Small Intestinal Bacterial Overgrowth (SIBO) and Twelve Groups of Related Diseases—Current State of Knowledge
Abstract
1. Introduction
2. Gut Microbiota
3. Methodology
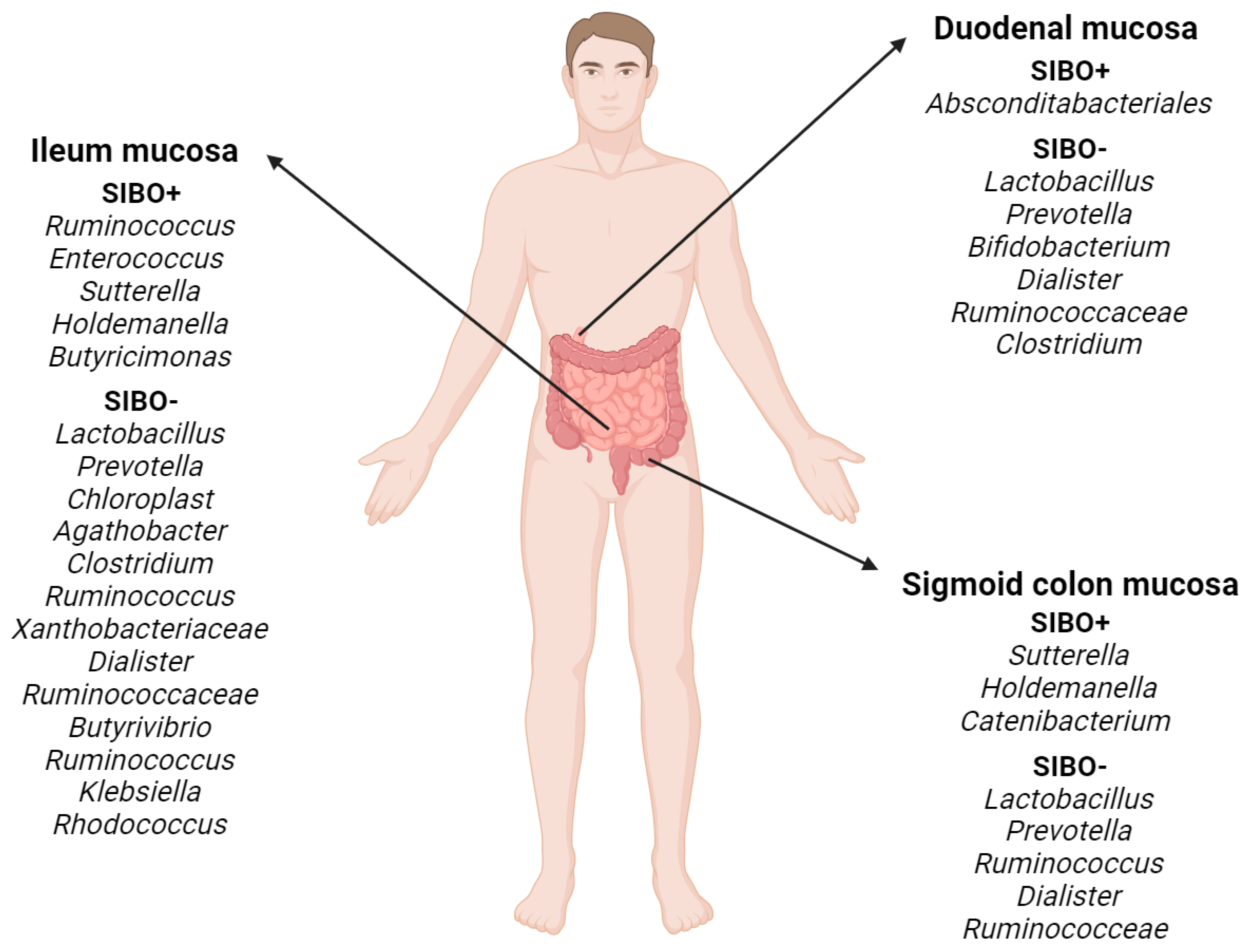
4. Small Intestinal Bacterial Overgrowth (SIBO)
4.1. General Characteristics
4.2. Diagnostics
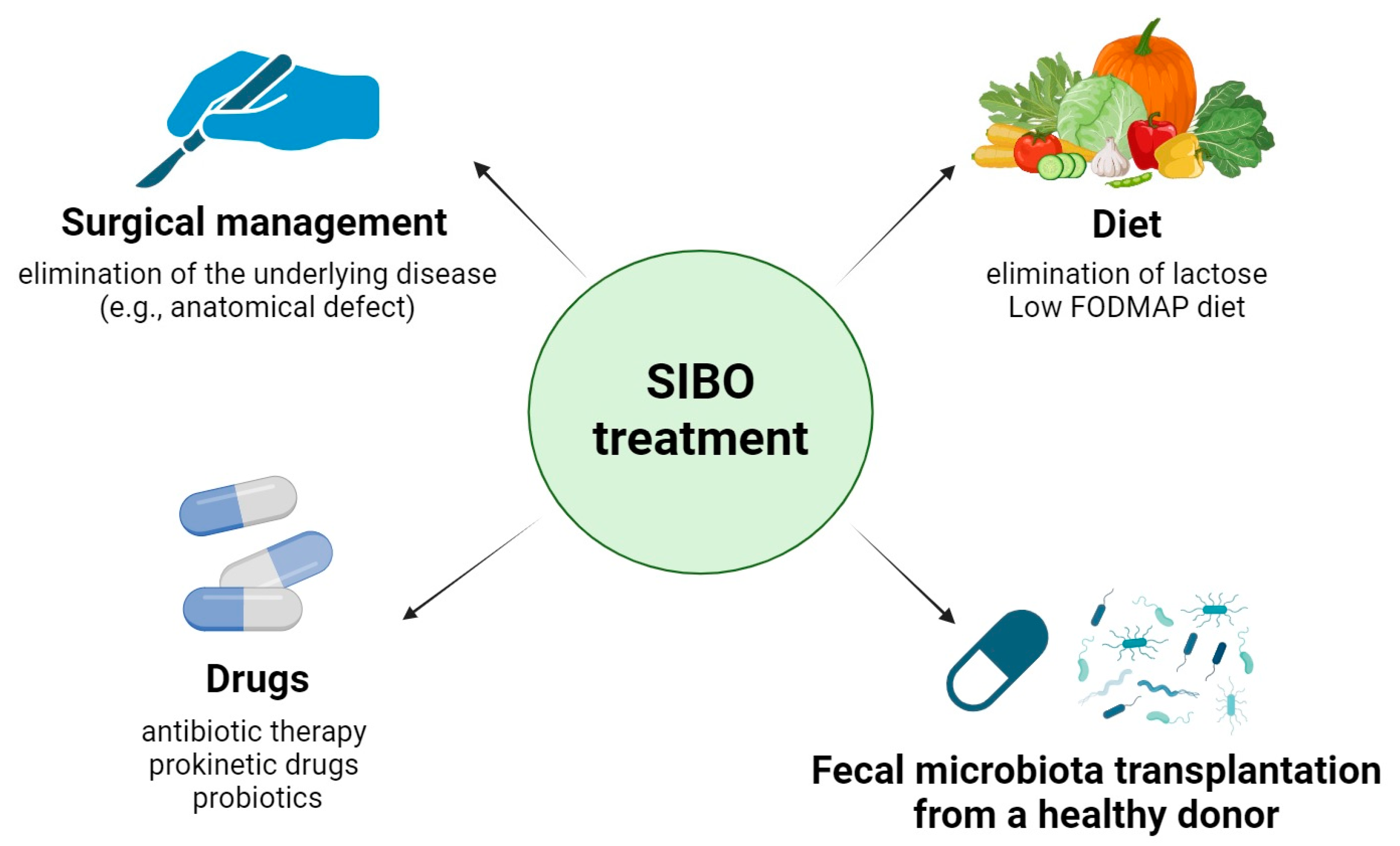
4.3. Treatment and Diet
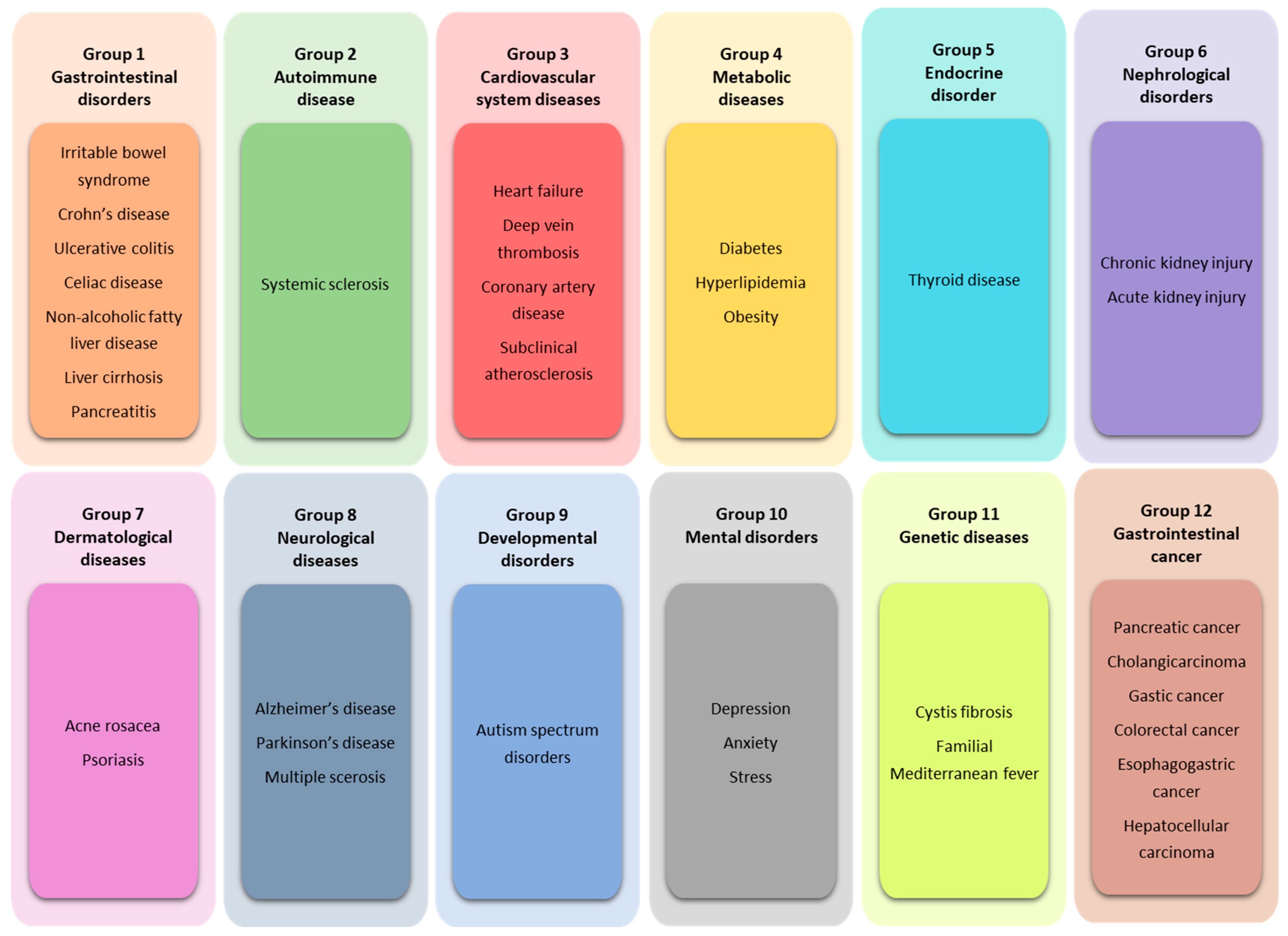
5. SIBO-Related Diseases
5.1. Group 1: Gastrointestinal Disorders
5.1.1. Irritable Bowel Syndrome (IBS)
5.1.2. Crohn’s Disease (CD) and Ulcerative Colitis (UC)
5.1.3. Celiac Disease (CeD)
5.1.4. Non-Alcoholic Fatty Liver Disease (NAFLD)
5.1.5. Liver Cirrhosis
5.1.6. Pancreatitis
5.2. Group 2: Autoimmune Disease
Systemic Sclerosis (SSc)
5.3. Group 3: Cardiovascular System Diseases
5.3.1. Heart Failure (HF)
5.3.2. Deep Vein Thrombosis (DVT)
5.3.3. Coronary Artery Disease (CAD)
5.3.4. Subclinical Atherosclerosis
5.4. Group 4: Metabolic Diseases
5.4.1. Diabetes
5.4.2. Hyperlipidemia
5.4.3. Obesity
5.5. Group 5: Endocrine Disorder
Thyroid Disease
5.6. Group 6: Nephrological Disorders
5.6.1. Chronic Kidney Injury
5.6.2. Acute Kidney Injury
5.7. Group 7. Dermatological Diseases
5.7.1. Acne Rosacea
5.7.2. Psoriasis
5.8. Group 8. Neurological Diseases
5.8.1. Alzheimer’s Disease (AD)
5.8.2. Parkinson’s Disease (PD)
5.8.3. Multiple Sclerosis (MS)
5.9. Group 9: Developmental Disorders
Autism Spectrum Disorders (ASDs)
5.10. Group 10: Mental Disorders
5.11. Group 11: Genetic Diseases
5.11.1. Cystic Fibrosis (CF)
5.11.2. Familial Mediterranean Fever (FMF)
5.12. Group 12: Gastrointestinal Cancer
| Groups | Diseases | The Impact of SIBO on the Disease |
|---|---|---|
| Group 1 | Irritable bowel syndrome (IBS) |
|
Inflammatory bowel disease (IBD)
|
| |
| Celiac disease (CeD) |
| |
| Non-alcoholic fatty liver disease (NAFLD) |
| |
| Liver cirrhosis |
| |
| Pancreatitis |
| |
| Group 2 | Systemic sclerosis (SSc) |
|
| Group 3 | Heart failure (HF) |
|
| Deep vein thrombosis (DVT) |
| |
| Coronary artery disease (CAD) |
| |
| Subclinical atherosclerosis |
| |
| Group 4 | Diabetes |
|
| Hyperlipidemia |
| |
| Obesity |
| |
| Group 5 | Thyroid disease |
|
| Group 6 | Chronic kidney injury (CKI) |
|
| Acute kidney injury (AKI) |
| |
| Group 7 | Acne rosacea |
|
| Psoriasis |
| |
| Group 8 | Alzheimer’s disease (AD) |
|
| Parkinson’s disease (PD) |
| |
| Multiple sclerosis (MS) |
| |
| Group 9 | Autism spectrum disorders (ASDs) |
|
| Group 10 | Mental disorders
|
|
| Group 11 | Cystic fibrosis (CF) |
|
| Familial Mediterranean Fever (FMF) |
| |
| Group 12 | Gastrointestinal cancer |
|
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnstone, C.; Hendry, C.; Farley, A.; McLafferty, E. The digestive system: Part 1. Nurs. Stand. 2014, 28, 37–45. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M. Gut bacteria in health and disease. Gastroenterol. Hepatol. 2013, 9, 560–569. [Google Scholar]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Achufusi, T.G.O.; Sharma, A.; Zamora, E.A.; Manocha, D. Small Intestinal Bacterial Overgrowth: Comprehensive Review of Diagnosis, Prevention, and Treatment Methods. Cureus 2020, 12, e8860. [Google Scholar] [CrossRef] [PubMed]
- Efremova, I.; Maslennikov, R.; Poluektova, E.; Vasilieva, E.; Zharikov, Y.; Suslov, A.; Letyagina, Y.; Kozlov, E.; Levshina, A.; Ivashkin, V. Epidemiology of small intestinal bacterial overgrowth. World J. Gastroenterol. 2023, 29, 3400–3421. [Google Scholar] [CrossRef]
- Bushyhead, D.; Quigley, E.M. Small Intestinal Bacterial Overgrowth. Gastroenterol. Clin. N. Am. 2021, 50, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Bushyhead, D.; Quigley, E.M.M. Small Intestinal Bacterial Overgrowth-Pathophysiology and Its Implications for Definition and Management. Gastroenterology 2022, 163, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Judkins, T.C.; Archer, D.L.; Kramer, D.C.; Solch, R.J. Probiotics, Nutrition, and the Small Intestine. Curr. Gastroenterol. Rep. 2020, 22, 2. [Google Scholar] [CrossRef]
- Volk, N.; Lacy, B. Anatomy and Physiology of the Small Bowel. Gastrointest. Endosc. 2017, 27, 1–13. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J.; McCray, A.T. Ome Sweet ‘Omics—A Genealogical Treasury of Words. Science 2001, 15, 8. [Google Scholar]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Eckburg, P.B.; Bik, E.M.; Relman, D.A. Assembly of the human intestinal microbiota. Trends Ecol. Evol. 2006, 21, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Losurdo, G.; Salvatore D’Abramo, F.; Indellicati, G.; Lillo, C.; Ierardi, E.; Di Leo, A. The Influence of Small Intestinal Bacterial Overgrowth in Digestive and Extra-Intestinal Disorders. Int. J. Mol. Sci. 2020, 21, 3531. [Google Scholar] [CrossRef]
- Bohm, M.; Shin, A.; Teagarden, S.; Xu, H.; Gupta, A.; Siwiec, R.; Nelson, D.; Wo, J.M. Risk Factors Associated with Upper Aerodigestive Tract or Coliform Bacterial Overgrowth of the Small Intestine in Symptomatic Patients. J. Clin. Gastroenterol. 2020, 54, 150–157. [Google Scholar] [CrossRef]
- Li, J.; Zhang, R.; Ma, J.; Tang, S.; Li, Y.; Li, Y.; Wan, J. Mucosa-Associated Microbial Profile Is Altered in Small Intestinal Bacterial Overgrowth. Front. Microbiol. 2021, 12, 710940. [Google Scholar] [CrossRef]
- Pimentel, M.; Saad, R.J.; Long, M.D.; Rao, S.S.C. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, A.; Lauritano, E.C.; Gabrielli, M.; Scarpellini, E.; Lupascu, A.; Ojetti, V.; Gasbarrini, G. Small intestinal bacterial overgrowth: Diagnosis and treatment. Dig. Dis. 2007, 25, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.D. Macrocytic anemia associated with intestinal strictures and anastomoses: Report of two cases. Ann. Intern. Med. 1954, 40, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.S.; Lin, H.C. Double-blind, placebo-controlled antibiotic treatment study of small intestinal bacterial overgrowth in children with chronic abdominal pain. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 382–386. [Google Scholar] [CrossRef] [PubMed]
- de Boissieu, D.; Chaussain, M.; Badoual, J.; Raymond, J.; Dupont, C. Small-bowel bacterial overgrowth in children with chronic diarrhea, abdominal pain, or both. J. Pediatr. 1996, 128, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Korterink, J.J.; Benninga, M.A.; van Wering, H.M.; Deckers-Kocken, J.M. Glucose hydrogen breath test for small intestinal bacterial overgrowth in children with abdominal pain-related functional gastrointestinal disorders. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Siniewicz-Luzenczyk, K.; Bik-Gawin, A.; Zeman, K.; Bak-Romaniszyn, L. Small intestinal bacterial overgrowth syndrome in children. Gastroenterol. Rev. 2015, 10, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef]
