Joint Effects of Exercise and Ramadan Fasting on Telomere Length: Implications for Cellular Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Selection
2.2. Physical Activity Description
2.3. Clinical Trait Measurements
2.4. Cytokine Profiling
2.5. Antioxidant Enzyme Activity Measurements
2.6. Measurement of Telomere Length
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Telomere Length before and after Intervention in 4W and 4W + F
3.3. Comparing the Percentage Change in Participants’ Markers in 4W versus 4W + F
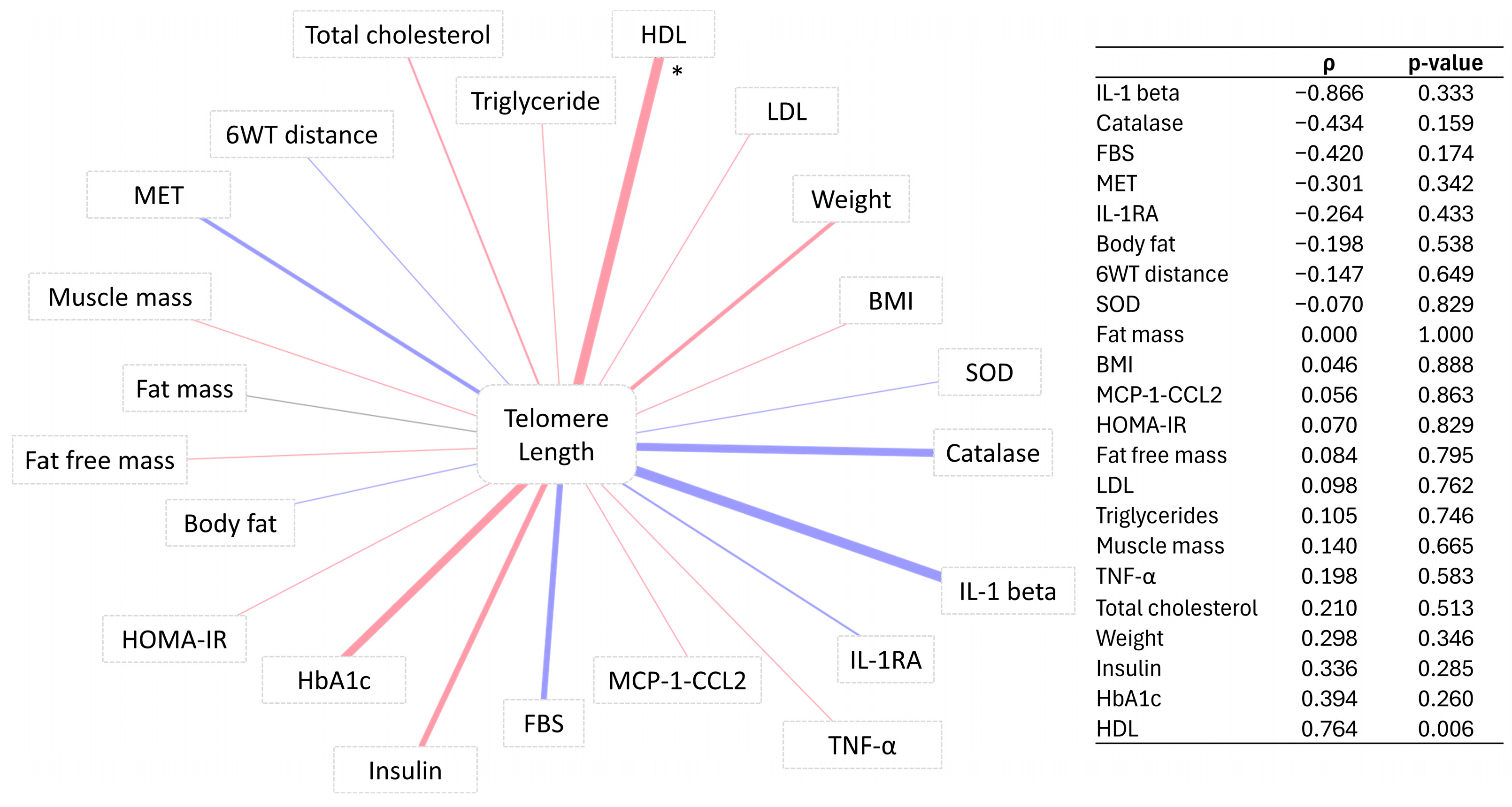
3.4. Correlation of Telomere Length Change with Percentage Change in Participants’ Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Cao, J.; Chen, M.; Chen, M.; Chen, W.; Chen, X.; Chen, Y.; Chen, Y.; Chen, Y.; Chen, Z.; et al. Biomarkers of aging. Sci. China Life Sci. 2023, 66, 893–1066. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2020, 11, 630186. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, S.M.; Chandola, H.M.; Ravishankar, B. Effect of dietary, social, and lifestyle determinants of accelerated aging and its common clinical presentation: A survey study. AYU 2011, 32, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martínez, M.E.; Villaseñor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Collier, R. Intermittent fasting: The science of going without. Can. Med. Assoc. J. 2013, 185, E363–E364. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.D.; Knapp, G.; Reimers, A.K. Does physical activity increase life expectancy? A review of the literature. J. Aging Res. 2012, 2012, 243958. [Google Scholar] [CrossRef]
- Garatachea, N.; Santos-Lozano, A.; Sanchis-Gomar, F.; Fiuza-Luces, C.; Pareja-Galeano, H.; Emanuele, E.; Lucia, A. Elite athletes live longer than the general population: A meta-analysis. Mayo Clin. Proc. 2014, 89, 1195–1200. [Google Scholar] [CrossRef]
- Chakravarty, E.F.; Hubert, H.B.; Lingala, V.B.; Fries, J.F. Reduced disability and mortality among aging runners: A 21-year longitudinal study. Arch. Intern. Med. 2008, 168, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, E.L.; Torres-Acosta, N.; O’Keefe, J.H.; Lavie, C.J. Training for Longevity: The Reverse J-Curve for Exercise. Mo. Med. 2020, 117, 355–361. [Google Scholar] [PubMed]
- Carapeto, P.V.; Aguayo-Mazzucato, C. Effects of exercise on cellular and tissue aging. Aging 2021, 13, 14522–14543. [Google Scholar] [CrossRef] [PubMed]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [PubMed]
