Safety Profile of the Trastuzumab-Based ADCs: Analysis of Real-World Data Registered in EudraVigilance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Extraction Criteria
2.2. Data Analysis
2.2.1. ADRs Reported for ADCs in EV
2.2.2. Descriptive Analysis of ADRs Related to T-DM1 and T-DXd
2.2.3. Disproportionality Analysis
2.3. Ethics
3. Results
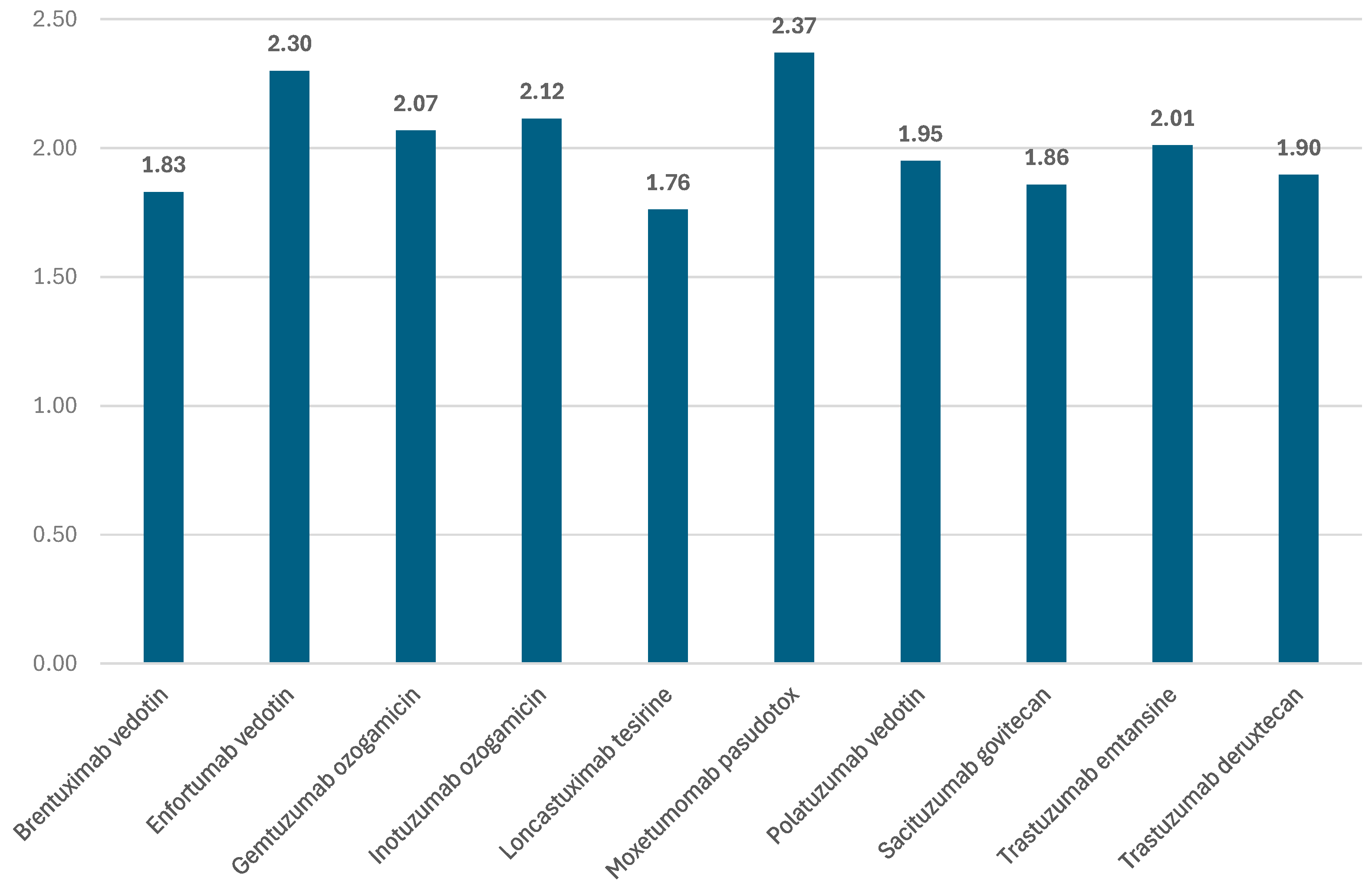
