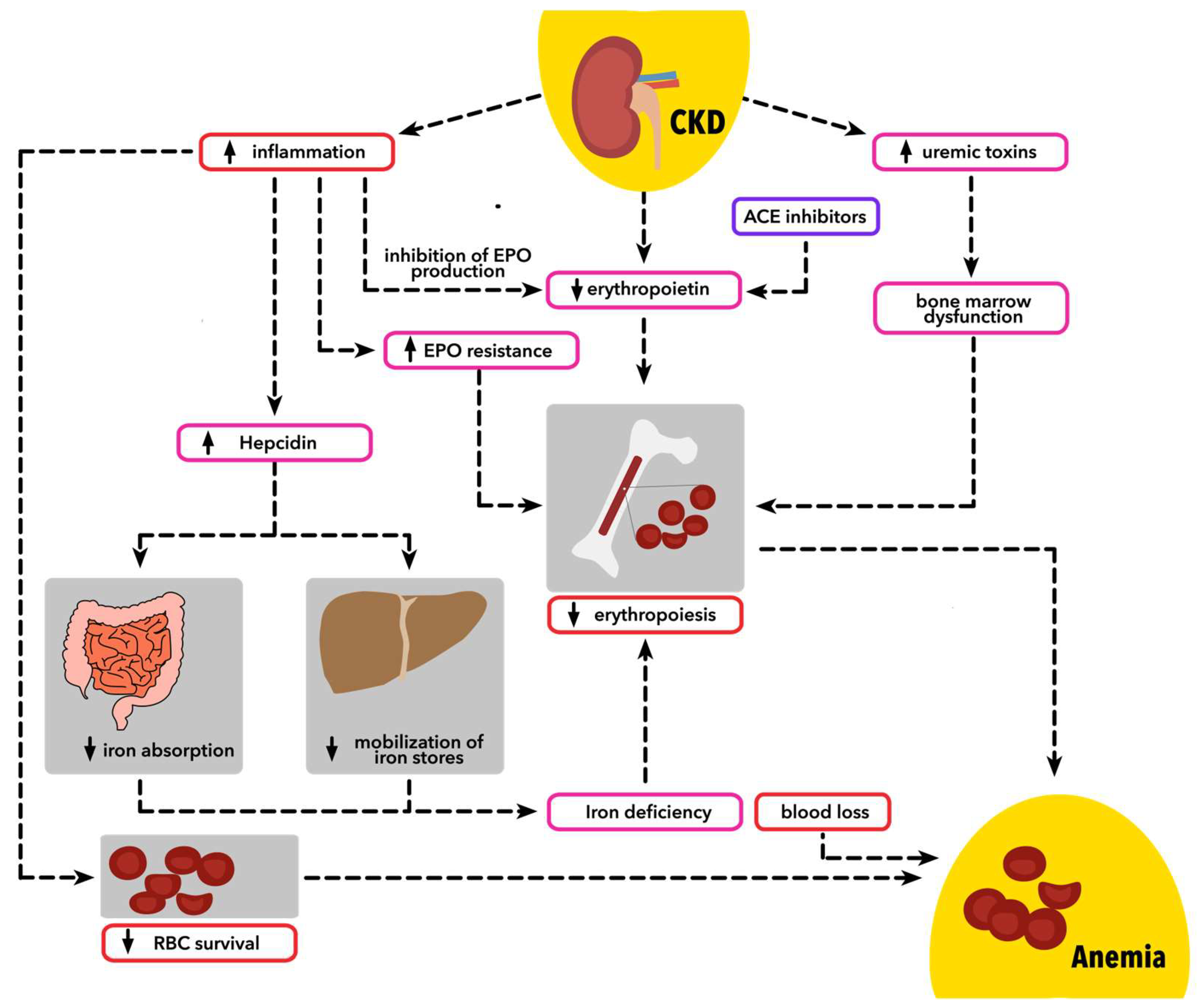
2. Pathophysiology
2.1. Erythropoetin Dysregulation
2.2. Iron Dysregulation and Inflammation
2.2.1. General Mechanisms of Iron Stores Disturbances
2.2.2. Chronic Inflammation
2.2.3. Hepcidin
2.3. Bone Marrow Dysfunction
2.4. Vitamin B, Folic Acid, and Hyperhomocysteinemia
2.5. Bone Morphogenetic Protein 6 (BMP-6)
2.6. Fibroblast Growth Factor 23 (FGF-23)
2.7. Other Factors
3. Diagnosis
3.1. Screening for Anemia
3.2. Diagnosis of Anemia
3.3. Evaluation of Anemia
- complete blood count (CBC), including mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), white blood cell count, and platelet count.
- absolute reticulocyte count.
- TSAT.
- serum ferritin.