- Mather, K.A.; Jorm, A.F.; Parslow, R.A.; Christensen, H. Is telomere length a biomarker of aging? A review. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, B.; Riat, A.; Ghashang, S.K.; Eljurnazi, L.; Gutenbrunner, C. A Prospective Clinical Trial of Prolonged Fasting in Healthy Young Males and Females-Effect on Fatigue, Sleepiness, Mood and Body Composition. Nutrients 2020, 12, 2281. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Mujika, I.; Sharp, M.A.; Foulis, S.A. Maintaining Physical Performance: The Minimal Dose of Exercise Needed to Preserve Endurance and Strength over Time. J. Strength Cond. Res. 2021, 35, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Küüsmaa-Schildt, M.; Eklund, D.; Avela, J.; Rytkönen, T.; Newton, R.; Izquierdo, M.; Häkkinen, K. Neuromuscular Adaptations to Combined Strength and Endurance Training: Order and Time-of-Day. Int. J. Sports Med. 2017, 38, 707–716. [Google Scholar] [CrossRef]
- Mallol, M.; Mejuto, G.; Bentley, D.; Norton, L.; Torres-Unda, J.; Arrieta, H.; Otxoteko, I. Effects of 4 Weeks High-Intensity Training on Running and Cycling Performance in Well-Trained Triathletes. Sports Exerc. Med.-Open J. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Ahmadabadi, S.; Rjabi, H.; Gharakhanlou, R.; Talebian, S.; Basereh, A. Effects of a 4-week plyometric training on activity patterns during different phases of one-leg drop jump with focus on jump height. Sci. Rep. 2023, 13, 9192. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Pepke, M.L.; Kvalnes, T.; Wright, J.; Araya-Ajoy, Y.G.; Ranke, P.S.; Boner, W.; Monaghan, P.; Sæther, B.-E.; Jensen, H.; Ringsby, T.H. Longitudinal telomere dynamics within natural lifespans of a wild bird. Sci. Rep. 2023, 13, 4272. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.; Felix, D.A.; Gutiérrez-Gutiérrez, Ó.; De Miguel-Bonet, M.D.M.; Sahu, S.; Fernández-Varas, B.; Perona, R.; Aboobaker, A.A.; Flores, I.; González-Estévez, C. Downregulation of mTOR Signaling Increases Stem Cell Population Telomere Length during Starvation of Immortal Planarians. Stem Cell Rep. 2019, 13, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Vera, E.; Bernardes de Jesus, B.; Foronda, M.; Flores, J.M.; Blasco, M.A. Telomerase reverse transcriptase synergizes with calorie restriction to increase health span and extend mouse longevity. PLoS ONE 2013, 8, e53760. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Lin, J.; Chan, J.M.; Epel, E.; Kemp, C.; Weidner, G.; Marlin, R.; Frenda, S.J.; Magbanua, M.J.M.; Daubenmier, J.; et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013, 14, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, T.; Yang, C.; Li, J.; Zhang, Y.; Zhao, Y. Persistent dyslipidemia increases the longitudinal changes in telomere length. Lipids Health Dis. 2023, 22, 173. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Iozzo, P.; Salonen, M.; Kajantie, E.; Eriksson, J.G. Rate of telomere shortening and metabolic and cardiovascular risk factors: A longitudinal study in the 1934–44 Helsinki Birth Cohort Study. Ann. Med. 2015, 47, 499–505. [Google Scholar] [CrossRef]