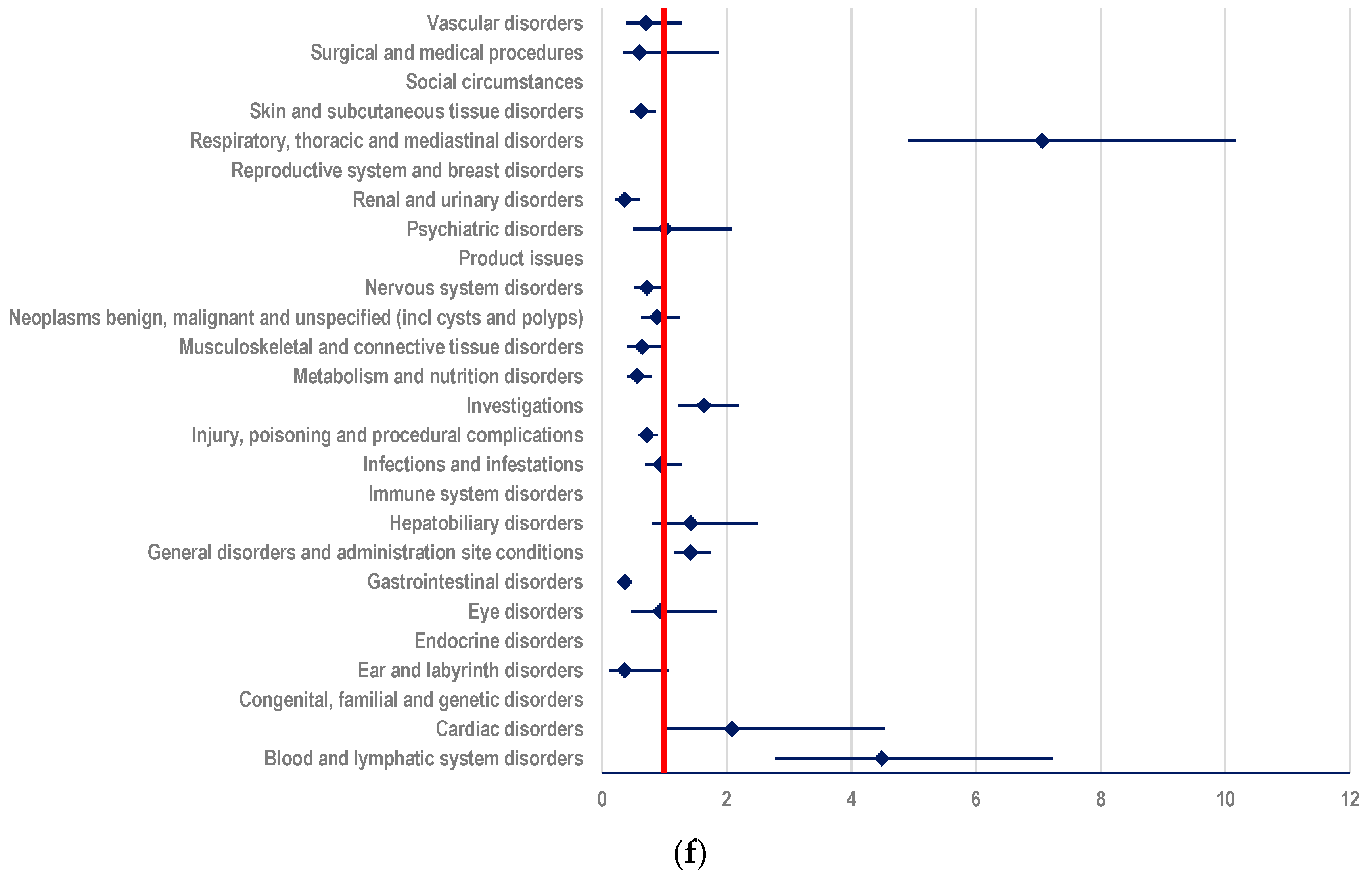
3.1. ADRs Reported for ADCs in EV
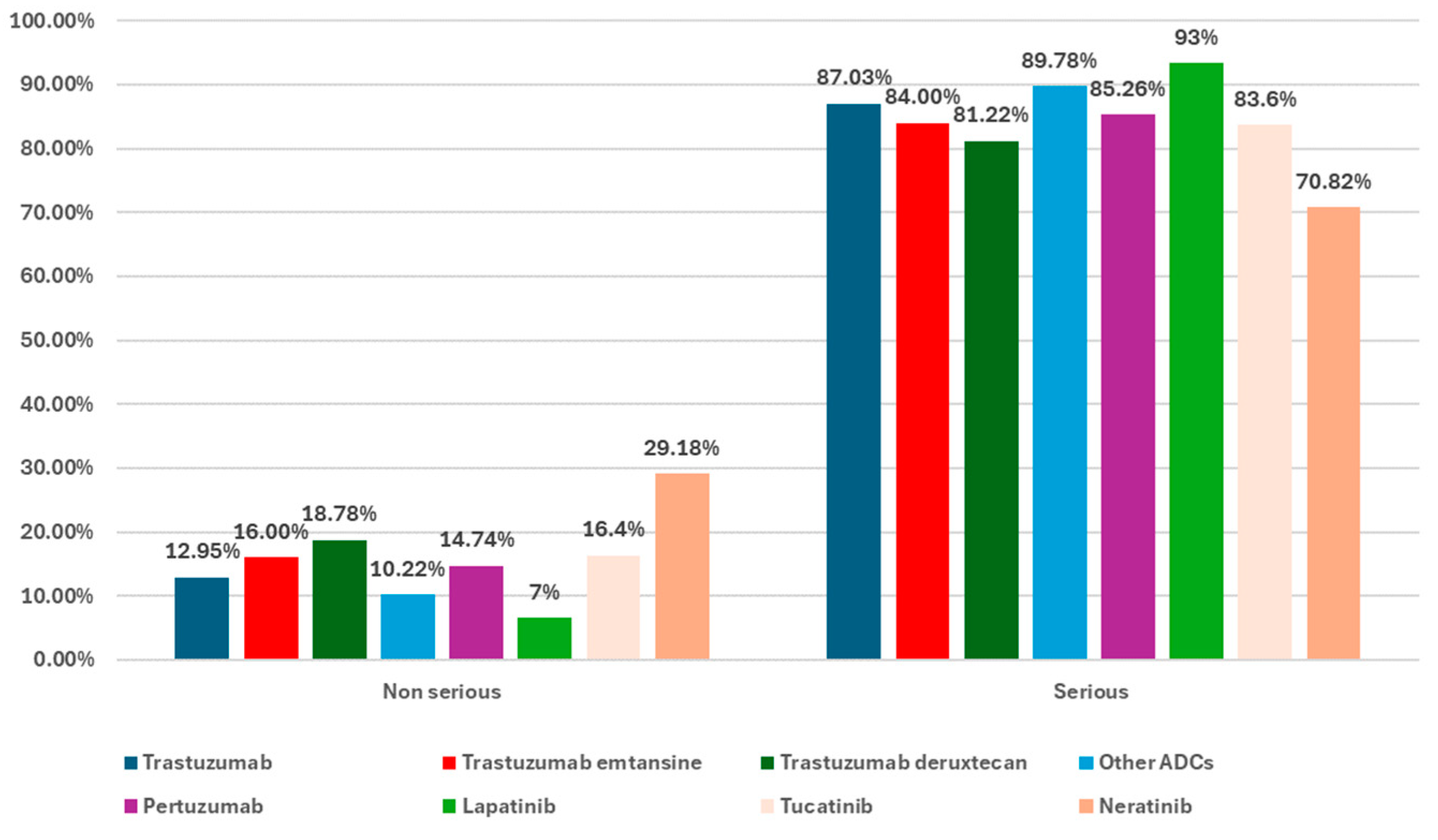
3.2. Descriptive Analysis of ICSRs Reported in the EV Database for Trastuzumab, Trastuzumab Emtansine, and Trastuzumab Deruxtecan
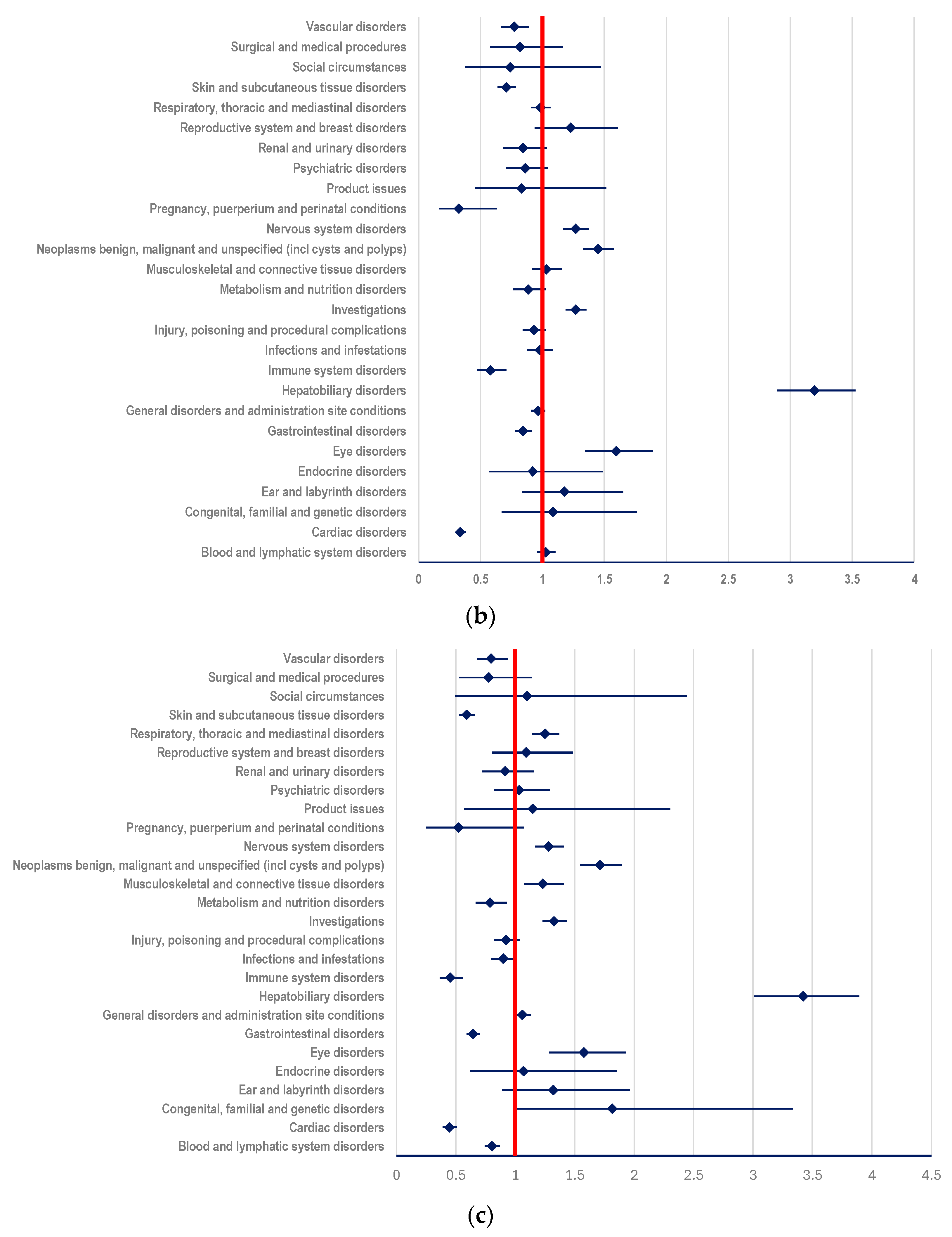
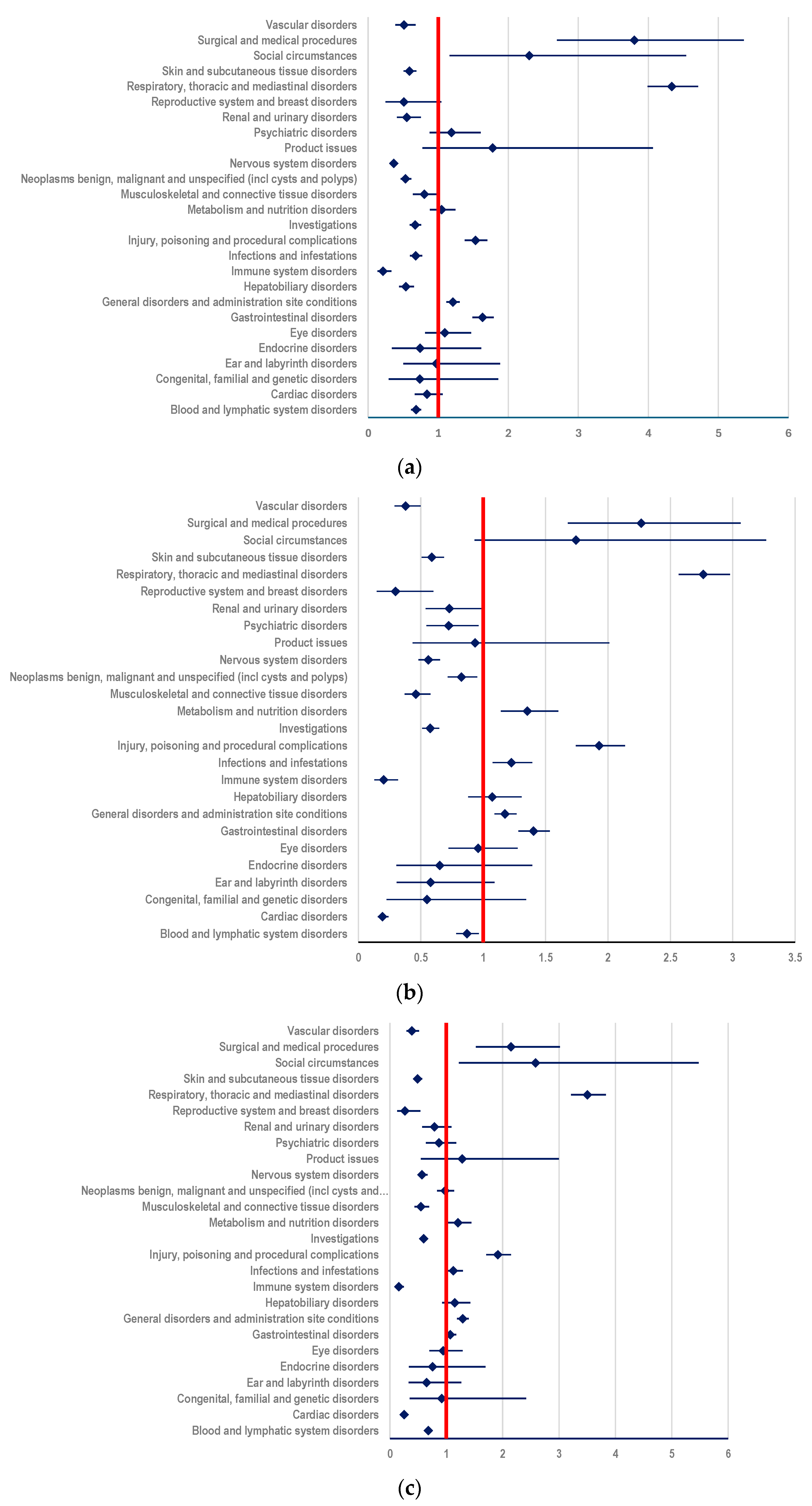
3.3. Disproportionality Analysis
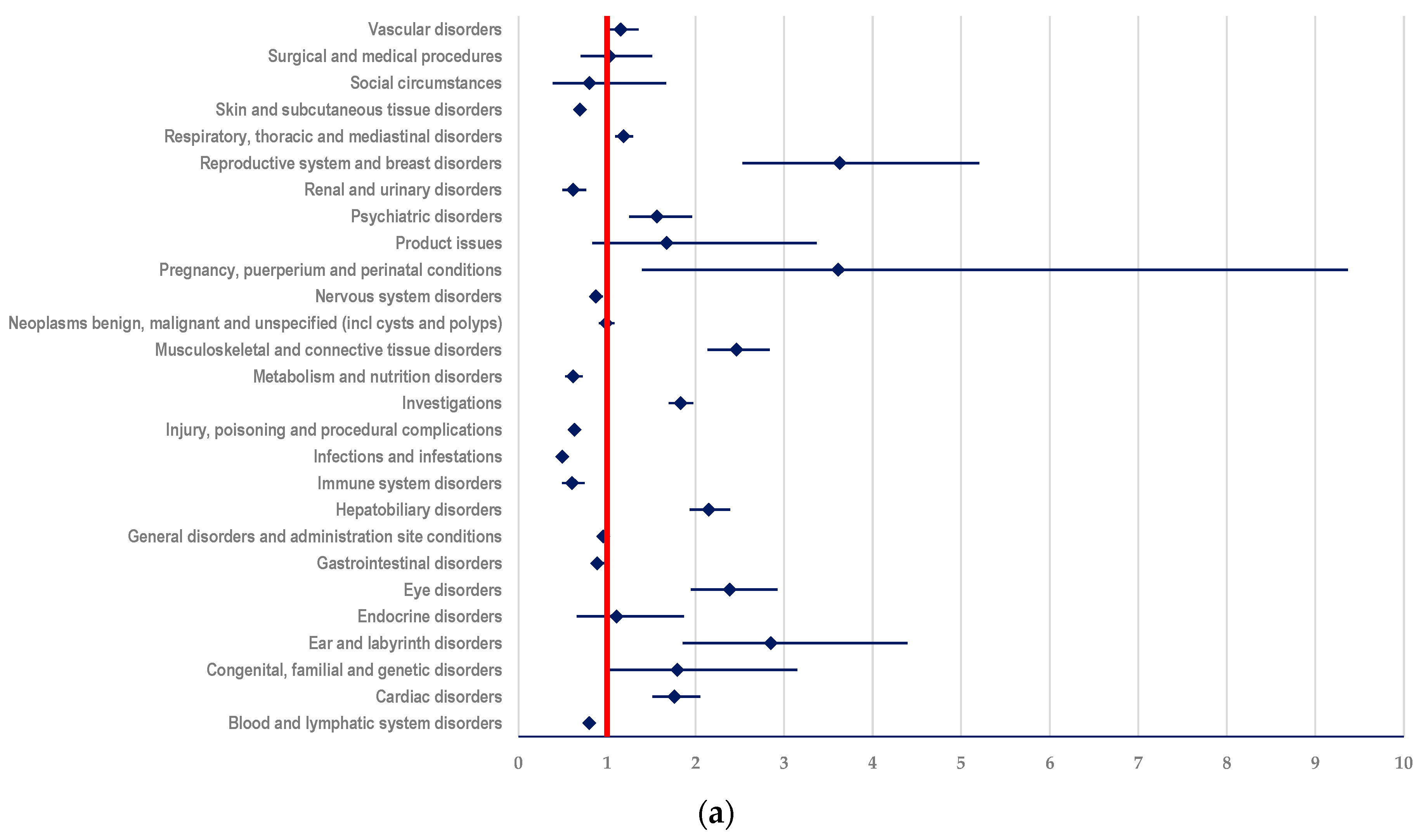
3.3.1. Probability of Reporting ADRs Related to T-DM1
- (i)
- ADCs (ROR: 2.1496; 95% CI: 1.9338-2.3894) (Figure 6a);
- (ii)
- mAbs: T (ROR: 3.1958; 95% CI: 2.8956-3.5270) and PER (ROR: 3.4217; 95% CI: 3.0069-3.8938) (Figure 6b,c)
- (iii)
- TKIs: LAP (ROR: 2.3419; 95% CI: 2.0155-2.7212), TUC (ROR: 2.5647; 95% CI: 2.0131-3.2676), and NER (ROR: 4.2442; 95% CI: 2.4878-7.2408) (Figure 6d–f).