- Saad, R.J.; Chey, W.D. Breath testing for small intestinal bacterial overgrowth: Maximizing test accuracy. Clin. Gastroenterol. Hepatol. 2014, 12, 1964–1972; quiz e1119–1920. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Bhagatwala, J. Small Intestinal Bacterial Overgrowth: Clinical Features and Therapeutic Management. Clin. Transl. Gastroenterol. 2019, 10, e00078. [Google Scholar] [CrossRef]
- Shaker, A.; Peng, B.; Soffer, E. Pattern of methane levels with lactulose breath testing; can we shorten the test duration? JGH Open 2021, 5, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Dukowicz, A.C.; Lacy, B.E.; Levine, G.M. Small intestinal bacterial overgrowth: A comprehensive review. Gastroenterol. Hepatol. 2007, 3, 112–122. [Google Scholar]
- Adike, A.; DiBaise, J.K. Small Intestinal Bacterial Overgrowth: Nutritional Implications, Diagnosis, and Management. Gastroenterol. Clin. N. Am. 2018, 47, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Vanderhoof, J.A.; Young, R.J.; Murray, N.; Kaufman, S.S. Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Sessarego, M.; Greco, A.; Bazzica, M.; Filaci, G.; Setti, M.; Savarino, E.; Indiveri, F.; Savarino, V.; Ghio, M. Small intestinal bacterial overgrowth in patients suffering from scleroderma: Clinical effectiveness of its eradication. Am. J. Gastroenterol. 2008, 103, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Frissora, C.L.; Cash, B.D. Review article: The role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2007, 25, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.L.; DuPont, H.L. Rifaximin: A unique gastrointestinal-selective antibiotic for enteric diseases. Curr. Opin. Gastroenterol. 2010, 26, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.; Scarpignato, C. Systematic review with meta-analysis: Rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment. Pharmacol. Ther. 2017, 45, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, H.R.; Low, K.; Chatterjee, S.; Pimentel, M. Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig. Dis. Sci. 2008, 53, 169–174. [Google Scholar] [CrossRef]
- Rabenstein, T.; Fromm, M.F.; Zolk, O. Rifaximin--a non-resorbable antibiotic with many indications in gastroenterology. Z. Gastroenterol. 2011, 49, 211–224. [Google Scholar] [CrossRef]
- Di Stefano, M.; Strocchi, A.; Malservisi, S.; Veneto, G.; Ferrieri, A.; Corazza, G.R. Non-absorbable antibiotics for managing intestinal gas production and gas-related symptoms. Aliment. Pharmacol. Ther. 2000, 14, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, N.; Wang, C.; Xing, H.; Chen, D.; Wei, Y. Clinical efficacy of fecal microbiota transplantation for patients with small intestinal bacterial overgrowth: A randomized, placebo-controlled clinic study. BMC Gastroenterol. 2021, 21, 54. [Google Scholar] [CrossRef]
- Nickles, M.A.; Hasan, A.; Shakhbazova, A.; Wright, S.; Chambers, C.J.; Sivamani, R.K. Alternative Treatment Approaches to Small Intestinal Bacterial Overgrowth: A Systematic Review. J. Altern. Complement. Med. 2021, 27, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, A.R.; Khalighi, M.R.; Behdani, R.; Jamali, J.; Khosravi, A.; Kouhestani, S.; Radmanesh, H.; Esmaeelzadeh, S.; Khalighi, N. Evaluating the efficacy of probiotic on treatment in patients with small intestinal bacterial overgrowth (SIBO)—A pilot study. Indian J. Med. Res. 2014, 140, 604–608. [Google Scholar] [PubMed]
- Leventogiannis, K.; Gkolfakis, P.; Spithakis, G.; Tsatali, A.; Pistiki, A.; Sioulas, A.; Giamarellos-Bourboulis, E.J.; Triantafyllou, K. Effect of a Preparation of Four Probiotics on Symptoms of Patients with Irritable Bowel Syndrome: Association with Intestinal Bacterial Overgrowth. Probiotics Antimicrob. Proteins 2019, 11, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; Rocha, R.; Cotrim, H.P. Diet and intestinal bacterial overgrowth: Is there evidence? World J. Clin. Cases 2022, 10, 4713–4716. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.; Whelan, K. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Ginnebaugh, B.; Chey, W.D.; Saad, R. Small Intestinal Bacterial Overgrowth: How to Diagnose and Treat (and Then Treat Again). Gastroenterol. Clin. N. Am. 2020, 49, 571–587. [Google Scholar] [CrossRef]
- Takakura, W.; Pimentel, M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome—An Update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef]
- Saltzman, J.R.; Kowdley, K.V.; Pedrosa, M.C.; Sepe, T.; Golner, B.; Perrone, G.; Russell, R.M. Bacterial overgrowth without clinical malabsorption in elderly hypochlorhydric subjects. Gastroenterology 1994, 106, 615–623. [Google Scholar] [CrossRef]
- Husebye, E.; Skar, V.; Hoverstad, T.; Melby, K. Fasting hypochlorhydria with gram positive gastric flora is highly prevalent in healthy old people. Gut 1992, 33, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Iivonen, M.K.; Ahola, T.O.; Matikainen, M.J. Bacterial overgrowth, intestinal transit, and nutrition after total gastrectomy. Comparison of a jejunal pouch with Roux-en-Y reconstruction in a prospective random study. Scand. J. Gastroenterol. 1998, 33, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Biavati, B.; Calabrese, C.; Granata, M.; Nannetti, A.; Mattarelli, P.; Di Febo, G.; Saccoccio, G.; Biasco, G. Urease-positive bacteria other than Helicobacter pylori in human gastric juice and mucosa. Am. J. Gastroenterol. 2006, 101, 1756–1761. [Google Scholar] [CrossRef]
- Shindo, K.; Fukumura, M. Effect of H2-receptor antagonists on bile acid metabolism. J. Investig. Med. 1995, 43, 170–177. [Google Scholar] [PubMed]
- Kim, D.B.; Paik, C.N.; Kim, Y.J.; Lee, J.M.; Jun, K.H.; Chung, W.C.; Lee, K.M.; Yang, J.M.; Choi, M.G. Positive Glucose Breath Tests in Patients with Hysterectomy, Gastrectomy, and Cholecystectomy. Gut Liver 2017, 11, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, T.; Rhyman, N.; Gauthier, C.; Paris, J.; Lang, A.S.; Lepers-Tassy, S.; Manfredi, S.; Lepage, C.; Leloup, C.; Jacquin-Piques, A.; et al. Study of Small Intestinal Bacterial Overgrowth in a Cohort of Patients with Abdominal Symptoms Who Underwent Bariatric Surgery. Obes. Surg. 2020, 30, 2331–2337. [Google Scholar] [CrossRef] [PubMed]