- Muniesa, C.A.; Verde, Z.; Diaz-Ureña, G.; Santiago, C.; Gutiérrez, F.; Díaz, E.; Gómez-Gallego, F.; Pareja-Galeano, H.; Soares-Miranda, L.; Lucia, A. Telomere Length in Elite Athletes. Int. J. Sports Physiol. Perform. 2017, 12, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, J.L.; Sánchez-Rodríguez, J.L.; Martín-Vallejo, J.; Martel-Martel, A.; González-Sarmiento, R. Effects of Physical Exercise on Cognition and Telomere Length in Healthy Older Women. Brain Sci. 2021, 11, 1417. [Google Scholar] [CrossRef]
- Werner, C.M.; Hecksteden, A.; Morsch, A.; Zundler, J.; Wegmann, M.; Kratzsch, J.; Thiery, J.; Hohl, M.; Bittenbring, J.T.; Neumann, F.; et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur. Heart J. 2019, 40, 34–46. [Google Scholar] [CrossRef]
- Fernández de la Puente, M.; Hernández-Alonso, P.; Canudas, S.; Marti, A.; Fitó, M.; Razquin, C.; Salas-Salvadó, J. Modulation of Telomere Length by Mediterranean Diet, Caloric Restriction, and Exercise: Results from PREDIMED-Plus Study. Antioxidants 2021, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Fernández de la Puente, M.; Canudas, S.; Zalba, G.; Razquin, C.; Valle-Hita, C.; Fitó, M.; Martínez-González, M.; García-Calzón, S.; Salas-Salvadó, J. Effect of a 3-year lifestyle intervention on telomere length in participants from PREDIMED-Plus: A randomized trial. Clin. Nutr. 2023, 42, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, P.M. Sex differences in telomere length, lifespan, and embryonic dyskerin levels. Aging Cell 2022, 21, e13614. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Al-Muraikhy, S.; Al-Jaber, H.; Al-Amri, H.; Al-Mansoori, L.; Mazloum, N.A.; Donati, F.; Botre, F.; Elrayess, M.A. Age and Sport Intensity-Dependent Changes in Cytokines and Telomere Length in Elite Athletes. Antioxidants 2021, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Brandao, C.F.C.; Nonino, C.B.; de Carvalho, F.G.; Nicoletti, C.F.; Noronha, N.Y.; San Martin, R.; de Freitas, E.C.; Junqueira-Franco, M.V.M.; Marchini, J.S. The effects of short-term combined exercise training on telomere length in obese women: A prospective, interventional study. Sports Med. Open 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- García-Calzón, S.; Moleres, A.; Marcos, A.; Campoy, C.; Moreno, L.A.; Azcona-Sanjulián, M.C.; Martínez-González, M.A.; Martínez, J.A.; Zalba, G.; Marti, A. Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: The EVASYON study. PLoS ONE 2014, 9, e89828. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.A.; Lee, J.H.; Song, W.; Jun, T.W. Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech. Ageing Dev. 2008, 129, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Nickels, M.; Mastana, S.; Hunter, D.; Denniff, M.; Codd, V.; Akam, E. The effect of a 12-week resistance training intervention on leukocyte telomere length. Heliyon 2020, 6, e04151. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.M.; Amaral, M.A.; Mundstock, E.; Barbé-Tuana, F.M.; Guma, F.T.C.R.; Jones, M.H.; Machado, D.C.; Sarria, E.E.; Marques e Marques, M.; Preto, L.T.; et al. Effects of Diet on Telomere Length: Systematic Review and Meta-Analysis. Public Health Genom. 2018, 20, 286–292. [Google Scholar] [CrossRef]
- Mason, C.; Risques, R.A.; Xiao, L.; Duggan, C.R.; Imayama, I.; Campbell, K.L.; Kong, A.; Foster-Schubert, K.E.; Wang, C.Y.; Alfano, C.M.; et al. Independent and combined effects of dietary weight loss and exercise on leukocyte telomere length in postmenopausal women. Obesity 2013, 21, E549–E554. [Google Scholar] [CrossRef]
- Steenstrup, T.; Hjelmborg, J.v.B.; Kark, J.D.; Christensen, K.; Aviv, A. The telomere lengthening conundrum—Artifact or biology? Nucleic Acids Res. 2013, 41, e131. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhou, K.W.; Yang, G.Z.; Chen, C. Association between lipoproteins and telomere length in US adults: Data from the NHANES 1999–2002. Lipids Health Dis. 2019, 18, 80. [Google Scholar] [CrossRef]
- Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S.; Aviv, A. Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis 2009, 205, 620–625. [Google Scholar] [CrossRef]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Coluzzi, E.; Colamartino, M.; Cozzi, R.; Leone, S.; Meneghini, C.; O’Callaghan, N.; Sgura, A. Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PLoS ONE 2014, 9, e110963. [Google Scholar] [CrossRef]
- Chen, X.; Andresen, B.T.; Hill, M.; Zhang, J.; Booth, F.; Zhang, C. Role of Reactive Oxygen Species in Tumor Necrosis Factor-alpha Induced Endothelial Dysfunction. Curr. Hypertens. Rev. 2008, 4, 245–255. [Google Scholar] [CrossRef]
- Kemp, G.M.; Altimimi, H.F.; Nho, Y.; Heir, R.; Klyczek, A.; Stellwagen, D. Sustained TNF signaling is required for the synaptic and anxiety-like behavioral response to acute stress. Mol. Psychiatry 2022, 27, 4474–4484. [Google Scholar] [CrossRef]
- Mushtaq, R.; Akram, A.; Mushtaq, R.; Khwaja, S.; Ahmed, S. The role of inflammatory markers following Ramadan Fasting. Pak. J. Med. Sci. 2019, 35, 77–81. [Google Scholar] [CrossRef]
- Amin, M.N.; El-Mowafy, M.; Mobark, A.; Abass, N.; Elgaml, A. Exercise-induced downregulation of serum interleukin-6 and tumor necrosis factor-alpha in Egyptian handball players. Saudi J. Biol. Sci. 2021, 28, 724–730. [Google Scholar] [CrossRef]
