Combination of Neovestitol and Vestitol Modifies the Profile of Periodontitis-Related Subgingival Multispecies Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Biofilm Formation
2.2. CNV Obtainment
2.3. 24 h Treatment with CNV on Mature Biofilm
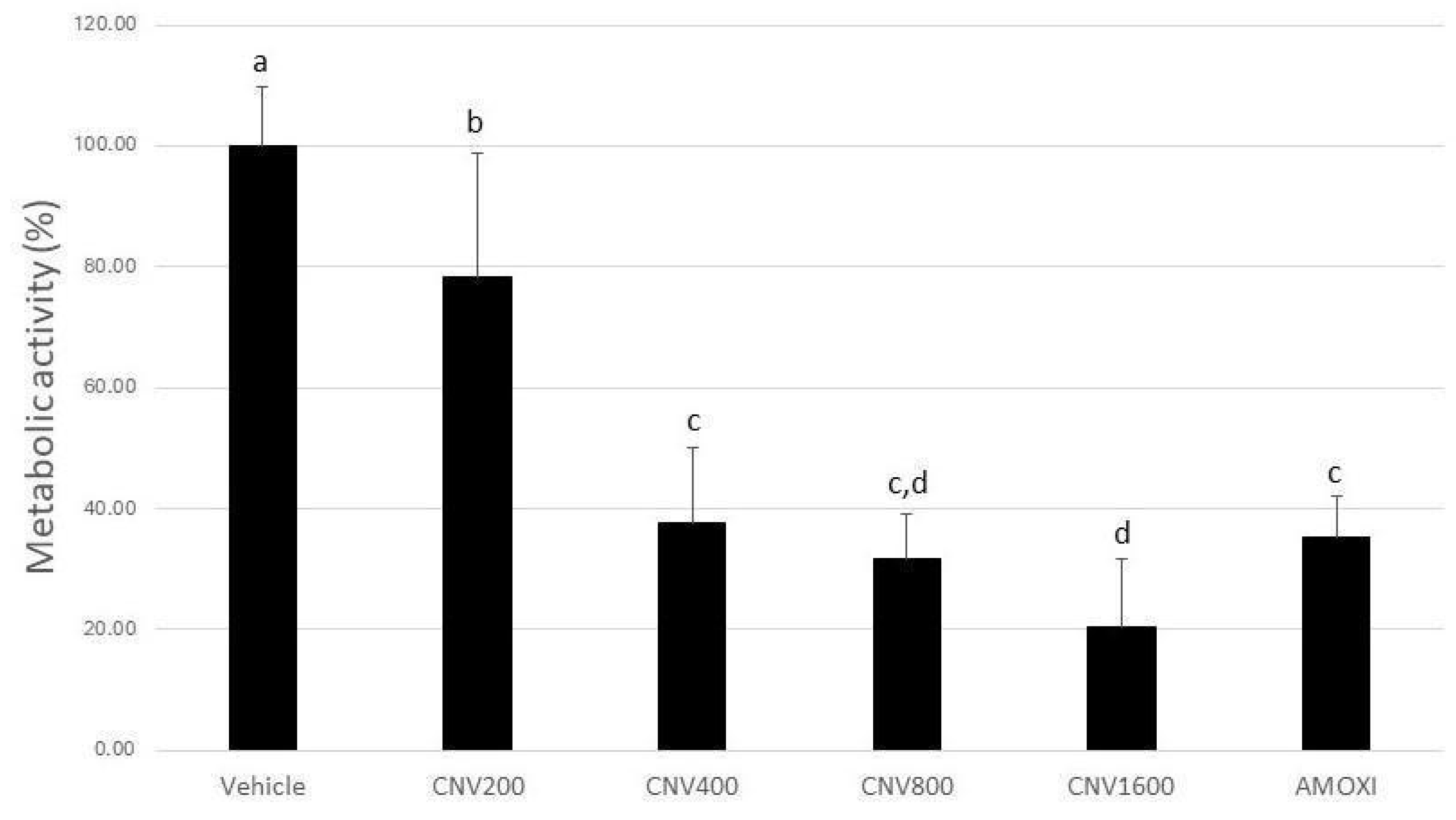
2.4. Biofilm Metabolic Activity
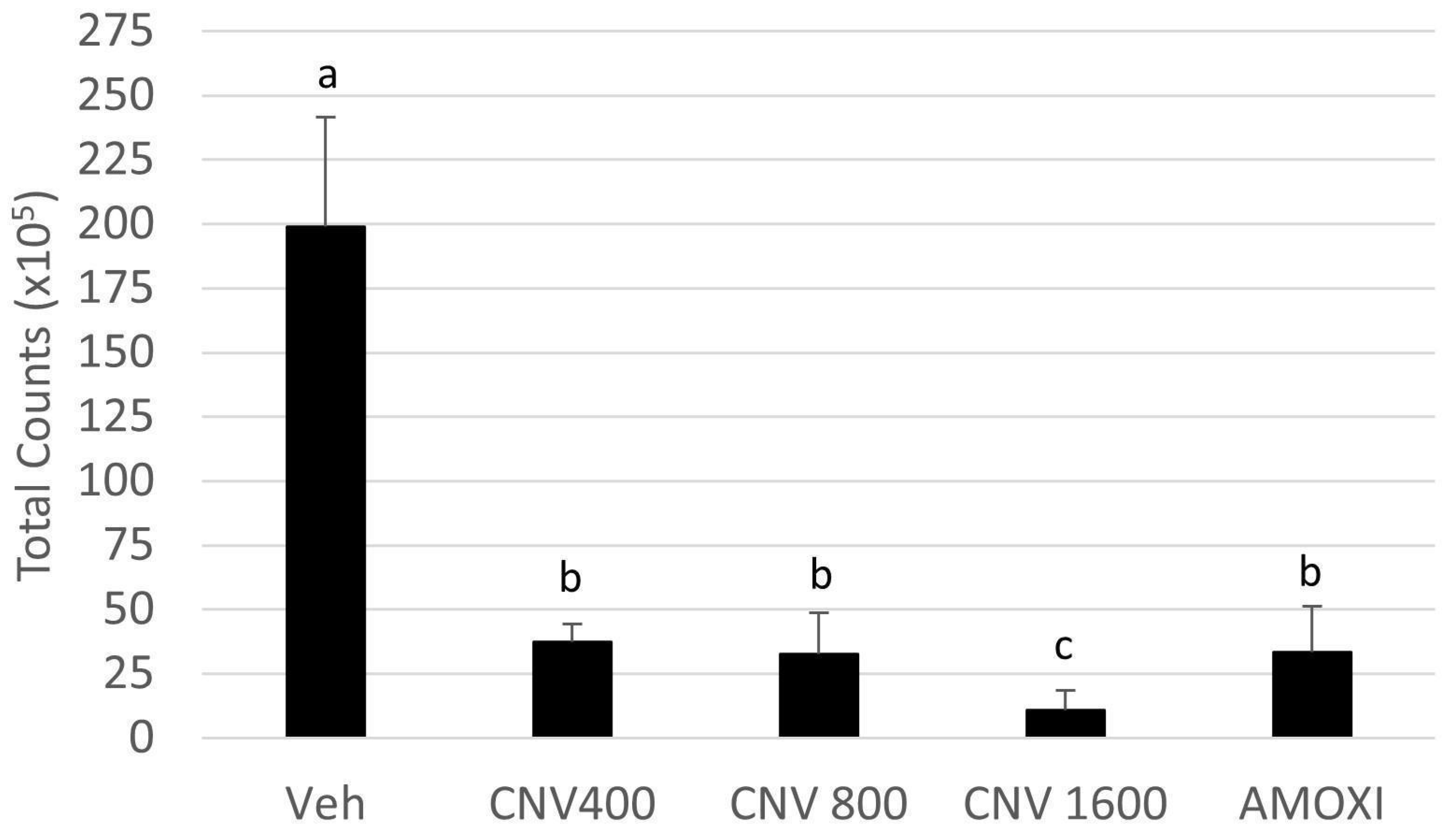
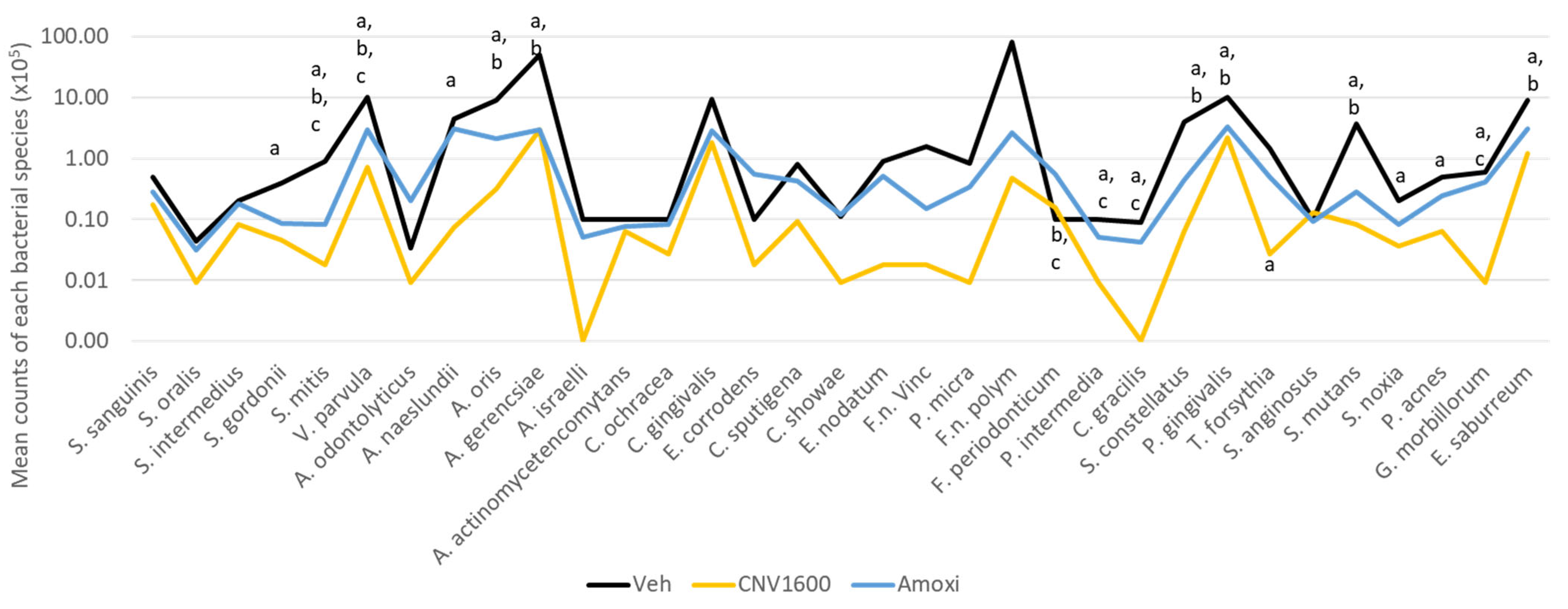
2.5. DNA–DNA Hybridization (Checkerboard)
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, E.; Tay, J.R.H.; Ong, M.M.A. Minimally invasive periodontology: A treatment philosophy and suggested approach. Int. J. Dent. 2021, 2021, 2810264. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus between periodontal inflammation and dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Feres, M.; Oud, V.; Martin, C.; Matesanz, P.; Herrera, D. Adjunctive effect of systemic antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. S20), 257–281. [Google Scholar] [CrossRef]
- Ng, E.; Tay, J.R.H.; Saffari, S.E.; Lim, L.P.; Chung, K.M.; Ong, M.M.A. Adjunctive probiotics after periodontal debridement versus placebo: A systematic review and meta-analysis. Acta Odontol. Scand. 2022, 80, 81–90. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Zhou, Y.; Buckley, T.; Wang, H.H. Antibiotic administration routes significantly influence the levels of antibiotic resistance in gut microbiota. Antimicrob. Agents Chemother. 2013, 57, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Ng, E.; Tay, J.R.H.; Boey, S.K.; Laine, M.L.; Ivanovski, S.; Seneviratne, C.J. Antibiotic resistance in the microbiota of periodontitis patients: An update of current findings. Crit. Rev. Microbiol. 2024, 50, 329–340. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Kawamoto, D.; Ando-Suguimoto, E.S.; Alencar, S.M.; Rosalen, P.L.; Mayer, M.P.A. Brazilian red propolis attenuates inflammatory signaling cascade in lps-activated macrophages. PLoS ONE 2015, 10, e0144954. [Google Scholar] [CrossRef]