- Onana Ndong, P.; Boutallaka, H.; Marine-Barjoan, E.; Ouizeman, D.; Mroue, R.; Anty, R.; Vanbiervliet, G.; Piche, T. Prevalence of small intestinal bacterial overgrowth in irritable bowel syndrome (IBS): Correlating H(2) or CH(4) production with severity of IBS. JGH Open 2023, 7, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Xiang, F.; Feng, J. Correlation between small intestinal bacterial overgrowth and irritable bowel syndrome and the prognosis of treatment. Ann. Palliat. Med. 2021, 10, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Q.; Sun, W.J.; Li, N.; Chen, Y.Q.; Wei, Y.L.; Chen, D.F. Small intestinal bacterial overgrowth is associated with Diarrhea-predominant irritable bowel syndrome by increasing mainly Prevotella abundance. Scand. J. Gastroenterol. 2019, 54, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C. Small intestinal bacterial overgrowth: A framework for understanding irritable bowel syndrome. JAMA 2004, 292, 852–858. [Google Scholar] [CrossRef]
- Pimentel, M.; Chow, E.J.; Lin, H.C. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 2003, 98, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Wallace, D.; Hallegua, D.; Chow, E.; Kong, Y.; Park, S.; Lin, H.C. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann. Rheum. Dis. 2004, 63, 450–452. [Google Scholar] [CrossRef]
- Posserud, I.; Stotzer, P.O.; Bjornsson, E.S.; Abrahamsson, H.; Simren, M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007, 56, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Bratten, J.R.; Spanier, J.; Jones, M.P. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am. J. Gastroenterol. 2008, 103, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Kumar, S.; Mehrotra, M.; Lakshmi, C.; Misra, A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J. Neurogastroenterol. Motil. 2010, 16, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Rawat, A.K.; Reddy, R.S.; Puri, A.S. Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: Frequency and predictors. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. S3), 135–138. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.H.; Zahedi, M.; Darvish Moghadam, S.; Shafieipour, S.; HayatBakhsh Abbasi, M. Small bowel bacterial overgrowth in patients with irritable bowel syndrome: The first study in iran. Middle East J. Dig. Dis. 2015, 7, 36–40. [Google Scholar] [PubMed]
- Parodi, A.; Dulbecco, P.; Savarino, E.; Giannini, E.G.; Bodini, G.; Corbo, M.; Isola, L.; De Conca, S.; Marabotto, E.; Savarino, V. Positive glucose breath testing is more prevalent in patients with IBS-like symptoms compared with controls of similar age and gender distribution. J. Clin. Gastroenterol. 2009, 43, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Yadav, A.; Fatima, B.; Agrahari, A.P.; Misra, A. Small intestinal bacterial overgrowth in patients with inflammatory bowel disease: A case-control study. Indian J. Gastroenterol. 2022, 41, 96–103. [Google Scholar] [CrossRef]
- Aziz, I.; Tornblom, H.; Simren, M. Small intestinal bacterial overgrowth as a cause for irritable bowel syndrome: Guilty or not guilty? Curr. Opin. Gastroenterol. 2017, 33, 196–202. [Google Scholar] [CrossRef]
- Wei, J.; Feng, J.; Chen, L.; Yang, Z.; Tao, H.; Li, L.; Xuan, J.; Wang, F. Small intestinal bacterial overgrowth is associated with clinical relapse in patients with quiescent Crohn’s disease: A retrospective cohort study. Ann. Transl. Med. 2022, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Kulygina, Y.; Osipenko, M.; Skalinskaya, M.; Alikina, T.; Kabilov, M.; Lukinov, V.; Sitkin, S. Small intestinal bacterial overgrowth in patients with Crohn’s disease is not only associated with a more severe disease, but is also marked by dramatic changes in the gut microbiome. J. Crohn’s Colitis 2019, 13 (Suppl. S1), S544. [Google Scholar] [CrossRef]
- Bertges, E.R.; Chebli, J.M.F. Prevalence and Factors Associated with Small Intestinal Bacterial Overgrowth in Patients with Crohn’s Disease: A Retrospective Study at a Referral Center. Arq. Gastroenterol. 2020, 57, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Wanzl, J.; Grohl, K.; Kafel, A.; Nagl, S.; Muzalyova, A.; Golder, S.K.; Ebigbo, A.; Messmann, H.; Schnoy, E. Impact of Small Intestinal Bacterial Overgrowth in Patients with Inflammatory Bowel Disease and Other Gastrointestinal Disorders-A Retrospective Analysis in a Tertiary Single Center and Review of the Literature. J. Clin. Med. 2023, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Wang, S.; Huo, X.; Wang, J. Small intestinal bacterial overgrowth and evaluation of intestinal barrier function in patients with ulcerative colitis. Am. J. Transl. Res. 2021, 13, 6605–6610. [Google Scholar]
- Pimentel, M.; Mathur, R.; Chang, C. Gas and the microbiome. Curr. Gastroenterol. Rep. 2013, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Mekelburg, S.; Tafesh, Z.; Coburn, E.; Weg, R.; Malik, N.; Webb, C.; Hammad, H.; Scherl, E.; Bosworth, B.P. Testing and Treating Small Intestinal Bacterial Overgrowth Reduces Symptoms in Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2018, 63, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Morrison, M.; Burger, D.; Martin, N.; Rich, J.; Jones, M.; Koloski, N.; Walker, M.M.; Talley, N.J.; Holtmann, G.J. Systematic review with meta-analysis: The prevalence of small intestinal bacterial overgrowth in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 49, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Guo, X.; Wang, J.; Fan, H.; Huo, X.; Dong, L.; Duan, Z. Relationship between Small Intestinal Bacterial Overgrowth and Peripheral Blood ET, TLR2 and TLR4 in Ulcerative Colitis. J. Coll. Physicians Surg. Pak. 2020, 30, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Safi, M.A.; Jiman-Fatani, A.A.; Saadah, O.I. Small intestinal bacterial overgrowth among patients with celiac disease unresponsive to a gluten free diet. Turk. J. Gastroenterol. 2020, 31, 767–774. [Google Scholar] [CrossRef]
- Shah, A.; Thite, P.; Hansen, T.; Kendall, B.J.; Sanders, D.S.; Morrison, M.; Jones, M.P.; Holtmann, G. Links between celiac disease and small intestinal bacterial overgrowth: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2022, 37, 1844–1852. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Giorgetti, G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am. J. Gastroenterol. 2003, 98, 839–843. [Google Scholar] [CrossRef]