3.4. Iron Status Assessment
3.4.1. Serum Ferritin
3.4.2. Transferrin Saturation (TSAT)
3.4.3. Reticulocyte Hemoglobin Count (CHr)
3.4.4. Percentage of Hypochromic Red Cells (%HRC)
3.5. Other and Novel Biomarkers in Anemia of Chronic Kidney Disease Evaluation
3.5.1. Soluble Transferrin Receptor (sTfR)
3.5.2. Hepcidin
3.5.3. Neutrophil-Gelatinase-Associated Lipocalin
3.5.4. Bone Metabolic Biomarkers
4. Management
4.1. Erythropoiesis-Stimulating Agents
4.2. Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitors
4.3. Anti-Bone Morphogenetic Protein 6 Antibodies
4.4. Sodium-Glucose Transport Protein 2 Inhibitors
4.5. Novel Iron Therapies
4.6. Ziltivekimab
4.7. Unaddressed Questions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zadrazil, J.; Horak, P. Pathophysiology of Anemia in Chronic Kidney Diseases: A Review. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2015, 159, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Natale, P.; Palmer, S.C.; Jaure, A.; Hodson, E.M.; Ruospo, M.; Cooper, T.E.; Hahn, D.; Saglimbene, V.M.; Craig, J.C.; Strippoli, G.F. Hypoxia-Inducible Factor Stabilisers for the Anaemia of Chronic Kidney Disease. Cochrane Database Syst. Rev. 2022, 2022, CD013751. [Google Scholar] [CrossRef] [PubMed]
- Portolés, J.; Martín, L.; Broseta, J.J.; Cases, A. Anemia in Chronic Kidney Disease: From Pathophysiology and Current Treatments, to Future Agents. Front. Med. 2021, 8, 642296. [Google Scholar] [CrossRef] [PubMed]
- Asada, N.; Takase, M.; Nakamura, J.; Oguchi, A.; Asada, M.; Suzuki, N.; Yamamura, K.; Nagoshi, N.; Shibata, S.; Rao, T.N.; et al. Dysfunction of Fibroblasts of Extrarenal Origin Underlies Renal Fibrosis and Renal Anemia in Mice. J. Clin. Investig. 2011, 121, 3981–3990. [Google Scholar] [CrossRef] [PubMed]
- Arezes, J.; Nemeth, E. Hepcidin and Iron Disorders: New Biology and Clinical Approaches. Int. J. Lab. Hematol. 2015, 37, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Lin, H.Y. Mechanisms of Anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yanagita, M. Renal Anemia: From Incurable to Curable. Am. J. Physiol.-Ren. Physiol. 2013, 305, F1239–F1248. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.M.; Streja, E.; Kalantar-Zadeh, K. Burden of Anemia in Chronic Kidney Disease: Beyond Erythropoietin. Adv. Ther. 2021, 38, 52–75. [Google Scholar] [CrossRef] [PubMed]
- Coyne, D.W.; Goldsmith, D.; Macdougall, I.C. New Options for the Anemia of Chronic Kidney Disease. Kidney Int. Suppl. 2017, 7, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-M.; Wu, C.-J.; Lin, S.-L. Physiology and Pathophysiology of Renal Erythropoietin-Producing Cells. J. Formos. Med. Assoc. 2018, 117, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Lankhorst, C.E.; Wish, J.B. Anemia in Renal Disease: Diagnosis and Management. Blood Rev. 2010, 24, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Ferguson, D.J.P.; Nicholls, L.G.; Iredale, J.P.; Pugh, C.W.; Johnson, M.H.; Ratcliffe, P.J. Sites of Erythropoietin Production. Kidney Int. 1997, 51, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Regulation of Erythropoietin Production. J. Physiol. 2011, 589, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N. Erythropoietin: Physiology and Molecular Mechanisms. Heart Fail. Rev. 2008, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Kular, D.; Macdougall, I.C. HIF Stabilizers in the Management of Renal Anemia: From Bench to Bedside to Pediatrics. Pediatr. Nephrol. 2019, 34, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Warady, B.A. Anemia in Chronic Kidney Disease. Pediatr. Nephrol. 2018, 33, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.; Palmer, S.C.; Saglimbene, V.M.; Craig, J.C.; Tonelli, M.; Strippoli, G.F. Erythropoiesis-Stimulating Agents for Anaemia in Adults with Chronic Kidney Disease: A Network Meta-Analysis. Cochrane Database Syst. Rev. 2023, 2023, CD010590. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.V.; Singh, A.K. Anemia in Chronic Kidney Disease: New Advances. Heart Fail. Clin. 2010, 6, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-C.; Tarng, D.-C. Bone Marrow Iron in CKD: Correlation with Functional Iron Deficiency. Am. J. Kidney Dis. 2010, 55, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Regulation of Iron Metabolism by Hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Solak, Y.; Covic, A.; Goldsmith, D.; Kanbay, M. Renal Anemia of Inflammation: The Name Is Self-Explanatory. Blood Purif. 2011, 32, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Gluba-Brzózka, A.; Franczyk, B.; Olszewski, R.; Rysz, J. The Influence of Inflammation on Anemia in CKD Patients. Int. J. Mol. Sci. 2020, 21, 725. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K. Iron Metabolism and Management: Focus on Chronic Kidney Disease. Kidney Int. Suppl. 2021, 11, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xing, J.; Zhu, X.; Xie, X.; Wang, L.; Zhang, X. Effects of Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitors vs. Erythropoiesis-Stimulating Agents on Iron Metabolism in Non-Dialysis-Dependent Anemic Patients with CKD: A Network Meta-Analysis. Front. Endocrinol. 2023, 14, 1131516. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Ashby, D. Hepcidin—A Well-Known Iron Biomarker with Prognostic Implications in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2013, 28, 2936–2939. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Yee, J. Hepcidin. Adv. Chronic Kidney Dis. 2019, 26, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, A.; Ribeiro, S.; Reis, F.; Belo, L. Hepcidin in chronic kidney disease anemia. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2019; Volume 110, pp. 243–264. ISBN 978-0-12-817842-3. [Google Scholar]