- Tiveron, A.P.; Rosalen, P.L.; Franchin, M.; Lacerda, R.C.C.; Bueno-Silva, B.; Benso, B.; Denny, C.; Ikegaki, M.; de Alencar, S.M. Chemical characterization and antioxidant, antimicrobial, and anti-inflammatory activities of south brazilian organic propolis. PLoS ONE 2016, 11, e0165588. [Google Scholar] [CrossRef] [PubMed]
- Banzato, T.P.; Gubiani, J.R.; Bernardi, D.I.; Nogueira, C.R.; Monteiro, A.F.; Juliano, F.F.; de Alencar, S.M.; Pilli, R.A.; de Lima, C.A.; Longato, G.B.; et al. Antiproliferative flavanoid dimers isolated from brazilian red propolis. J. Nat. Prod. 2020, 83, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.B.; Rosalen, P.L.; Cury, J.A.; Ikegaki, M.; Souza, V.C.; Esteves, A.; Alencar, S.M. Chemical composition and botanical origin of red propolis, a new type of brazilian propolis. Evid. Based Complement. Altern. Med. 2008, 5, 313–316. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Rosalen, P.L.; Alencar, S.M.; Mayer, M.P. Anti-inflammatory mechanisms of neovestitol from Brazilian red propolis in LPS-activated macrophages. J. Funct. Foods 2017, 36, 440–447. [Google Scholar] [CrossRef]
- Silva, N.B.S.; de Souza, J.H.; Santiago, M.B.; Aguiar, J.R.d.S.; Martins, D.O.S.; da Silva, R.A.; Santos, I.d.A.; Aldana-Mejía, J.A.; Jardim, A.C.G.; Pedroso, R.d.S.; et al. Potential in vitro anti-periodontopathogenic, anti-Chikungunya activities and in vivo toxicity of Brazilian red propolis. Sci. Rep. 2022, 12, 21165. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; de Alencar, S.M.; Rosalen, P.L. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur. J. Med. Chem. 2016, 110, 267–279. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.C.D.; de Miranda, M.B.; de Oliveira, D.T.; Vieira-Filho, S.A.; Caligiorne, R.B.; de Figueiredo, S.M. Biological activities of red propolis: A review. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017, 11, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Alencar, S.M.; Koo, H.; Ikegaki, M.; Silva, G.V.J.; Napimoga, M.H.; Rosalen, P.L. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from brazilian red propolis. J. Agric. Food Chem. 2013, 61, 4546–4550. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Koo, H.; Falsetta, M.; Alencar, S.; Ikegaki, M.; Rosalen, P. Effect of neovestitol–vestitol containing Brazilian red propolis on accumulation of biofilm in vitro and development of dental caries in vivo. Biofouling 2013, 29, 1233–1242. [Google Scholar] [CrossRef]
- Soares, G.M.S.; Teles, F.; Starr, J.R.; Feres, M.; Patel, M.; Martin, L.; Teles, R. Effects of azithromycin, metronidazole, amoxicillin, and metronidazole plus amoxicillin on an in vitro polymicrobial subgingival biofilm model. Antimicrob. Agents Chemother. 2015, 59, 2791–2798. [Google Scholar] [CrossRef]
- Miranda, S.L.F.; Damasceno, J.T.; Faveri, M.; Figueiredo, L.; da Silva, H.D.; Alencar, S.M.d.A.; Rosalen, P.L.; Feres, M.; Bueno-Silva, B. Brazilian red propolis reduces orange-complex periodontopathogens growing in multispecies biofilms. Biofouling 2019, 35, 308–319. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, K.A.; da Silva, H.D.P.; Miranda, S.L.F.; Gonçalves, F.J.d.S.; de Sousa, A.P.; de Figueiredo, L.C.; Feres, M.; Bueno-Silva, B. Brazilian red propolis is as effective as amoxicillin in controlling red-complex of multispecies subgingival mature biofilm in vitro. Antibiotics 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Dzink, J.L. Relationship of subgingival microbial complexes to clinical features at the sampled sites. J. Clin. Periodontol. 1988, 15, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Jiao, Y.; Hasegawa, M.; Inohara, N. The role of oral pathobionts in dysbiosis during periodontitis development. J. Dent. Res. 2014, 93, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.; Tay, J.R.H.; Balan, P.; Ong, M.M.A.; Bostanci, N.; Belibasakis, G.N.; Seneviratne, C.J. Metagenomic