- Fraquelli, M.; Bardella, M.T.; Peracchi, M.; Cesana, B.M.; Bianchi, P.A.; Conte, D. Gallbladder emptying and somatostatin and cholecystokinin plasma levels in celiac disease. Am. J. Gastroenterol. 1999, 94, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Bardella, M.T.; Fraquelli, M.; Peracchi, M.; Cesana, B.M.; Bianchi, P.A.; Conte, D. Gastric emptying and plasma neurotensin levels in untreated celiac patients. Scand. J. Gastroenterol. 2000, 35, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Barton, S.H.; Rosenblatt, J.E.; Murray, J.A. Prevalence of small intestine bacterial overgrowth diagnosed by quantitative culture of intestinal aspirate in celiac disease. J. Clin. Gastroenterol. 2009, 43, 157–161. [Google Scholar] [CrossRef]
- Chang, M.S.; Minaya, M.T.; Cheng, J.; Connor, B.A.; Lewis, S.K.; Green, P.H. Double-blind randomized controlled trial of rifaximin for persistent symptoms in patients with celiac disease. Dig. Dis. Sci. 2011, 56, 2939–2946. [Google Scholar] [CrossRef]
- Charlesworth, R.P.G.; Winter, G. Small intestinal bacterial overgrowth and Celiac disease—Coincidence or causation? Expert Rev. Gastroenterol. Hepatol. 2020, 14, 305–306. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Goel, A.; Quigley, E.M.M. Gut microbiota abnormalities, small intestinal bacterial overgrowth, and non-alcoholic fatty liver disease: An emerging paradigm. Indian J. Gastroenterol. 2020, 39, 9–21. [Google Scholar] [CrossRef]
- Gudan, A.; Kozlowska-Petriczko, K.; Wunsch, E.; Bodnarczuk, T.; Stachowska, E. Small Intestinal Bacterial Overgrowth and Non-Alcoholic Fatty Liver Disease: What Do We Know in 2023? Nutrients 2023, 15, 1323. [Google Scholar] [CrossRef]
- Augustyn, M.; Grys, I.; Kukla, M. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease. Clin. Exp. Hepatol. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gudan, A.; Jamiol-Milc, D.; Hawrylkowicz, V.; Skonieczna-Zydecka, K.; Stachowska, E. The Prevalence of Small Intestinal Bacterial Overgrowth in Patients with Non-Alcoholic Liver Diseases: NAFLD, NASH, Fibrosis, Cirrhosis-A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 5261. [Google Scholar] [CrossRef]
- Rafiei, R.; Bemanian, M.; Rafiei, F.; Bahrami, M.; Fooladi, L.; Ebrahimi, G.; Hemmat, A.; Torabi, Z. Liver disease symptoms in non-alcoholic fatty liver disease and small intestinal bacterial overgrowth. Rom. J. Intern. Med. 2018, 56, 85–89. [Google Scholar] [CrossRef]
- Gkolfakis, P.; Tziatzios, G.; Leite, G.; Papanikolaou, I.S.; Xirouchakis, E.; Panayiotides, I.G.; Karageorgos, A.; Millan, M.J.; Mathur, R.; Weitsman, S.; et al. Prevalence of Small Intestinal Bacterial Overgrowth Syndrome in Patients with Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis: A Cross-Sectional Study. Microorganisms 2023, 11, 723. [Google Scholar] [CrossRef]
- Fialho, A.; Fialho, A.; Thota, P.; McCullough, A.J.; Shen, B. Small Intestinal Bacterial Overgrowth Is Associated with Non-Alcoholic Fatty Liver Disease. J. Gastrointest. Liver Dis. 2016, 25, 159–165. [Google Scholar] [CrossRef]
- Shi, H.; Mao, L.; Wang, L.; Quan, X.; Xu, X.; Cheng, Y.; Zhu, S.; Dai, F. Small intestinal bacterial overgrowth and orocecal transit time in patients of nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2021, 33, e535–e539. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Delija, B.; Mijic, A.; Stevanovic, T.; Skenderevic, N.; Sosa, I.; Krznaric-Zrnic, I.; Abram, M.; Krznaric, Z.; Domislovic, V.; et al. Small intestinal bacterial overgrowth and non-alcoholic fatty liver disease diagnosed by transient elastography and liver biopsy. Int. J. Clin. Pract. 2021, 75, e13947. [Google Scholar] [CrossRef]
- Stepanov, Y.M.; Zavhorodnia, N.Y.; Yagmur, V.B.; Lukianenko, O.Y.; Zygalo, E.V. Association of nonalcoholic fatty liver disease with small intestine bacterial overgrowth in obese children. Wiad. Lek. 2019, 72, 350–356. [Google Scholar] [CrossRef]
- Troisi, J.; Pierri, L.; Landolfi, A.; Marciano, F.; Bisogno, A.; Belmonte, F.; Palladino, C.; Guercio Nuzio, S.; Campiglia, P.; Vajro, P. Urinary Metabolomics in Pediatric Obesity and NAFLD Identifies Metabolic Pathways/Metabolites Related to Dietary Habits and Gut-Liver Axis Perturbations. Nutrients 2017, 9, 485. [Google Scholar] [CrossRef]
- Belei, O.; Olariu, L.; Dobrescu, A.; Marcovici, T.; Marginean, O. The relationship between non-alcoholic fatty liver disease and small intestinal bacterial overgrowth among overweight and obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2017, 30, 1161–1168. [Google Scholar] [CrossRef]
- Kuang, L.; Zhou, W.; Jiang, Y. Association of small intestinal bacterial overgrowth with nonalcoholic fatty liver disease in children: A meta-analysis. PLoS ONE 2021, 16, e0260479. [Google Scholar] [CrossRef]
- Bakhshimoghaddam, F.; Alizadeh, M. Contribution of gut microbiota to nonalcoholic fatty liver disease: Pathways of mechanisms. Clin. Nutr. ESPEN 2021, 44, 61–68. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Masciana, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- GuimarAes, V.M.; Santos, V.N.; Borges, P.S.A.; JLR, D.E.F.; Grillo, P.; Parise, E.R. Peripheral Blood Endotoxin Levels Are Not Associated with Small Intestinal Bacterial Overgrowth in Nonalcoholic Fatty Liver Disease without Cirrhosis. Arq. Gastroenterol. 2020, 57, 471–476. [Google Scholar] [CrossRef]
- Gunnarsdottir, S.A.; Sadik, R.; Shev, S.; Simren, M.; Sjovall, H.; Stotzer, P.O.; Abrahamsson, H.; Olsson, R.; Bjornsson, E.S. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am. J. Gastroenterol. 2003, 98, 1362–1370. [Google Scholar] [CrossRef]
- Chesta, J.; Silva, M.; Thompson, L.; del Canto, E.; Defilippi, C. Bacterial overgrowth in small intestine in patients with liver cirrhosis. Rev. Med. Chile 1991, 119, 626–632. [Google Scholar]
- Maslennikov, R.; Pavlov, C.; Ivashkin, V. Small intestinal bacterial overgrowth in cirrhosis: Systematic review and meta-analysis. Hepatol. Int. 2018, 12, 567–576. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Efremova, I.; Poluektova, E.; Kudryavtseva, A.; Krasnov, G. Gut dysbiosis and small intestinal bacterial overgrowth as independent forms of gut microbiota disorders in cirrhosis. World J. Gastroenterol. 2022, 28, 1067–1077. [Google Scholar] [CrossRef]