- Dallalio, G.; Law, E.; Means, R.T. Hepcidin Inhibits in Vitro Erythroid Colony Formation at Reduced Erythropoietin Concentrations. Blood 2006, 107, 2702–2704. [Google Scholar] [CrossRef] [PubMed]
- Van Swelm, R.P.L.; Wetzels, J.F.M.; Swinkels, D.W. The Multifaceted Role of Iron in Renal Health and Disease. Nat. Rev. Nephrol. 2020, 16, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-C.; Chan, M.-J.; Su, Y.-J.; Fu, J.-F.; Wang, I.-K.; Chen, C.-Y.; Weng, C.-H.; Huang, W.-H.; Hsu, C.-W.; Yen, T.-H. Bone Marrow Hypocellularity in Patients with End-Stage Kidney Disease. Healthcare 2021, 9, 1452. [Google Scholar] [CrossRef]
- Weng, C.-H.; Lu, K.-Y.; Hu, C.-C.; Huang, W.-H.; Wang, I.-K.; Yen, T.-H. Bone Marrow Pathology Predicts Mortality in Chronic Hemodialysis Patients. BioMed Res. Int. 2015, 2015, 60382. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.-M.; Elsurer Afsar, R.; Sussman-Dabach, E.J.; White, J.A.; MacLaughlin, H.; Ikizler, T.A. Vitamin Supplement Use in Patients with CKD: Worth the Pill Burden? Am. J. Kidney Dis. 2024, 83, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.L.; McDonnell, T.; Chinnadurai, R. Physiological Associations between Vitamin B Deficiency and Diabetic Kidney Disease. Biomedicines 2023, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Badri, S.; Vahdat, S.; Seirafian, S.; Pourfarzam, M.; Gholipur-Shahraki, T.; Ataei, S. Homocysteine-Lowering Interventions in Chronic Kidney Disease. J. Res. Pharm. Pract. 2021, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Cappuccilli, M.L.; Magnoni, G.; Croci Chiocchini, A.L.; Aiello, V.; Napoletano, A.; Iacovella, F.; Troiano, A.; Mancini, R.; Capelli, I.; et al. The Link between Homocysteine, Folic Acid and Vitamin B12 in Chronic Kidney Disease. G. Ital. Nefrol. 2021, 38, 1–17. [Google Scholar]
- Ratajczak, A.E.; Szymczak-Tomczak, A.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Does Folic Acid Protect Patients with Inflammatory Bowel Disease from Complications? Nutrients 2021, 13, 4036. [Google Scholar] [CrossRef] [PubMed]
- Kandel, R.; Singh, K.P. Higher Concentrations of Folic Acid Cause Oxidative Stress, Acute Cytotoxicity, and Long-Term Fibrogenic Changes in Kidney Epithelial Cells. Chem. Res. Toxicol. 2022, 35, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Huang, F.W.; Xia, Y.; Sidis, Y.; Andrews, N.C.; Lin, H.Y. Modulation of Bone Morphogenetic Protein Signaling in Vivo Regulates Systemic Iron Balance. J. Clin. Investig. 2007, 117, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Lowery, J.W.; Rosen, V. Bone Morphogenetic Protein–Based Therapeutic Approaches. Cold Spring Harb. Perspect. Biol. 2018, 10, a022327. [Google Scholar] [CrossRef]
- Agoro, R.; White, K.E. Anemia and Fibroblast Growth Factor 23 Elevation in Chronic Kidney Disease: Homeostatic Interactions and Emerging Therapeutics. Curr. Opin. Nephrol. Hypertens. 2022, 31, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Sliem, H.; Tawfik, G.; Moustafa, F.; Zaki, H. Relationship of Associated Secondary Hyperparathyroidism to Serum Fibroblast Growth Factor-23 in End Stage Renal Disease: A Case-Control Study. Indian J. Endocr. Metab. 2011, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Le Henaff, C.; Sitara, D. Administration of α-Klotho Does Not Rescue Renal Anemia in Mice. Front. Pediatr. 2022, 10, 924915. [Google Scholar] [CrossRef] [PubMed]
- Hanudel, M.R.; Laster, M.L.; Portale, A.A.; Dokras, A.; Quigley, R.P.; Guzman, G.A.L.; Zaritsky, J.J.; Hayde, N.A.; Kaskel, F.J.; Mitsnefes, M.M.; et al. A Review of Ferric Citrate Clinical Studies, and the Rationale and Design of the Ferric Citrate and Chronic Kidney Disease in Children (FIT4KiD) Trial. Pediatr. Nephrol. 2022, 37, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.A.; Clinkenbeard, E.L. Regulation of Fibroblast Growth Factor 23 by Iron, EPO, and HIF. Curr. Mol. Biol. Rep. 2019, 5, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.C.; Cho, M.E.; Cai, X.; Lee, J.; Chen, J.; He, J.; Flack, J.; Shafi, T.; Saraf, S.L.; David, V.; et al. Iron Status, Fibroblast Growth Factor 23 and Cardiovascular and Kidney Outcomes in Chronic Kidney Disease. Kidney Int. 2021, 100, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Koch, T.A.; Bregman, D.B. Effects of Iron Deficiency Anemia and Its Treatment on Fibroblast Growth Factor 23 and Phosphate Homeostasis in Women. J. Bone Miner. Res. 2013, 28, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Abu-Alfa, A.K.; Cruz, D.; Perazella, M.A.; Mahnensmith, R.L.; Simon, D.; Bia, M.J. Ace Inhibitors Do Not Induce Recombinant Human Erythropoietin Resistance in Hemodialysis Patients. Am. J. Kidney Dis. 2000, 35, 1076–1082. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Waters, D.; Kwok, C.S. Gastrointestinal Bleeding in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. Ren. Fail. 2023, 45, 2276908. [Google Scholar] [CrossRef]
- Pavord, S.; Myers, B. Bleeding and Thrombotic Complications of Kidney Disease. Blood Rev. 2011, 25, 271–278. [Google Scholar] [CrossRef]