sequencing provides new insights into the subgingival bacteriome and aetiopathology of periodontitis. J. Periodontal Res. 2021, 56, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.L.; Lourenco, T.G.B.; Colombo, A.P.V. Periodontal disease severity is associated to pathogenic consortia comprising putative and candidate periodontal pathogens. J. Appl. Oral Sci. 2023, 31, e20220359. [Google Scholar] [CrossRef]
- Aja, E.; Mangar, M.; Fletcher, H.; Mishra, A. Filifactor alocis: Recent insights and advances. J. Dent. Res. 2021, 100, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J.; Koo, H. Oral polymicrobial communities: Assembly, function, and impact on diseases. Cell Host Microbe 2023, 31, 528–538. [Google Scholar] [CrossRef]
- Hajishengallis, G. Oral bacteria and leaky endothelial junctions in remote extraoral sites. FEBS J. 2021, 288, 1475–1478. [Google Scholar] [CrossRef]
- Lim, Y.; Kim, H.Y.; Han, D.; Choi, B. Proteome and immune responses of extracellular vesicles derived from macrophages infected with the periodontal pathogen Tannerella forsythia. J. Extracell. Vesicles 2023, 12, e12381. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E. Oral Microbial Communities: Biofilms, Interactions, and Genetic Systems. Annu. Rev. Microbiol. 2000, 54, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.P.V.; Teles, R.P.; Torres, M.C.; Souto, R.; Rosalém, W., Jr.; Mendes, M.C.S.; Uzeda, M. Subgingival microbiota of brazilian subjects with untreated chronic periodontitis. J. Periodontol. 2002, 73, 360–369. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.D.P.; Moreno, J.d.A.; Roque, S.M.; Chan, D.C.H.; Torrez, W.B.; Stipp, R.N.; Bueno-Silva, B.; de Lima, P.O.; Cogo-Müller, K. Statins and oral biofilm: Simvastatin as a promising drug to control periodontal dysbiosis. Oral Dis. 2024, 30, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Kiausinus, K.R.; Goncalves, F.J.; Moreira, M.V.C.; de Oliveira, E.G.; Junior, A.B.; Feres, M.; Figueiredo, L.C. Antimicrobial activity of Desplac® oral gel in the subgingival multispecies biofilm formation. Front. Microbiol. 