- Ghosh, G.; Jesudian, A.B. Small Intestinal Bacterial Overgrowth in Patients With Cirrhosis. J. Clin. Exp. Hepatol. 2019, 9, 257–267. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Zhang, X.; Chen, W.; Tian, Y.; Yang, Q.; Yang, Y.; Pan, H.; Jiang, Z. Hepatic Encephalopathy in Cirrhotic Patients and Risk of Small Intestinal Bacterial Overgrowth: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2022, 2022, 2469513. [Google Scholar] [CrossRef]
- Kim, D.B.; Paik, C.N.; Sung, H.J.; Chung, W.C.; Lee, K.M.; Yang, J.M.; Choi, M.G. Breath hydrogen and methane are associated with intestinal symptoms in patients with chronic pancreatitis. Pancreatology 2015, 15, 514–518. [Google Scholar] [CrossRef]
- Signoretti, M.; Stigliano, S.; Valente, R.; Piciucchi, M.; Delle Fave, G.; Capurso, G. Small intestinal bacterial overgrowth in patients with chronic pancreatitis. J. Clin. Gastroenterol. 2014, 48 (Suppl. S1), S52–S55. [Google Scholar] [CrossRef]
- Ni Chonchubhair, H.M.; Bashir, Y.; Dobson, M.; Ryan, B.M.; Duggan, S.N.; Conlon, K.C. The prevalence of small intestinal bacterial overgrowth in non-surgical patients with chronic pancreatitis and pancreatic exocrine insufficiency (PEI). Pancreatology 2018, 18, 379–385. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, H.M.; He, F.; Li, B.Y.; Li, X.C. Association between acute pancreatitis and small intestinal bacterial overgrowth assessed by hydrogen breath test. World J. Gastroenterol. 2017, 23, 8591–8596. [Google Scholar] [CrossRef]
- Kim, D.B.; Paik, C.N.; Lee, J.M.; Kim, Y.J. Association between increased breath hydrogen methane concentration and prevalence of glucose intolerance in acute pancreatitis. J. Breath Res. 2020, 14, 026006. [Google Scholar] [CrossRef]
- El Kurdi, B.; Babar, S.; El Iskandarani, M.; Bataineh, A.; Lerch, M.M.; Young, M.; Singh, V.P. Factors That Affect Prevalence of Small Intestinal Bacterial Overgrowth in Chronic Pancreatitis: A Systematic Review, Meta-Analysis, and Meta-Regression. Clin. Transl. Gastroenterol. 2019, 10, e00072. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Cui, M.Y.; Jiang, Q.L.; Wang, J.J.; Fan, M.Y.; Lu, Y.Y. Characterization of Duodenal Microbiota in Patients with Acute Pancreatitis and Healthy Controls. Dig. Dis. Sci. 2023, 68, 3341–3353. [Google Scholar] [CrossRef]
- Lee, A.A.; Baker, J.R.; Wamsteker, E.J.; Saad, R.; DiMagno, M.J. Small Intestinal Bacterial Overgrowth Is Common in Chronic Pancreatitis and Associates With Diabetes, Chronic Pancreatitis Severity, Low Zinc Levels, and Opiate Use. Am. J. Gastroenterol. 2019, 114, 1163–1171. [Google Scholar] [CrossRef]
- Gyger, G.; Baron, M. Gastrointestinal manifestations of scleroderma: Recent progress in evaluation, pathogenesis, and management. Curr. Rheumatol. Rep. 2012, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, R.W. Gastrointestinal motility disorders in scleroderma. Arthritis Rheum. 1994, 37, 1265–1282. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Tandon, P.; Gohel, T.; Corrigan, M.L.; Coughlin, K.L.; Shatnawei, A.; Chatterjee, S.; Kirby, D.F. Gastrointestinal Manifestations, Malnutrition, and Role of Enteral and Parenteral Nutrition in Patients With Scleroderma. J. Clin. Gastroenterol. 2015, 49, 559–564. [Google Scholar] [CrossRef]
- Garcia-Collinot, G.; Madrigal-Santillan, E.O.; Martinez-Bencomo, M.A.; Carranza-Muleiro, R.A.; Jara, L.J.; Vera-Lastra, O.; Montes-Cortes, D.H.; Medina, G.; Cruz-Dominguez, M.P. Effectiveness of Saccharomyces boulardii and Metronidazole for Small Intestinal Bacterial Overgrowth in Systemic Sclerosis. Dig. Dis. Sci. 2020, 65, 1134–1143. [Google Scholar] [CrossRef]
- Morrisroe, K.; Baron, M.; Frech, T.; Nikpour, M. Small intestinal bacterial overgrowth in systemic sclerosis. J. Scleroderma Relat. Disord. 2020, 5, 33–39. [Google Scholar] [CrossRef]
- Sawadpanich, K.; Soison, P.; Chunlertrith, K.; Mairiang, P.; Sukeepaisarnjaroen, W.; Sangchan, A.; Suttichaimongkol, T.; Foocharoen, C. Prevalence and associated factors of small intestinal bacterial overgrowth among systemic sclerosis patients. Int. J. Rheum. Dis. 2019, 22, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.; De Palma, G.; Zou, H.; Bazzaz, A.H.Z.; Verdu, E.; Baker, B.; Pinto-Sanchez, M.I.; Khalidi, N.; Larche, M.J.; Beattie, K.A.; et al. Fecal microbiome differs between patients with systemic sclerosis with and without small intestinal bacterial overgrowth. J. Scleroderma Relat. Disord. 2021, 6, 290–298. [Google Scholar] [CrossRef]
- Shah, A.; Pakeerathan, V.; Jones, M.P.; Kashyap, P.C.; Virgo, K.; Fairlie, T.; Morrison, M.; Ghoshal, U.C.; Holtmann, G.J. Small Intestinal Bacterial Overgrowth Complicating Gastrointestinal Manifestations of Systemic Sclerosis: A Systematic Review and Meta-analysis. J. Neurogastroenterol. Motil. 2023, 29, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Fynne, L.; Worsoe, J.; Gregersen, T.; Schlageter, V.; Laurberg, S.; Krogh, K. Gastrointestinal transit in patients with systemic sclerosis. Scand. J. Gastroenterol. 2011, 46, 1187–1193. [Google Scholar] [CrossRef]
- Savarino, E.; Mei, F.; Parodi, A.; Ghio, M.; Furnari, M.; Gentile, A.; Berdini, M.; Di Sario, A.; Bendia, E.; Bonazzi, P.; et al. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology 2013, 52, 1095–1100. [Google Scholar] [CrossRef]
- Fiorentini, E.; Russo, E.; Amedei, A.; Bellando Randone, S. Fecal microbiome in systemic sclerosis, in search for the best candidate for microbiota-targeted therapy for small intestinal bacterial overgrowth control. J. Scleroderma Relat. Disord. 2022, 7, 163–167. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.Q.; Jiang, Z. Prevalence and predictors of small intestinal bacterial overgrowth in systemic sclerosis: A systematic review and meta-analysis. Clin. Rheumatol. 2021, 40, 3039–3051. [Google Scholar] [CrossRef]
- Tian, X.P.; Zhang, X. Gastrointestinal complications of systemic sclerosis. World J. Gastroenterol. 2013, 19, 7062–7068. [Google Scholar] [CrossRef]
- Marie, I.; Leroi, A.M.; Menard, J.F.; Levesque, H.; Quillard, M.; Ducrotte, P. Fecal calprotectin in systemic sclerosis and review of the literature. Autoimmun. Rev. 2015, 14, 547–554. [Google Scholar] [CrossRef]
- Polkowska-Pruszynska, B.; Gerkowicz, A.; Rawicz-Pruszynski, K.; Krasowska, D. The Role of Fecal Calprotectin in Patients with Systemic Sclerosis and Small Intestinal Bacterial Overgrowth (SIBO). Diagnostics 2020, 10, 587. [Google Scholar] [CrossRef]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef]