- Parker, K.; Hartemink, J.; Saha, A.; Mitra, R.; Lewis, P.; Power, A.; Choudhuri, S.; Mitra, S.; Thachil, J. A Systematic Review of the Efficacy and Safety of Anticoagulants in Advanced Chronic Kidney Disease. J. Nephrol. 2022, 35, 2015–2033. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Menke, J.; Sollinger, D.; Schinzel, H.; Thürmel, K. Haemostasis in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2014, 29, 29–40. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.; Parfrey, P.; Adamson, J.W.; Aljama, P.; Berns, J.S.; Bohlius, J.; Drüeke, T.B.; Finkelstein, F.O.; Fishbane, S.; Ganz, T.; et al. Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- Sofue, T.; Nakagawa, N.; Kanda, E.; Nagasu, H.; Matsushita, K.; Nangaku, M.; Maruyama, S.; Wada, T.; Terada, Y.; Yamagata, K.; et al. Prevalence of Anemia in Patients with Chronic Kidney Disease in Japan: A Nationwide, Cross-Sectional Cohort Study Using Data from the Japan Chronic Kidney Disease Database (J-CKD-DB). PLoS ONE 2020, 15, e0236132. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, M.E.; Fan, T. Prevalence of Anemia in Chronic Kidney Disease in the United States. PLoS ONE 2014, 9, e84943. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, A.; Brown, C.; Williams, J.A.; Mathrani, V.; Shrivastava, R.; Evans, J.; Isaac, H.; Bhandari, S. Renal Association Clinical Practice Guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol. 2017, 18, 345. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline on Haemoglobin Cutoffs to Define Anaemia in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Executive Summary. Am. J. Kidney Dis. 2006, 47, S11–S15. [CrossRef] [PubMed]
- Locatelli, F.; Bárány, P.; Covic, A.; De Francisco, A.; Del Vecchio, L.; Goldsmith, D.; Hörl, W.; London, G.; Vanholder, R.; Van Biesen, W.; et al. Kidney Disease: Improving Global Outcomes Guidelines on Anaemia Management in Chronic Kidney Disease: A European Renal Best Practice Position Statement. Nephrol. Dial. Transplant. 2013, 28, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Locatelli, F.; Gallieni, M.; Bonofiglio, R.; Fuiano, G.; Oldrizzi, L.; Conte, G.; De Nicola, L.; Mangione, F.; Esposito, P.; et al. Anaemia Management in Non-Dialysis Chronic Kidney Disease (CKD) Patients: A Multicentre Prospective Study in Renal Clinics. Nephrol. Dial. Transplant. 2013, 28, 3035–3045. [Google Scholar] [CrossRef]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and Inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Comin-Colet, J.; De Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron Deficiency across Chronic Inflammatory Conditions: International Expert Opinion on Definition, Diagnosis, and Management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Gafter-Gvili, A.; Schechter, A.; Rozen-Zvi, B. Iron Deficiency Anemia in Chronic Kidney Disease. Acta Haematol. 2019, 142, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Gaweda, A.E. Markers of Iron Status in Chronic Kidney Disease. Hemodial. Int. 2017, 21, S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Paragas, N.; Ned, R.M.; Qiu, A.; Viltard, M.; Leete, T.; Drexler, I.R.; Chen, X.; Sanna-Cherchi, S.; Mohammed, F.; et al. Scara5 Is a Ferritin Receptor Mediating Non-Transferrin Iron Delivery. Dev. Cell 2009, 16, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Plays, M.; Müller, S.; Rodriguez, R. Chemistry and Biology of Ferritin. Metallomics 2021, 13, mfab021. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Mondorf, A.; Beifuß, J.; Jung, M.; Brüne, B. Hypoxia Inhibits Ferritinophagy, Increases Mitochondrial Ferritin, and Protects from Ferroptosis. Redox Biol. 2020, 36, 101670. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cui, Y.; Ren, Q.; Yan, B.; Zhao, Y.; Yu, P.; Gao, G.; Shi, H.; Chang, S.; Chang, Y.-Z. Mitochondrial Ferritin Attenuates Cerebral Ischaemia/Reperfusion Injury by Inhibiting Ferroptosis. Cell Death Dis. 2021, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative Proteomics Identifies NCOA4 as the Cargo Receptor Mediating Ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Wesselius, L.J.; Nelson, M.E.; Skikne, B.S. Increased Release of Ferritin and Iron by Iron-Loaded Alveolar Macrophages in Cigarette Smokers. Am. J. Respir. Crit. Care Med. 1994, 150, 690–695. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kalantar-Zadeh, K.; Lee, G.H. The Fascinating but Deceptive Ferritin: To Measure It or Not to Measure It in Chronic Kidney Disease? Clin. J. Am. Soc. Nephrol. 2006, 1, S9–S18. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Höffken, B.; Wünsch, H.; Fink, H.; Kleiner, M.; Luft, F.C. Diagnosis of Iron Deficiency Anemia in Renal Failure Patients during the Post-Erythropoietin Era. Am. J. Kidney Dis. 1995, 26, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Domrongkitchaiporn, S.; Jirakranont, B.; Atamasrikul, K.; Ungkanont, A.; Bunyaratvej, A. Indices of Iron Status in Continuous Ambulatory Peritoneal Dialysis Patients. Am. J. Kidney Dis. 1999, 34, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Canavese, C.; Bergamo, D.; Ciccone, G.; Longo, F.; Fop, F.; Thea, A.; Martina, G.; Piga, A. Validation of Serum Ferritin Values by Magnetic Susceptometry in Predicting Iron Overload in Dialysis Patients. Kidney Int. 2004, 65, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, G.; Griuncelli, M.; Loridon, C.; Magna, T.; Machado, G.; Drahi, G.; Dahan, H.; Janklewicz, P.; Cohen, Y. Reassessment of Iron Biomarkers for Prediction of Dialysis Iron Overload: An MRI Study. PLoS ONE 2015, 10, e0132006. [Google Scholar] [CrossRef] [PubMed]