2023, 14, 1122051. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Rocha, T.F.; Coelho, F.; de Oliveira Capote, T.S.; Saska, S.; Melo, M.A.; Pingueiro, J.M.S.; de Faveri, M.; Bueno-Silva, B. Metabolic activity of hydro-carbon-oxo-borate on a multispecies subgingival periodontal biofilm: A short communication. Clin. Oral Investig. 2021, 25, 5945–5953. [Google Scholar] [CrossRef] [PubMed]
- Saliba, A.S.M.C.; Quirino, D.J.G.; Favaro-Trindade, C.S.; de Oliveira Sartori, A.G.; Massarioli, A.P.; Lazarini, J.G.; de Souza Silva, A.P.; de Alencar, S.M. Effects of simulated gastrointestinal digestion/epithelial transport on phenolics and bioactivities of particles of brewer’s spent yeasts loaded with Brazilian red propolis. Food Res. Int. 2023, 173, 113345. [Google Scholar] [CrossRef]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.S.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontology 2000 2015, 67, 131–186. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontology 2000 2016, 71, 82–112. [Google Scholar] [CrossRef]
- de Nies, L.; Kobras, C.M.; Stracy, M. Antibiotic-induced collateral damage to the microbiota and associated infections. Nat. Rev. Microbiol. 2023, 21, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.-H.; Harkins, C.P.; Schwardt, N.H.; Portillo, J.A.; Zimmerman, M.D.; Carter, C.L.; Hossen, A.; Peer, C.J.; Polley, E.C.; Dartois, V.; et al. Alterations of human skin microbiome and expansion of antimicrobial resistance after systemic antibiotics. Sci. Transl. Med. 2021, 13, eabd8077. [Google Scholar] [CrossRef] [PubMed]
- Franchin, M.; Colón, D.F.; da Cunha, M.G.; Castanheira, F.V.S.; Saraiva, A.L.L.; Bueno-Silva, B.; Alencar, S.M.; Cunha, T.M.; Rosalen, P.L. Neovestitol, an isoflavonoid isolated from Brazilian red propolis, reduces acute and chronic inflammation: Involvement of nitric oxide and IL-6. Sci. Rep. 2016, 6, 36401. [Google Scholar] [CrossRef] [PubMed]