- Sandek, A.; Rauchhaus, M.; Anker, S.D.; von Haehling, S. The emerging role of the gut in chronic heart failure. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 632–639. [Google Scholar] [CrossRef]
- Anker, S.D.; von Haehling, S. Inflammatory mediators in chronic heart failure: An overview. Heart 2004, 90, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, Y.; Qi, B.; Cui, X.; Dong, X.; Wang, Y.; Han, X.; Li, F.; Shen, D.; Zhang, X.; et al. Association of Small Intestinal Bacterial Overgrowth With Heart Failure and Its Prediction for Short-Term Outcomes. J. Am. Heart Assoc. 2021, 10, e015292. [Google Scholar] [CrossRef] [PubMed]
- Mollar, A.; Marrachelli, V.G.; Nunez, E.; Monleon, D.; Bodi, V.; Sanchis, J.; Navarro, D.; Nunez, J. Bacterial metabolites trimethylamine N-oxide and butyrate as surrogates of small intestinal bacterial overgrowth in patients with a recent decompensated heart failure. Sci. Rep. 2021, 11, 6110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chu, M.; Wang, D.; Luo, Y.; Liu, Z.; Zhao, J. Risk factors for small intestinal bacterial overgrowth in patients with acute ischaemic stroke. J. Med. Microbiol. 2023, 72, 001666. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, L.; Xie, N.C.; Xu, H.L.; Lian, Y.J. Association between small-intestinal bacterial overgrowth and deep vein thrombosis in patients with spinal cord injuries. J. Thromb. Haemost. 2017, 15, 304–311. [Google Scholar] [CrossRef]
- He, Z.; Ding, R.; Wu, F.; Wu, Z.; Liang, C. Excess Alcohol Consumption: A Potential Mechanism Behind the Association Between Small Intestinal Bacterial Overgrowth and Coronary Artery Disease. Dig. Dis. Sci. 2018, 63, 3516–3517. [Google Scholar] [CrossRef]
- Dong, C.; Wang, G.; Xian, R.; Li, C.; Wang, S.; Cui, L. Association between Small Intestinal Bacterial Overgrowth and Subclinical Atheromatous Plaques. J. Clin. Med. 2022, 12, 314. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Pompili, M.; Di Stasio, E.; Zocco, M.A.; Gasbarrini, A.; Flore, R. Subclinical atherosclerosis is linked to small intestinal bacterial overgrowth via vitamin K2-dependent mechanisms. World J. Gastroenterol. 2017, 23, 1241–1249. [Google Scholar] [CrossRef]
- Ojetti, V.; Pitocco, D.; Scarpellini, E.; Zaccardi, F.; Scaldaferri, F.; Gigante, G.; Gasbarrini, G.; Ghirlanda, G.; Gasbarrini, A. Small bowel bacterial overgrowth and type 1 diabetes. Eur. Rev. Med. Pharmacol. Sci. 2009, 13, 419–423. [Google Scholar]
- Rana, S.; Bhansali, A.; Bhadada, S.; Sharma, S.; Kaur, J.; Singh, K. Orocecal transit time and small intestinal bacterial overgrowth in type 2 diabetes patients from North India. Diabetes Technol. Ther. 2011, 13, 1115–1120. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.Q. The prevalence of small intestinal bacterial overgrowth in diabetes mellitus: A systematic review and meta-analysis. Aging 2022, 14, 975–988. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Stratigou, T.; Tsagarakis, S. Microbiome and diabetes: Where are we now? Diabetes Res. Clin. Pract. 2018, 146, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, K.E.; Cummings, B.P. Rethinking Bile Acid Metabolism and Signaling for Type 2 Diabetes Treatment. Curr. Diabetes Rep. 2018, 18, 109. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Zhang, J.; Sun, Z.; Ban, Y.; Wang, B.; Hou, X.; Cai, Y.; Li, J.; Wang, M.; et al. Application of methane and hydrogen-based breath test in the study of gestational diabetes mellitus and intestinal microbes. Diabetes Res. Clin. Pract. 2021, 176, 108818. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.H.; Mu, B.; Pan, D.; Shi, Y.N.; Yuan, J.H.; Guan, Y.; Li, W.; Zhu, X.Y.; Guo, L. Association between small intestinal bacterial overgrowth and beta-cell function of type 2 diabetes. J. Int. Med. Res. 2020, 48, 300060520937866. [Google Scholar] [CrossRef] [PubMed]
- Kvit, K.B.; Kharchenko, N.V.; Kharchenko, V.V.; Chornenka, O.I.; Chornovus, R.I.; Dorofeeva, U.S.; Draganchuk, O.B.; Slaba, O.M. The role of small intestinal bacterial overgrowth in the pathogenesis of hyperlipidemia. Wiad. Lek. 2019, 72, 645–649. [Google Scholar] [CrossRef]
- John, G.K.; Mullin, G.E. The Gut Microbiome and Obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef]
- Sun, L.; Ma, L.; Ma, Y.; Zhang, F.; Zhao, C.; Nie, Y. Insights into the role of gut microbiota in obesity: Pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell 2018, 9, 397–403. [Google Scholar] [CrossRef]
- Nawrocka, M.; Szulińska, M.; Bogdański, P. Rola mikroflory jelitowej w patogenezie i leczeniu otyłości oraz zespołu metabolicznego. Forum Zaburzeń Metab. 2015, 6, 95–102. [Google Scholar]
- Wijarnpreecha, K.; Werlang, M.E.; Watthanasuntorn, K.; Panjawatanan, P.; Cheungpasitporn, W.; Gomez, V.; Lukens, F.J.; Ungprasert, P. Obesity and Risk of Small Intestine Bacterial Overgrowth: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2020, 65, 1414–1422. [Google Scholar] [CrossRef]
- Roland, B.C.; Lee, D.; Miller, L.S.; Vegesna, A.; Yolken, R.; Severance, E.; Prandovszky, E.; Zheng, X.E.; Mullin, G.E. Obesity increases the risk of small intestinal bacterial overgrowth (SIBO). Neurogastroenterol. Motil. 2018, 30, e13199. [Google Scholar] [CrossRef]
- Madrid, A.M.; Poniachik, J.; Quera, R.; Defilippi, C. Small intestinal clustered contractions and bacterial overgrowth: A frequent finding in obese patients. Dig. Dis. Sci. 2011, 56, 155–160. [Google Scholar] [CrossRef]
- Ierardi, E.; Losurdo, G.; Sorrentino, C.; Giorgio, F.; Rossi, G.; Marinaro, A.; Romagno, K.R.; Di Leo, A.; Principi, M. Macronutrient intakes in obese subjects with or without small intestinal bacterial overgrowth: An alimentary survey. Scand. J. Gastroenterol. 2016, 51, 277–280. [Google Scholar] [CrossRef]
- Mushref, M.A.; Srinivasan, S. Effect of high fat-diet and obesity on gastrointestinal motility. Ann. Transl. Med. 2013, 1, 14. [Google Scholar]
- Xing, J.; Chen, J.D. Alterations of gastrointestinal motility in obesity. Obes. Res. 2004, 12, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, L.; Dumitrascu, D.L. Gastrointestinal motility disorders in endocrine diseases. Pol. Arch. Med. Wewn. 2011, 121, 129–136. [Google Scholar] [CrossRef]