- Bartnikas, T.B. Known and Potential Roles of Transferrin in Iron Biology. Biometals 2012, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Gomme, P.T.; McCann, K.B.; Bertolini, J. Transferrin: Structure, Function and Potential Therapeutic Actions. Drug Discov. Today 2005, 10, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Besarab, A.; Drueke, T.B. The Problem with Transferrin Saturation as an Indicator of Iron ‘Sufficiency’ in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2021, 36, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Hamano, T.; Fujii, N.; Hayashi, T.; Yamamoto, H.; Iseki, K.; Tsubakihara, Y. Thresholds of Iron Markers for Iron Deficiency Erythropoiesis-Finding of the Japanese Nationwide Dialysis Registry. Kidney Int. Suppl. 2015, 5, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kuragano, T.; Joki, N.; Hase, H.; Kitamura, K.; Murata, T.; Fujimoto, S.; Fukatsu, A.; Inoue, T.; Itakura, Y.; Nakanishi, T. Low Transferrin Saturation (TSAT) and High Ferritin Levels Are Significant Predictors for Cerebrovascular and Cardiovascular Disease and Death in Maintenance Hemodialysis Patients. PLoS ONE 2020, 15, e0236277. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C. Reticulocyte Cellular Indices: A New Approach in the Diagnosis of Anemias and Monitoring of Erythropoietic Function. Crit. Rev. Clin. Lab. Sci. 2000, 37, 93–130. [Google Scholar] [CrossRef]
- Fishbane, S.; Shapiro, W.; Dutka, P.; Valenzuela, O.F.; Faubert, J. A Randomized Trial of Iron Deficiency Testing Strategies in Hemodialysis patients. Kidney Int. 2001, 60, 2406–2411. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, C.; Tsuchiya, K.; Maeda, K. Reticulocyte Hemoglobin Content. Clin. Chim. Acta 2020, 504, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Galgano, C.; Langley, R.C.; Canfield, W.; Maesaka, J.K. Reticulocyte Hemoglobin Content in the Evaluation of Iron Status of Hemodialysis Patients. Kidney Int. 1997, 52, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, N.; Solero, G.P.; Lippi, G.; Bassi, A.; Faccini, G.B.; Bedogna, V.; Gammaro, L.; Brocco, G.; Restivo, G.; Bernich, P.; et al. The Role of Iron Status Markers in Predicting Response to Intravenous Iron in Haemodialysis Patients on Maintenance Erythropoietin. Nephrol. Dial. Transplant. 2001, 16, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Mitsuiki, K.; Miyata, Y. Assessment of Iron Deficiency in Chronic Hemodialysis Patients: Investigation of Cutoff Values for Reticulocyte Hemoglobin Content. Clin. Exp. Nephrol. 2003, 7, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Ihm, C.H.; Kim, H.J. Evaluation of Reticulocyte Haemoglobin Content as Marker of Iron Deficiency and Predictor of Response to Intravenous Iron in Haemodialysis Patients. Int. J. Lab. Hematol. 2008, 30, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kee, Y.K.; Jeon, H.J.; Oh, J.; Shin, D.H. Hypochromic Red Cells as Predictors of Anemia in Patients Undergoing Hemodialysis: An Observational Retrospective Study. Sci. Rep. 2021, 11, 24215. [Google Scholar] [CrossRef] [PubMed]
- Dinh, N.H.; Cheanh Beaupha, S.M.; Tran, L.T.A. The Validity of Reticulocyte Hemoglobin Content and Percentage of Hypochromic Red Blood Cells for Screening Iron-Deficiency Anemia among Patients with End-Stage Renal Disease: A Retrospective Analysis. BMC Nephrol. 2020, 21, 142. [Google Scholar] [CrossRef] [PubMed]
- Nahm, C.H.; Lee, M.H.; Fujii, T.; Fujii, N.; Choi, J.W. Lipocalin-2, Soluble Transferrin Receptor, and Erythropoietin in Anemia During Mild Renal Dysfunction. Int. J. Gen. Med. 2023, 16, 3603–3612. [Google Scholar] [CrossRef] [PubMed]
- Chua, E. Serum Transferrin Receptor Assay in Iron Deficiency Anaemia and Anaemia of Chronic Disease in the Elderly. QJM 1999, 92, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.-C.; Tsai, T.-J.; Chen, Y.-M.; Lin, S.-L.; Hsieh, B.-S. Serum Soluble Transferrin Receptor Reflects Erythropoiesis but Not Iron Availability in Erythropoietin-Treated Chronic Hemodialysis Patients. Clin. Nephrol. 2002, 58, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Munaretto, G.; Spinello, M.; Rebeschini, M.; Amici, G.; Gallieni, M.; Piccoli, A. Soluble Transferrin Receptors and Reticulocyte Hemoglobin Concentration in the Assessment of Iron Deficiency in Hemodialysis Patients. J. Nephrol. 2005, 18, 72–79. [Google Scholar] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.; Kaiser, T. Beyond Soluble Transferrin Receptor: Old Challenges and New Horizons. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Nemeth, E.; Swinkels, D.W. Hepcidin in the Diagnosis of Iron Disorders. Blood 2016, 127, 2809–2813. [Google Scholar] [CrossRef] [PubMed]
- Troutt, J.S.; Butterfield, A.M.; Konrad, R.J. Hepcidin-25 Concentrations Are Markedly Increased in Patients with Chronic Kidney Disease and Are Inversely Correlated with Estimated Glomerular Filtration Rates: Human Hepcidin-25 in Chronic Kidney Disease. J. Clin. Lab. Anal. 2013, 27, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Niihata, K.; Tomosugi, N.; Uehata, T.; Shoji, T.; Mitsumoto, K.; Shimizu, M.; Kawabata, H.; Sakaguchi, Y.; Suzuki, A.; Hayashi, T.; et al. Serum Hepcidin-25 Levels Predict the Progression of Renal Anemia in Patients with Non-Dialysis Chronic Kidney Disease. Nephrol. Dial. Transplant. 2012, 27, 4378–4385. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Hu, Y.; Gao, Y.; Ma, X.; Hu, Z. The Association of Hepcidin, Reticulocyte Hemoglobin Equivalent and Anemia-Related Indicators on Anemia in Chronic Kidney Disease. Medicine 2023, 102, e33558. [Google Scholar] [CrossRef] [PubMed]