- Franchin, M.; Cólon, D.F.; Castanheira, F.V.S.; da Cunha, M.G.; Bueno-Silva, B.; Alencar, S.M.; Cunha, T.M.; Rosalen, P.L. Vestitol isolated from brazilian red propolis inhibits neutrophils migration in the inflammatory process: Elucidation of the mechanism of action. J. Nat. Prod. 2016, 79, 954–960. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo, T.T.; Malavazi, L.M.; Vargas, G.Q.; Gonçalves, F.J.d.S.; Gomes, A.P.d.A.P.; Bueno, M.R.; Aguiar da Silva, L.D.; Figueiredo, L.C.; Bueno-Silva, B. Combination of Neovestitol and Vestitol Modifies the Profile of Periodontitis-Related Subgingival Multispecies Biofilm. Biomedicines 2024, 12, 1189. https://doi.org/10.3390/biomedicines12061189
Macedo TT, Malavazi LM, Vargas GQ, Gonçalves FJdS, Gomes APdAP, Bueno MR, Aguiar da Silva LD, Figueiredo LC, Bueno-Silva B. Combination of Neovestitol and Vestitol Modifies the Profile of Periodontitis-Related Subgingival Multispecies Biofilm. Biomedicines. 2024; 12(6):1189. https://doi.org/10.3390/biomedicines12061189
Chicago/Turabian StyleMacedo, Tatiane Tiemi, Larissa Matias Malavazi, Gustavo Quilles Vargas, Francisco Jerfeson dos Santos Gonçalves, Aline Paim de Abreu Paulo Gomes, Manuela Rocha Bueno, Lucas Daylor Aguiar da Silva, Luciene Cristina Figueiredo, and Bruno Bueno-Silva. 2024. "Combination of Neovestitol and Vestitol Modifies the Profile of Periodontitis-Related Subgingival Multispecies Biofilm" Biomedicines 12, no. 6: 1189. https://doi.org/10.3390/biomedicines12061189
APA StyleMacedo, T. T., Malavazi, L. M., Vargas, G. Q., Gonçalves, F. J. d. S., Gomes, A. P. d. A. P., Bueno, M. R., Aguiar da Silva, L. D., Figueiredo, L. C., & Bueno-Silva, B. (2024). Combination of Neovestitol and Vestitol Modifies the Profile of Periodontitis-Related Subgingival Multispecies Biofilm. Biomedicines, 12(6), 1189. https://doi.org/10.3390/biomedicines12061189