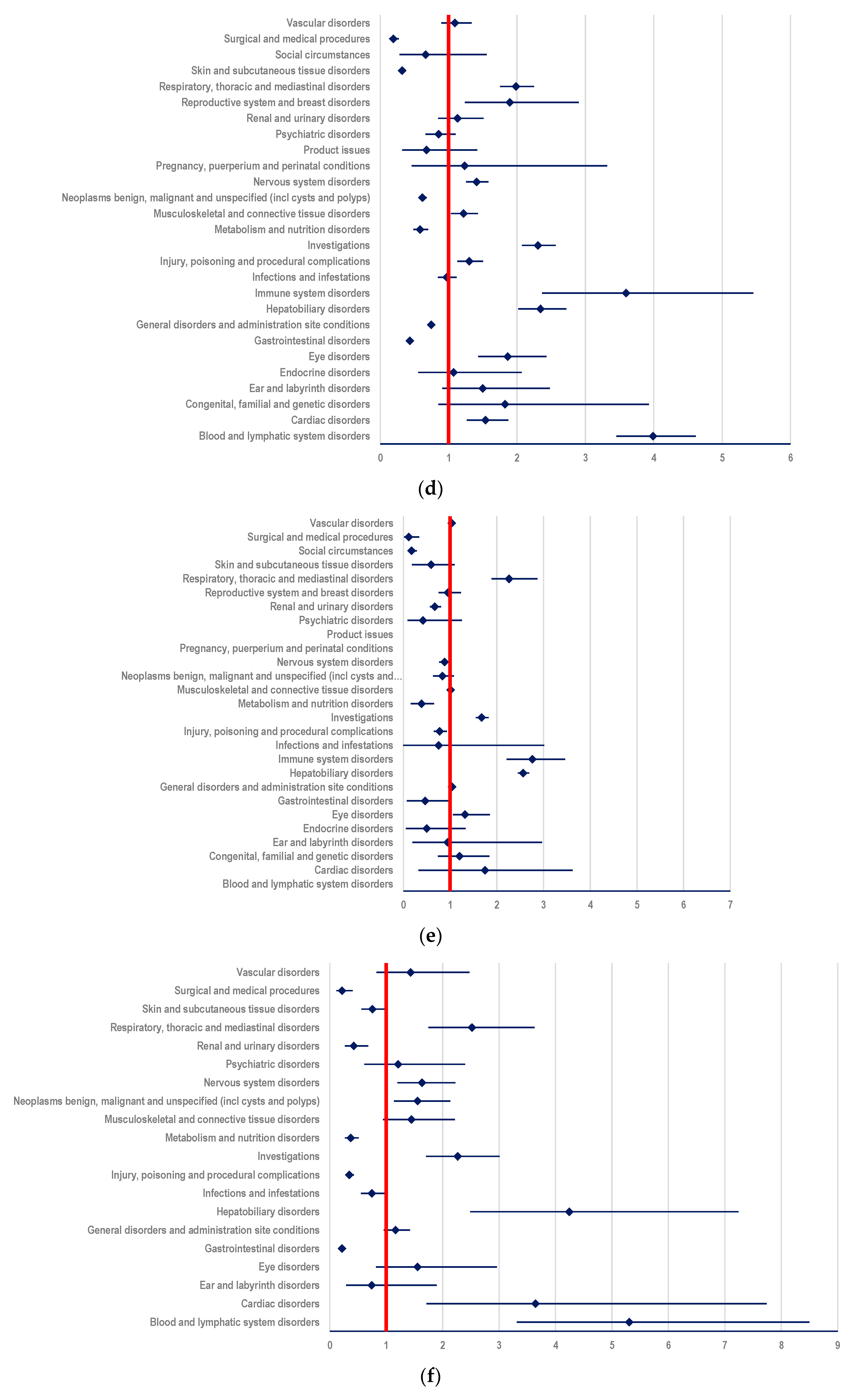
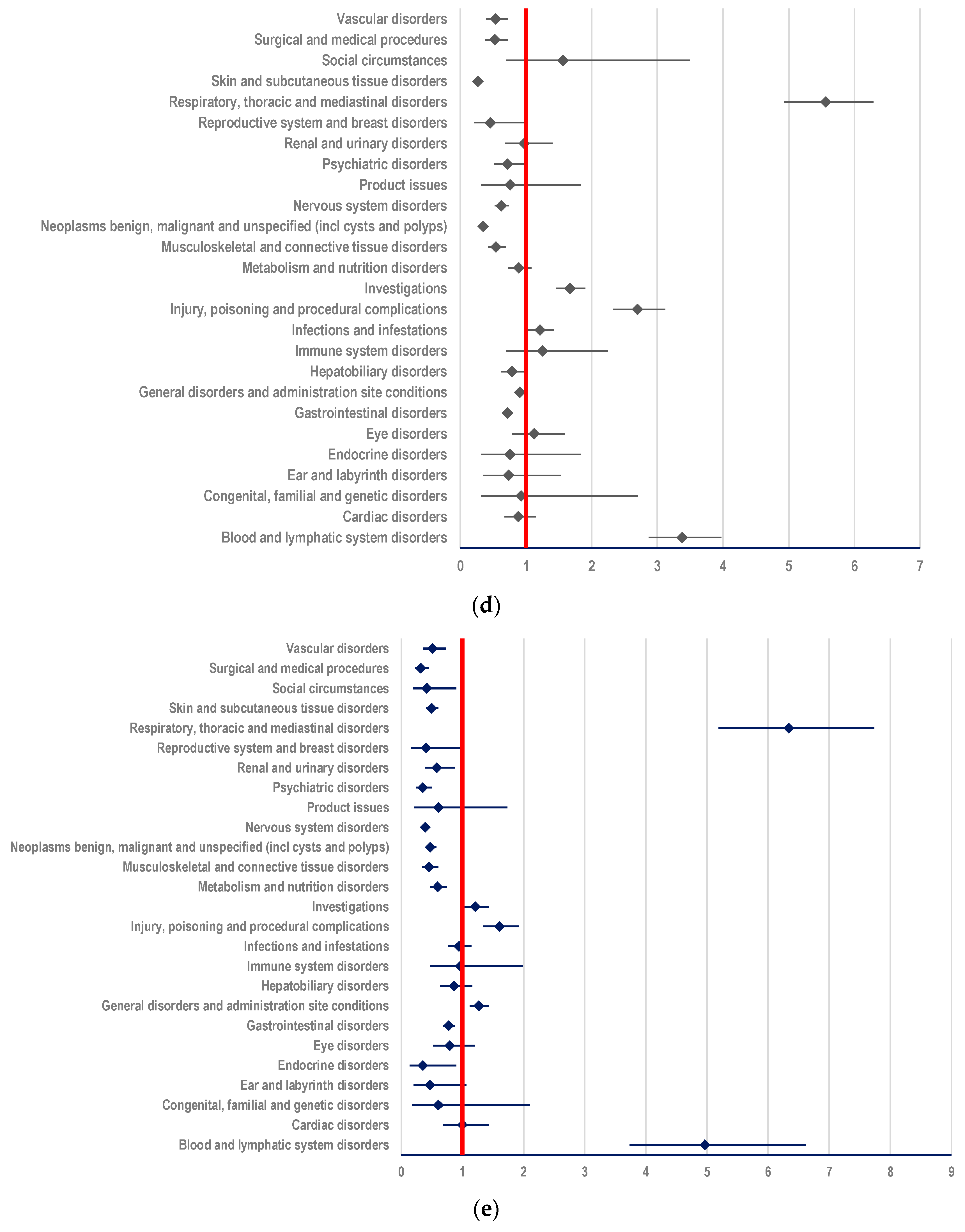
3.3.2. Probability of Reporting ADRs Related to T-DXd
- (i)
- other ADCs: ROR-4.3343; 95% CI: 3.9893-4.7091 (Figure 7a);
- (ii)
- mAbs: T: ROR-2.7646; 95% CI: 2.5669-2.9776; PER: ROR-3.5059; 95% CI: 3.2112-3.8276 (Figure 7b,c);
- (iii)
- TKIs: LAP: ROR-5.5659; 95% CI: 4.9248-6.2905; TUC: ROR-6.3382; 95% CI: 5.1930-7.7360; NER: ROR-7.0641; 95% CI 4.9065-10.1703 (Figure 7d–f);
3.3.3. Comparison between T-DM1 and T-DXd
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mckertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Pysz, I.; Jackson, P.J.M.; Thurston, D.E. Introduction to Antibody–Drug Conjugates (ADCs). In Cytotoxic Payloads for Antibody—Drug Conjugates; Royal Society of Chemistry: London, UK, 2019; pp. 1–30. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; De Azambuja, E.; Demichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer 5 Behalf of the ESMO Guidelines Committee. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Albagoush, S.A.; Zubair, M.; Limaiem, F. Tissue Evaluation for HER2 Tumor Marker; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537134/ (accessed on 26 February 2024).
- Rugo, H.S.; Krop, I.E.; Chu, Y.W. Trastuzumab Emtansine (T-DM1) for the Treatment of HER2-Positive Cancer with a Focus on Breast Cancer. In Antibody-Drug Conjugates and Immunotoxins from Pre-Clinical Development to Therapeutic Applications; Humana: New York, NY, USA, 2013; pp. 179–210. [Google Scholar]
- Ashman, N.; Bargh, J.D.; Spring, D.R. Non-Internalising Antibody–Drug Conjugates. Chem. Soc. Rev. 2022, 51, 9182–9202. [Google Scholar] [CrossRef] [PubMed]
- Joubert, N.; Beck, A.; Dumontet, C.; Denevault-Sabourin, C. Antibody–Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245. [Google Scholar] [CrossRef]
- Yu, J.; Fang, T.; Yun, C.; Liu, X.; Cai, X. Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers. Front. Mol. Biosci. 2022, 9, 847835. [Google Scholar] [CrossRef] [PubMed]
- Merlino, F.; Bellavita, R.; Balamkundu, S.; Liu, C.-F. Lysosomal-Cleavable Peptide Linkers in Antibody–Drug Conjugates. Biomedicines 2023, 11, 3080. [Google Scholar] [CrossRef]
- Singh, D.; Dheer, D.; Samykutty, A.; Shankar, R. Antibody Drug Conjugates in Gastrointestinal Cancer: From Lab to Clinical Development. J. Control. Release 2021, 340, 1–34. [Google Scholar] [CrossRef]
- Maadi, H.; Soheilifar, M.H.; Choi, W.S.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 3540. [Google Scholar] [CrossRef]
- Jackson, C.; Finikarides, L.; Freeman, A.L.J. The Adverse Effects of Trastuzumab-Containing Regimes as a Therapy in Breast Cancer: A Piggy-Back Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0275321. [Google Scholar] [CrossRef]
- Moulder, S.L.; Borges, V.F.; Baetz, T.; Mcspadden, T.; Fernetich, G.; Murthy, R.K.; Chavira, R.; Guthrie, K.; Barrett, E.; Chia, S.K. Phase I Study of ONT-380, a HER2 Inhibitor, in Patients with HER2+-Advanced Solid Tumors, with an Expansion Cohort in HER2+ Metastatic Breast Cancer (MBC). Clin. Cancer Res. 2017, 23, 3529–3536. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, P.A.; Neuberger, E.; Schwartz, N.R.M.; Wang, S.; Liu, Y.; Hsu, L.I.; Bartley, K.; Blahna, M.T.; Pittner, B.T.; Wong, G.; et al. Real-World Patient Characteristics, Treatment Patterns, and Clinical Outcomes Associated with Tucatinib Therapy in HER2-Positive Metastatic Breast Cancer. Front. Oncol. 2023, 13, 1264861. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shao, W.; Zhou, C.; Yang, H.; Yang, L.; Cai, Q.; Wang, J.; Shi, Y.; Huang, L.; Zhang, J. Neratinib for HER2-Positive Breast Cancer with an Overlooked Option. Mol. Med. 2023, 29, 134. [Google Scholar] [CrossRef] [PubMed]