- Niikura, T.; Maruyama, Y.; Nakashima, S.; Matsuo, N.; Tanno, Y.; Ohkido, I.; Yokoyama, K.; Yamamoto, H.; Yokoo, T. Hepcidin/Ferritin Ratios Differ Among Non-Dialyzed Chronic Kidney Disease Patients, and Patients on Hemodialysis and Peritoneal Dialysis. Ther. Apher. Dial. 2019, 23, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.P.E.; Rumjon, A.; Bansal, S.S.; Laarakkers, C.M.M.; Van Den Brand, J.A.J.G.; Sarafidis, P.; Musto, R.; Malyszko, J.; Swinkels, D.W.; Wetzels, J.F.M.; et al. Intra-Individual Variability of Serum Hepcidin-25 in Haemodialysis Patients Using Mass Spectrometry and ELISA. Nephrol. Dial. Transplant. 2012, 27, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Aghsaeifard, Z.; Alizadeh, R.; Bagheri, N. Association between Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Iron Profile in Chronic Renal Disease. Arch. Physiol. Biochem. 2022, 128, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Kim, J.H.; Lee, D.W.; Lee, S.B.; Rhee, H.; Song, S.H.; Seong, E.Y.; Kwak, I.S. Plasma Neutrophil Gelatinase-Associated Lipocalin Is Associated with Iron Status in Anemic Patients with Pre-Dialysis Chronic Kidney Disease. Clin. Exp. Nephrol. 2018, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wang, X.; Liu, P.; Pan, Y.; Zhang, Q.; Chi, X.; Jing, Y.; Duan, X.; Wei, Q.; Wang, J.; et al. Increased NGAL Level Associated with Iron Store in Chronic Kidney Disease with Anemia. Clin. Exp. Med. 2018, 18, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Pazianas, M.; Miller, P.D. Osteoporosis and Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD): Back to Basics. Am. J. Kidney Dis. 2021, 78, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Napoletano, A.; Provenzano, M.; Garofalo, C.; Bini, C.; Comai, G.; La Manna, G. Mineral Bone Disorders in Kidney Disease Patients: The Ever-Current Topic. Int. J. Mol. Sci. 2022, 23, 12223. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Kim, H.; An, S.Y.; Lee, M.; Cha, M.-U.; Park, J.T.; Yoo, T.-H.; Lee, K.-B.; Kim, Y.-H.; Sung, S.-A.; et al. Circulating Fibroblast Growth Factor-23 Levels Are Associated with an Increased Risk of Anemia Development in Patients with Nondialysis Chronic Kidney Disease. Sci. Rep. 2018, 8, 7294. [Google Scholar] [CrossRef] [PubMed]
- Clinkenbeard, E.L.; Hanudel, M.R.; Stayrook, K.R.; Appaiah, H.N.; Farrow, E.G.; Cass, T.A.; Summers, L.J.; Ip, C.S.; Hum, J.M.; Thomas, J.C.; et al. Erythropoietin Stimulates Murine and Human Fibroblast Growth Factor-23, Revealing Novel Roles for Bone and Bone Marrow. Haematologica 2017, 102, e427–e430. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Tanaka, K.; Michihata, T.; Shibagaki, K.; Yuza, T.; Hirao, K.; Tomosugi, N.; Ganz, T.; Higashimoto, Y. Erythropoiesis Stimulating Agents Are Associated with Serum Fibroblast Growth Factor 23 Metabolism in Patients on Hemodialysis. Clin. Kidney J. 2021, 14, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Noonan, M.L.; Ni, P.; Agoro, R.; Sacks, S.A.; Swallow, E.A.; Wheeler, J.A.; Clinkenbeard, E.L.; Capitano, M.L.; Prideaux, M.; Atkins, G.J.; et al. The HIF-PHI BAY 85-3934 (Molidustat) Improves Anemia and Is Associated with Reduced Levels of Circulating FGF23 in a CKD Mouse Model. J. Bone Miner. Res. 2020, 36, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, C.; Tsuchiya, K.; Nitta, K.; Maeda, K. Significance of Content of the Reticulocyte Hemoglobin in the Management of Renal Anemia. Blood Purif. 2019, 47 (Suppl. 2), 70–73. [Google Scholar] [CrossRef]
- Bovy, C.; Tsobo, C.; Crapanzano, L.; Rorive, G.; Beguin, Y.; Albert, A.; Paulus, J.M. Factors Determining the Percentage of Hypochromic Red Blood Cells in Hemodialysis Patients. Kidney Int. 1999, 56, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.M.; Speeckaert, R.; Delanghe, J.R. Biological and Clinical Aspects of Soluble Transferrin Receptor. Crit. Rev. Clin. Lab. Sci. 2010, 47, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Markó, L.; Paragas, N.; Barasch, J.; Dragun, D.; Müller, D.N.; Budde, K.; Schmidt-Ott, K.M. Neutrophil Gelatinase-associated Lipocalin: Pathophysiology and Clinical Applications. Acta Physiol. 2013, 207, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Drüeke, T.B.; Parfrey, P.S. Summary of the KDIGO Guideline on Anemia and Comment: Reading between the (Guide)Line(s). Kidney Int. 2012, 82, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, A.V.; Levin, A.; Locatelli, F.; Małyszko, J.; Swinkels, D.W.; Tarng, D.-C.; Cheung, M.; et al. Controversies in Optimal Anemia Management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron Balance and the Role of Hepcidin in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Wada, A.; Masakane, I. Types of Erythropoietin-Stimulating Agents and Mortality among Patients Undergoing Hemodialysis. J. Am. Soc. Nephrol. 2019, 30, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, R.; Qadri, S.M.; Artunc, F. Eryptosis: A Driver of Anemia in Chronic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2024, 33, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Borzych-Duzalka, D.; Bilginer, Y.; Ha, I.S.; Bak, M.; Rees, L.; Cano, F.; Munarriz, R.L.; Chua, A.; Pesle, S.; Emre, S.; et al. Management of Anemia in Children Receiving Chronic Peritoneal Dialysis. J. Am. Soc. Nephrol. 2013, 24, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Cernaro, V.; Coppolino, G.; Visconti, L.; Rivoli, L.; Lacquaniti, A.; Santoro, D.; Buemi, A.; Loddo, S.; Buemi, M. Erythropoiesis and Chronic Kidney Disease–Related Anemia: From Physiology to New Therapeutic Advancements. Med. Res. Rev. 2019, 39, 427–460. [Google Scholar] [CrossRef] [PubMed]