- Brechmann, T.; Sperlbaum, A.; Schmiegel, W. Levothyroxine therapy and impaired clearance are the strongest contributors to small intestinal bacterial overgrowth: Results of a retrospective cohort study. World J. Gastroenterol. 2017, 23, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, X.; Ahmed, A.; Wu, D.; Liu, L.; Qiu, J.; Yan, Y.; Jin, M.; Xin, Y. Gut microbe analysis between hyperthyroid and healthy individuals. Curr. Microbiol. 2014, 69, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Penhale, W.J.; Young, P.R. The influence of the normal microbial flora on the susceptibility of rats to experimental autoimmune thyroiditis. Clin. Exp. Immunol. 1988, 72, 288–292. [Google Scholar]
- Lauritano, E.C.; Bilotta, A.L.; Gabrielli, M.; Scarpellini, E.; Lupascu, A.; Laginestra, A.; Novi, M.; Sottili, S.; Serricchio, M.; Cammarota, G.; et al. Association between hypothyroidism and small intestinal bacterial overgrowth. J. Clin. Endocrinol. Metab. 2007, 92, 4180–4184. [Google Scholar] [CrossRef] [PubMed]
- Konrad, P.; Chojnacki, J.; Kaczka, A.; Pawlowicz, M.; Rudnicki, C.; Chojnacki, C. Thyroid dysfunction in patients with small intestinal bacterial overgrowth. Pol. Merkur. Lek. 2018, 44, 15–18. [Google Scholar] [PubMed]
- Wang, B.; Xu, Y.; Hou, X.; Li, J.; Cai, Y.; Hao, Y.; Ouyang, Q.; Wu, B.; Sun, Z.; Zhang, M.; et al. Small Intestinal Bacterial Overgrowth in Subclinical Hypothyroidism of Pregnant Women. Front. Endocrinol. 2021, 12, 604070. [Google Scholar] [CrossRef]
- Rukavina Mikusic, N.L.; Kouyoumdzian, N.M.; Choi, M.R. Gut microbiota and chronic kidney disease: Evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pflugers Arch. 2020, 472, 303–320. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Dure-Smith, B.; Miller, R.; Mirahmadi, M.K. Pathology of gastrointestinal tract in chronic hemodialysis patients: An autopsy study of 78 cases. Am. J. Gastroenterol. 1985, 80, 608–611. [Google Scholar] [PubMed]
- Barrios, C.; Beaumont, M.; Pallister, T.; Villar, J.; Goodrich, J.K.; Clark, A.; Pascual, J.; Ley, R.E.; Spector, T.D.; Bell, J.T.; et al. Gut-Microbiota-Metabolite Axis in Early Renal Function Decline. PLoS ONE 2015, 10, e0134311. [Google Scholar] [CrossRef]
- Strid, H.; Simren, M.; Stotzer, P.O.; Ringstrom, G.; Abrahamsson, H.; Bjornsson, E.S. Patients with chronic renal failure have abnormal small intestinal motility and a high prevalence of small intestinal bacterial overgrowth. Digestion 2003, 67, 129–137. [Google Scholar] [CrossRef]
- Nallu, A.; Sharma, S.; Ramezani, A.; Muralidharan, J.; Raj, D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl. Res. 2017, 179, 24–37. [Google Scholar] [CrossRef]
- Satoh, M.; Hayashi, H.; Watanabe, M.; Ueda, K.; Yamato, H.; Yoshioka, T.; Motojima, M. Uremic toxins overload accelerates renal damage in a rat model of chronic renal failure. Nephron Exp. Nephrol. 2003, 95, e111–e118. [Google Scholar] [CrossRef]
- Thadhani, R.; Pascual, M.; Bonventre, J.V. Acute renal failure. N. Engl. J. Med. 1996, 334, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, C.J.; Go, Y.S.; Lee, H.Y.; Kim, M.G.; Oh, S.W.; Cho, W.Y.; Im, S.H.; Jo, S.K. Intestinal microbiota control acute kidney injury severity by immune modulation. Kidney Int. 2020, 98, 932–946. [Google Scholar] [CrossRef]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Magdaleno-Tapial, J.; Lopez-Marti, C.; Garcia-Legaz-Martinez, M.; Martinez-Domenech, A.; Partarrieu-Mejias, F.; Casanova-Esquembre, A.; Lorca-Sprohnle, J.; Labrandero-Hoyos, C.; Penuelas-Leal, R.; Sierra-Talamantes, C.; et al. Contact Allergy in Patients with Rosacea. Actas Dermosifiliogr. 2022, 113, 550–554. [Google Scholar] [CrossRef]
- Daou, H.; Paradiso, M.; Hennessy, K.; Seminario-Vidal, L. Rosacea and the Microbiome: A Systematic Review. Dermatol. Ther. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Wang, F.Y.; Chi, C.C. Rosacea, Germs, and Bowels: A Review on Gastrointestinal Comorbidities and Gut-Skin Axis of Rosacea. Adv. Ther. 2021, 38, 1415–1424. [Google Scholar] [CrossRef]
- Parodi, A.; Paolino, S.; Greco, A.; Drago, F.; Mansi, C.; Rebora, A.; Parodi, A.; Savarino, V. Small intestinal bacterial overgrowth in rosacea: Clinical effectiveness of its eradication. Clin. Gastroenterol. Hepatol. 2008, 6, 759–764. [Google Scholar] [CrossRef]
- Branisteanu, D.E.; Cojocaru, C.; Diaconu, R.; Porumb, E.A.; Alexa, A.I.; Nicolescu, A.C.; Brihan, I.; Bogdanici, C.M.; Branisteanu, G.; Dimitriu, A.; et al. Update on the etiopathogenesis of psoriasis (Review). Exp. Ther. Med. 2022, 23, 201. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Ciccarese, G.; Cordara, V.; Paudice, M.; Herzum, A.; Parodi, A. Oral psoriasis and SIBO: Is there a link? J. Eur. Acad. Dermatol. Venereol. 2018, 32, e368–e369. [Google Scholar] [CrossRef]
- Drago, F.; Ciccarese, G.; Indemini, E.; Savarino, V.; Parodi, A. Psoriasis and small intestine bacterial overgrowth. Int. J. Dermatol. 2018, 57, 112–113. [Google Scholar] [CrossRef]
- Lombardi, N.; Moneghini, L.; Varoni, E.M.; Lodi, G. Nodular migratory tongue lesions: Atypical geographic tongue or a new entity? Oral. Dis. 2023, 29, 1791–1794. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef]
- Sim, J.; Wang, Y.T.; Mamun, K.; Tay, S.Y.; Doshi, K.; Hameed, S.; Ting, S.K. Assessment of small intestinal bacterial overgrowth in Alzheimer’s disease. Acta Neurol. Taiwanica 2021, 30, 102–107. [Google Scholar]
- Kowalski, K.; Mulak, A. Small intestinal bacterial overgrowth in Alzheimer’s disease. J. Neural Transm. 2022, 129, 75–83. [Google Scholar] [CrossRef]
- Li, X.; Feng, X.; Jiang, Z.; Jiang, Z. Association of small intestinal bacterial overgrowth with Parkinson’s disease: A systematic review and meta-analysis. Gut Pathog. 2021, 13, 25. [Google Scholar] [CrossRef]
- Niu, X.L.; Liu, L.; Song, Z.X.; Li, Q.; Wang, Z.H.; Zhang, J.L.; Li, H.H. Prevalence of small intestinal bacterial overgrowth in Chinese patients with Parkinson’s disease. J. Neural Transm. 2016, 123, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Bove, F.; Gabrielli, M.; Petracca, M.; Zocco, M.A.; Ragazzoni, E.; Barbaro, F.; Piano, C.; Fortuna, S.; Tortora, A.; et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2013, 28, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Mahadeva, S.; Thalha, A.M.; Gibson, P.R.; Kiew, C.K.; Yeat, C.M.; Ng, S.W.; Ang, S.P.; Chow, S.K.; Tan, C.T.; et al. Small intestinal bacterial overgrowth in Parkinson’s disease. Park. Relat. Disord. 2014, 20, 535–540. [Google Scholar] [CrossRef]