- Tevaarwerk, A.J.; Kolesar, J.M. Lapatinib: A Small-Molecule Inhibitor of Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor-2 Tyrosine Kinases Used in the Treatment of Breast Cancer. Clin. Ther. 2009, 31 Pt 2, 2332–2348. [Google Scholar] [CrossRef] [PubMed]
- Nami, B.; Maadi, H.; Wang, Z. Mechanisms Underlying the Action and Synergism of Trastuzumab and Pertuzumab in Targeting HER2-Positive Breast Cancer. Cancers 2018, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Oostra, D.R.; Macrae, E.R. Role of Trastuzumab Emtansine in the Treatment of HER2-Positive Breast Cancer. Breast Cancer 2014, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S.M.F. Mechanisms of Resistance to Trastuzumab Emtansine (T-DM1) in HER2-Positive Breast Cancer. Br. J. Cancer 2019, 122, 603–612. [Google Scholar] [CrossRef]
- Tamura, K.; Tsurutani, J.; Takahashi, S.; Iwata, H.; Krop, I.E.; Redfern, C.; Sagara, Y.; Doi, T.; Park, H.; Murthy, R.K.; et al. Trastuzumab Deruxtecan (DS-8201a) in Patients with Advanced HER2-Positive Breast Cancer Previously Treated with Trastuzumab Emtansine: A Dose-Expansion, Phase 1 Study. Lancet. Oncol. 2019, 20, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, a Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results from the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47. [Google Scholar] [CrossRef]
- Nader-Marta, G.; Molinelli, C.; Debien, V.; Martins-Branco, D.; Aftimos, P.; de Azambuja, E.; Awada, A. Antibody–Drug Conjugates: The Evolving Field of Targeted Chemotherapy for Breast Cancer Treatment. Ther. Adv. Med. Oncol. 2023, 15, 17588359231183679. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.M.; Rus, L.L.; Frum, A.; Morgovan, C.; Butuca, A.; Totan, M.; Juncan, A.M.; Gligor, F.G.; Arseniu, A.M. Dendrimers as Non-Viral Vectors in Gene-Directed Enzyme Prodrug Therapy. Molecules 2021, 26, 5976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, Y.; Li, W.; Jeanty, C.; Xiang, G.; Dong, Y. Recent Advances of Antibody Drug Conjugates for Clinical Applications. Acta Pharm. Sin. B 2020, 10, 1589–1600. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, E.; Gandullo-Sánchez, L.; Ocaña, A.; Pandiella, A. Novel ADCs and Strategies to Overcome Resistance to Anti-HER2 ADCs. Cancers 2021, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Wynn, C.S.; Tang, S.C. Anti-HER2 Therapy in Metastatic Breast Cancer: Many Choices and Future Directions. Cancer Metastasis Rev. 2022, 41, 193–209. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Hegg, R.; Chung, W.P.; Im, S.A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine in Patients with HER2-Positive Metastatic Breast Cancer: Updated Results from DESTINY-Breast03, a Randomised, Open-Label, Phase 3 Trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Tarantino, P.; Pestana, R.C.; Corti, C.; Modi, S.; Bardia, A.; Tolaney, S.M.; Cortes, J.; Soria, J.-C.; Curigliano, G. Antibody–Drug Conjugates: Smart Chemotherapy Delivery across Tumor Histologies. CA Cancer J. Clin. 2022, 72, 165–182. [Google Scholar] [CrossRef]
- Gogia, P.; Ashraf, H.; Bhasin, S.; Xu, Y. Antibody-Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence. Cancers 2023, 15, 3886. [Google Scholar] [CrossRef]
- Morgovan, C.; Dobrea, C.M.; Chis, A.A.; Juncan, A.M.; Arseniu, A.M.; Rus, L.L.; Gligor, F.G.; Ardelean, S.A.; Stoicescu, L.; Ghibu, S.; et al. A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharmaceuticals 2023, 16, 455. [Google Scholar] [CrossRef] [PubMed]
- Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins;A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef]
- MedCalc Software Ltd. Odds Ratio Calculator, MedCalc Software Ltd.: Ostend, Belgium.