- Sturiale, A.; Campo, S.; Crasci, E.; Coppolino, G.; Bolignano, D.; Grasso, G.; Buemi, M. Erythropoietin and Its Lost Receptor. Nephrol. Dial. Transplant. 2007, 22, 1484–1485. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Grandaliano, G.; Di Rienzo, P.; Snijder, R.; Degli Esposti, L.; Perrone, V.; Todorova, L. Prevalence, Incidence, and Treatment of Anaemia in Patients with Non-Dialysis-Dependent Chronic Kidney Disease: Findings from a Retrospective Real-World Study in Italy. J. Nephrol. 2022, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, L.; Minutolo, R.; Conte, G. Anaemia Management in Non-Dialysis Chronic Kidney Disease: Flexibility of Target to Target Stability? Nephron Clin. Pract. 2010, 114, c236–c241. [Google Scholar] [CrossRef] [PubMed]
- Sanghani, N.S.; Haase, V.H. Hypoxia-Inducible Factor Activators in Renal Anemia: Current Clinical Experience. Adv. Chronic Kidney Dis. 2019, 26, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Haase, V.H. Hypoxia-Inducible Factor–Prolyl Hydroxylase Inhibitors in the Treatment of Anemia of Chronic Kidney Disease. Kidney Int. Suppl. 2021, 11, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Hao, C.; Peng, X.; Lin, H.; Yin, A.; Hao, L.; Tao, Y.; Liang, X.; Liu, Z.; Xing, C.; et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N. Engl. J. Med. 2019, 381, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Hao, C.; Liu, B.-C.; Lin, H.; Wang, C.; Xing, C.; Liang, X.; Jiang, G.; Liu, Z.; Li, X.; et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N. Engl. J. Med. 2019, 381, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Qian, J.; Chen, J.; Yu, X.; Mei, C.; Hao, C.; Jiang, G.; Lin, H.; Zhang, X.; Zuo, L.; et al. Phase 2 Studies of Oral Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor FG-4592 for Treatment of Anemia in China. Nephrol. Dial. Transplant. 2017, 32, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Koiwa, F.; Akizawa, T. Anemia in Conventional Hemodialysis: Finding the Optimal Treatment Balance. Semin. Dial. 2018, 31, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, R.; Zheng, Z.; Jiang, L.; Xu, Y.; Raj, A.; Sun, D. Effect of Roxadustat on Iron Metabolism in Patients with Peritoneal Dialysis: A Real-World 24-Week Study. Eur. J. Med. Res. 2023, 28, 489. [Google Scholar] [CrossRef] [PubMed]
- Joharapurkar, A.A.; Pandya, V.B.; Patel, V.J.; Desai, R.C.; Jain, M.R. Prolyl Hydroxylase Inhibitors: A Breakthrough in the Therapy of Anemia Associated with Chronic Diseases. J. Med. Chem. 2018, 61, 6964–6982. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, C.; Tsuchiya, K.; Maeda, K. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors and Iron Metabolism. Int. J. Mol. Sci. 2023, 24, 3037. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shou, X.; Xu, Y.; Jin, L.; Zhu, C.; Ye, X.; Mei, Z.; Chen, P. A Network Meta-Analysis of the Efficacy of Hypoxia-Inducible Factor Prolyl-Hydroxylase Inhibitors in Dialysis Chronic Kidney Disease. Aging 2023, 15, 2237–2274. [Google Scholar] [CrossRef] [PubMed]
- Coyne, D.W.; Singh, A.K.; Lopes, R.D.; Bailey, C.K.; DiMino, T.L.; Huang, C.; Connaire, J.; Rastogi, A.; Kim, S.-G.; Orias, M.; et al. Three Times Weekly Dosing of Daprodustat versus Conventional Epoetin for Treatment of Anemia in Hemodialysis Patients: ASCEND-TD: A Phase 3 Randomized, Double-Blind, Noninferiority Trial. Clin. J. Am. Soc. Nephrol. 2022, 17, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Cobitz, A.R.; Singh, A.K.; Macdougall, I.C.; Lopes, R.D.; Obrador, G.T.; Kovesdy, C.P.; Israni, R.; Jha, V.; Okoro, T.; et al. The ASCEND-NHQ Randomized Trial Found Positive Effects of Daprodustat on Hemoglobin and Quality of Life in Patients with Non-Dialysis Chronic Kidney Disease. Kidney Int. 2023, 103, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, Y.; Yang, H.; Sun, L.; Zhang, P.; Zhang, X.; Guo, J.; Liu, Y.N.; Liu, W.J. Cardiac and Kidney Adverse Effects of HIF Prolyl-Hydroxylase Inhibitors for Anemia in Patients with CKD Not Receiving Dialysis: A Systematic Review and Meta-Analysis. Am. J. Kidney Dis. 2023, 81, 434–445.e1. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K. HIF-α Prolyl Hydroxylase Inhibitors and Their Implications for Biomedicine: A Comprehensive Review. Biomedicines 2021, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, H.; Hagiwara, H.; Kamiya, T. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors for Anemia in Heart Failure Patients: A Protocol for Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0275311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, P.; Zhang, H.-L.; Jia, L. An Updated Meta-Analysis on the Efficacy and Safety of Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor Treatment of Anemia in Nondialysis-Dependent Chronic Kidney Disease. Ren. Fail. 2023, 45, 2258986. [Google Scholar] [CrossRef] [PubMed]