- Tsukui, S.; Kanda, T.; Nara, M.; Nishino, M.; Kondo, T.; Kobayashi, I. Moderate-intensity regular exercise decreases serum tumor necrosis factor-alpha and HbA1c levels in healthy women. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1207–1211. [Google Scholar] [CrossRef]
- Lyu, L.; He, S.; Zhang, H.; Li, W.; Zeng, J.; Ping, F.; Li, Y.X. TNFα Mediates the Interaction of Telomeres and Mitochondria Induced by Hyperglycemia: A Rural Community-Based Cross-Sectional Study. Oxid. Med. Cell. Longev. 2020, 2020, 8235873. [Google Scholar] [CrossRef]
- Maekawa, T.; Liu, B.; Nakai, D.; Yoshida, K.; Nakamura, K.I.; Yasukawa, M.; Koike, M.; Takubo, K.; Chatton, B.; Ishikawa, F.; et al. ATF7 mediates TNF-α-induced telomere shortening. Nucleic Acids Res. 2018, 46, 4487–4504. [Google Scholar] [CrossRef]
- Liu, B.; Maekawa, T.; Yoshida, K.; Ly, N.H.; Inoue, K.; Hasegawa, A.; Chatton, B.; Ogura, A.; Ishii, S. Telomere shortening by transgenerational transmission of TNF-α-induced TERRA via ATF7. Nucleic Acids Res. 2019, 47, 283–298. [Google Scholar] [CrossRef]
- Li, P.; Gan, Y.; Xu, Y.; Song, L.; Wang, L.; Ouyang, B.; Zhang, C.; Zhou, Q. The inflammatory cytokine TNF-α promotes the premature senescence of rat nucleus pulposus cells via the PI3K/Akt signaling pathway. Sci. Rep. 2017, 7, 42938. [Google Scholar] [CrossRef]
- Anton, S.; Leeuwenburgh, C. Fasting or caloric restriction for healthy aging. Exp. Gerontol. 2013, 48, 1003–1005. [Google Scholar] [CrossRef]
- Schellnegger, M.; Lin, A.C.; Hammer, N.; Kamolz, L.P. Physical Activity on Telomere Length as a Biomarker for Aging: A Systematic Review. Sports Med. Open 2022, 8, 111. [Google Scholar] [CrossRef]

| 4W (n = 16) | 4W + F (n = 13) | p-Value | |
|---|---|---|---|
| Age | 21 (20–26) | 22 (20.75–22.25) | 0.700 |
| BMI | 24.78 (5.87) | 24.42 (4.54) | 0.862 |
| TL (T/S ratio) | 0.33 (0.09) | 0.28 (0.08) | 0.153 |
| Height (m) | 1.6 (0.06) | 1.59 (0.05) | 0.611 |
| Weight (kg) | 65.1 (49.1–71.3) | 58.75 (51.8–65.72) | 0.913 |
| Body fat (%) | 0.31 (0.11) | 0.32 (0.09) | 0.659 |
| Fat free mass (kg) | 42.95 (4.65) | 43.85 (4.49) | 0.629 |
| Fat mass (kg) | 20.54 (11.68) | 21.86 (11.36) | 0.777 |
| Muscle mass (kg) | 40.77 (4.43) | 41.59 (4.27) | 0.641 |
| MET | 975 (622–1480) | 1251.25 (826.5–2708.25) | 0.265 |
| 6WT Distance (m) | 531 (62.71) | 587.83 (70.68) | 0.045 |
| Handgrip (L) | 21.83 (4.04) | 21.92 (5.6) | 0.966 |
| Handgrip (R) | 23.44 (5.1) | 22.18 (5.37) | 0.556 |
| Insulin (mU/L) | 13.03 (7.69) | 12.13 (5.13) | 0.731 |
| FBS (mmol/L) | 5.1 (4.7–5.3) | 5.3 (4.8–5.32) | 0.284 |
| HOMA-IR | 2.39 (1.76–3.94) | 3.73 (1.99–4) | 0.496 |
| Total cholesterol (g/dL) | 184 (159–202) | 161.5 (147.75–174.5) | 0.103 |
| Triglycerides (g/dL) | 64 (50–75) | 59.5 (48.5–66) | 0.549 |
| HDL (g/dL) | 68.31 (13.81) | 55.45 (14.7) | 0.040 |