- Grundmark, B.; Holmberg, L.; Garmo, H.; Zethelius, B. Reducing the Noise in Signal Detection of Adverse Drug Reactions by Standardizing the Background: A Pilot Study on Analyses of Proportional Reporting Ratios-by-Therapeutic Area. Eur. J. Clin. Pharmacol. 2014, 70, 627–635. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Screening for Adverse Reactions in EudraVigilance; European Medicines Agency: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-Positive Breast Cancer: Advances and Future Directions. Nat. Rev. Drug Discov. 2023, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Arabloo, J.; Azari, S.; Gorji, H.A.; Rezapour, A.; Alipour, V.; Ehsanzadeh, S.J. Cost-Effectiveness of Brentuximab Vedotin in Hodgkin Lymphoma: A Systematic Review. Eur. J. Clin. Pharmacol. 2023, 79, 1443–1452. [Google Scholar] [PubMed]
- Stainthorpe, A.; Fleeman, N.; Houten, R.; Chaplin, M.; Boland, A.; Beale, S.; Dundar, Y.; McEntee, J.; Syndikus, I. Brentuximab Vedotin for Treating Relapsed or Refractory CD30-Positive Cutaneous T-Cell Lymphoma: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics—Open 2020, 4, 563–574. [Google Scholar] [PubMed]
- Shea, L.; Mehta-Shah, N. Brentuximab Vedotin in the Treatment of Peripheral T Cell Lymphoma and Cutaneous T Cell Lymphoma. Curr. Hematol. Malig. Rep. 2020, 15, 9–19. [Google Scholar] [PubMed]
- Andrade-Gonzalez, X.; Ansell, S.M. Novel Therapies in the Treatment of Hodgkin Lymphoma. Curr. Treat. Options Oncol. 2021, 22, 42. [Google Scholar] [PubMed]
- Velasco, R.; Domingo-Domenech, E.; Sureda, A. Brentuximab-Induced Peripheral Neurotoxicity: A Multidisciplinary Approach to Manage an Emerging Challenge in Hodgkin Lymphoma Therapy. Cancers 2021, 13, 6125. [Google Scholar] [CrossRef]
- Ansell, S.M.; Radford, J.; Connors, J.M.; Długosz-Danecka, M.; Kim, W.-S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.; Hutchings, M.; et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2022, 387, 310–320. [Google Scholar] [CrossRef]
- Liu, F.; Yin, G.; Xue, S.; Rehman, F.U.L.; Liao, D.; Pan, Y. Adverse Event Profile Differences between Trastuzumab Emtansine and Trastuzumab Deruxtecan: A Real-World, Pharmacovigilance Study. J. Cancer 2023, 14, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Speirs, V.; Shaaban, A.M. Male Breast Cancer: An Update. Virchows Arch. 2022, 480, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Huang, T.W.; Tam, K.W. Treatment of Male Breast Cancer: Meta-Analysis of Real-World Evidence. Br. J. Surg. 2021, 108, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Deklerck, E.; Denys, H.; Kreps, E.O. Corneal Features in Trastuzumab Emtansine Treatment: Not a Rare Occurrence. Breast Cancer Res. Treat. 2019, 175, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, L.; Lixi, F.; Coco, G.; Giannaccare, G. Ocular Surface Side Effects of Novel Anticancer Drugs. Cancers 2024, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Llamas, S.; Caro-Magdaleno, M.; Mataix-Albert, B.; Avilés-Prieto, J.; Romero-Barranca, I.; Rodríguez-de-la-Rúa, E. Adverse Events of Antibody–Drug Conjugates on the Ocular Surface in Cancer Therapy. Clin. Transl. Oncol. 2023, 25, 3086–3100. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Kim, N.; Choung, H.K.; In Khwarg, S. Lacrimal Drainage System Stenosis Associated with Trastuzumab Emtansine (Kadcyla®, T-DM1) Administration: A Case Report. BMC Cancer 2019, 19, 774. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, C.; Zhang, C.; Xu, X.; Tian, F.; Wu, B.; Xu, T. Cardiotoxicity with Human Epidermal Growth Factor Receptor-2 Inhibitors in Breast Cancer: Disproportionality Analysis of the FDA Adverse Event Reporting System. Int. J. Cardiol. 2023, 375, 87–93. [Google Scholar] [CrossRef]
- Pondé, N.; Amaye, L.; Lambertini, M.; Paesmans, M.; Piccart, M.; de Azambuja, E. Trastuzumab Emtansine (T-DM1)-Associated Cardiotoxicity: Pooled Analysis in Advanced HER2-Positive Breast Cancer. Eur. J. Cancer 2020, 126, 65–73. [Google Scholar] [CrossRef]
- Delgado, J.; Vleminckx, C.; Sarac, S.; Sosa, A.; Bergh, J.; Giuliani, R.; Enzmann, H.; Pignatti, F. The EMA Review of Trastuzumab Emtansine (T-DM1) for the Adjuvant Treatment of Adult Patients with HER2-Positive Early Breast Cancer. ESMO Open 2021, 6, 100074. [Google Scholar] [CrossRef]