- Petzer, V.; Tymoszuk, P.; Asshoff, M.; Carvalho, J.; Papworth, J.; Deantonio, C.; Bayliss, L.; Wake, M.S.; Seifert, M.; Brigo, N.; et al. A Fully Human Anti-BMP6 Antibody Reduces the Need for Erythropoietin in Rodent Models of the Anemia of Chronic Disease. Blood 2020, 136, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Zinman, B.; Fitchett, D.; Wanner, C.; Ferrannini, E.; Schumacher, M.; Schmoor, C.; Ohneberg, K.; Johansen, O.E.; George, J.T.; et al. How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2018, 41, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Neal, B.; Perkovic, V.; De Zeeuw, D.; Neuen, B.L.; Arnott, C.; Simpson, R.; Oh, R.; Mahaffey, K.W.; Heerspink, H.J.L. Mediators of the Effects of Canagliflozin on Kidney Protection in Patients with Type 2 Diabetes. Kidney Int. 2020, 98, 769–777. [Google Scholar] [CrossRef]
- Baruah, M.; Makkar, B.; Ghatnatti, V.; Mandal, K. Sodium Glucose Co-Transporter-2 Inhibitor: Benefits beyond Glycemic Control. Indian J. Endocr. Metab. 2019, 23, 140. [Google Scholar] [CrossRef] [PubMed]
- Barrand, M.A.; Callingham, B.A.; Hider, R.C. Effects of the Pyrones, Maltol and Ethyl Maltol, on Iron Absorption from the Rat Small Intestine. J. Pharm. Pharmacol. 2011, 39, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Fishbane, S.; Ganz, T. Novel Oral Iron Therapies for Iron Deficiency Anemia in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Kopyt, N.P. Oral Ferric Maltol for the Treatment of Iron-Deficiency Anemia in Patients with CKD: A Randomized Trial and Open-Label Extension. Am. J. Kidney Dis. 2021, 78, 846–856.e1. [Google Scholar] [CrossRef] [PubMed]
- Brilli, E.; Romano, A.; Fabiano, A.; Zambito, Y.; Di Raimondo, F.; Tarantino, G. Sucrosomial Technology Is Able to Promote Ferric Iron Absorption: Pre-Clinical and Clinical Evidences. Blood 2016, 128, 3618. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Muñoz, M. Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation. Pharmaceuticals 2018, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Riccio, E.; Sabbatini, M.; Andreucci, M.; Del Rio, A.; Visciano, B. Effect of Oral Liposomal Iron versus Intravenous Iron for Treatment of Iron Deficiency Anaemia in CKD Patients: A Randomized Trial. Nephrol. Dial. Transplant. 2015, 30, 645–652. [Google Scholar] [CrossRef]
- Shepshelovich, D.; Rozen-Zvi, B.; Avni, T.; Gafter, U.; Gafter-Gvili, A. Intravenous Versus Oral Iron Supplementation for the Treatment of Anemia in CKD: An Updated Systematic Review and Meta-Analysis. Am. J. Kidney Dis. 2016, 68, 677–690. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Davidson, M.; Jensen, C.; Mohseni Zonoozi, A.A.; Raj, D.S.; Andreas Schytz, P.; Tuttle, K.R.; Perkovic, V. Effect of Ziltivekimab on Determinants of Hemoglobin in Patients with CKD Stage 3–5: An Analysis of a Randomized Trial (RESCUE). J. Am. Soc. Nephrol. 2024, 35, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-R.; Park, S.K.; Jung, J.Y.; Kim, Y.H.; Oh, Y.K.; Yoo, T.H.; Sung, S. The Prevalence and Management of Anemia in Chronic Kidney Disease Patients: Result from the KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). J. Korean Med. Sci. 2017, 32, 249. [Google Scholar] [CrossRef] [PubMed]
- Raichoudhury, R.; Spinowitz, B.S. Treatment of Anemia in Difficult-to-Manage Patients with Chronic Kidney Disease. Kidney Int. Suppl. 2021, 11, 26–34. [Google Scholar] [CrossRef] [PubMed]
| CKD Stage | Testing Frequency (at Least) |
|---|---|
| G3 | Once per year |
| G4–G5 non-dialyzed | Twice per year |
| G4–G5 dialyzed | Every 3 months |
| Guideline | Hb * | TSAT ** | Ferritin ** | CHr ** | %HRC ** |
|---|---|---|---|---|---|
| KDIGO 2012 [54] | F: <12 g/dL M: <13 g/dL | <30% *** | <500 ng/mL *** | - | - |
| KDOQI 2006 4 [59] | F: <12 g/dL M: <13.5 g/dL | ≤20% | ≤100 ng/mL in PD and ND ≤200 ng/mL in HD | - | - |
| Renal Association 2017 [57] | Men and postmenopausal F: <13 g/dL Premenopausal F: <12 g/dL | ≤20% | <100 ng/mL in PD and ND <200 ng/mL in HD | ≤29 pg | ≥6% |
| ERBP 2017 [60] | F: <12 g/dL M: <13.5 g/dL (in males >70 years <13.2 g/dL) | <20% in ND *** <25% in HD and PD *** <30% in patients on ESA *** | <100 ng/mL in ND *** <300 ng/mL in HD, PD *** <300 ng/mL in patients on ESA *** | - | - |
| Increase | Decrease | |
|---|---|---|
| Serum ferritin [67] |
|
|
| TSAT [77,78,79] | As a result of TIBC decrease:
| As a result of iron decrease:
|
| CHr [84,112,113] | Iron supplementation | Iron deficiency |
| %HRC [113] |
| Iron supplementation |
| sTFR [96,114] |
|
|
| Hepcidin-25 [97] |
|
|
| NGAL [115] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badura, K.; Janc, J.; Wąsik, J.; Gnitecki, S.; Skwira, S.; Młynarska, E.; Rysz, J.; Franczyk, B. Anemia of Chronic Kidney Disease—A Narrative Review of Its Pathophysiology, Diagnosis, and Management. Biomedicines 2024, 12, 1191. https://doi.org/10.3390/biomedicines12061191
Badura K, Janc J, Wąsik J, Gnitecki S, Skwira S, Młynarska E, Rysz J, Franczyk B. Anemia of Chronic Kidney Disease—A Narrative Review of Its Pathophysiology, Diagnosis, and Management. Biomedicines. 2024; 12(6):1191. https://doi.org/10.3390/biomedicines12061191
Chicago/Turabian StyleBadura, Krzysztof, Jędrzej Janc, Joanna Wąsik, Szymon Gnitecki, Sylwia Skwira, Ewelina Młynarska, Jacek Rysz, and Beata Franczyk. 2024. "Anemia of Chronic Kidney Disease—A Narrative Review of Its Pathophysiology, Diagnosis, and Management" Biomedicines 12, no. 6: 1191. https://doi.org/10.3390/biomedicines12061191
APA StyleBadura, K., Janc, J., Wąsik, J., Gnitecki, S., Skwira, S., Młynarska, E., Rysz, J., & Franczyk, B. (2024). Anemia of Chronic Kidney Disease—A Narrative Review of Its Pathophysiology, Diagnosis, and Management. Biomedicines, 12(6), 1191. https://doi.org/10.3390/biomedicines12061191