- van Kessel, S.P.; El Aidy, S. Contributions of Gut Bacteria and Diet to Drug Pharmacokinetics in the Treatment of Parkinson’s Disease. Front. Neurol. 2019, 10, 1087. [Google Scholar] [CrossRef]
- Danau, A.; Dumitrescu, L.; Lefter, A.; Tulba, D.; Popescu, B.O. Small Intestinal Bacterial Overgrowth as Potential Therapeutic Target in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 11663. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Duan, Y.; Han, X.; Dong, H.; Geng, J. Prevalence of Small Intestinal Bacterial Overgrowth in Multiple Sclerosis: A Case-Control Study from China. J. Neuroimmunol. 2016, 301, 83–87. [Google Scholar] [CrossRef]
- Geier, D.A.; Kern, J.K.; Geier, M.R. A Comparison of the Autism Treatment Evaluation Checklist (ATEC) and the Childhood Autism Rating Scale (CARS) for the Quantitative Evaluation of Autism. J. Ment. Health Res. Intellect. Disabil. 2013, 6, 255–267. [Google Scholar] [CrossRef]
- Finegold, S.M.; Summanen, P.H.; Downes, J.; Corbett, K.; Komoriya, T. Detection of Clostridium perfringens toxin genes in the gut microbiota of autistic children. Anaerobe 2017, 45, 133–137. [Google Scholar] [CrossRef]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.M.; Zhang, Y.Q.; Zhang, J.; Lu, N.; Liu, N. Hydrogen breath test to detect small intestinal bacterial overgrowth: A prevalence case-control study in autism. Eur. Child Adolesc. Psychiatry 2018, 27, 233–240. [Google Scholar] [CrossRef]
- Wei, F.; Zhou, L.; Wang, Q.; Zheng, G.; Su, S. Effect of Compound Lactic Acid Bacteria Capsules on the Small Intestinal Bacterial Overgrowth in Patients with Depression and Diabetes: A Blinded Randomized Controlled Clinical Trial. Dis. Markers 2022, 2022, 6721695. [Google Scholar] [CrossRef]
- Kossewska, J.; Bierlit, K.; Trajkovski, V. Personality, Anxiety, and Stress in Patients with Small Intestine Bacterial Overgrowth Syndrome. The Polish Preliminary Study. Int. J. Environ. Res. Public Health 2022, 20, 93. [Google Scholar] [CrossRef]
- Chojnacki, C.; Konrad, P.; Blonska, A.; Medrek-Socha, M.; Przybylowska-Sygut, K.; Chojnacki, J.; Poplawski, T. Altered Tryptophan Metabolism on the Kynurenine Pathway in Depressive Patients with Small Intestinal Bacterial Overgrowth. Nutrients 2022, 14, 3217. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Poplawski, T.; Konrad, P.; Fila, M.; Blasiak, J.; Chojnacki, J. Antimicrobial treatment improves tryptophan metabolism and mood of patients with small intestinal bacterial overgrowth. Nutr. Metab. 2022, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, A.; Wojtowicz, J.; Walkowiak, J. Small intestine bacterial overgrowth is frequent in cystic fibrosis: Combined hydrogen and methane measurements are required for its detection. Acta Biochim. Pol. 2009, 56, 631–634. [Google Scholar] [CrossRef] [PubMed]
- De Lisle, R.C. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G104–G111. [Google Scholar] [CrossRef] [PubMed]
- Furnari, M.; De Alessandri, A.; Cresta, F.; Haupt, M.; Bassi, M.; Calvi, A.; Haupt, R.; Bodini, G.; Ahmed, I.; Bagnasco, F.; et al. The role of small intestinal bacterial overgrowth in cystic fibrosis: A randomized case-controlled clinical trial with rifaximin. J. Gastroenterol. 2019, 54, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Verrecchia, E.; Sicignano, L.L.; La Regina, M.; Nucera, G.; Patisso, I.; Cerrito, L.; Montalto, M.; Gasbarrini, A.; Manna, R. Small Intestinal Bacterial Overgrowth Affects the Responsiveness to Colchicine in Familial Mediterranean Fever. Mediat. Inflamm. 2017, 2017, 7461426. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, H.; Zhang, P.; Xu, L.; Tian, Z. Association between small intestinal bacterial overgrowth and toll-like receptor 4 in patients with pancreatic carcinoma and cholangiocarcinoma. Turk. J. Gastroenterol. 2019, 30, 177–183. [Google Scholar] [CrossRef]
- Liang, S.; Xu, L.; Zhang, D.; Wu, Z. Effect of probiotics on small intestinal bacterial overgrowth in patients with gastric and colorectal cancer. Turk. J. Gastroenterol. 2016, 27, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liu, Y.; Zhang, D.; Li, Y.; Xu, L. Prevalence and treatment of small intestinal bacterial overgrowth in postoperative patients with colorectal cancer. Mol. Clin. Oncol. 2016, 4, 883–887. [Google Scholar] [CrossRef]
- Savva, K.V.; Hage, L.; Belluomo, I.; Gummet, P.; Boshier, P.R.; Peters, C.J. Assessment of the Burden of Small Intestinal Bacterial Overgrowth (SIBO) in Patients After Oesophagogastric (OG) Cancer Resection. J. Gastrointest. Surg. 2022, 26, 924–926. [Google Scholar] [CrossRef]
- Zhou, D.X.; Ma, Y.J.; Chen, G.Y.; Gao, X.; Yang, L. Relationship of TLR2 and TLR4 expressions on the surface of peripheral blood mononuclear cells to small intestinal bacteria overgrowth in patients with hepatocellular carcinoma. Chin. J. Hepatol. 2019, 27, 286–290. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roszkowska, P.; Klimczak, E.; Ostrycharz, E.; Rączka, A.; Wojciechowska-Koszko, I.; Dybus, A.; Cheng, Y.-H.; Yu, Y.-H.; Mazgaj, S.; Hukowska-Szematowicz, B. Small Intestinal Bacterial Overgrowth (SIBO) and Twelve Groups of Related Diseases—Current State of Knowledge. Biomedicines 2024, 12, 1030. https://doi.org/10.3390/biomedicines12051030
Roszkowska P, Klimczak E, Ostrycharz E, Rączka A, Wojciechowska-Koszko I, Dybus A, Cheng Y-H, Yu Y-H, Mazgaj S, Hukowska-Szematowicz B. Small Intestinal Bacterial Overgrowth (SIBO) and Twelve Groups of Related Diseases—Current State of Knowledge. Biomedicines. 2024; 12(5):1030. https://doi.org/10.3390/biomedicines12051030
Chicago/Turabian StyleRoszkowska, Paulina, Emilia Klimczak, Ewa Ostrycharz, Aleksandra Rączka, Iwona Wojciechowska-Koszko, Andrzej Dybus, Yeong-Hsiang Cheng, Yu-Hsiang Yu, Szymon Mazgaj, and Beata Hukowska-Szematowicz. 2024. "Small Intestinal Bacterial Overgrowth (SIBO) and Twelve Groups of Related Diseases—Current State of Knowledge" Biomedicines 12, no. 5: 1030. https://doi.org/10.3390/biomedicines12051030
APA StyleRoszkowska, P., Klimczak, E., Ostrycharz, E., Rączka, A., Wojciechowska-Koszko, I., Dybus, A., Cheng, Y.-H., Yu, Y.-H., Mazgaj, S., & Hukowska-Szematowicz, B. (2024). Small Intestinal Bacterial Overgrowth (SIBO) and Twelve Groups of Related Diseases—Current State of Knowledge. Biomedicines, 12(5), 1030. https://doi.org/10.3390/biomedicines12051030