- Montemurro, F.; Ellis, P.; Anton, A.; Wuerstlein, R.; Delaloge, S.; Bonneterre, J.; Quenel-Tueux, N.; Linn, S.C.; Irahara, N.; Donica, M.; et al. Safety of Trastuzumab Emtansine (T-DM1) in Patients with HER2-Positive Advanced Breast Cancer: Primary Results from the KAMILLA Study Cohort 1. Eur. J. Cancer 2019, 109, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Duret-Aupy, N.; Lagarce, L.; Blouet, A.; Kettani, S.; Conte, C.; Bourneau-Martin, D.; Drablier, G.; Umlil, A.; Briet, M. Liver Sinusoidal Obstruction Syndrome Associated with Trastuzumab Emtansine Treatment for Breast Cancer. Therapie 2019, 74, 675–677. [Google Scholar] [CrossRef]
- Curigliano, G.; Dunton, K.; Rosenlund, M.; Janek, M.; Cathcart, J.; Liu, Y.; Fasching, P.A.; Iwata, H. Patient-Reported Outcomes and Hospitalization Data in Patients with HER2-Positive Metastatic Breast Cancer Receiving Trastuzumab Deruxtecan or Trastuzumab Emtansine in the Phase III DESTINY-Breast03 Study. Ann. Oncol. 2023, 34, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Shi, Y.; Yan, R.; Yin, S.; Bu, H.; Huang, J. Efficacy and Safety of Trastuzumab Deruxtecan in Treating Human Epidermal Growth Factor Receptor 2-Low/Positive Advanced Breast Cancer: A Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Oncol. Hematol. 2024, 196, 104305. [Google Scholar] [CrossRef] [PubMed]
- Wuerstlein, R.; Ellis, P.; Montemurro, F.; Antón Torres, A.; Delaloge, S.; Zhang, Q.; Wang, X.; Wang, S.; Shao, Z.; Li, H.; et al. Final Results of the Global and Asia Cohorts of KAMILLA, a Phase IIIB Safety Trial of Trastuzumab Emtansine in Patients with HER2-Positive Advanced Breast Cancer. ESMO Open 2022, 7, 100561. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.R.; Vilbert, M.; Rosa, V.D.L.; Oliveira, J.L.; Deus, M.M.; Freitas-Junior, R. Incidence of Interstitial Lung Disease and Cardiotoxicity with Trastuzumab Deruxtecan in Breast Cancer Patients: A Systematic Review and Single-Arm Meta-Analysis. ESMO Open 2023, 8, 101613. [Google Scholar] [CrossRef]
- Riudavets, M.; Azarine, A.; Smaali, S.; Kim, Y.W.; Thomas de Montpréville, V.; Grecea, A.M.; Naltet, C.; Gazzah, A.; Planchard, D. Unexpected Cardiotoxicity in Patients With HER2-Mutant NSCLC Treated With Trastuzumab Deruxtecan: A Case Report. JTO Clin. Res. Rep. 2022, 3, 100432. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Swain, S.M.; Nishino, M.; Lancaster, L.H.; Li, B.T.; Nicholson, A.G.; Bartholmai, B.J.; Naidoo, J.; Schumacher-Wulf, E.; Shitara, K.; Tsurutani, J.; et al. Multidisciplinary Clinical Guidance on Trastuzumab Deruxtecan (T-DXd)–Related Interstitial Lung Disease/Pneumonitis—Focus on Proactive Monitoring, Diagnosis, and Management. Cancer Treat. Rev. 2022, 106, 102378. [Google Scholar] [CrossRef]
- Bellon, J.R.; Tayob, N.; Yang, D.D.; Tralins, J.; Dang, C.T.; Isakoff, S.J.; DeMeo, M.; Burstein, H.J.; Partridge, A.H.; Winer, E.P.; et al. Local Therapy Outcomes and Toxicity From the ATEMPT Trial (TBCRC 033): A Phase II Randomized Trial of Adjuvant Trastuzumab Emtansine Versus Paclitaxel in Combination with Trastuzumab in Women with Stage I HER2-Positive Breast Cancer. Int. J. Radiat. Oncol. 2022, 113, 117–124. [Google Scholar] [CrossRef]
- Salvestrini, V.; Kim, K.; Caini, S.; Alkner, S.; Ekholm, M.; Skyttä, T.; Becherini, C.; Coles, C.E.; Kaidar-Person, O.; Offersen, B.; et al. Safety Profile of Trastuzumab-Emtansine (T-DM1) with Concurrent Radiation Therapy: A Systematic Review and Meta-Analysis. Radiother. Oncol. 2023, 186, 109805. [Google Scholar] [CrossRef] [PubMed]
- Crestan, D.; Trojniak, M.P.; Francescon, S.; Fornasier, G.; Baldo, P. Pharmacovigilance of Anti-Cancer Medicines: Opportunities and Challenges. Expert Opin. Drug Saf. 2020, 19, 849–860. [Google Scholar] [CrossRef] [PubMed]
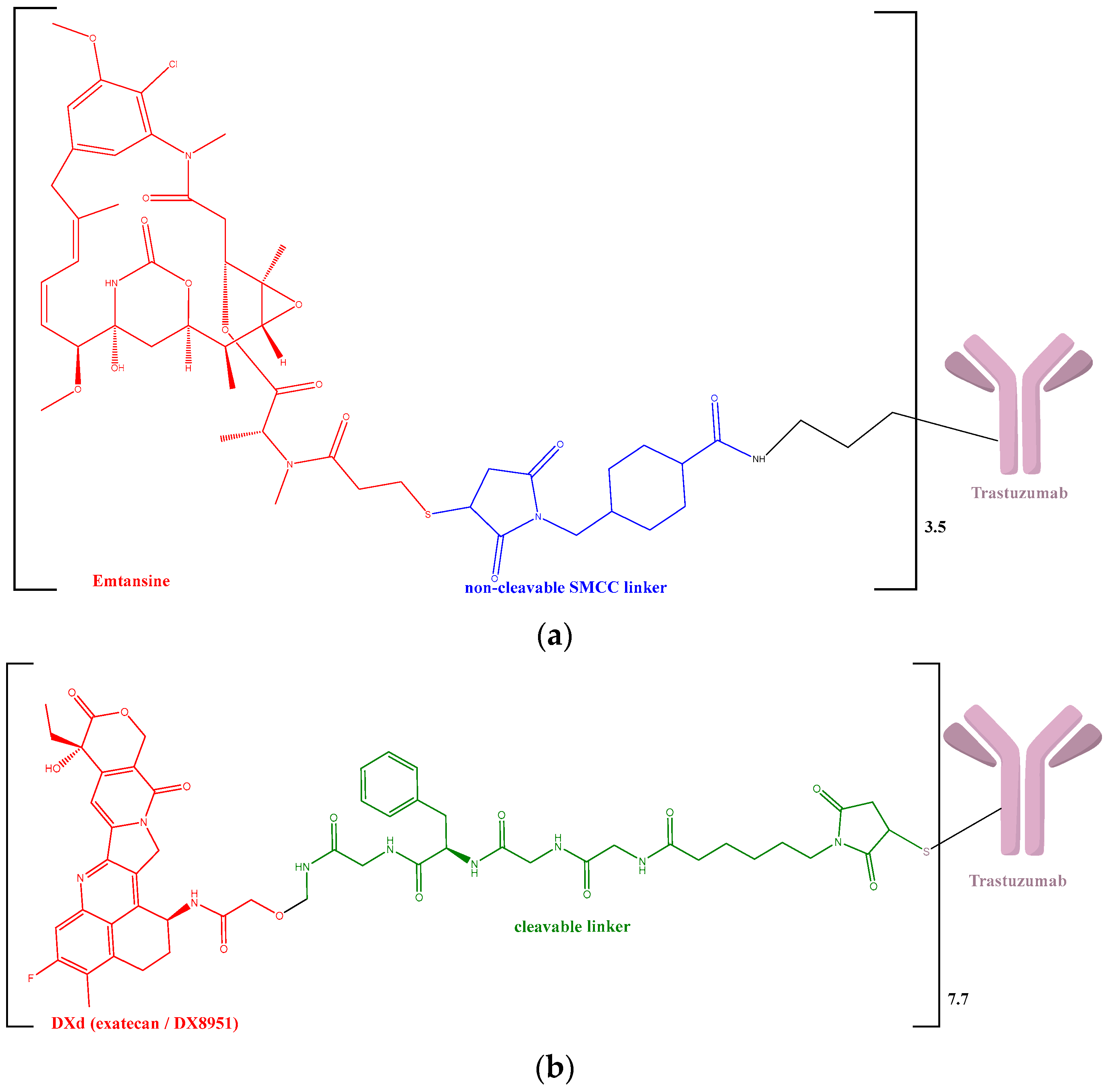



| ADC | Year of Approval on the Market | Indication | Reports Registered in the EV Database |
|---|---|---|---|
| Gemtuzumab ozogamicin * | 2000 | acute myelogenous leukaemia | Yes |
| Brentuximab vedotin | 2011 | Hodgkin lymphoma | Yes |
| Trastuzumab emtansine | 2013 | HER2-positive breast cancer | Yes |
| Inotuzumab ozogamicin | 2017 | acute lymphoblastic leukaemia | Yes |
| Moxetumomab pasudotox | 2018 | hairy cell leukaemia | Yes |
| Enfortumab vedotin | 2019 | metastatic urothelial cancer | Yes |
| Polatuzumab vedotin | 2019 | beta-cell lymphoma | Yes |
| Trastuzumab deruxtecan | 2019 | HER2-positive breast cancer | Yes |
| Sacituzumab govitecan | 2020 | triple-negative breast cancer | Yes |