| LDL (g/dL) | 101.46 (24.37) | 101.92 (35.19) | 0.971 |
| HbA1C % | 4.9 (4.7–5.5) | 5.25 (4.82–5.38) | 0.935 |
| SOD (U/mL) | 0.97 (0.87–1.22) | 0.92 (0.83–1.08) | 0.463 |
| Catalase (U/mL) | 19.61 (18.78–19.92) | 20.09 (18.69–20.21) | 0.586 |
| IL-1 beta (pg/mL) | 0.14 (0.14–0.14) | 0.14 (0.14–0.32) | 0.210 |
| IL-6 (pg/mL) | 24.77 (24.77–24.77) | 24.77 (24.77–30.68) | 0.765 |
| IL-8 CXCL8 (pg/mL) | 1.37 (0.83) | 2.4 (1.72) | 0.195 |
| IL-1RA (pg/mL) | 294.02 (176.92–607.44) | 457.1 (204.11–1068.14) | 0.518 |
| TNF-α (pg/mL) | 0.43 (0.43–3.95) | 0.43 (0.43–3.95) | 0.635 |
| MCP-1 (CCL2) (pg/mL) | 345.82 (226.46) | 271.42 (172.68) | 0.363 |
| Physical function | 0.69 (0.26) | 0.77 (0.25) | 0.471 |
| Physical health limitation | 0.75 (0.5–1) | 0.62 (0.25–1) | 0.932 |
| Emotional problems limitation | 0.67 (0.33–1) | 0.5 (0–1) | 0.632 |
| Energy/Fatigue | 0.55 (0.2) | 0.54 (0.1) | 0.851 |
| Emotional well being | 0.72 (0.64–0.76) | 0.66 (0.58–0.72) | 0.459 |
| Social function | 0.58 (0.24) | 0.6 (0.23) | 0.846 |
| Pain | 0.76 (0.23) | 0.64 (0.28) | 0.255 |
| General health | 0.71 (0.18) | 0.66 (0.12) | 0.422 |
| Health change | 0.75 (0.75–1) | 0.75 (0.5–0.75) | 0.141 |
| Group | Before | After | p-Value |
|---|---|---|---|
| 4W | 0.335 (0.094) | 0.328 (0.137) | 0.87 |
| 4W + F | 0.284 (0.24–0.34) | 0.393 (0.22–0.55) | 0.048 |
| Percentage Change | Estimate | SE | p-Value |
|---|---|---|---|
| TNF-α | −568.854 | 215.301 | 0.013 |
| Telomere length | 113.493 | 46.995 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuraikhy, S.; Sellami, M.; Naja, K.; Al-Amri, H.S.; Anwardeen, N.; Aden, A.; Dömling, A.; Elrayess, M.A. Joint Effects of Exercise and Ramadan Fasting on Telomere Length: Implications for Cellular Aging. Biomedicines 2024, 12, 1182. https://doi.org/10.3390/biomedicines12061182
Almuraikhy S, Sellami M, Naja K, Al-Amri HS, Anwardeen N, Aden A, Dömling A, Elrayess MA. Joint Effects of Exercise and Ramadan Fasting on Telomere Length: Implications for Cellular Aging. Biomedicines. 2024; 12(6):1182. https://doi.org/10.3390/biomedicines12061182
Chicago/Turabian StyleAlmuraikhy, Shamma, Maha Sellami, Khaled Naja, Hadaia Saleh Al-Amri, Najeha Anwardeen, Amina Aden, Alexander Dömling, and Mohamed A. Elrayess. 2024. "Joint Effects of Exercise and Ramadan Fasting on Telomere Length: Implications for Cellular Aging" Biomedicines 12, no. 6: 1182. https://doi.org/10.3390/biomedicines12061182
APA StyleAlmuraikhy, S., Sellami, M., Naja, K., Al-Amri, H. S., Anwardeen, N., Aden, A., Dömling, A., & Elrayess, M. A. (2024). Joint Effects of Exercise and Ramadan Fasting on Telomere Length: Implications for Cellular Aging. Biomedicines, 12(6), 1182. https://doi.org/10.3390/biomedicines12061182