| Tisotumab vedotin | 2021 | cervical cancer | No |
| Disitamab vedotin | 2021 | advanced breast cancer | No |
| Loncastuximab tesirine | 2021 | beta-cell lymphoma | Yes |
| Mirvetuximab soravtansine | 2022 | ovarian cancer | No |
| T-DXd n (%) | T-DM1 n (%) | Other ADCs n (%) | T n (%) | PER n (%) | LAP n (%) | TUC n (%) | NER n (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Total ICSR | 2753 | 4994 | 13,768 | 37,461 | 11,452 | 4268 | 1015 | 377 | |
| Age category | Not specified | 1350 | 1823 | 3996 | 10,722 | 3063 | 1576 | 649 | 155 |
| (49.04) | (36.50) | (29.02) | (28.62) | (26.75) | (36.93) | (63.94) | (41.11) | ||
| 0–1 month | 0 | 0 | 2 | 16 | 2 | 0 | 2 | 0 | |
| (0.00) | (0.00) | (0.01) | (0.04) | (0.02) | (0.00) | (0.20) | (0.00) | ||
| 2 months–2 years | 3 | 1 | 18 | 16 | 3 | 0 | 0 | 0 | |
| (0.11) | (0.02) | (0.13) | (0.04) | (0.03) | (0.00) | (0.00) | (0.00) | ||
| 3–11 years | 0 | 0 | 122 | 4 | 1 | 3 | 0 | 0 | |
| (0.00) | (0.00) | (0.89) | (0.01) | (0.01) | (0.07) | (0.00) | (0.00) | ||
| 12–17 years | 0 | 0 | 185 | 6 | 1 | 3 | 0 | 0 | |
| (0.00) | (0.00) | (1.34) | (0.02) | (0.01) | (0.07) | (0.00) | (0.00) | ||
| 18–64 years | 950 | 2366 | 4814 | 19,439 | 6341 | 2062 | 269 | 174 | |
| (34.51) | (47.38) | (34.97) | (51.89) | (55.37) | (48.31) | (26.50) | (46.15) | ||
| 65–85 years | 433 | 769 | 4162 | 6994 | 1980 | 598 | 95 | 48 | |
| (15.73) | (15.40) | (30.23) | (18.67) | (17.29) | (14.01) | (9.36) | (12.73) | ||
| More than 85 years | 17 | 35 | 469 | 264 | 61 | 26 | 0 | 0 | |
| (0.62) | (0.70) | (3.41) | (0.70) | (0.53) | (0.61) | (0.00) | (0.00) | ||
| Sex | Female | 2469 | 4679 | 5619 | 33,081 | 10,637 | 3979 | 942 | 350 |
| (89.68) | (93.69) | (40.81) | (88.31) | (92.88) | (93.23) | (92.81) | (92.84) | ||
| Male | 214 | 68 | 6624 | 2491 | 246 | 118 | 28 | 11 | |
| (7.77) | (1.36) | (48.11) | (6.65) | (2.15) | (2.76) | (2.76) | (2.92) | ||
| Not specified | 70 | 247 | 1525 | 1889 | 569 | 171 | 45 | 16 | |
| (2.54) | (4.95) | (11.08) | (5.04) | (4.97) | (4.01) | (4.43) | (4.24) | ||
| T-DM1 n (%) | T-DXd n (%) | Other ADCs n (%) | T n (%) | PER n (%) | LAP n (%) | TUC n (%) | NER n (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Geographical origin of the reporter | EA | 1920 | 1073 | 4760 | 14,238 | 4111 | 1118 | 293 | 180 |
| (38.45) | (38.98) | (34.57) | (38.01) | (35.90) | (26.19) | (28.87) | (47.75) | ||
| NON-EEA | 3074 | 1680 | 9008 | 23,223 | 7341 | 3150 | 722 | 197 | |
| (61.55) | (61.02) | (65.43) | (61.99) | (64.10) | (73.81) | (71.13) | (52.25) | ||
| Reporter Category | HP | 4522 | 2600 | 13,195 | 34,256 | 10,417 | 3544 | 742 | 322 |
| (90.55) | (94.44) | (95.84) | (91.44) | (90.96) | (83.04) | (73.10) | (85.41) | ||
| Non-HP | 472 | 153 | 572 | 3182 | 1035 | 724 | 273 | 55 | |
| (9.45) | (5.56) | (4.15) | (8.49) | (9.04) | (16.96) | (26.90) | (14.59) | ||
| NS | 0 | 0 | 1 | 23 | 0 | 0 | 0 | 0 | |
| (0.00) | (0.00) | (0.01) | (0.06) | (0.00) | (0.00) | (0.00) | (0.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgovan, C.; Dobrea, C.M.; Butuca, A.; Arseniu, A.M.; Frum, A.; Rus, L.L.; Chis, A.A.; Juncan, A.M.; Gligor, F.G.; Georgescu, C.; et al. Safety Profile of the Trastuzumab-Based ADCs: Analysis of Real-World Data Registered in EudraVigilance. Biomedicines 2024, 12, 953. https://doi.org/10.3390/biomedicines12050953
Morgovan C, Dobrea CM, Butuca A, Arseniu AM, Frum A, Rus LL, Chis AA, Juncan AM, Gligor FG, Georgescu C, et al. Safety Profile of the Trastuzumab-Based ADCs: Analysis of Real-World Data Registered in EudraVigilance. Biomedicines. 2024; 12(5):953. https://doi.org/10.3390/biomedicines12050953
Chicago/Turabian StyleMorgovan, Claudiu, Carmen Maximiliana Dobrea, Anca Butuca, Anca Maria Arseniu, Adina Frum, Luca Liviu Rus, Adriana Aurelia Chis, Anca Maria Juncan, Felicia Gabriela Gligor, Cecilia Georgescu, and et al. 2024. "Safety Profile of the Trastuzumab-Based ADCs: Analysis of Real-World Data Registered in EudraVigilance" Biomedicines 12, no. 5: 953. https://doi.org/10.3390/biomedicines12050953
APA StyleMorgovan, C., Dobrea, C. M., Butuca, A., Arseniu, A. M., Frum, A., Rus, L. L., Chis, A. A., Juncan, A. M., Gligor, F. G., Georgescu, C., Ghibu, S., & Vonica-Tincu, A. L. (2024). Safety Profile of the Trastuzumab-Based ADCs: Analysis of Real-World Data Registered in EudraVigilance. Biomedicines, 12(5), 953. https://doi.org/10.3390/biomedicines12050953








